Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments
Abstract
1. Introduction
2. Results and Discussion
2.1. Pharmacokinetic Profile (ADMET) and Key Pharmacokinetic Parameters
2.2. Tools for the Prediction of ADMET Parameters
- Websites change frequently because they are continuously being improved. Such mutability causes two types of problems: on the one hand, they are not always available to be used, and, on the other hand, the mathematical models can be modified, so the predictions can vary, as Sheridan pointed out in a recent paper [9]. In some cases, the web pages have simply disappeared during the preparation of this manuscript.
- Some websites, particularly those that allow for uploading a database including several molecules, may take a long time to complete the calculations (in one of the cases tested, 3 h were needed to complete the prediction of 24 compounds).
- In the final list, it is possible to find specific platforms for a specific pharmacokinetic category, such as MetaTox, NERDD, or XenoSite, which only predict metabolic properties, or DrugLogit, which predicts drug-likeness character. The opposite happens with the ADMETlab, admetSAR, and pkCSM platforms, which predict at least one parameter from each ADMET category.
| Drug-Likeness | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMES | ||||||||||||||||||
| hERG | ||||||||||||||||||
| Carcinogenicity | ||||||||||||||||||
| Acute toxicity | ||||||||||||||||||
| t1/2 | ||||||||||||||||||
| Cl | ||||||||||||||||||
| Sites | ||||||||||||||||||
| HLMS | ||||||||||||||||||
| Metabolites | ||||||||||||||||||
| CYP450 | ||||||||||||||||||
| PPB | ||||||||||||||||||
| BBB | ||||||||||||||||||
| Pgp | ||||||||||||||||||
| HOB | ||||||||||||||||||
| HIA | ||||||||||||||||||
| Caco-2 | ||||||||||||||||||
| pKa | ||||||||||||||||||
| log S | ||||||||||||||||||
| log P | ||||||||||||||||||
| WEBSERVERS | ADMETlab [10,11,12] | admetSAR [13] | BioTransformer 3.0 [14,15] | CYP Rules [16] | Drug Logit [17] | FAF-Drugs4 [18,19,20] | Lazar [21] | MetaTox [22] | NERDD [23,24] | OCHEM [25,26] | OSCADD [27] | pKCSM [28] | PreADMET [29,30] | SmartCyp [31,32] | SwissADME [33] | vNN-ADMET [34] | Way2Drug [35] | XenoSite [36] |
- 4.
- A quick look at Table 2 shows that none of the web servers predict the pKa of a molecule. This is probably one of the most difficult parameters to be found on a free website. In the past years, there are many examples of web pages that were very useful for the prediction of pKa that simply disappeared from one day to another, probably as a result of them being included as a part of commercial software. Recently, the web server MolGpka [37], for the prediction of the pKa of small molecules using a graph-convolutional neural network, has come to help academic researchers, but, for instance, it is not capable of predicting the pKa of an α-carbonyl methylene group.
- 5.
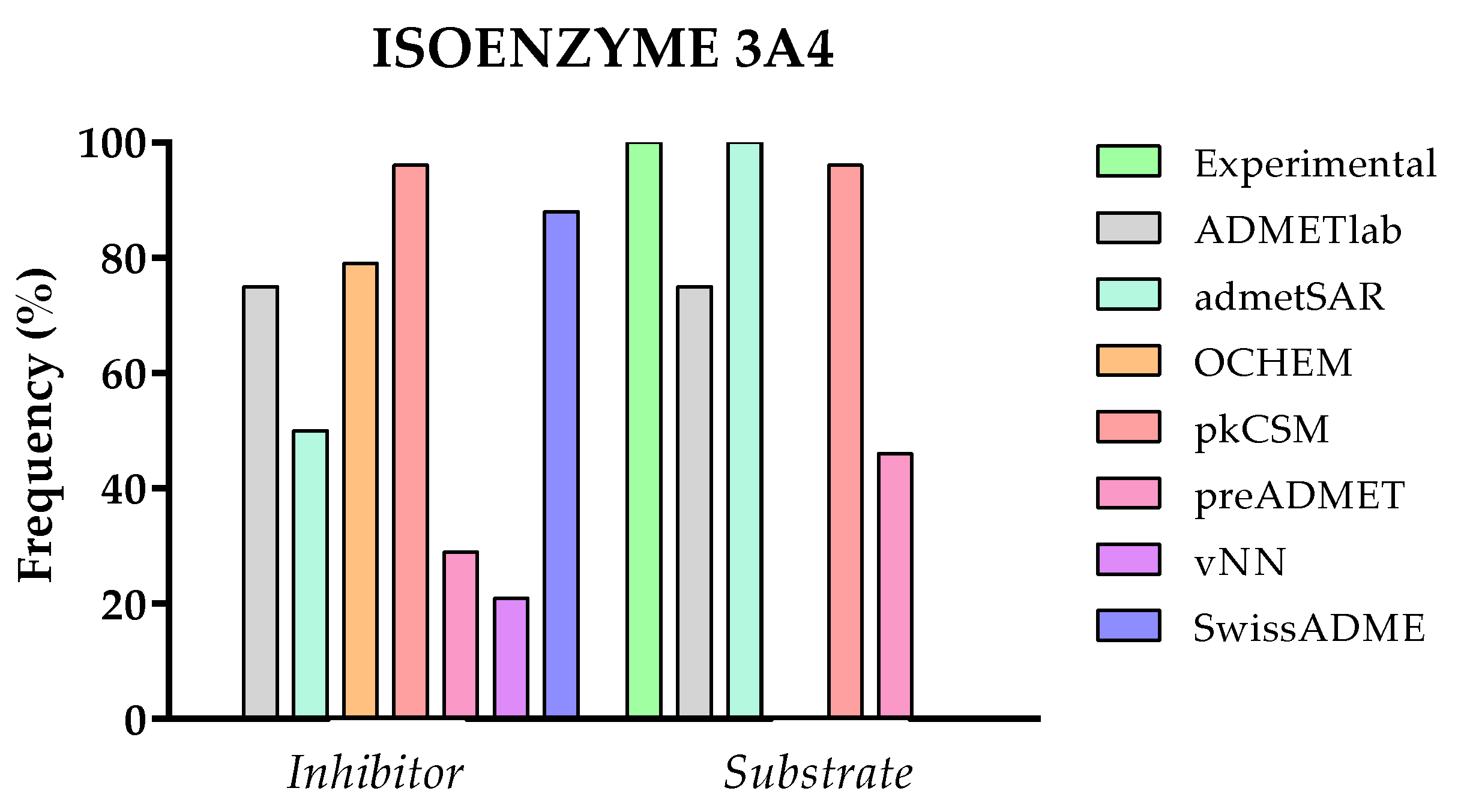
- From Table 2, it is also possible to conclude that metabolism is the most predicted category and, within this, the ability of a drug to interact with CYP450 enzymes as a substrate or inhibitor. However, during the early stages of the drug development process, the information related to metabolic stability and the site of metabolism is more relevant for researchers. This type of information is only available on a few specific websites (BioTransformer 3.0, NERDD, and SmartCyp).
- 6.
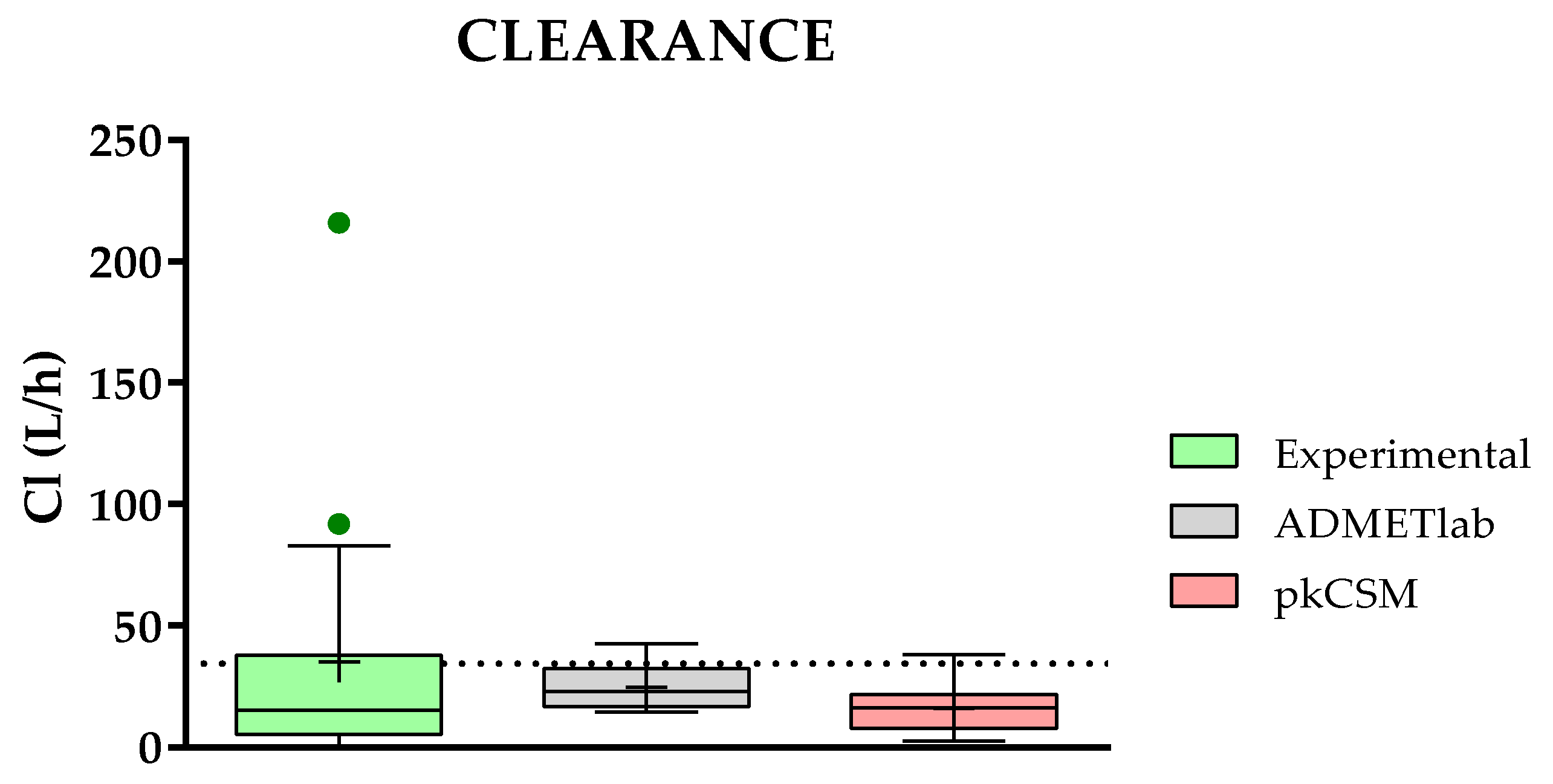
- Just the opposite situation happens with the prediction of the elimination parameters (clearance and half-life time) that are only available on two web servers: ADMETlab and pkCSM.
- 7.
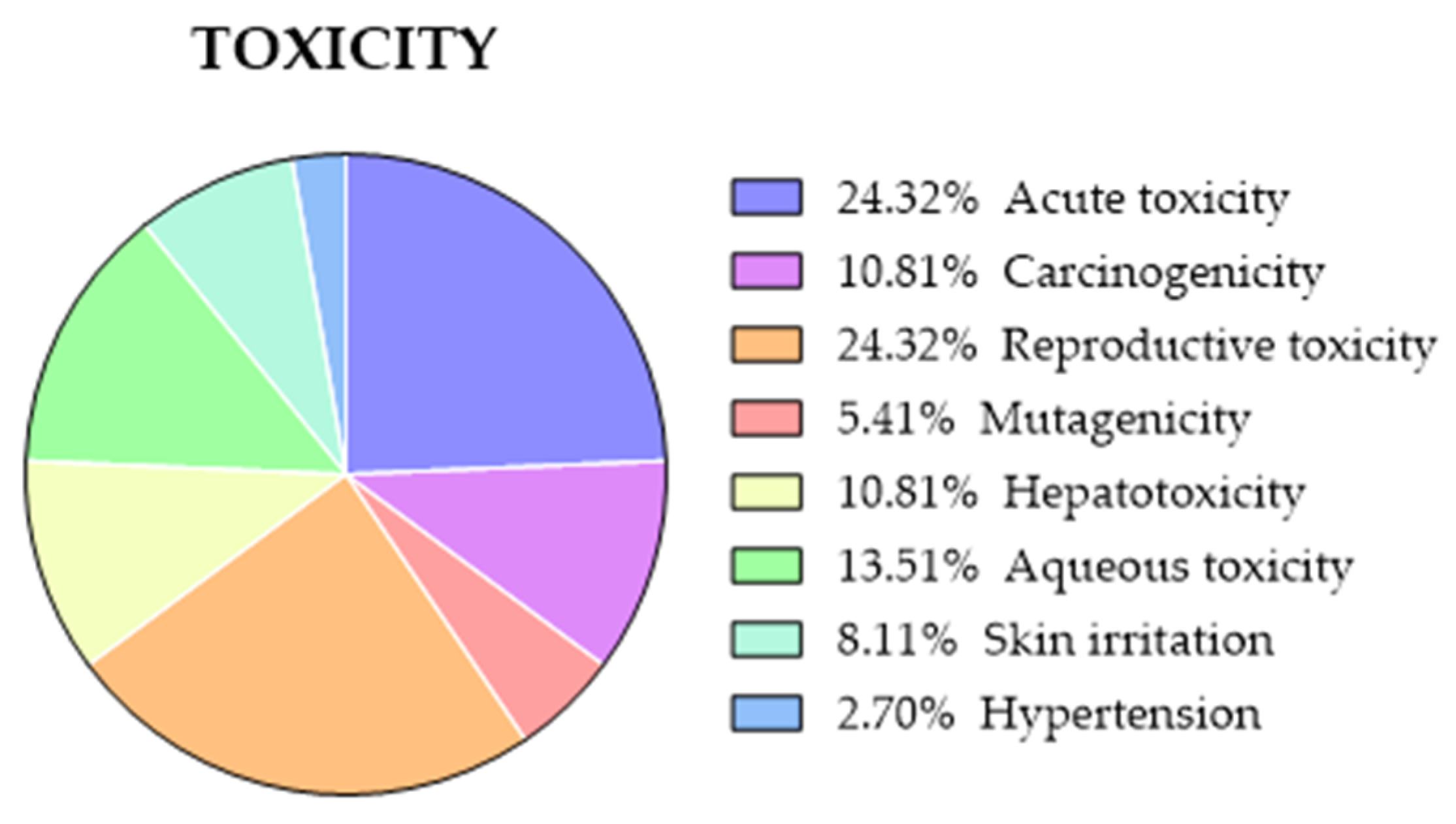
- Many websites offer several two-dimensional descriptors (number of heavy atoms, number of rotatable bonds, H-bridges, total polar surface area (TPSA), etc.) that would not be relevant in many cases. Moreover, other websites, especially those that predict toxicity, include long lists of toxicity parameters of difficult interpretation. So, for this research, we have only considered four toxicity parameters: acute toxicity, carcinogenicity, mutagenicity (Ames), and cardiotoxicity (hERG).
- 8.
- It is worth mentioning that few web servers predict pharmacodynamic properties. For example, Lazar predicts the lowest observed adverse effect level and the maximum recommended daily dose, which are not considered for this study. In this sense, the main toxicity parameters mentioned in point 7 are included in the study of the pharmacodynamic drug profile.
- 9.
- While the use of such websites for the teaching of Medicinal Chemistry is straightforward, the prediction of ADMET properties for compounds under investigation could be problematic due to confidentiality issues. The truth is that only a few platforms include a disclaimer statement saying that the uploaded structures will not be used in any way by the provider of the service. For instance, in the Terms of Use section of SwissADME (provided by the Swiss Institute of Bioinformatics, SIB), it is clearly stated that “SIB is committed to ensuring your privacy and the confidentiality of your personal data”, and a message on the pkCSM website announces that “no molecule information will be retained on the system after being uploaded by the user”.
2.3. Comparative Evaluation of the Goodness of the Predictions of the Different Websites
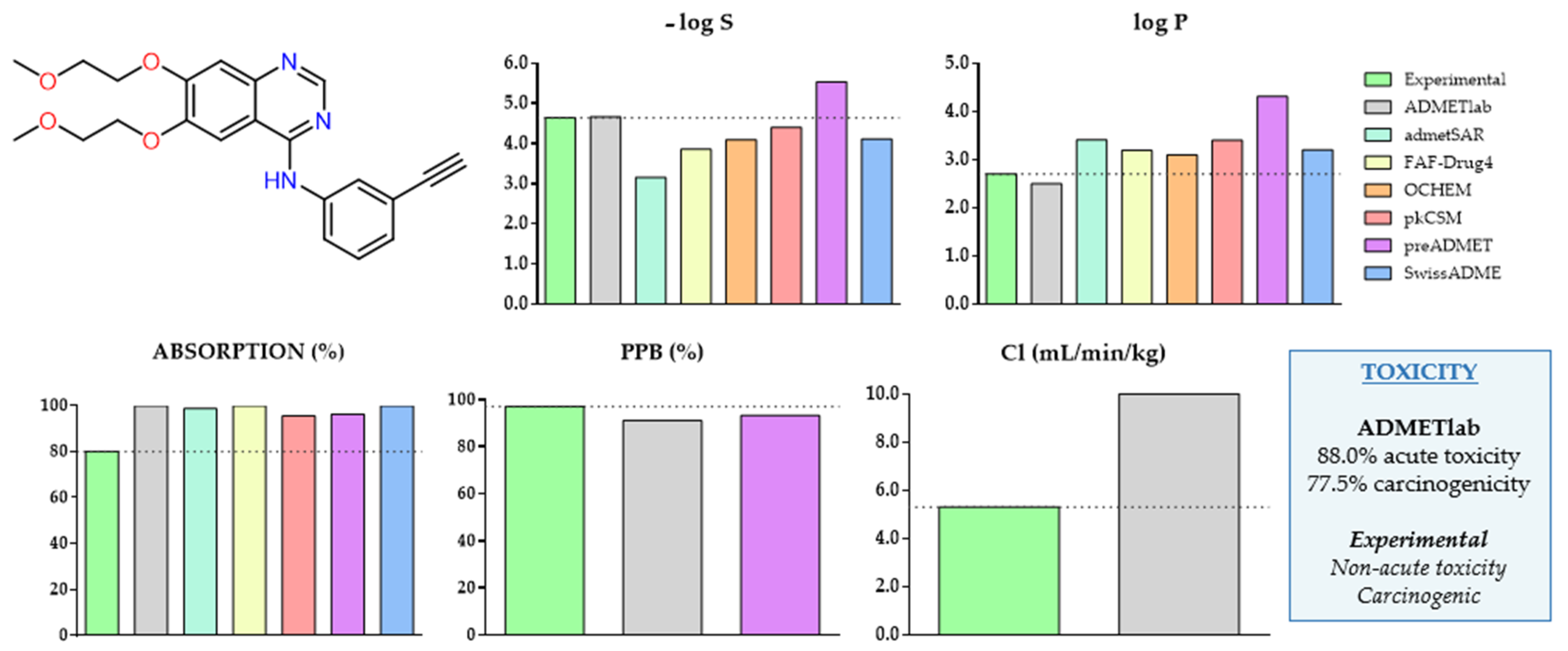
2.3.1. Erlotinib as a Case Study
2.3.2. Comparison of Predicted and Experimental Solubilities (log S) for a Group of TKIs
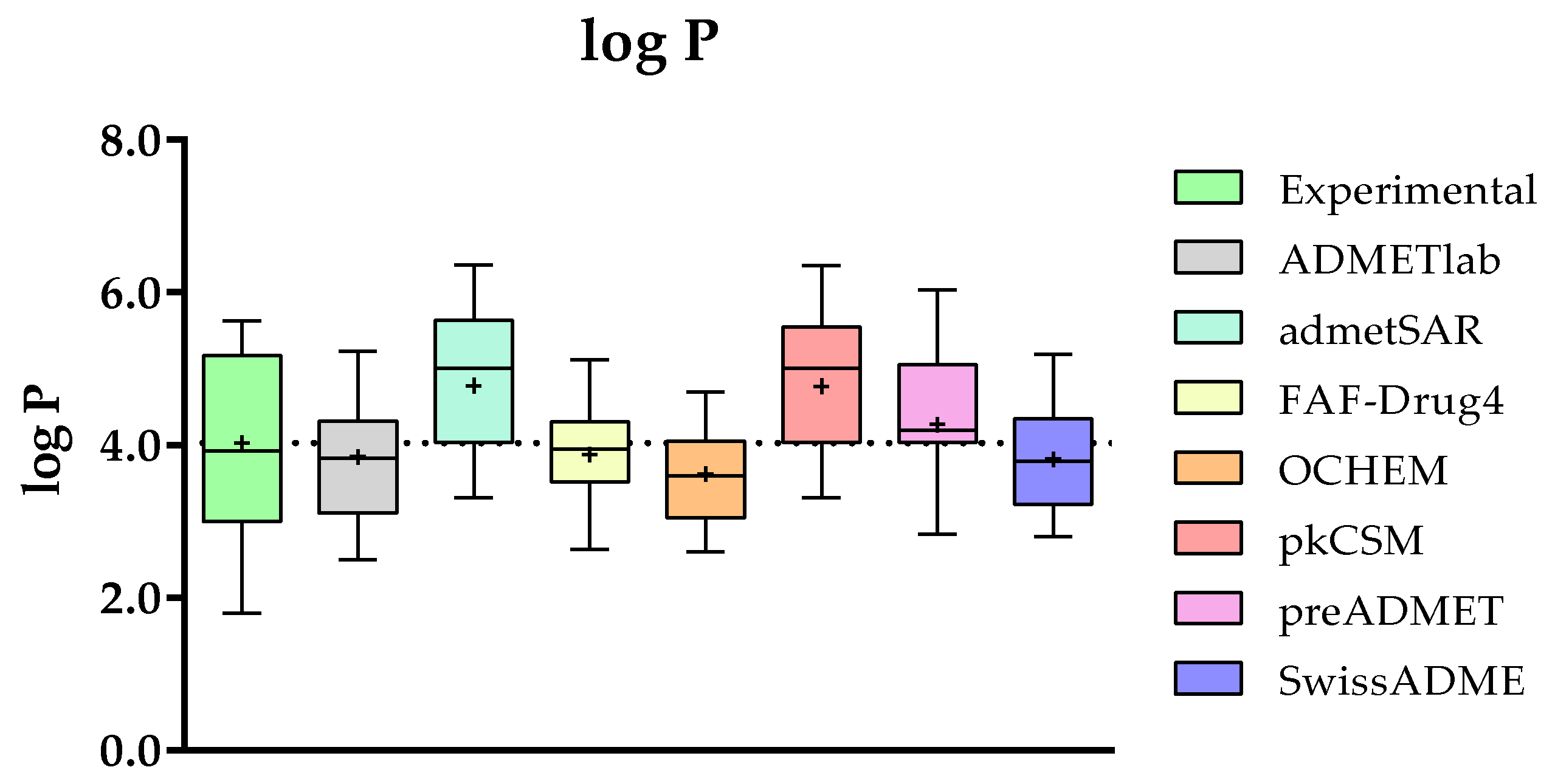
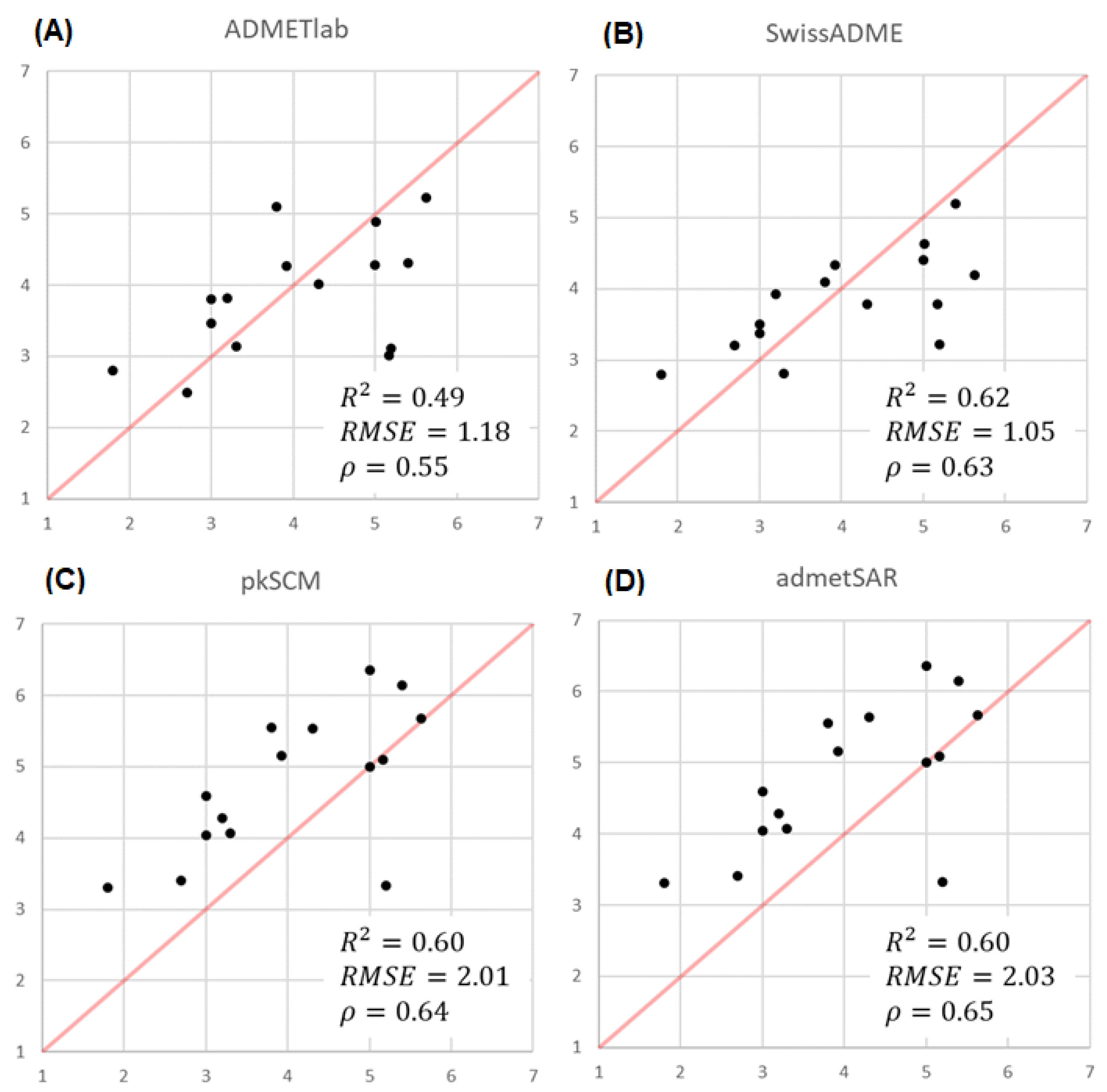
2.3.3. Comparison of the Predicted and Experimental log P for a Group of TKIs
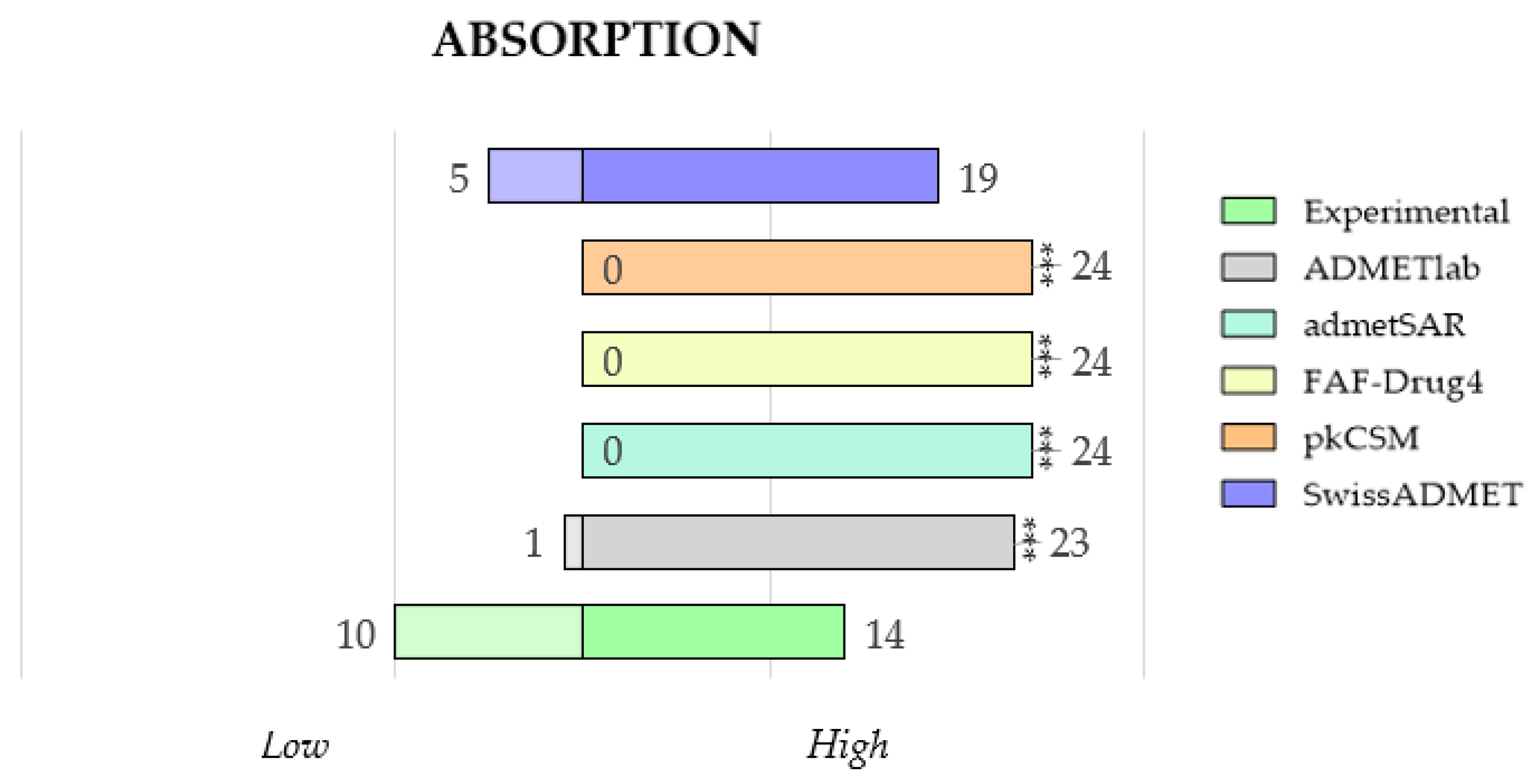
2.3.4. Comparison of Predicted and Experimental Absorption (HIA) for a Group of TKIs
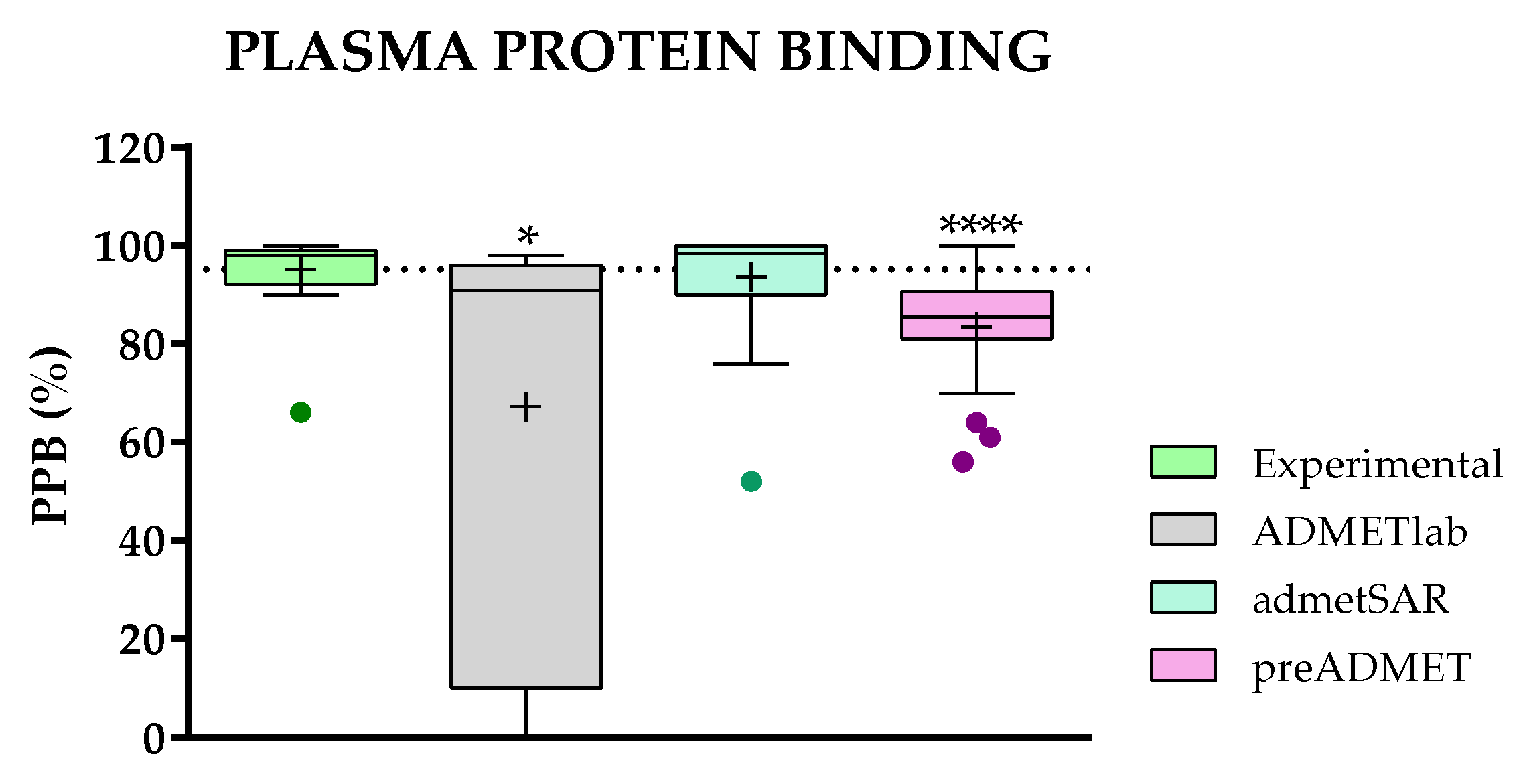
2.3.5. Comparison of Predicted and Experimental Plasma Protein Binding (PPB) for a Group of TKIs
2.3.6. Comparison of Predicted and Experimental Metabolism for a Group of TKIs
2.3.7. Comparison of Predicted and Experimental Clearance (Cl) for a Group of TKIs
2.3.8. Comparison of Predicted and Experimental Toxicity for a Group of TKIs
3. Materials and Methods
4. Conclusions
- None of the webservers cover all the physicochemical and pharmacokinetic parameters considered relevant in this study (Table 1). So, in many cases, the simultaneous use of several platforms for the prediction of the required parameters will be needed. ADMETLab is, without a doubt, the one that offers the best coverage of these parameters and the one that offers the best accuracy and precision in the predictions.
- As mentioned above, the volatility of such free web servers is extremely high, both due to changes in the calculation methodologies and due to the conversion of some of them into commercial software. Each academic or small biotech group interested in using such platforms must carefully select those more stable and more appropriate for their research and monitor the possible changes in the calculation methods.
- Only a few of the 18 web servers considered guarantee the confidentiality of the structures uploaded for prediction, a condition that is important when developing a new drug candidate. Such a lack of an explicit compromise of confidentiality can set back many academic research groups in the use of these platforms. In our opinion, if only a structure of a molecule is uploaded, it is difficult for it to damage the confidentiality of the project because hundreds are probably uploaded each day; however, if a database containing several similar structures is uploaded, the risk of the unauthorized use of such structures is certainly higher. We strongly suggest that all web servers include a confidentiality statement. If confidentiality is an unnegotiable requirement, two options are available: pkCSM and SwissADME. pkCSM covers a larger range of parameters, at least one of each pharmacokinetic category, but has a lower predictive ability than SwissADME, which is extremely good at covering physicochemical and absorption properties.
- Concerning the results of such comparisons, octanol/water coefficient and plasma protein binding are the two best-predicted parameters, followed by clearance. On the contrary, solubility and absorption show the worst predicted results due to the complexity of the phenomena involved.
- Predictions will not avoid the experimental evaluation of candidates during the drug development process, but they can help reduce the number of compounds being studied by offering a global vision of the pharmacokinetic profile of a drug at a low cost and in a short time. More importantly, if a compound is going to fail as a drug, predictions can help in doing it early as possible.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocci, G.; Carosati, E.; Vayer, P.; Arrault, A.; Lozano, S.; Cruciani, G. ADME-Space: A New Tool for Medicinal Chemists to Explore ADME Properties. Sci. Rep. 2017, 7, 6359. [Google Scholar] [CrossRef] [PubMed]
- Pantaleão, S.Q.; Fernandes, P.O.; Gonçalves, J.E.; Maltarollo, V.G.; Honorio, K.M. Recent Advances in the Prediction of Pharmacokinetics Properties in Drug Design Studies: A Review. ChemMedChem 2022, 17, e202100542. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Kola, I. The State of Innovation in Drug Development. Clin. Pharmacol. Ther. 2008, 83, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.D.Y.; Terry, D.B.; Smith, L.H. In Vitro and In Vivo Assessment of ADME and PK Properties During Lead Selection and Lead Optimization-Guidelines, Benchmarks and Rules of Thumb. 2015. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company: Indianapolis, IN, USA; The National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Issa, N.T.; Wathieu, H.; Ojo, A.; Byers, S.W.; Dakshanamurthy, S. Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools. Curr. Drug Metab. 2017, 18, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Desai, V.; Arimoto, R.; Desino, K.E.; Fischer, H.; Keefer, C.E.; Petersson, C.; Winiwarter, S.; Broccatelli, F.; Desai, P.V. In Silico Absorption, Distribution, Metabolism, Excretion and Pharmacokinetics (ADME-PK): Utility and Best Practices-An Industry Perspective from the International Consortium for Innovation through Quality in Pharmaceutical Development. J. Med. Chem. 2017, 60, 9097–9113. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S. In Silico ADME-Tox Modeling: Progress and Prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef]
- Sheridan, R.P. Stability of Prediction in Production ADMET Models as a Function of Version: Why and When Predictions Change. J. Chem. Inf. Model. 2022, 62, 3477–3485. [Google Scholar] [CrossRef]
- ADMETlab 2.0 Documentation. Available online: https://admetmesh.scbdd.com/ (accessed on 1 January 2022).
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.N.; Yao, Z.J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.P.; Cao, D.S. Admetlab: A Platform for Systematic ADMET Evaluation Based on a Comprehensively Collected ADMET Database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef]
- User Guide for AdmetSAR 2.0. Available online: http://lmmd.ecust.edu.cn/admetsar2/ (accessed on 1 January 2022).
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A Comprehensive Computational Tool for Small Molecule Metabolism Prediction and Metabolite Identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Willighagen, E.L.; Mayfield, J.W.; Alvarsson, J.; Berg, A.; Carlsson, L.; Jeliazkova, N.; Kuhn, S.; Pluskal, T.; Rojas-Chertó, M.; Spjuth, O.; et al. The Chemistry Development Kit (CDK) v2.0: Atom Typing, Depiction, Molecular Formulas, and Substructure Searching. J. Cheminform. 2017, 9, 33. [Google Scholar] [CrossRef]
- Shao, C.-Y.; Su, B.-H.; Tu, Y.-S.; Lin, C.; Lin, O.A.; Tseng, Y.J. CypRules: A Rule-Based P450 Inhibition Prediction Server. Bioinformatics 2015, 31, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- García-Sosa, A.T.; Oja, M.; Hetényi, C.; Maran, U. DrugLogit: Logistic Discrimination between Drugs and Nondrugs Including Disease-Specificity by Assigning Probabilities Based on Molecular Properties. J. Chem. Inf. Model. 2012, 52, 2165–2180. [Google Scholar] [CrossRef]
- Miteva, M.A.; Violas, S.; Montes, M.; Gomez, D.; Tuffery, P.; Villoutreix, B.O. FAF-Drugs: Free ADME/Tox Filtering of Compound Collections. Nucleic Acids Res. 2006, 34, W738–W744. [Google Scholar] [CrossRef]
- Lagorce, D.; Sperandio, O.; Galons, H.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs2: Free ADME/Tox Filtering Tool to Assist Drug Discovery and Chemical Biology Projects. BMC Bioinform. 2008, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A Web Server for Compound Property Calculation and Chemical Library Design. Nucleic Acids Res. 2015, 43, W200–W207. [Google Scholar] [CrossRef] [PubMed]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. Lazar: A Modular Predictive Toxicology Framework. Front. Pharmacol. 2013, 4, 38. [Google Scholar] [CrossRef]
- Rudik, A.v.; Bezhentsev, V.M.; Dmitriev, A.v.; Druzhilovskiy, D.S.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. MetaTox: Web Application for Predicting Structure and Toxicity of Xenobiotics’ Metabolites. J. Chem. Inf. Model. 2017, 57, 638–642. [Google Scholar] [CrossRef]
- Šícho, M.; Stork, C.; Mazzolari, A.; de Bruyn Kops, C.; Pedretti, A.; Testa, B.; Vistoli, G.; Svozil, D.; Kirchmair, J. FAME 3: Predicting the Sites of Metabolism in Synthetic Compounds and Natural Products for Phase 1 and Phase 2 Metabolic Enzymes. J. Chem. Inf. Model. 2019, 59, 3400–3412. [Google Scholar] [CrossRef]
- Stork, C.; Embruch, G.; Šícho, M.; de Bruyn Kops, C.; Chen, Y.; Svozil, D.; Kirchmair, J. NERDD: A Web Portal Providing Access to in Silico Tools for Drug Discovery. Bioinformatics 2019, 36, 1291–1292. [Google Scholar] [CrossRef]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.v.; Tanchuk, V.Y.; et al. Online Chemical Modeling Environment (OCHEM): Web Platform for Data Storage, Model Development and Publishing of Chemical Information. J. Comput. Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Sushko, I.; Salmina, E.; Potemkin, V.A.; Poda, G.; Tetko, I.V. ToxAlerts: A Web Server of Structural Alerts for Toxic Chemicals and Compounds with Potential Adverse Reactions. J. Chem. Inf. Model. 2012, 52, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.K.; Agarwal, S.; Raghava, G.P. Prediction of Cytochrome P450 Isoform Responsible for Metabolizing a Drug Molecule. BMC Pharmacol. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, I.H.; Kim, H.J.; Chang, G.S.; Chung, J.E. The PreADMET Approach: Web-Based Program for Rapid Prediction of Physico-Chemical, Drug Absorption and Drug-like Properties. EuroQSAR 2003, 2003, 418–420. [Google Scholar]
- Lee, S.K.; Chang, G.S.; Lee, I.H.; Chung, J.E.; Sung, K.Y.; No, K.T. The PreADMET: PC-Based Program for Batch Prediction of ADME Properties. EuroQSAR 2004, 9, 5–10. [Google Scholar]
- Rydberg, P.; Gloriam, D.E.; Zaretzki, J.; Breneman, C.; Olsen, L. SMARTCyp: A 2D Method for Prediction of Cytochrome P450-Mediated Drug Metabolism. ACS Med. Chem. Lett. 2010, 1, 96–100. [Google Scholar] [CrossRef]
- Rydberg, P.; Olsen, L. Predicting Drug Metabolism by Cytochrome P450 2C9: Comparison with the 2D6 and 3A4 Isoforms. ChemMedChem 2012, 7, 1202–1209. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Schyman, P.; Liu, R.; Desai, V.; Wallqvist, A. VNN Web Server for ADMET Predictions. Front. Pharmacol. 2017, 8, 889. [Google Scholar] [CrossRef]
- Rudik, A.; Dimitriev, A.; Lagunin, A.; Filimonov, D.; Poroikov, V. SOMP: Web-Service for in Silico Prediction of Sites of Metabolism for Drug-like Compounds. Bioinformatics 2015, 31, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Zaretzki, J.; Matlock, M.; Swamidass, S.J. XenoSite: Accurately Predicting Cyp-Mediated Sites of Metabolism with Neural Networks. J. Chem. Inf. Model. 2013, 53, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpka: A Web Server for Small Molecule PKaPrediction Using a Graph-Convolutional Neural Network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Schettino, C.; Bareschino, M.A.; Ricci, V.; Ciardiello, F. Erlotinib: An EGF Receptor Tyrosine Kinase Inhibitor in Non-Small-Cell Lung Cancer Treatment. Expert Rev. Respir. Med. 2008, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Wang, R.; Gao, Y.; Lai, L. Calculating Partition Coefficient by Atom-Additive Method. Perspect. Drug Discov. Des. 2000, 19, 47–66. [Google Scholar] [CrossRef]
- Tetko, I.; Poda, G.; Ostermann, C.; Mannhold, R. Large-Scale Evaluation of Log P Predictors: Local Corrections May Compensate Insufficient Accuracy and Need of Experimentally Testing Every Other Compound. Chem. Biodivers. 2009, 6, 1837–1844. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of Log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. ILOGP: A Simple, Robust, and Efficient Description of n -Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Hollenberg, P.F. Characteristics and Common Properties of Inhibitors, Inducers, and Activators of CYP Enzymes. Drug Metab. Rev. 2002, 34, 17–35. [Google Scholar] [CrossRef]
- Van Waterschoot, R.A.B.; Schinkel, A.H. A Critical Analysis of the Interplay between Cytochrome P450 3A and P-Glycoprotein: Recent Insights from Knockout and Transgenic Mice. Pharmacol. Rev. 2011, 63, 390–410. [Google Scholar] [CrossRef]
- Testa, B.; Krämer, S.D. The Biochemistry of Drug Metabolism-An Introduction. Chem. Biodivers. 2007, 4, 257–405. [Google Scholar] [CrossRef]
- Kirchmair, J.; Göller, A.H.; Lang, D.; Kunze, J.; Testa, B.; Wilson, I.D.; Glen, R.C.; Schneider, G. Predicting Drug Metabolism: Experiment and/or Computation? Nat. Rev. Drug Discov. 2015, 14, 387–404. [Google Scholar] [CrossRef]
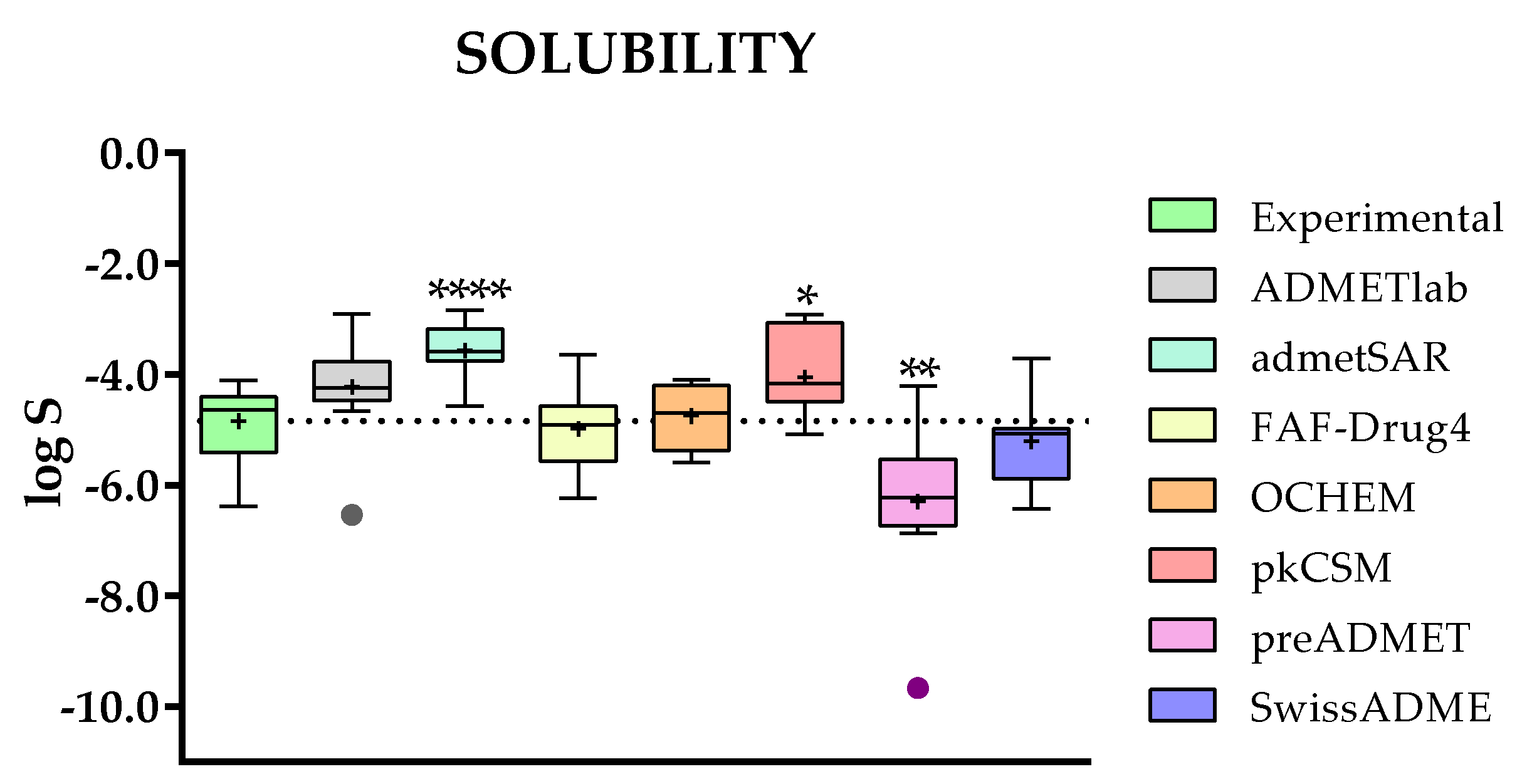
| ADMET | Description | Parameters |
|---|---|---|
| Physicochemical properties [a] | Intrinsic physical and chemical characteristics of a substance | log P, log S, pKa |
| Absorption [b] | Transportation of the unmetabolized drug from the site of administration to the body circulation system | Caco-2, HIA, HOB, Pgp |
| Distribution [c] | Reversible transfer of an unmetabolized drug through the body’s blood and tissues | BBB, PPB |
| Metabolism [d] | Biotransformation of pharmaceutical substances in the body so that they can be easily eliminated | CYP450, Metabolites, HLMS, Sites |
| Elimination [e] | The removal of an administered drug from the body | Cl, t1/2 |
| Toxicity [f] | The level of damage that a compound can inflict on an organism | Acute toxicity, carcinogenicity, hERG, Ames |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. https://doi.org/10.3390/molecules28020776
Dulsat J, López-Nieto B, Estrada-Tejedor R, Borrell JI. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules. 2023; 28(2):776. https://doi.org/10.3390/molecules28020776
Chicago/Turabian StyleDulsat, Júlia, Blanca López-Nieto, Roger Estrada-Tejedor, and José I. Borrell. 2023. "Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments" Molecules 28, no. 2: 776. https://doi.org/10.3390/molecules28020776
APA StyleDulsat, J., López-Nieto, B., Estrada-Tejedor, R., & Borrell, J. I. (2023). Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules, 28(2), 776. https://doi.org/10.3390/molecules28020776






