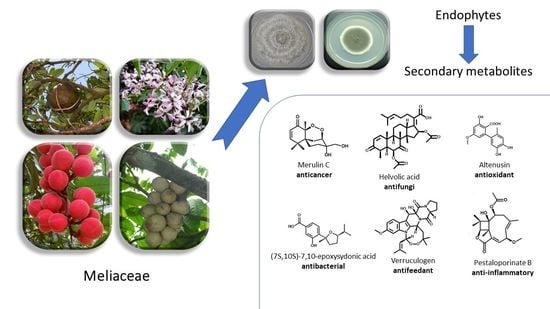
Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family
Abstract
1. Introduction
2. Methodology and Botany
3. Phytochemistry
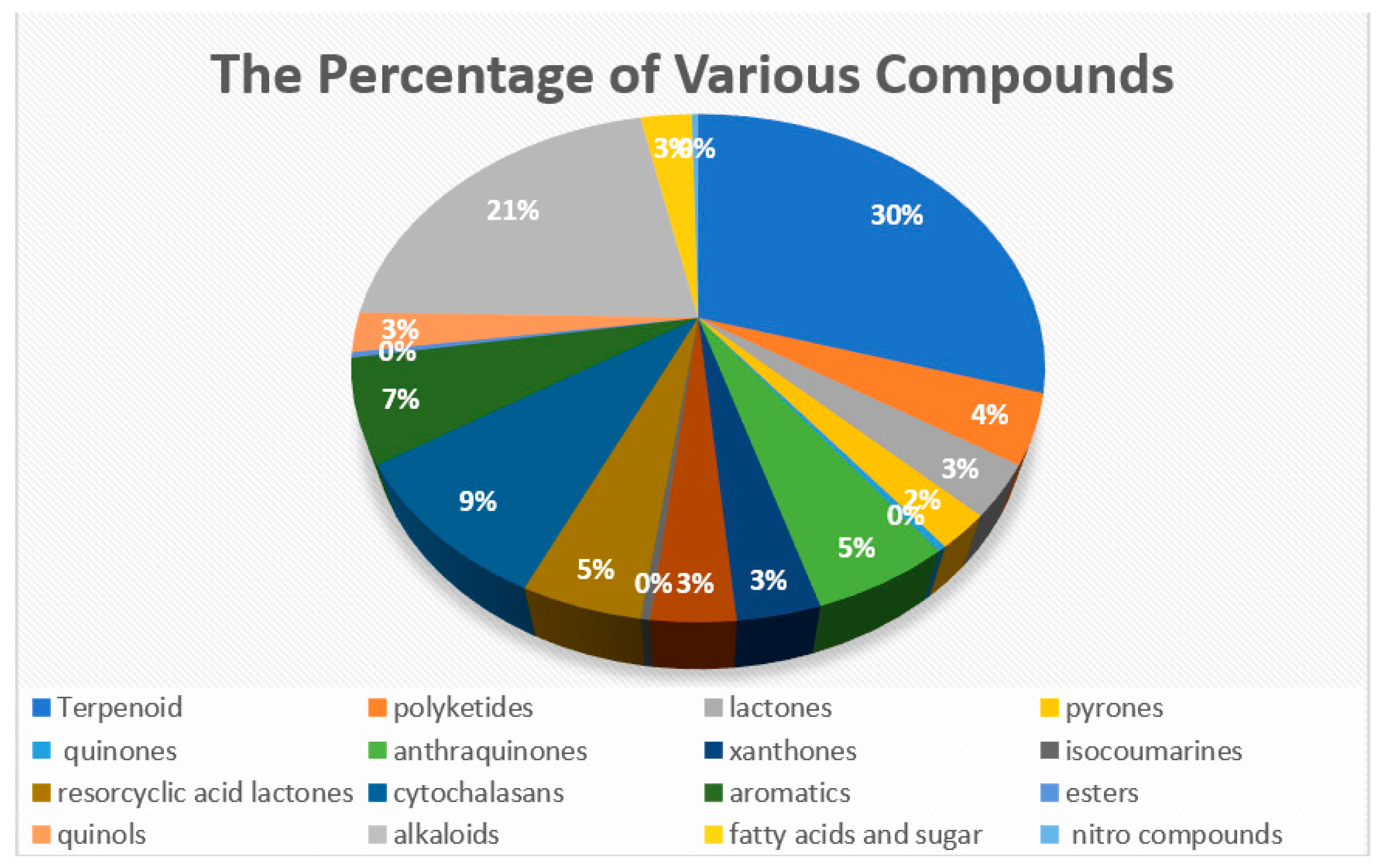
3.1. Overview of Isolated Compounds Derived from Endophytic Fungi Meliaceae
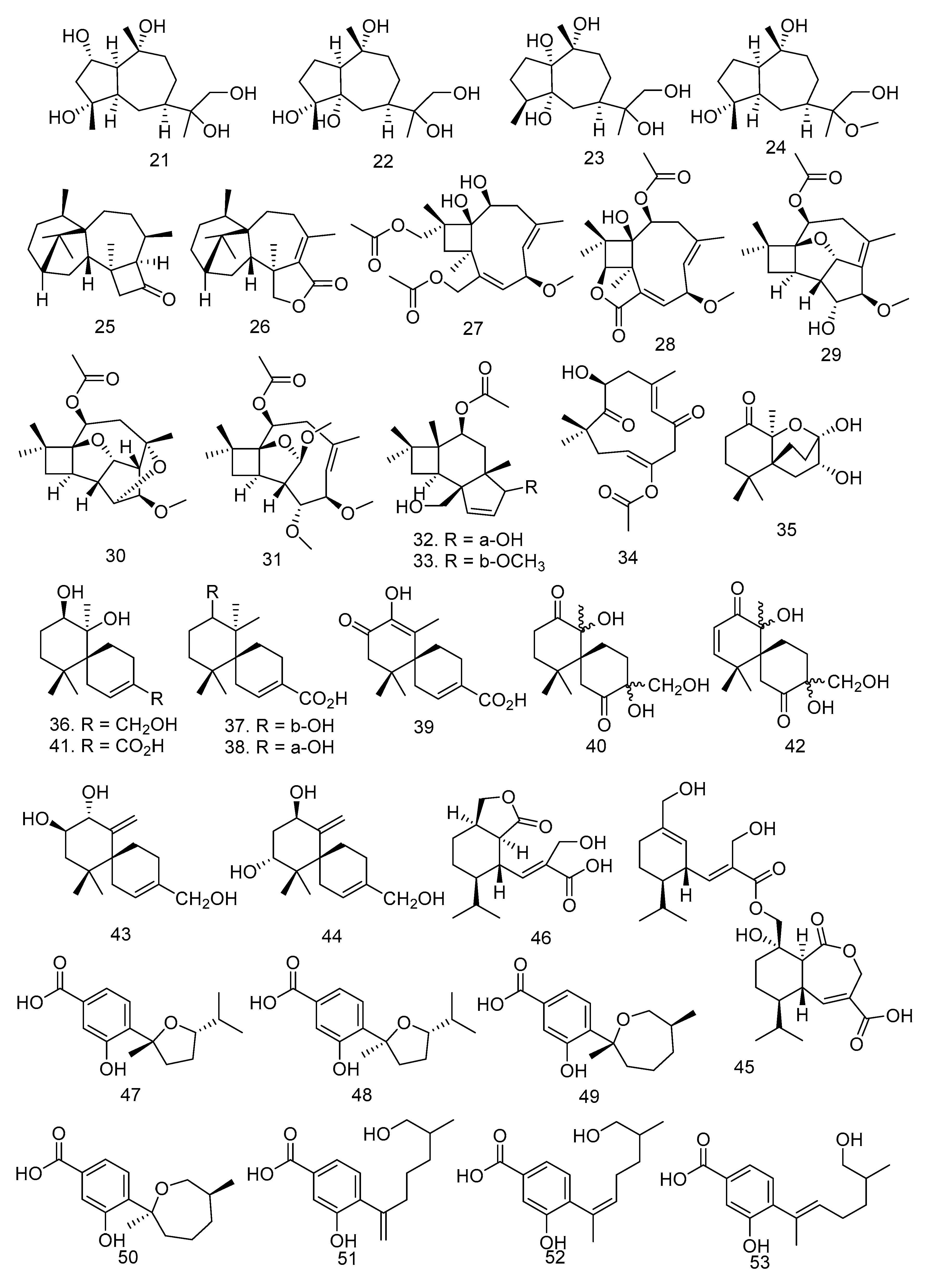
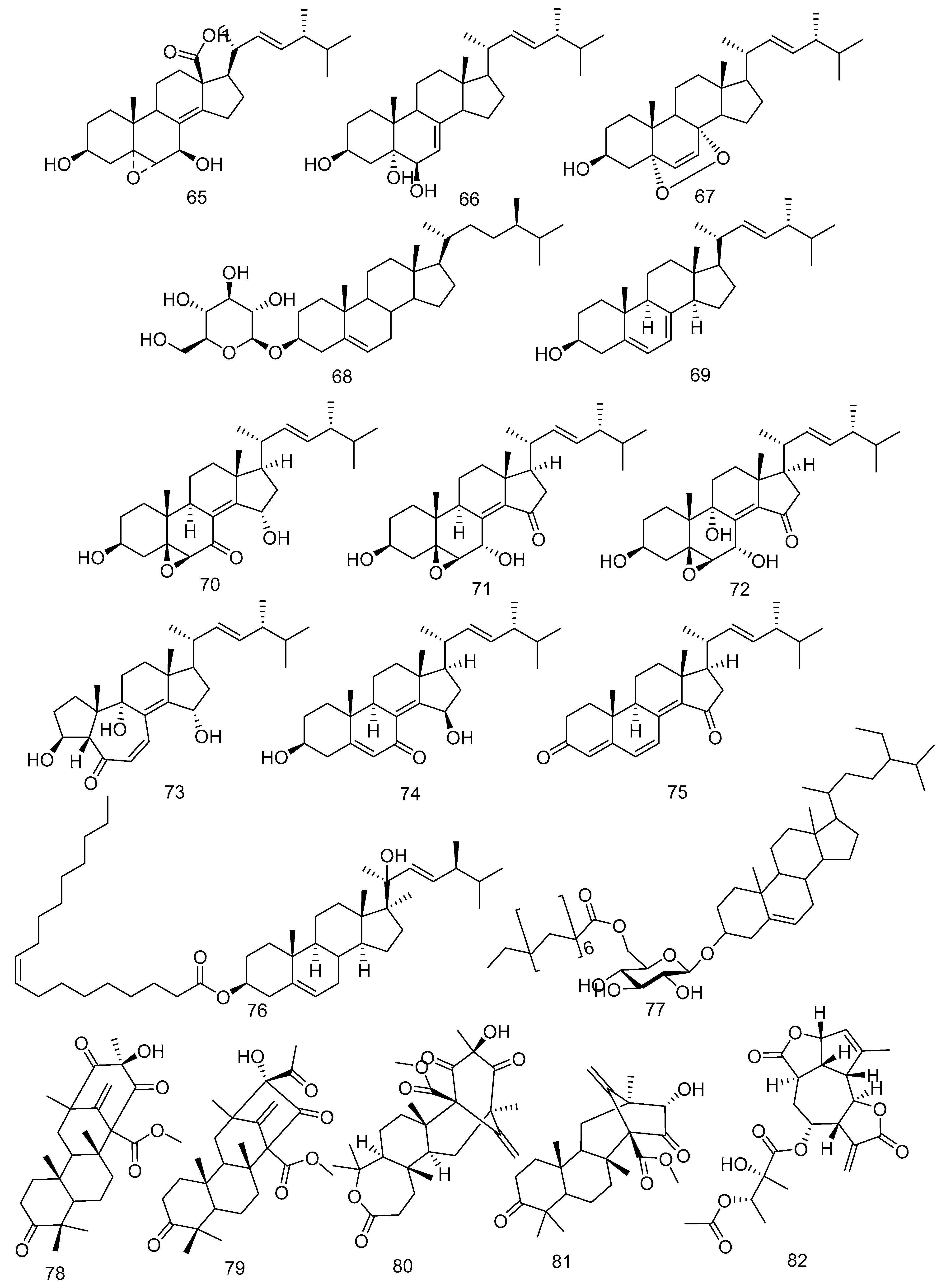
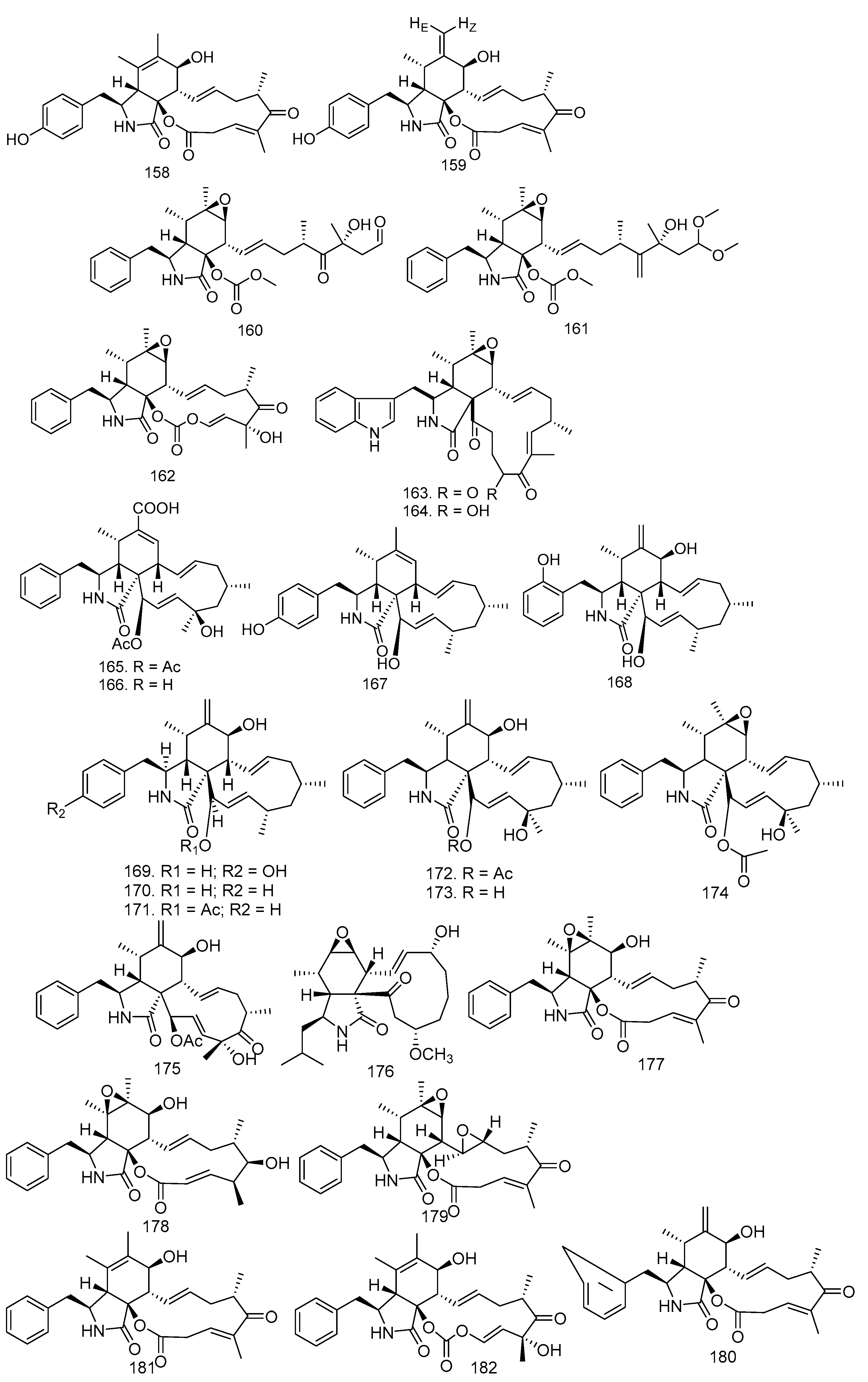
3.2. Triterpenoid and Sesquiterpenoid Compounds
3.3. Steroid Compounds
3.4. Meroterpenoid Compounds
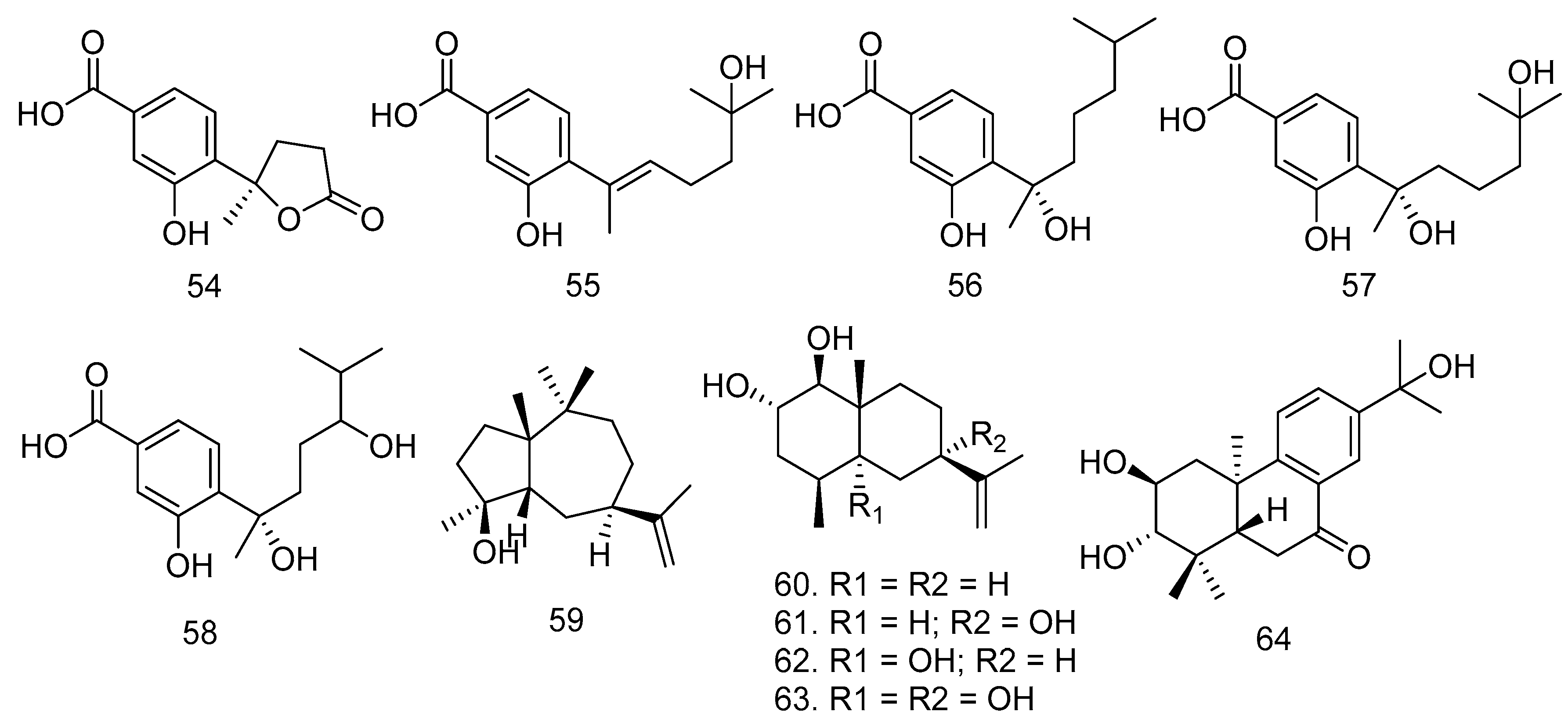
3.5. Polyketides
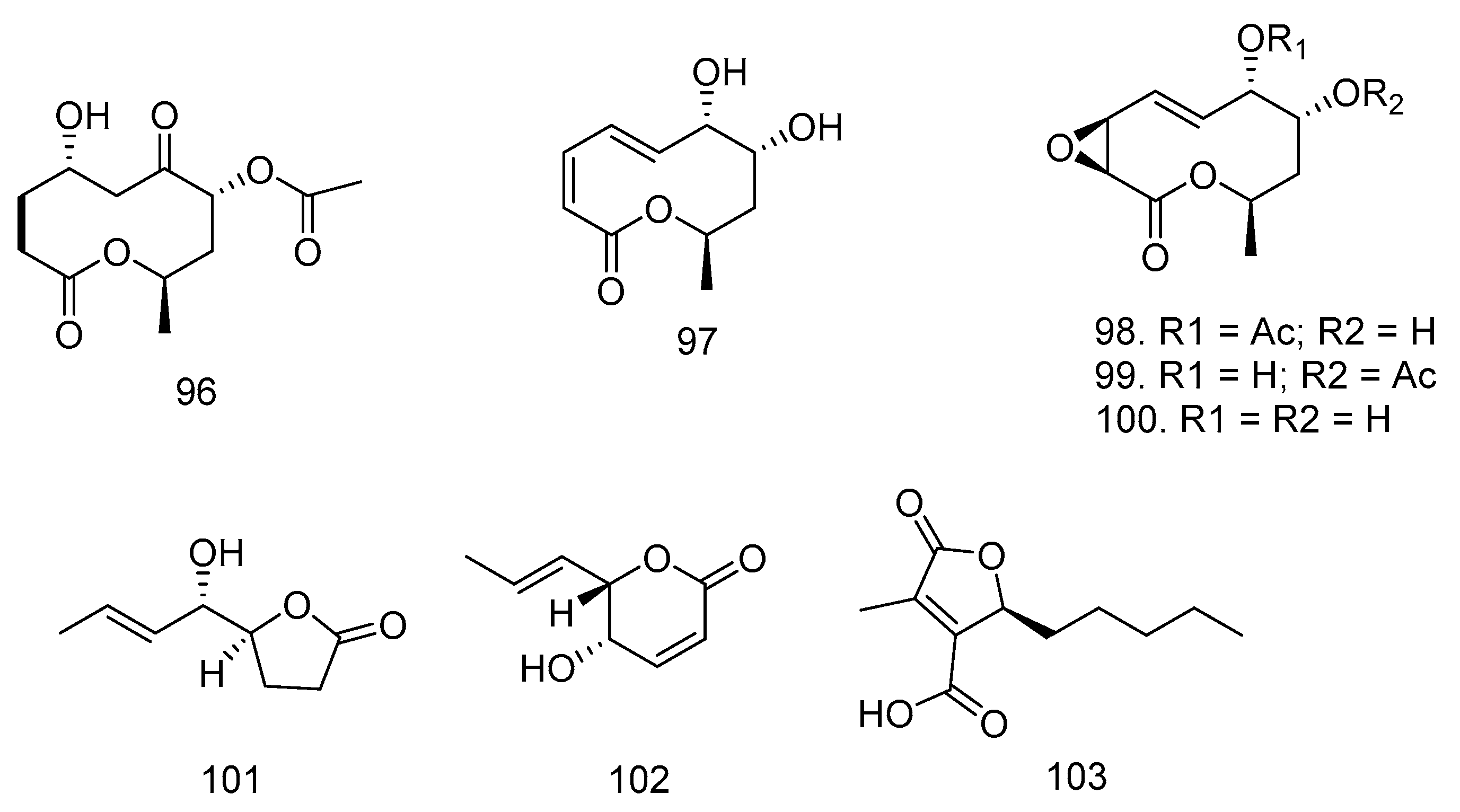
3.6. Lactones
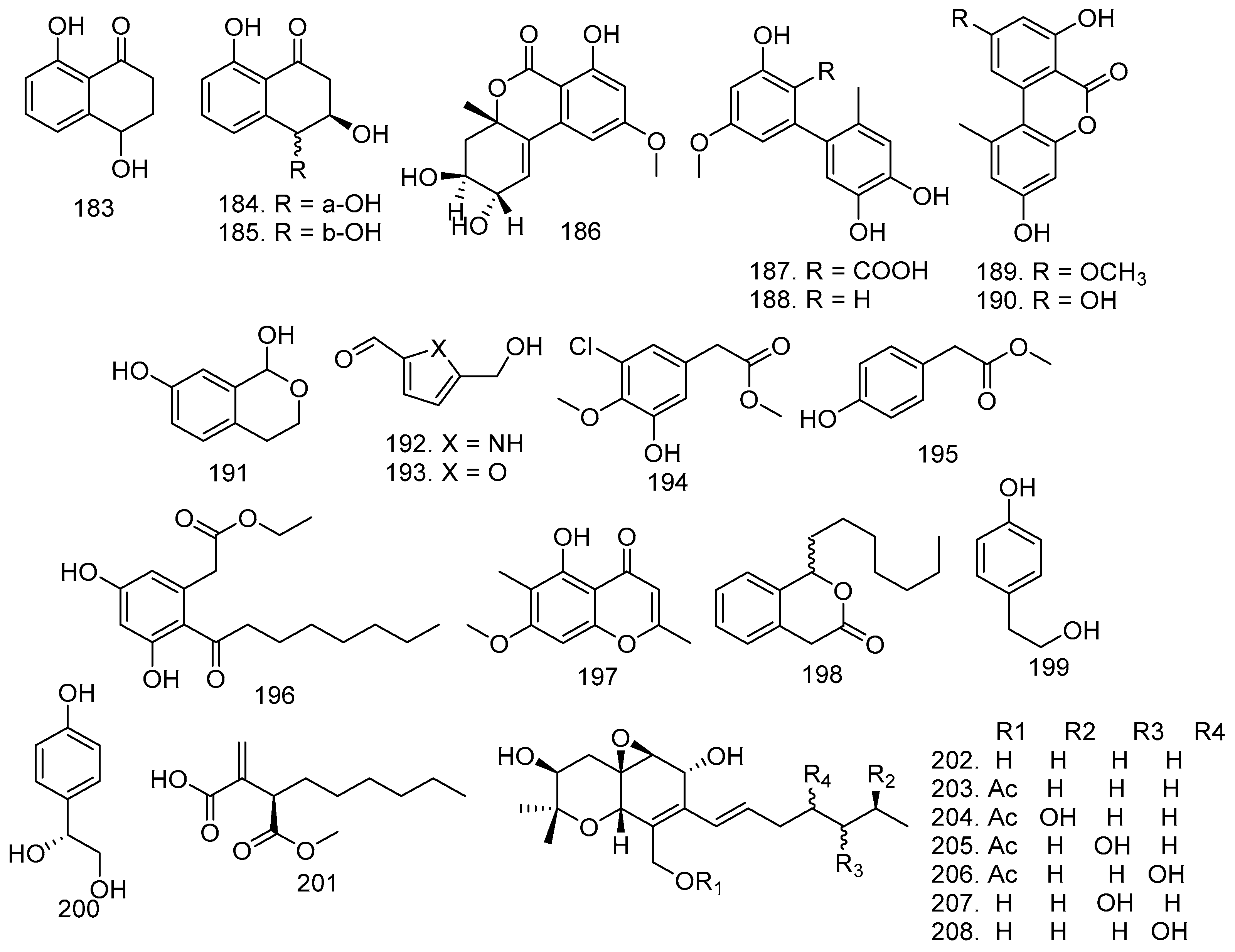
3.7. Pyrones, Quinone, and Anthraquinones
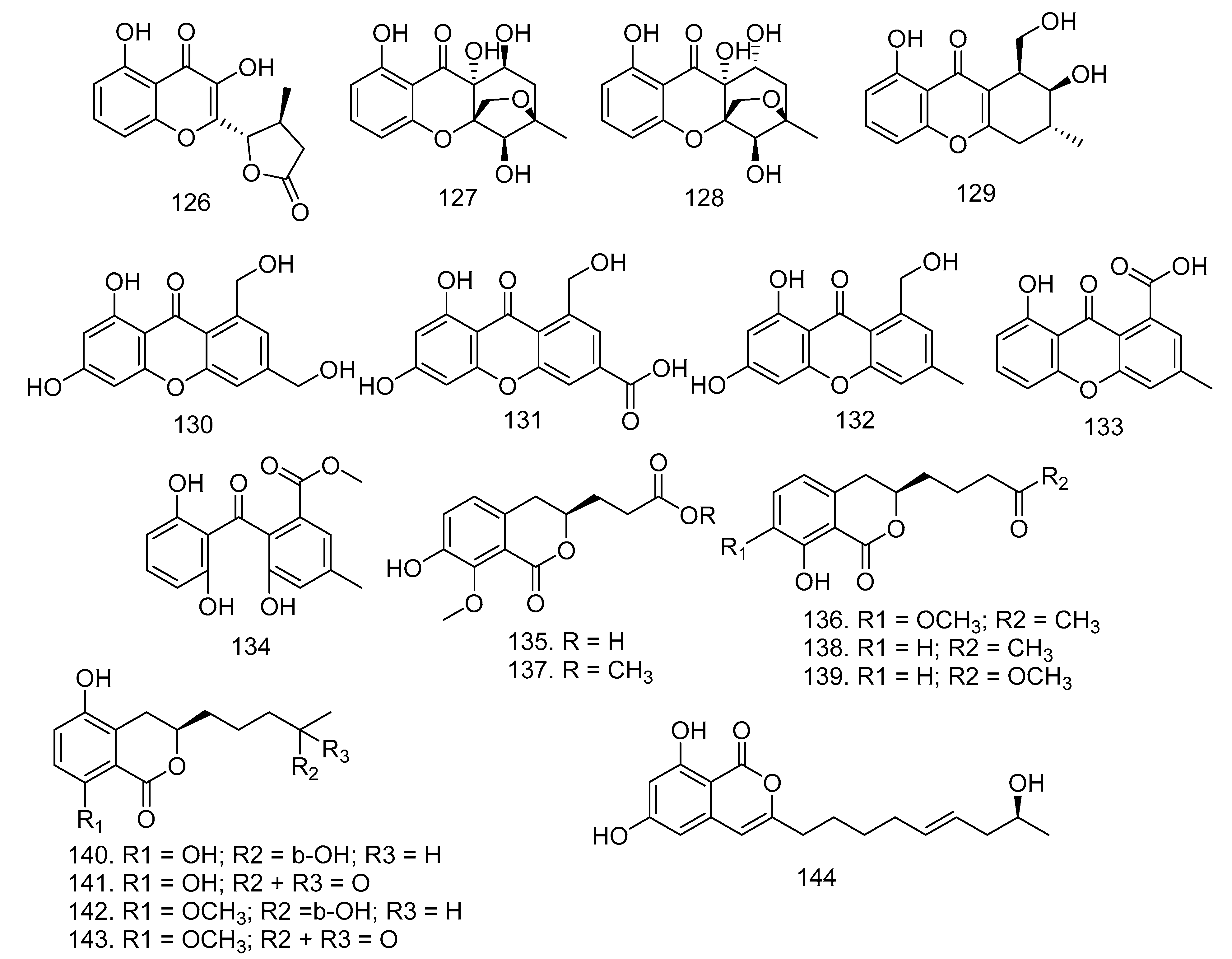
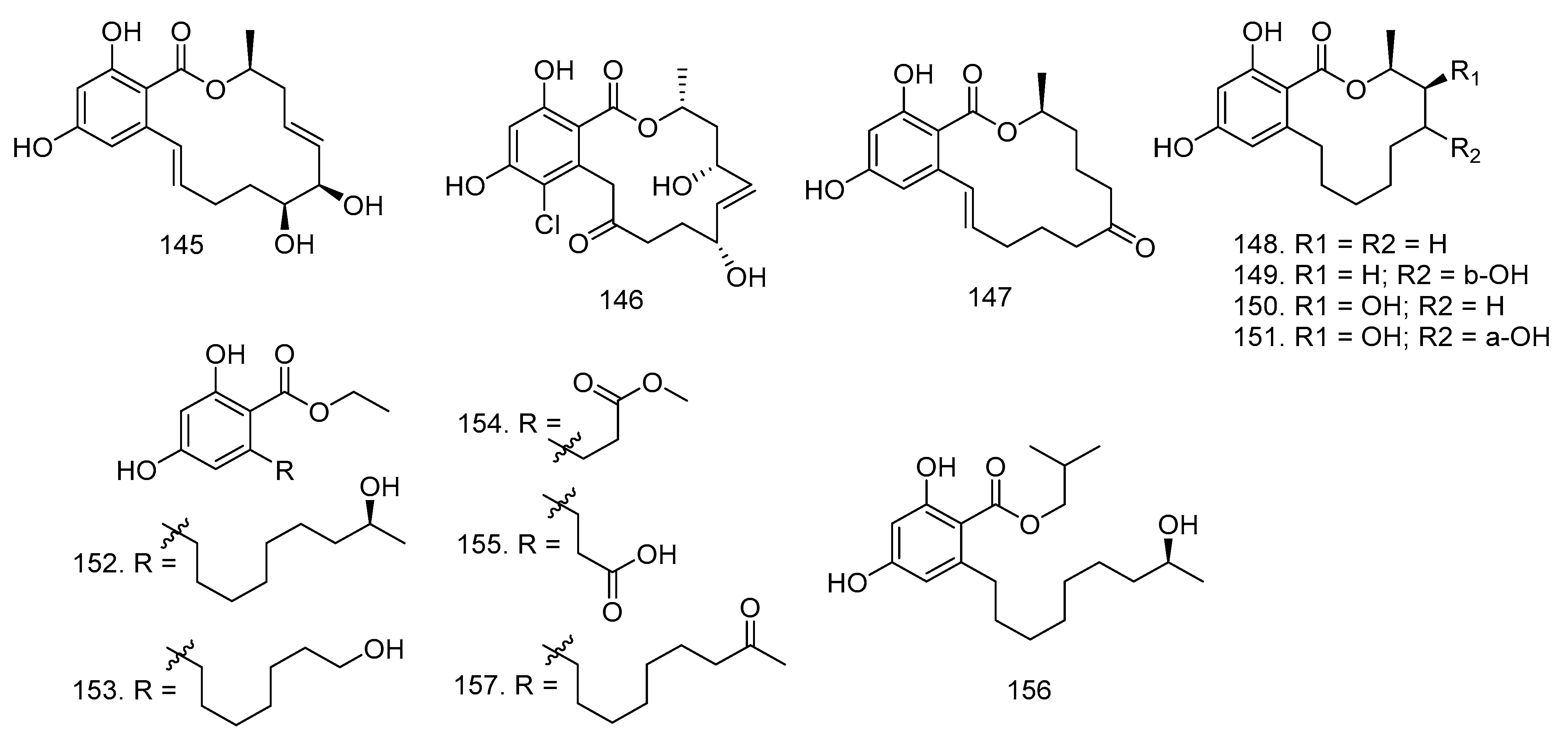
3.8. Xanthones, Isocoumarin, and Resorcylic Acids
3.9. Cytochalasins
3.10. Aromatics, Ester, Quinols
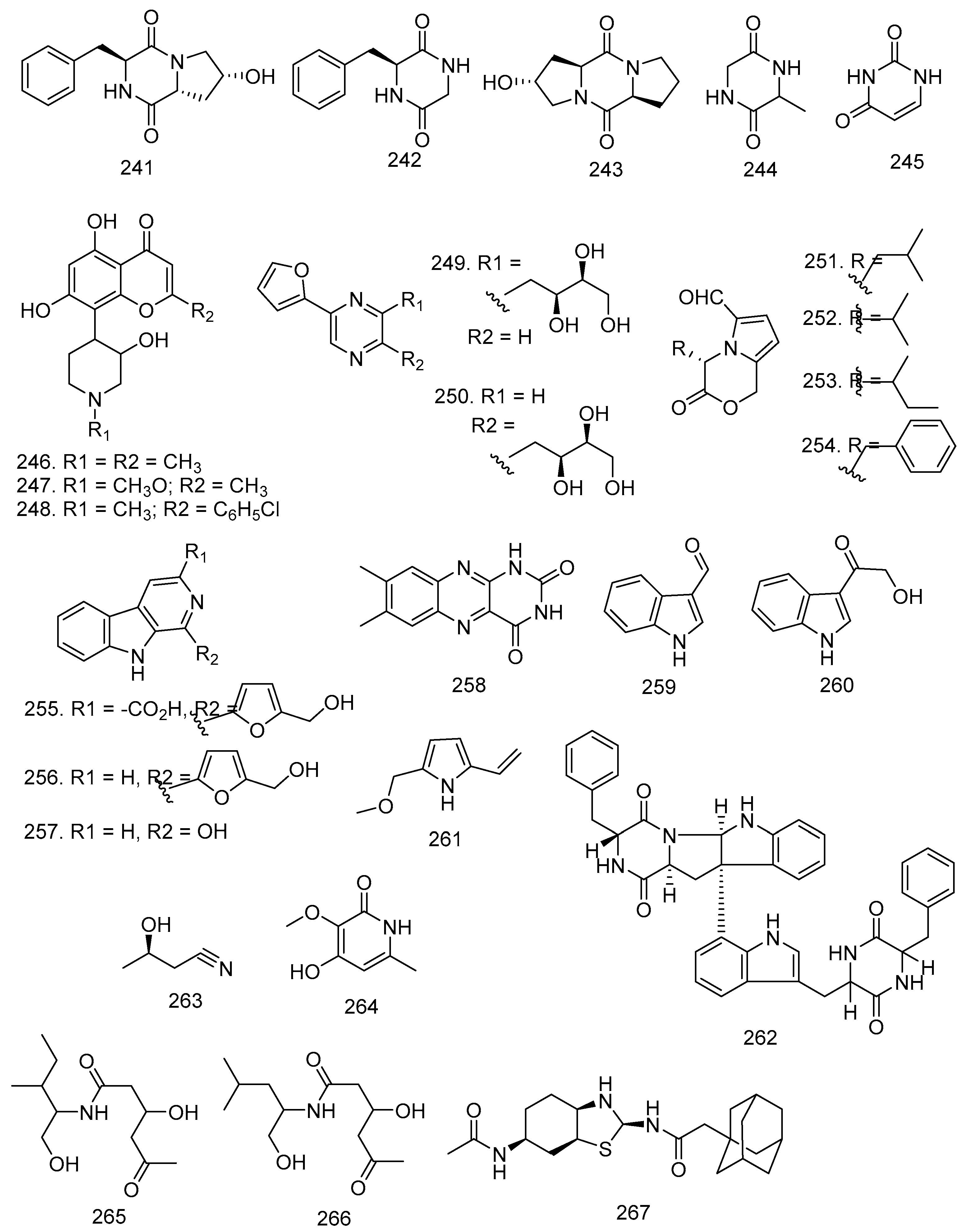
3.11. Alkaloids
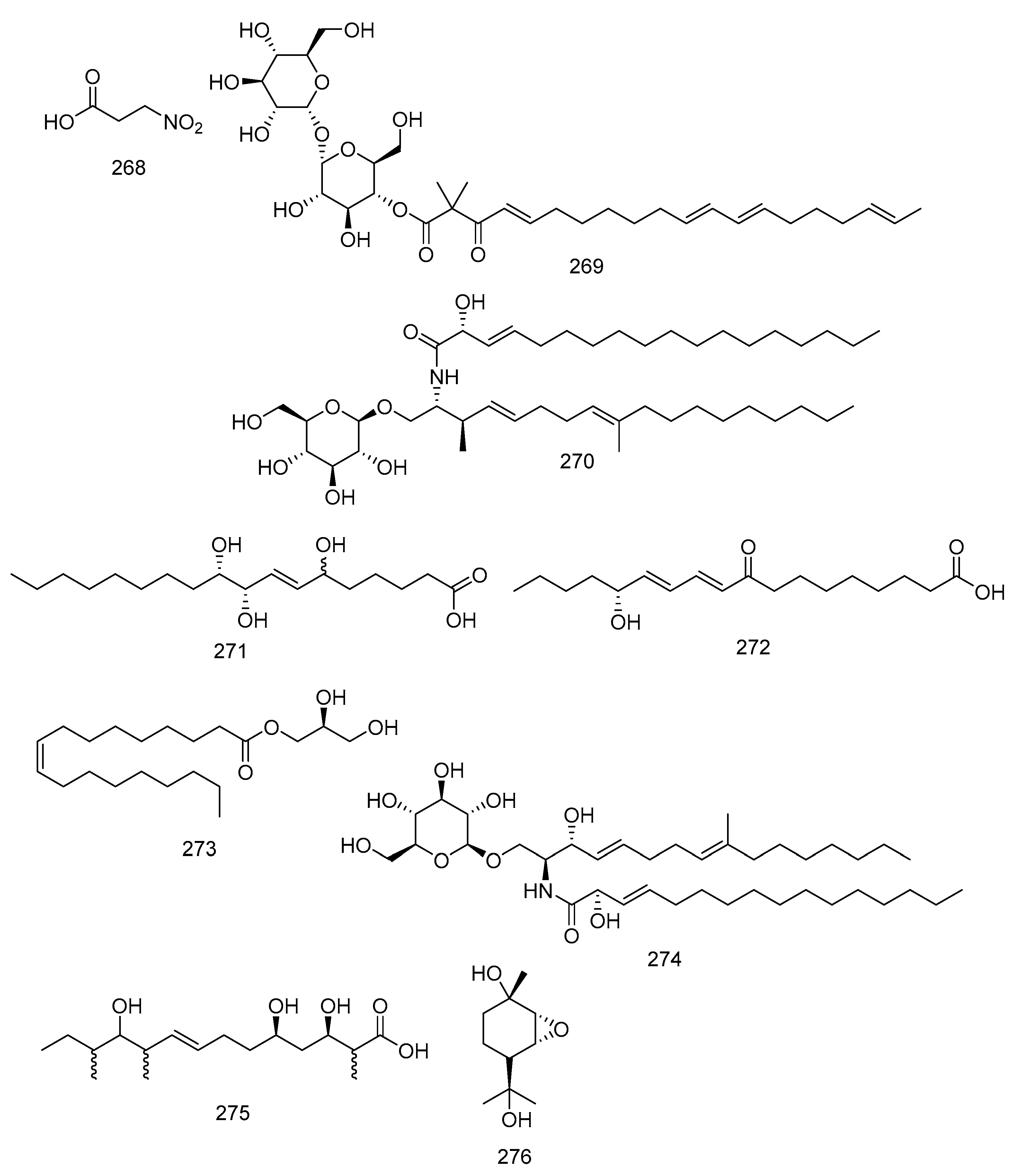
3.12. Nitro Compound, Fatty Acid, and Sugars
4. Bioactivity of Secondary Metabolites Isolated from Endophytic Fungi
4.1. Antimicrobial
4.2. Cytotoxic Activity
4.3. Antioxidant and α-Glucoside Inhibitory Activity
4.4. Anti-Inflammatory and Anti-Influenza
4.5. Brine Shrimp Lethality Test (BSLT)
4.6. Allelophatic Effects on Wheat Triticum Aestivum
4.7. Antifeedant
4.8. Neuroprotective Activity
4.9. Anti-HIV Activity
4.10. Phytotoxic Activity
4.11. Enhanced Root Elongation Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, R.; Pednekar, A.; Avalaskar, A.; Rathi, M.; Rewachandani, Y. A comprehensive review on Meliaceae family. World J. Pharm. Sci. 2015, 3, 1572–1577. [Google Scholar]
- Sinaga, S.E.; Mayanti, T.; Naini, A.A.; Harneti, D.; Nurlelasari, N.; Maharani, R.; Farabi, K.; Supratman, U.; Fajriah, S.; Azmi, M.N. 1035 Sesquiterpenoids from the Stem Bark of Lansium domesticum Corr. Cv. Kokossan and Their Cytotoxic Activity against MCF-7 Breast Cancer Cell Lines. J. Pharm. Pharmacol. 2022, 22, 1035–1042. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Odebunmi, C.A.; Adetunji, A.E. Biological activities of limonoids in the Genus Khaya (Meliaceae): A review. Futur. J. Pharm. Sci. 2021, 7, 74. [Google Scholar] [CrossRef]
- Suzuki, T.; Ariefta, N.R.; Koseki, T.; Furuno, H.; Kwon, E.; Momma, H.; Harneti, D.; Maharani, R.; Supratman, U.; Kimura, K.; et al. New polyketides, paralactonic acids A–E produced by Paraconiothyrium sp. SW-B-1, an endophytic fungus associated with a seaweed, Chondrus ocellatus Holmes. Fitoterapia 2019, 132, 75–81. [Google Scholar] [CrossRef]
- Shiono, Y.; Sasaki, T.; Shibuya, F.; Yasuda, Y.; Koseki, T.; Supratman, U. Isolation of a Phomoxanthone A Derivative, a New Metabolite of Tetrahydroxanthone, from a Phomopsis sp. Isolated from the Mangrove, Rhizhopora mucronata. Nat. Prod. Commun. 2013, 8, 1735–1737. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q. Endophytic fungal community in stems and leaves of plants from desert areas in China. Mycol. Prog. 2012, 11, 781–790. [Google Scholar] [CrossRef]
- Sofian, F.F.; Suzuki, T.; Supratman, U.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Yosida, J.; Ito, Y.; Koseki, T.; et al. The 2,3-epoxy naphtoquinol produced by endophyte Arthrinium marii M-211. Nat. Prod. Res. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Res. 2001, 18, 448–459. [Google Scholar]
- De Battista, J.P.; Bacon, C.W.; Severson, R.; Plattner, R.D.; Bouton, J.H. Indole Acetic Acid Production by the Fungal Endophyte of Tall Fescue (AJ). Agron 1990, 880, 878–880. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Khan, A.L.; Shinwari, Z.K. Isolation of plant growth promoting endophytic fungi from dicots inhabiting coastal sand dunes of korea. Pak. J. Bot. 2012, 44, 1453–1460. [Google Scholar]
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res. 2014, 169, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Fiorentino, A. Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Kusari, S.; Spiteller, M. Are we ready for industrial production of bioactive plant secondary. Nat. Prod. Rep. 2015, 28, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-harrasi, A.; Al-rawahi, A.; Lee, J.; Latif, A.; Hussain, J.; Al-harrasi, A.; Al-rawahi, A. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Mabberley, D.J. Florae Malesianae PraecursoresLXVII Meliaceae (Divers Genera). Blumea 1985, 31, 129–152. [Google Scholar]
- van der Nat, J.M.; van der Sluis, W.G.; de Silva, K.T.D.; Labadie, R.P. Ethnopharmacognostical survey of Azadirachta indica A. Juss (Meliaceae). J. Ethnopharmacol. 1991, 35, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mayanti, T.; Sinaga, S.E.; Supratman, U. Phytochemistry and biological activity of Lansium domesticum Corr. species: A review. J. Pharm. Pharmacol. 2022, 74, 1568–1587. [Google Scholar] [CrossRef]
- Samaranada, V.A.; Wijekumar, P.J.; Nirmani, D.; Samarakoon, A.W.; Perera, P.K.; Wijekumar, J. The phytochemical constituents and pharmacological properties of Munronia pinnata: A review. Int. J. Herb. Med. 2021, 9, 85–91. [Google Scholar]
- Ogbuewu, I.P.; Odoemenam, Y.U.; Obikaonu, H.O.; Opara, M.N.; Emenalom, O.O.; Uchegbu, M.C.; Okoli, I.C.; Esonu, B.O.; Iloeje, M.U. The growing importance of neem (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: A review. Res. J. Med. Plant 2011, 5, 230–245. [Google Scholar] [CrossRef]
- Yang, S.X.; Wang, H.P.; Gao, J.M.; Zhang, Q.; Laatsch, H.; Kuang, Y. Fusaroside, a unique glycolipid from Fusarium sp., an endophytic fungus isolated from Melia azedarach. Org. Biomol. Chem. 2012, 10, 819–824. [Google Scholar] [CrossRef]
- Mei, R.Q.; Nong, X.H.; Wang, B.; Sun, X.P.; Huang, G.L.; Luo, Y.P.; Zheng, C.J.; Chen, G.Y. A new phenol derivative isolated from mangrove-derived fungus Eupenicillium sp. HJ002. Nat. Prod. Res. 2021, 35, 4051–4057. [Google Scholar] [CrossRef]
- Geris Dos Santos, R.M.; Rodrigues-Fo, E.; Caldas Rocha, W.; Simas Teixeira, M.F. Endophytic fungi from Melia azedarach. World J. Microbiol. Biotechnol. 2003, 19, 767–770. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of cytochalasins from the endophytic Xylaria sp. and their biological functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, P.; Liao, G.; Zeng, Y.; Cai, C.; Kong, F.; Guo, Z.; Proksch, P.; Dai, H.; Mei, W. New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54. Mar. Drugs 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chen, Y.W.; Shao, S.C.; Wang, L.D.; Li, Z.Y.; Yang, L.Y.; Li, S.L.; Huang, R. Ten-membered lactones from Phomopsis sp., an endophytic fungus of Azadirachta indica. J. Nat. Prod. 2008, 71, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Kemda, P.N.; Akone, S.H.; Tontsa, A.T.; Zhen, L.; Müller, W.E.G.; Proksch, P.; Nkengfack, A.E. Colletotrin: A sesquiterpene lactone from the endophytic fungus Colletotrichum gloeosporioides associated with Trichilia monadelpha. Z. Für Nat. B 2017, 72, 697–703. [Google Scholar] [CrossRef]
- Flores, A.C.; Pamphile, J.A.; Sarragiotto, M.H.; Clemente, E. Production of 3-nitropropionic acid by endophytic fungus Phomopsis longicolla isolated from Trichilia elegans A. JUSS ssp. elegans and evaluation of biological activity. World J. Microbiol. Biotechnol. 2013, 29, 923–932. [Google Scholar] [CrossRef]
- Mohana Kumara, P.; Zuehlke, S.; Priti, V.; Ramesha, B.T.; Shweta, S.; Ravikanth, G.; Vasudeva, R.; Santhoshkumar, T.R.; Spiteller, M.; Uma Shaanker, R. Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Antonie Van Leeuwenhoek 2012, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Value, N. Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients 2022, 147, 1531. [Google Scholar]
- Chokpaiboon, S.; Sommit, D.; Teerawatananond, T.; Muangsin, N.; Bunyapaiboonsri, T.; Pudhom, K. Cytotoxic nor-chamigrane and chamigrane endoperoxides from a basidiomycetous fungus. J. Nat. Prod. 2010, 73, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Zhang, A.; Gao, J. Metabolites from Aspergillus an endophytic fungus associated with Melia azedarach, and Their Antifungal, Antifeedant and Toxic Activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Verma, V.C.; Lamshoeft, M.; Spiteller, M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J. Microbiol. Biotechnol. 2012, 28, 1287–1294. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; He, J.; Li, X.N.; Huang, R.; Song, F.; Chen, Y.W.; Miao, C.P. Guaiane sesquiterpenes and isopimarane diterpenes from an endophytic fungus Xylaria sp. Phytochemistry 2014, 105, 197–204. [Google Scholar] [CrossRef]
- Huang, R.; Xie, X.S.; Fang, X.W.; Ma, K.X.; Wu, S.H. Five new guaiane sesquiterpenes from the endophytic fungus Xylaria sp. YM 311647 of Azadirachta indica. Chem. Biodivers. 2015, 12, 1281–1286. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.M.; Zhao, J.L.; Li, N.; Chen, R.D.; Xie, K.B.; Zhang, W.J.; Feng, K.P.; Yan, Z.; Wang, N.; et al. Two new diterpenoids from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Chin. Chem. Lett. 2016, 27, 957–960. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.H.; Wang, X.B.; Li, T.X.; Kong, L.Y. Caryophyllene sesquiterpenoids from the endophytic fungus, Pestalotiopsis sp. Fitoterapia 2016, 109, 119–124. [Google Scholar] [CrossRef]
- Choodej, S.; Teerawatananond, T.; Mitsunaga, T.; Pudhom, K. Chamigrane sesquiterpenes from a basidiomycetous endophytic fungus XG8D associated with Thai mangrove Xylocarpus granatum. Mar. Drugs 2016, 14, 132. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.H.; Zhu, K.; Wang, Y.; Cheng, Z.Q.; Jiang, C.S.; Dai, J.G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Cai, C.H.; Kong, F.D.; Chen, H.Q.; Zhou, L.M.; Song, X.M.; Mei, W.L.; Dai, H.F. A new cytochalasin derivative from the mangrove-derived endophytic fungus Xylaria sp. HNWSW-2. J. Asian Nat. Prod. Res. 2018, 20, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Han, W.B.; Zhai, Y.J.; Gao, Y.; Zhou, H.Y.; Xiao, J.; Pescitelli, G.; Gao, J.M. Cytochalasins and an Abietane-Type Diterpenoid with Allelopathic Activities from the Endophytic Fungus Xylaria Species. J. Agric. Food Chem. 2019, 67, 3643–3650. [Google Scholar] [CrossRef]
- Sari, A.P.; Nurlelasari; Azhari, A.; Harneti, D.; Maharani, R.; Mayanti, T.; Farabi, K.; Darwati; Supratman, U.; Fajriah, S.; et al. New Ergostane-Type Sterol Produced by an Endophytic Fungus Fusarium phaseoli Isolated from Chisocheton macrophyllus (Meliaceae). Rec. Nat. Prod. 2022, 16, 614–621. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Shi, X.W.; Gao, J.M. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 2014, 28, 1388–1392. [Google Scholar] [CrossRef]
- Zhu, X.C.; Huang, G.L.; Mei, R.Q.; Wang, B.; Sun, X.P.; Luo, Y.P.; Xu, J.; Zheng, C.J. One new α,β-unsaturated 7-ketone sterol from the mangrove-derived fungus Phomopsis sp. MGF222. Nat. Prod. Res. 2021, 35, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Geris dos Santos, R.M.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Fill, T.P.; dos Santos, R.M.G.; Barisson, A.; Rodrigues-Filho, E.; Souza, A.Q.L. Co-Production of bisphenylpropanoid amides and meroterpenes by an endophytic Penicillium brasilianum found in the root bark of Melia azedarach. Z. Für Nat. C 2009, 64, 355–360. [Google Scholar] [CrossRef]
- Campos, F.R.; Barison, A.; Daolio, C.; Ferreira, A.G.; Rodrigues-Fo, E. Complete 1H and 13C NMR assignments of aurasperone A and fonsecinone A, two bis-naphthopyrones produced by Aspergillus aculeatus. Magn. Reson. Chem. 2005, 43, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Hu, H.B.; Huang, Z.H.; Yan, R.J.; Tian, L.W.; Wu, J. Phomopsols A and B from the Mangrove Endophytic Fungus Phomopsis sp. xy21: Structures, Neuroprotective Effects, and Biogenetic Relationships. Org. Lett. 2019, 21, 7919–7922. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Du, S.T.; Xiao, J.; Wang, D.C.; Han, W.B.; Zhang, Q.; Gao, J.M. Isolation and Characterization of Antifungal Metabolites from the Melia Azedarach-Associated Fungus Diaporthe Eucalyptorum. J. Agric. Food Chem. 2020, 68, 2418–2425. [Google Scholar] [CrossRef]
- do, R.; Marinho, A.M.; Rodrigues-Filho, E.; Moitinho, M.D.L.R.; Santos, L. Biologically Active Polyketides Produced by Penicillium janthinellum Isolated as an Endophytic Fungus from Fruits of Melia azedarach. J. Braz. Chem. Soc. 2005, 16, 280–283. [Google Scholar]
- Wu, S.H.; Chen, Y.W.; Shao, S.C.; Wang, L.D.; Yu, Y.; Li, Z.Y.; Yang, L.Y.; Li, S.L.; Huang, R. Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem. Biodivers. 2009, 6, 79–85. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Verma, V.C.; Kumar, A.; Gond, S.K.; Harper, J.K.; Hess, W.M.; Lobkovosky, E.; Ma, C.; Ren, Y.; Strobel, G.A. Javanicin, an antibacterial naphthaquinone from an endophytic fungus of neem, Chloridium sp. Curr. Microbiol. 2009, 58, 233–238. [Google Scholar] [CrossRef]
- Rudiyansyah; Alimuddin, A.H.; Masriani; Muharini, R.; Liu, Z.; Lin, W.; Hartmann, R.; Proksch, P. Arugosins O-Q, new fungal metabolites from the fungus Xylariaceae sp. isolated from leaves of Lansium domesticum (Meliaceae). Nat. Prod. Commun. 2019, 14, 125–128. [Google Scholar] [CrossRef]
- Hu, H.B.; Luo, Y.F.; Wang, P.; Wang, W.J.; Wu, J. Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21. Fitoterapia 2018, 131, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.Q.; Wang, B.; Zeng, W.N.; Huang, G.L.; Chen, G.Y.; Zheng, C.J. Bioactive isocoumarins isolated from a mangrove-derived fungus Penicillium sp. MGP11. Nat. Prod. Res. 2022, 36, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Gao, J.M.; Zhang, Q.; Laatsch, H. Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Bioorganic Med. Chem. Lett. 2011, 21, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sofian, F.F.; Suehiro, W.; Harneti, D.; Maharani, R.; Supratman, U.; Abdullah, F.F.; Salam, S.; Koseki, T.; Shiono, Y. β-Resorcylic Acid Derivatives, with Their Phytotoxic Activities, from the Endophytic Fungus Lasiodiplodia theobromae in the Mangrove Plant Xylocarpus granatum. Chem. Biodivers. 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Zhang, M.; Dai, J.G.; Pedpradab, P.; Wang, W.J.; Wu, J. Cytochalasins from mangrove endophytic fungi Phomopsis spp. xy21 and xy22. Phytochem. Lett. 2016, 17, 162–166. [Google Scholar] [CrossRef]
- Fill, T.P.; Asenha, H.B.R.; Marques, A.S.; Ferreira, A.G.; Rodrigues-Fo, E. Time course production of indole alkaloids by an endophytic strain of Penicillium brasilianum cultivated in rice. Nat. Prod. Res. 2013, 27, 967–974. [Google Scholar] [CrossRef]
- Kumara, P.M.; Soujanya, K.N.; Ravikanth, G.; Vasudeva, R.; Ganeshaiah, K.N.; Shaanker, R.U. Rohitukine, a chromone alkaloid and a precursor of flavopiridol, is produced by endophytic fungi isolated from Dysoxylum binectariferum Hook.f and Amoora rohituka (Roxb). Wight & Arn. Phytomedicine 2014, 21, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kong, F.; Wei, J.; Wang, Y.; Wang, W.; Hong, K.; Zhu, W. Alkaloids from the mangrove-derived actinomycete Jishengella endophytica 161111. Mar. Drugs 2014, 12, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Shrivastava, D.; Govil, S.; Kumar, S.; Bisen, P.S. A Novel Anticandidal Compound Containing Sulfur from Endophytic Fungus Emericella sp. Nat. Prod. J. 2016, 6, 188–193. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.M.; Cui, C.M.; Li, C.S.; Sun, H.; Wang, B.G. Sesquiterpene and acetogenin derivatives from the marine red alga Laurencia okamurai. Mar. Drugs 2012, 10, 2817–2825. [Google Scholar] [CrossRef]
- Dorta, E.; Díaz-Marrero, A.R.; Cueto, M.; D’Croz, L.; Maté, J.L.; Darias, J. Chamigrenelactone, a polyoxygenated sesquiterpene with a novel structural type and devoid of halogen from Laurencia obtusa. Tetrahedron Lett. 2004, 45, 7065–7068. [Google Scholar] [CrossRef]
- Suzuki, M.; Daitoh, M.; Vairappan, C.S.; Tsuyoshi, A.; Masuda, M. Novel halogenated metabolites from the Malaysian Laurencia pannosa. J. Nat. Prod. 2001, 64, 597–602. [Google Scholar] [CrossRef]
- Guella, G.; Öztunç, A.; Mancini, I.; Pietra, F. Stereochemical features of sesquiterpene metabolites as a distinctive trait of red seaweeds in the genus Laurencia. Tetrahedron Lett. 1997, 38, 8261–8264. [Google Scholar] [CrossRef]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombrú, A.W.; Saldaña, J.; Domínguez, L.; Coll, J.; Fujii, M.T.; Manta, E. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J. Nat. Prod. 2001, 64, 1552–1555. [Google Scholar] [CrossRef]
- Juagdan, E.G.; Kalidindi, R.; Scheuer, P. Two new chamigranes from an Hawaiian red alga, Laurencia cartilaginea. Tetrahedron 1997, 53, 521–528. [Google Scholar] [CrossRef]
- De Carvalho, L.R.; Fujii, M.T.; Roque, N.F.; Kato, M.J.; Lago, J.H.G. Aldingenin A, new brominated sesquiterpene from red algae Laurencia aldingensis. Tetrahedron Lett. 2003, 44, 2637–2640. [Google Scholar] [CrossRef]
- Vrabcheva, T.; Usleber, E.; Dietrich, R.; Märtlbauer, E. Co-occurrence of ochratoxin A and citrinin in cereals from bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000, 48, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Beno, M.A.; Christoph, G.G.; Cox, R.H.; Wells, J.M.; Cole, R.J.; Kirksey, J.W. Structure of a New [11]Cytochalasin, Cytochalasin H or Kodo-cytochalasin-1. J. Am. Chem. Soc. 1977, 99, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Daletos, G.; Okoye, F.; Lai, D.; Dai, H.; Proksch, P. A new cytotoxic cytochalasin from the endophytic fungus Trichoderma harzianum. Nat. Prod. Commun. 2015, 10, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Elsässer, B.; Krohn, K.; Flörke, U.; Root, N.; Aust, H.J.; Draeger, S.; Schulz, B.; Antus, S.; Kurtán, T. X-ray structure determination, absolute configuration and biological activity of Phomoxanthone A. Eur. J. Org. Chem. 2005, 2005, 4563–4570. [Google Scholar] [CrossRef]
- Akone, S.H.; El Amrani, M.; Lin, W.; Lai, D.; Proksch, P. Cytosporins F-K, new epoxyquinols from the endophytic fungus Pestalotiopsis theae. Tetrahedron Lett. 2013, 54, 6751–6754. [Google Scholar] [CrossRef]
- Nischitha, R.; Shivanna, M.B. Antimicrobial activity and metabolite profiling of endophytic fungi in Digitaria bicornis (Lam) Roem. and Schult. and Paspalidium flavidum (Retz.) A. Camus. 3 Biotech 2021, 11, 1–15. [Google Scholar] [CrossRef]
- de Carvalho, C.R.; Maia, M.Q.; Sobral, M.; Pereira, G.M.D.; da Silva, K.; Vital, M.J.S.; Zilli, J.É.; Rosa, C.A.; Rosa, L.H. Diversity and antimicrobial activity of culturable endophytic fungi associated with the neotropical ethnomedicinal plants Copaifera langsdorffii and Copaifera pubiflora. S. Afr. J. Bot. 2021, 142, 305–315. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef]
- Sette, L.D.; Passarini, M.R.Z.; Delarmelina, C.; Salati, F.; Duarte, M.C.T. Molecular characterization and antimicrobial activity of endophytic fungi from coffee plants. World J. Microbiol. Biotechnol. 2006, 22, 1185–1195. [Google Scholar] [CrossRef]
- Ramos, H.P.; Braun, G.H.; Pupo, M.T.; Said, S. Antimicrobial activity from endophytic fungi Arthrinium state of Apiospora montagnei Sacc. and Papulaspora immersa. Braz. Arch. Biol. Technol. 2010, 53, 629–632. [Google Scholar] [CrossRef]
- Vaz, A.B.M.; Mota, R.C.; Bomfim, M.R.Q.; Vieira, M.L.A.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Antimicrobial activity of endophytic fungi associated with Orchidaceae in Brazil. Can. J. Microbiol. 2009, 55, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Phongpaichit, S.; Rungjindamai, N.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol. Med. Microbiol. 2006, 48, 367–372. [Google Scholar] [CrossRef]
- Sang, X.N.; Chen, S.F.; Tang, M.X.; Wang, H.F.; An, X.; Lu, X.J.; Zhao, D.; Wang, Y.B.; Bai, J.; Hua, H.M.; et al. α-Pyrone derivatives with cytotoxic activities, from the endophytic fungus Phoma sp. YN02-P-3. Bioorganic Med. Chem. Lett. 2017, 27, 3723–3725. [Google Scholar] [CrossRef]
- Wang, L.-W.; Zhang, Y.-L.; Lin, F.-C.; Hu, Y.-Z.; Zhang, C.-L. Natural Products with Antitumor Activity from Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 1056–1074. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zhang, X.; Wang, X.H.; Zhao, C.Q. Novel natural compounds from endophytic fungi with anticancer activity. Eur. J. Med. Chem. 2018, 156, 316–343. [Google Scholar] [CrossRef]
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N.; et al. Endophytic fungi-alternative sources of cytotoxic compounds: A review. Front. Pharmacol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Janakiraman, V.; Govindarajan, K.; Magesh, C.R. Biosynthesis of Silver Nanoparticles from Endophytic Fungi, and its Cytotoxic Activity. Bionanoscience 2019, 9, 573–579. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Xing, J.; Corke, H.; Sun, M. A potential antioxidant resource: Endophytic fungi from medicinal plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Praptiwi; Raunsai, M.; Wulansari, D.; Fathoni, A.; Agusta, A. Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. J. Appl. Pharm. Sci. 2018, 8, 069–074. [Google Scholar] [CrossRef]
- Yadav, M.; Yadav, A.; Yadav, J.P. In Vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, S256–S261. [Google Scholar] [CrossRef]
- Manjunath Hulikere, M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus—Cladosporium cladosporioides. Process. Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Abuzaid, H.; Amin, E.; Moawad, A.; Ramadan, U.; Abdelmohsen; Hetta, M.; Mohammedl, R. Liquid Chromatography High-Resolution Mass Spectrometry Analysis, Phytochemical and Biological Study of Two Aizoaceae Plants Plants: A New Kaempferol Derivative from Trianthema portulacastrum L. Pharmacogn. Res. 2020, 10, 24–30. [Google Scholar]
- Stankov, S.V. Definition of Inflammation, Causes of Inflammation and Possible Anti-inflammatory Strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Widiyastuti, Y.; Sholikhah, I.Y.M.; Haryanti, S. Cytotoxic activities of ethanolic and dichloromethane extract of leaves, stems, and flowers of Jarong [Stachytarpheta jamaicensis (L.) Vahl.] on HeLa and T47D cancer cell line. AIP Conf. Proc. 2019, 2202, 020101. [Google Scholar] [CrossRef]
- Chen, S.; Ding, M.; Liu, W.; Huang, X.; Liu, Z.; Lu, Y.; Liu, H.; She, Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry 2018, 146, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Proksch, P. Mangrove derived fungal endophytes—A chemical and biological perception. Fungal Divers. 2013, 61, 1–27. [Google Scholar] [CrossRef]
- Stephenson, I.; Nicholson, K.G.; Gluck, R.; Mischler, R. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073. Lancet 2003, 362, 1959–1966. [Google Scholar] [CrossRef]
- Moradi, M.T.; Karimi, A.; Lorigooini, Z. Alkaloids as the natural anti-influenza virus agents: A systematic review. Toxin Rev. 2018, 37, 11–18. [Google Scholar] [CrossRef]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological activities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Simorangkir, M.; Nainggolan, B.; Juwitaningsih, T.; Silaban, S. The Toxicity of n-Hexane, Ethyl Acetate and Ethanol Extracts of SarangBanua (Clerodendrumfragrans Vent Willd) Leaves by Brine Shrimp Lethality Test (BSLT) Method. J. Phys. Conf. Ser. 2021, 1811, 012053. [Google Scholar] [CrossRef]
- Koul, O. Phytochemicals and insect control: An antifeedant approach. CRC. Crit. Rev. Plant Sci. 2008, 27, 1–24. [Google Scholar] [CrossRef]
- Rawat, L.S.; Narwal, S.S.; Kadiyan, H.S.; Maikhuri, R.K.; Negi, V.S.; Pharswan, D.S. Allelopathic effects of sunflower on seed germination and seedling growth of Trianthema portulacastrum. Allelopath. J. 2012, 30, 11–22. [Google Scholar]
- Yin, H.Y.; Yang, X.Q.; Wang, D.L.; De Zhao, T.; Wang, C.F.; Yang, Y.B.; Ding, Z.T. Antifeedant and antiphytopathogenic metabolites from co-culture of endophyte Irpex lacteus, phytopathogen Nigrospora oryzae, and entomopathogen Beauveria bassiana. Fitoterapia 2021, 148, 104781. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- d’Errico, G.; Aloj, V.; Flematti, G.R.; Sivasithamparam, K.; Worth, C.M.; Lombardi, N.; Ritieni, A.; Marra, R.; Lorito, M.; Vinale, F. Metabolites of a Drechslera sp. endophyte with potential as biocontrol and bioremediation agent. Nat. Prod. Res. 2021, 35, 4508–4516. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyani, Y.; Sinaga, S.E.; Supratman, U. Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family. Molecules 2023, 28, 778. https://doi.org/10.3390/molecules28020778
Mulyani Y, Sinaga SE, Supratman U. Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family. Molecules. 2023; 28(2):778. https://doi.org/10.3390/molecules28020778
Chicago/Turabian StyleMulyani, Yeni, Siska Elisahbet Sinaga, and Unang Supratman. 2023. "Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family" Molecules 28, no. 2: 778. https://doi.org/10.3390/molecules28020778
APA StyleMulyani, Y., Sinaga, S. E., & Supratman, U. (2023). Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family. Molecules, 28(2), 778. https://doi.org/10.3390/molecules28020778