Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome—A Review
Abstract
1. Introduction
1.1. Pharmacokinetics and Bioavailability of Ginger Compounds
1.2. Antioxidant Activity/Capacity of Ginger Compounds
2. Materials and Methods
3. Introduction to thermal processing techniques
3.1. Cooking
3.1.1. Traditional Cooking
3.1.2. Stewing
3.2. Frying
3.2.1. Traditional Cooking
3.2.2. Stir-Frying
3.2.3. Blanching
3.3. Steaming (Hot Vapor Processing)
3.4. Drying
3.4.1. Drying in the Sun
3.4.2. Other Drying Methods
3.4.3. Oven and Hot-Air Drying
3.4.4. Blanching as Pretreatment and Drying in the Drying Chamber
3.5. Microwaves
Intermittent Microwave-Convection Drying
3.6. Carbonization
3.7. Other
3.7.1. Freeze-Dried/Lyophilization
3.7.2. Infrared Drying
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.B.; Czaczyk, K. The Industrial Potential of Herbs and Spices - a Mini Review. Acta Sci. Pol. Technol. Aliment 2016, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A Small Plant with Big Benefits: Fenugreek (Trigonella Foenum-Graecum Linn.) for Disease Prevention and Health Promotion. Mol. Nutr. Food. Res. 2017, 61. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health Benefits of Herbs and Spices: The Past, the Present, the Future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Haniadka, R.; Saldanha, E.; Sunita, V.; Palatty, P.L.; Fayad, R.; Baliga, M.S. A Review of the Gastroprotective Effects of Ginger (Zingiber Officinale Roscoe). Food Funct. 2013, 4, 845–855. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.-A.P.M.; Santos, J.V.D.O.; Filho, J.W.G.D.O.; Ferreira, J.R.D.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and Therapeutic Potential of Ginger (Zingiber Officinale) Extract and [6]-Gingerol in Cancer: A Comprehensive Review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef]
- Santos, S.H.S. Plants and Their Phytochemicals Bioactive Compounds: Characterization and Role in Chronic Diseases. Protein Pept. Lett. 2021, 28, 723–724. [Google Scholar] [CrossRef]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- De La Torre, J.E.; Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Spice Use in Food: Properties and Benefits. Crit. Rev. Food Sci. Nutr. 2017, 57, 1078–1088. [Google Scholar] [CrossRef]
- Patra, K.; Jana, S.; Mandal, D.P.; Bhattacharjee, S. Evaluation of the Antioxidant Activity of Extracts and Active Principles of Commonly Consumed Indian Spices. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-C.; Kwon, S.-J.; Eom, S.-H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia Faba L.) Leaves and Seeds. Antioxidants 2021, 10, 1207. [Google Scholar] [CrossRef]
- Zagórska, J.; Czernicka-Boś, L.; Kukula-Koch, W.; Szalak, R.; Koch, W. Impact of Thermal Processing on the Composition of Secondary Metabolites of Ginger Rhizome—A Review. Foods 2022, 11, 3484. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S. Processed Gingers: Current and Prospective Use in Food, Cosmetic, and Pharmaceutical Industry. Recent Pat. Food Nutr. Agric. 2019, 10, 20–26. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis Rhizoma: A Comprehensive Review on the Ginger Effect and Efficacy Profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef]
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis Rhizoma Recens: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement Altern. Med. 2021, 2021, 6668990. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber Officinale Rosc.) and Its Bioactive Components Are Potential Resources for Health Beneficial Agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Bekkouch, O.; Harnafi, M.; Touiss, I.; Khatib, S.; Harnafi, H.; Alem, C.; Amrani, S. In Vitro Antioxidant and In Vivo Lipid-Lowering Properties of Zingiber Officinale Crude Aqueous Extract and Methanolic Fraction: A Follow-Up Study. Evid. Based Complement Altern. Med. 2019, 2019, 9734390. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Lawal, B.; Abubakar, A.N.; Berinyuy, E.B.; Omonije, Y.O.; Umar, S.I.; Shebe, M.N.; Alhaji, Y.M. In-Vitro Antioxidants, Antimicrobial and Toxicological Evaluation of Nigerian Zingiber Officinale. Clin. Phytosci. 2018, 4, 12. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger Rhizomes (Zingiber Officinale): A Spice with Multiple Health Beneficial Potentials. PharmaNutrition 2017, 5. [Google Scholar] [CrossRef]
- Walstab, J.; Krüger, D.; Stark, T.; Hofmann, T.; Demir, I.E.; Ceyhan, G.O.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and Its Pungent Constituents Non-Competitively Inhibit Activation of Human Recombinant and Native 5-HT3 Receptors of Enteric Neurons. Neurogastroenterol. Motil. 2013, 25, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial Effects of Ginger Zingiber Officinale Roscoe on Obesity and Metabolic Syndrome: A Review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber Officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic Potential of Ginger and Its Compounds in Age-Related Neurological Disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber Officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zaki, L.; KarimiPourSaryazdi, A.; Tavakoli, P.; Tavajjohi, A.; Poursalehi, R.; Delavari, H.; Ghaffarifar, F. Efficacy of Green Synthesized Silver Nanoparticles via Ginger Rhizome Extract against Leishmania Major in Vitro. PLoS ONE 2021, 16, e0255571. [Google Scholar] [CrossRef]
- Pagano, E.; Souto, E.B.; Durazzo, A.; Sharifi-Rad, J.; Lucarini, M.; Souto, S.B.; Salehi, B.; Zam, W.; Montanaro, V.; Lucariello, G.; et al. Ginger (Zingiber Officinale Roscoe) as a Nutraceutical: Focus on the Metabolic, Analgesic, and Antiinflammatory Effects. Phytother Res 2020. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, N.; Mi, S. Plasma Pharmacokinetics and Tissue Distribution of [6]-Gingerol in Rats. Biopharm. Drug Dispos. 2008, 29, 529–537. [Google Scholar] [CrossRef]
- Levita, J.; Syafitri, D.M.; Supu, R.D.; Mutakin, M.; Megantara, S.; Febrianti, M.; Diantini, A. Pharmacokinetics of 10-Gingerol and 6-Shogaol in the Plasma of Healthy Subjects Treated with Red Ginger (Zingiber Officinale Var. Rubrum) Suspension. Biomed. Rep. 2018, 9, 474–482. [Google Scholar] [CrossRef]
- Li, L.-L.; Cui, Y.; Guo, X.-H.; Ma, K.; Tian, P.; Feng, J.; Wang, J.-M. Pharmacokinetics and Tissue Distribution of Gingerols and Shogaols from Ginger (Zingiber Officinale Rosc.) in Rats by UPLC−Q-Exactive−HRMS. Molecules 2019, 24, 512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, Q.; Yang, Q.; Cao, X.; Li, Q.; Shi, F.; Tong, S.S.; Feng, C.; Yu, Q.; Yu, J.; et al. A Novel Formulation of [6]-Gingerol: Proliposomes with Enhanced Oral Bioavailability and Antitumor Effect. Int. J. Pharm. 2018, 535, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lamela, C.; Franco, I.; Falqué, E. Impact of High-Pressure Processing on Antioxidant Activity during Storage of Fruits and Fruit Products: A Review. Molecules 2021, 26, 5265. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Makanjuola, S.A. Influence of Particle Size and Extraction Solvent on Antioxidant Properties of Extracts of Tea, Ginger, and Tea-Ginger Blend. Food Sci. Nutr. 2017, 5, 1179–1185. [Google Scholar] [CrossRef]
- Marak, N.R.; Malemnganbi, C.C.; Marak, C.R.; Mishra, L.K. Functional and Antioxidant Properties of Cookies Incorporated with Foxtail Millet and Ginger Powder. J. Food Sci. Technol. 2019, 56, 5087–5096. [Google Scholar] [CrossRef]
- Gunasena, B.V.A.P.; Rajapakse, R.P.N.P. Effect of Cooking Time and Cooking Temperature on Antioxidant Activity and Antimicrobial Activity of Cinnamon, Garlic, Ginger and Turmeric. Message Dean Fac. Agric. 2015. Available online: https://www.researchgate.net/publication/340933099_Effect_of_cooking_time_and_cooking_temperature_on_antioxidant_activity_and_antimicrobial_activity_of_cinnamon_garlic_ginger_and_turmeric (accessed on 17 November 2022).
- Koch, W.; Kukula-Koch, W.; Dziedzic, M.; Glowniak, K.; Asakawa, Y. Influence of Thermal Processing and in Vitro Digestion on the Antioxidant Potential of Ginger and Ginger Containing Products. Nat. Prod. Commun. 2016, 11, 1153–1156. [Google Scholar] [CrossRef]
- Chohan, M.; Forster-Wilkins, G.; Opara, E.I. Determination of the Antioxidant Capacity of Culinary Herbs Subjected to Various Cooking and Storage Processes Using the ABTS(*+) Radical Cation Assay. Plant Foods Hum. Nutr. 2008, 63, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Widelski, J.; Koch, W.; Głowniak, K. HPLC, Two-Dimensional TLC Determination of Phenolic Content, and an In Vitro Perspective to Antioxidant Potential of Euonymus Verrucosus Scop. Extracts. Acta Chromatogr. 2015. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical Characterization and Antioxidant Activities Comparison in Fresh, Dried, Stir-Frying and Carbonized Ginger. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kushwaha, R.; Goyal, A.; Tanwar, B.; Kaur, J. Process Optimization for the Preparation of Antioxidant Rich Ginger Candy Using Beetroot Pomace Extract. Food Chem. 2018, 245, 168–177. [Google Scholar] [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong, S.Y.; Lee, Y.-G.; Shim, J.H.; Yasmin, T.; Kim, N.W.; Koo, Y.T.; et al. Steamed Ginger May Enhance Insulin Secretion through KATP Channel Closure in Pancreatic β-Cells Potentially by Increasing 1-Dehydro-6-Gingerdione Content. Nutrients 2020, 12, 324. [Google Scholar] [CrossRef]
- Takahashi, M.; Inouye, S.; Abe, S. Anti-Candida and Radical Scavenging Activities of Essential Oils and Oleoresins of Zingiber Officinale Roscoe and Essential Oils of Other Plants Belonging to the Family Zingiberaceae. Drug Discov. Ther. 2011, 5, 238–245. [Google Scholar] [CrossRef]
- Teng, H.; Seuseu, K.T.; Lee, W.-Y.; Chen, L. Comparing the Effects of Microwave Radiation on 6-Gingerol and 6-Shogaol from Ginger Rhizomes (Zingiber Officinale Rosc). PLoS ONE 2019, 14, e0214893. [Google Scholar] [CrossRef]
- Amoah, R.E.; Kalakandan, S.; Wireko-Manu, F.D.; Oduro, I.; Saalia, F.K.; Owusu, E. The Effect of Vinegar and Drying (Solar and Open Sun) on the Microbiological Quality of Ginger (ZINGIBER OFFICINALE ROSCOE) Rhizomes. Food Sci. Nutr. 2020, 8, 6112–6119. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Khanprom, I.; Butkhup, L. Stability of Phytochemicals and Antioxidant Properties in Ginger (Zingiber Officinale Roscoe) Rhizome with Different Drying Methods. J. Herbs SpicesMed. Plants 2011, 17, 361–374. [Google Scholar] [CrossRef]
- Gümüşay, Ö.A.; Borazan, A.A.; Ercal, N.; Demirkol, O. Drying Effects on the Antioxidant Properties of Tomatoes and Ginger. Food Chem 2015, 173, 156–162. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially Processed Dry Ginger (Zingiber Officinale): Composition and Effects on LPS-Stimulated PGE2 Production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Shanker, R.; Srivastava, J.; Vankar, P.S. Change in Antioxidant Activity of Spices Turmeric and Ginger on Heat Treatment. EJEAFChe 2006, 5, 1313–1317. [Google Scholar]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of Different Drying Methods on Chinese Ginger (Zingiber Officinale Roscoe): Changes in Volatiles, Chemical Profile, Antioxidant Properties, and Microstructure. Food Chem. 2016, 197 Pt B, 1292–1300. [Google Scholar] [CrossRef]
- Khatun, M.; Eguchi, S.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effect of Thermal Treatment on Radical-Scavenging Activity of Some Spices. Food Sci. Technol. Res. 2006, 12, 178–185. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Phowong, C.; Jaisut, D.; Stencl, J. Effects of Pretreatments and Drying Temperatures on Drying Characteristics, Antioxidant Properties and Color of Ginger Slice. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2014, 62, 1125–1134. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-B.; Choi, A.-J.; Oh, J.-M. Zingiber Officinale Extract (ZOE) Incorporated with Layered Double Hydroxide Hybrid through Reconstruction to Preserve Antioxidant Activity of ZOE against Ultrasound and Microwave Irradiation. Nanomaterials 2019, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Murtijaya, J. Antioxidant Properties of Phyllanthus Amarus Extracts as Affected by Different Drying Methods. LWT—Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Kubra, R.; Rao, L. Effect of Microwave Drying on the Phytochemical Composition of Volatiles of Ginger. Int. J. Food Sci. Technol. 2011, 47, 53–60. [Google Scholar] [CrossRef]
- Ding, S.; An, K.; Zhao, C.P.; Li, Y.; Guo, Y.; Wang, Z. Effect of Drying Methods on Volatiles of Chinese Ginger (Zingiber Officinale Roscoe). Food Bioprod. Process. 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Gan, H.; Charters, E.; Driscoll, R.; Srzednicki, G. Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric. Horticulturae 2017, 3, 13. [Google Scholar] [CrossRef]
- Salem, M.A.; Zayed, A.; Alseekh, S.; Fernie, A.R.; Giavalisco, P. The Integration of MS-Based Metabolomics and Multivariate Data Analysis Allows for Improved Quality Assessment of Zingiber Officinale Roscoe. Phytochemistry 2021, 190, 112843. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Baranowska, M.; Namieśnik, J.; Bartoszek, A. Determination of Antioxidant activity of Phytochemicals in Cellular Models by Fluorescence/Luminescence Methods. Postepy Hig. Med. Dosw. 2017, 71, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cred turmeric (Curcuma longa) rhizomes. Ind. Crop. and Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Dahmke, I.N.; Boettcher, S.P.; Groh, M.; Mahlknecht, U. Cooking enhances curcumin anti-cancerogenic activity through pyrolytic formation of “deketene curcumin”. Food Chem. 2014, 151, 514–519. [Google Scholar] [CrossRef]
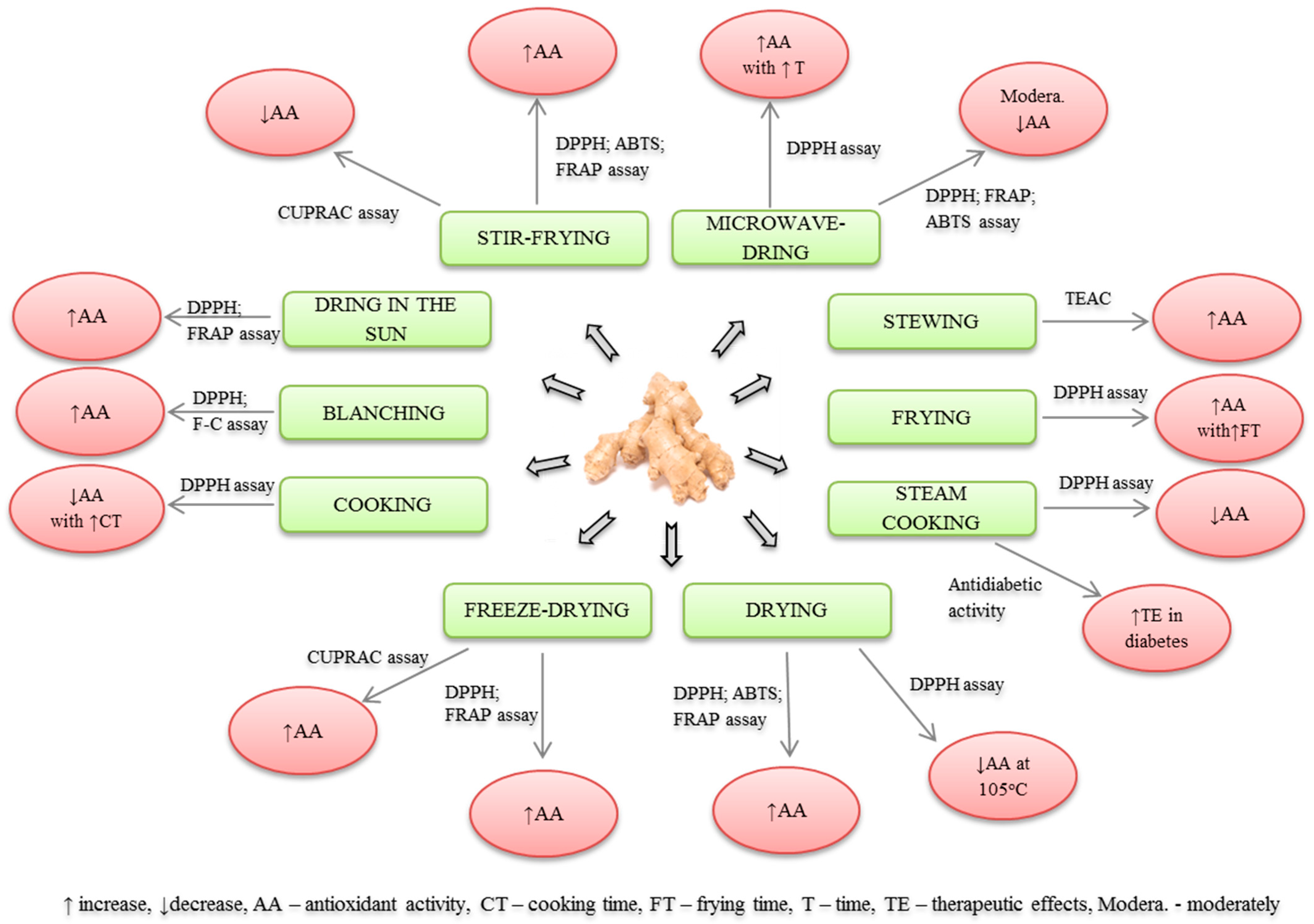
| Lp | Type of Processing | Processing Conditions | Tested Activity | Results | Reference |
|---|---|---|---|---|---|
| 1 | Cooking | 10, 20 and 30 min cooking at 40, 70 and 98 °C | Antioxidant activity (MDA assay) | ↑ or ↓ activity | [39] |
| 2 | 5, 10 and 15 min boiling followed by diethyl ether extr. | Antioxidant activity (DPPH assay) | ↓ activity with ↑ cooking time | [40] | |
| 3 | Stewing | 10 min boiling of GIN (1:100 w/v), followed by 1 h stewing, kept covered for 1 h and cooled | Antioxidant activity (TEAC) | ↑ antioxidant activity | [41] |
| 4 | Frying | 2, 5, 10 and 15 min frying in a ceramic pan followed by diethyl ether extr. | Antioxidant activity (DPPH assay) | ↑ activity with ↑ frying time | [40] |
| 5 | Blanching | 10 min blanching of dried GIN in boiling water with 2% citric acid followed by 60 min drying at 50 °C | Antioxidant activity of candies (DPPH and Folin assay) | ↑ activity by ginger candies | [44] |
| 6 | Steam cooking | 120 min steaming of GIN at 3 kgf/cm3 and 97 °C, followed by 40 h drying in oven at 45 °C and 5 h extr. with 70% ethanol at 70 °C | Antidiabetic activity | ↑ therapeutic effects in diabetes | [45] |
| 7 | 18 h steaming of GIN followed by freeze-drying at −70 °C for 7 days and 10 min microwave extr. at 180 W with 70% ethanol | Antioxidant activity (DPPH assay) | ↓ activity | [47] | |
| 8 | Steam heating | 120 min heating followed by drying and EO extr. | Anti-Candida activity. | = activity | [46] |
| 9 | Autoclaving | 30, 60 and 90 min heating at 128 °C and diethyl ether extr. | Antioxidant activity (DPPH assay) | ↑ activity | [40] |
| 10 | Stir-frying | 7 min frying of dried rhizomes (40 °C) mixed with sand (1:10 w/w) at 220 °C and extr. with methanol | Antioxidant activity (DPPH, ABTS, FRAP assays) | ↑ activity | [43] |
| 11 | Microwave-drying | 1, 3 and 5 min drying at 600 W and extr. with diethyl ether | Antioxidant activity (DPPH assay) | ↑ activity with ↑ time | [40] |
| 12 | 1 min drying of enzymatic extract with 10% DMSO at 700 W and 2.45 GHz | Antioxidant activity (DPPH assay) | ↓ activity | [56] | |
| 13 | Drying until 50% ↓ in GIN moisture at 5 w/g energy density followed by 1 w/g to dryness | Antioxidant activity (DPPH, FRAP, ABTS assays) | moderately ↓ activity | [53] | |
| 14 | Oven heating | 60 min drying of EO and powdered rhizome at 120 °C, followed by methanol: ethyl acetate 50:50 extr. (v/v) | Antioxidant activity (DPPH assay) of EO and powder | ↓ activity in both | [52] |
| 15 | 1, 3 or 6 h drying of GIN in 20% ethanol at 100 °C | Antioxidant activity (DPPH and peroxide scavenging assays) | ↓ activity in DPPH assay = / slightly ↑ activity in peroxide test | [54] | |
| 16 | Drying in the sun | 7 days drying and EO extr. | Candida inhibition | ↑ activity | [46] |
| 17 | Dried 9- and 12-months old GIN | Antioxidant activity (DPPH and FRAP assay) | ↑ activity | [49] | |
| 18 | 72 h drying at 25–30 °C. | Antioxidant activity (CUPRAC assay) | ↓ activity | [50] | |
| 19 | Fresh ginger rhizome immersed for 1 h in a 10% vinegar solution, then dried for 3 days at a temperature of 25.5–36.6 °C | Antimicrobial (Salmonella sp.) and antifungal activity (Aspergillus, Penicillium, Mucor and Rhizopus) | ↑ activity | [48] | |
| 20 | Solar dryer | 15 h drying of GIN, immersed for 1 h in 10% vinegar, at 44–58 °C | As above | ↑ activity | [48] |
| 21 | Concrete tent-type solar dryer | 5 days drying of GIN, immersed for 1 h in 10% vinegar, at 32–42 °C | As above | ↑ activity | [48] |
| 22 | Drying | Commercially dried ginger powder extr. with dichloromethane | Anti-inflammatory activity (PGE2 assay) | = activity | [51] |
| 23 | 2 h drying of GIN at 105 °C followed by diethyl ether extr. | Antioxidant activity (DPPH assay) | ↓ activity | [40] | |
| 24 | GIN drying at ca. 40 °C, methanol extr. | Antioxidant activity (DPPH, ABTS, FRAP assays) | ↑ activity | [43] | |
| 25 | Hot-air drying | GIN drying at 60 °C | Antioxidant activity (DPPH, FRAP, ABTS assays) | ↓ activity | [53] |
| 26 | Oven drying | 9- and 12-months GIN drying at 40 °C, 50 °C, 60 °C and 70 °C | Antioxidant activity (DPPH and FRAP assays) | ↑ activity, best for 60 °C and 70 °C | [49] |
| 27 | 36 h GIN drying at 60 °C | Antioxidant activity (CUPRAC assay) | ↓ activity | [50] | |
| 28 | Vacuum oven drying | 36 h drying at 60 °C at 0.025 mbar | Antioxidant activity (CUPRAC assay) | ↓ activity | [50] |
| 29 | Carbonization | Drying at ca. 40 ° C, followed by heating and frying until carbonization | Antioxidant activity (DPPH, ABTS, FRAP assays) | ↑ activity | [43] |
| 30 | Intermittent microwave-convection drying | GIN drying with 700 W with hot air drying at 60 °C, using pulse regulation (PR) of first PR = 2 (5 s on–5 s off) up to 50% water content, and PR = 6 (5 s on–25 s off) to the end of drying. | Antioxidant activity (DPPH, FRAP, ABTS assays). | ↑ activity | [53] |
| 31 | Blanching as pretreatment to drying chamber | 2 min blanching with 95 ± 1 °C water and drying at 60, 70 or 80 °C with 0.3 m/s air velocity | Antioxidant activity (DPPH and ABTS method) | Blanching ↓ activity Air drying ↑ activity | [55] |
| Lp | Type of Processing | Processing Conditions | Tested Activity | Results | Reference |
|---|---|---|---|---|---|
| 1 | Freeze-drying/Lyophilization | Freeze-drying of 9- and 12-months old at −40 °C | Antioxidant activity (DPPH and FRAP assays) | ↑ activity | [49] |
| 2 | 24 h freeze-drying of GIN −50 °C and 0.133 mbar | Antioxidant activity (CUPRAC assay). | ↑ activity | [50] | |
| 3 | 12 h freezing of GIN at −40 °C followed by freeze-drying at 20 Pa, 25 °C and −58 °C of heating plate and cold trap, respectively | Antioxidant activity (DPPH, FRAP, ABTS assays) | ↑ activity | [53] | |
| 4 | Infrared drying | Drying of GIN by three red glass lamps (225 W each) | Antioxidant activity (DPPH, FRAP, ABTS assays) | ↑ activity | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska, J.; Czernicka-Boś, L.; Kukula-Koch, W.; Iłowiecka, K.; Koch, W. Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome—A Review. Molecules 2023, 28, 412. https://doi.org/10.3390/molecules28010412
Zagórska J, Czernicka-Boś L, Kukula-Koch W, Iłowiecka K, Koch W. Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome—A Review. Molecules. 2023; 28(1):412. https://doi.org/10.3390/molecules28010412
Chicago/Turabian StyleZagórska, Justyna, Lidia Czernicka-Boś, Wirginia Kukula-Koch, Katarzyna Iłowiecka, and Wojciech Koch. 2023. "Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome—A Review" Molecules 28, no. 1: 412. https://doi.org/10.3390/molecules28010412
APA StyleZagórska, J., Czernicka-Boś, L., Kukula-Koch, W., Iłowiecka, K., & Koch, W. (2023). Impact of Thermal Processing on the Selected Biological Activities of Ginger Rhizome—A Review. Molecules, 28(1), 412. https://doi.org/10.3390/molecules28010412









