Abstract
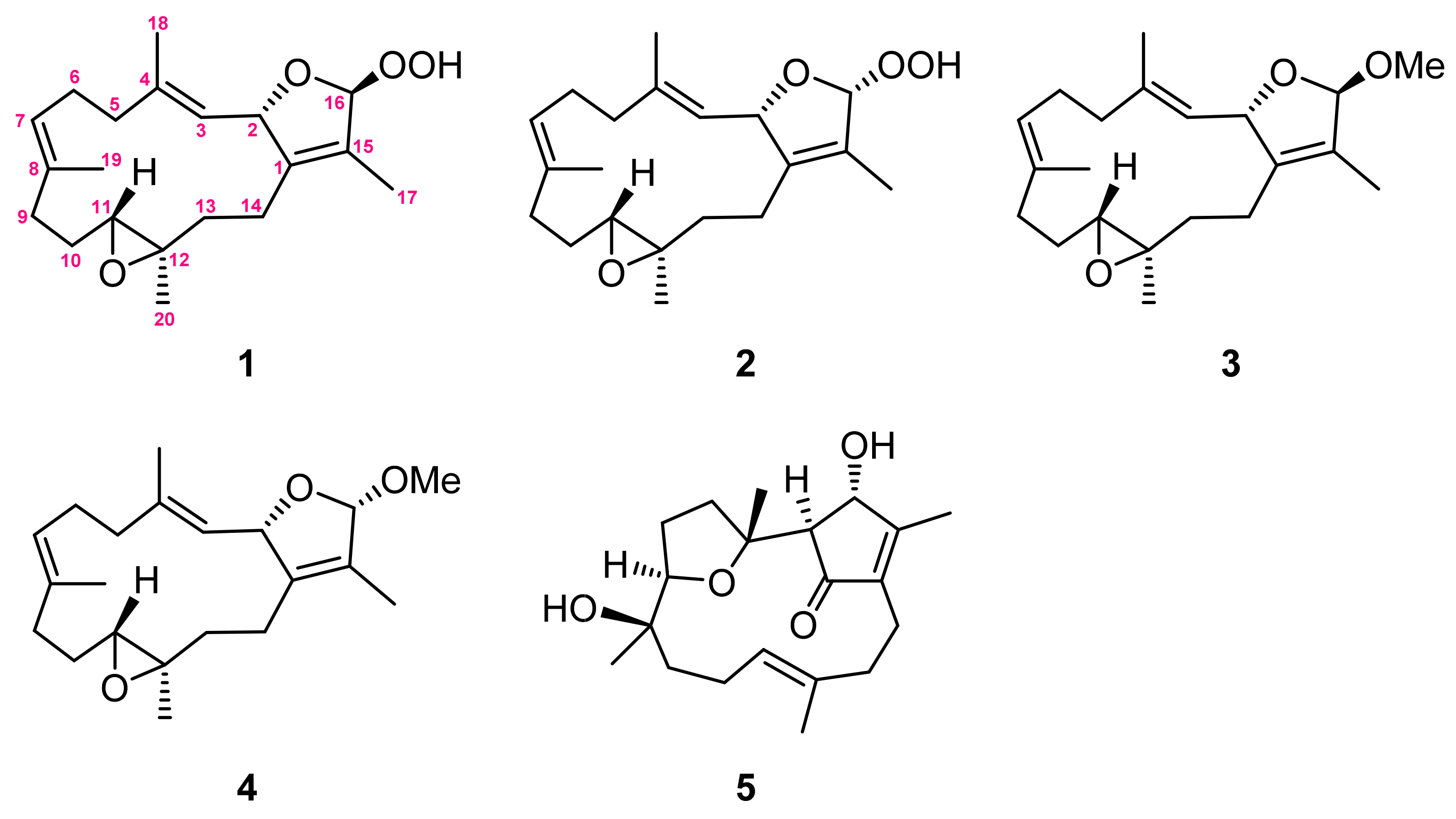
The present chemical investigation on the organic extract of the soft coral Sarcophyton cinereum has contributed to the isolation of four new cembranoids: 16β- and 16α-hydroperoxyisosarcophytoxides (1 and 2), 16β- and 16α-methoxyisosarcophytoxides (3 and 4), and a known cembranoid, lobocrasol (5). The structures of all isolates were elucidated by detailed spectroscopic analysis. Their structures were characterized by a 2,5-dihydrofuran moiety, of which the relative configuration was determined by DU8-based calculation for long-range coupling constants (4JH,H). The cytotoxicity and immunosuppressive activities of all isolates were evaluated in this study.
1. Introduction
Marine soft corals, in particular those of the Sarcophyton genus, have been considered to be a valuable reservoir of bioactive natural products, such as sesquiterpenoids, diterpenoids, steroids, and fatty acids [1]. Among them, the cembrane-type diterpenoids and related analogues are the most characteristic and abundant metabolites [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. These structurally diversified metabolites have been reported to possess various biological activities, such as cytotoxic [9,10,11], anti-inflammatory [9,10,11,12,13,14,15,16,17,18], neuroprotective [19], anti-bacterial [19,20,21], and anti-viral activities [19,22]. Also, some of the cembranids have shown the ability to protect the host against predators and stressful environments [7,8]. The chemical investigations of the Sarcophyton genus have been widely reported [1], and the high chemical diversity of cembranoids from this genus has been suggested as originating from different genetically distinct clades [4]. Recently, our chemical investigation led to the discovery of a new cembranoid with a novel skeleton, a new sarsolenone [10], and other cembranoids of various types, such as biscembranoids [11]. These results and the notable biological activities further prompted us to explore the unknown natural products from this soft coral. Our present chemical investigation of the S. cinereum has led to the isolation of four new cembranoids: 16β- and 16α-hydroperoxyisosarcophytoxides (1 and 2) and 16β- and 16α-methoxyisosarcophytoxides (3 and 4), as well as a previously reported one, lobocrasol (5) [23,24,25] (Figure 1). In this study, the cytotoxicity and immunosuppressive activity of all isolates are reported herein.
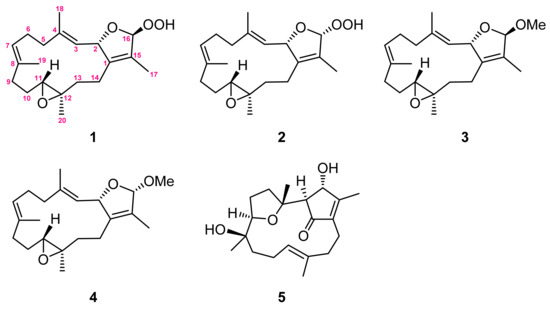
Figure 1.
Structures of metabolites 1–5.
2. Results and Discussion
The specimen of S. cinereum was extracted with EtOAc. The oily residue was repeatedly purified by column chromatography to afford four new compounds 1–4 and one known diterpene 5. The chemical structures of all metabolites were elucidated by analyzing IR, MS, 1D, and 2D NMR spectra, as well as J-based DU8 quantum chemical calculations (Supplementary Materials Figures S1–S32).
The HRESIMS of 16β-hydroperoxyisosarcophytoxide (1), white amorphous powder, showed a sodiated peak at m/z 357.2039 [M + Na]+ (calculated for 357.2037, C20H30O4Na), indicating a molecular formula of C20H30O4 and six degrees of unsaturation. IR spectrum displayed absorption bands at 3462 and 1660 cm−1, suggesting the presence of hydroxy and olefinic functional groups. The 13C NMR spectra showed 20 signals (Table 1), including four methyl groups, six sp3 methylenes, three sp3 methines, two sp2 methines, one sp3 quaternary carbon, and four sp2 quaternary carbons. The 1H and 13C NMR spectra revealed the signals characteristic of two tri-substituted double bonds (δH 4.95, d, J = 10.3 Hz; δC 126.5, CH; 141.0, C; and δH 4.84, d, J = 9.2 Hz; δC 125.9, CH; 133.8, C), one tetra-substituted double bond (δC 125.9, C; 141.9, C), one oxygen-bearing group (δH 5.59, d, J = 10.3 Hz; δC 83.2, CH), one acetal (δH 6.10, d, J = 3.3 Hz; δC 114.8, CH), and one tri-substituted epoxy group (δH 2.45, dd, J = 10.9, 2.7 Hz; δC 61.9, CH; 60.8, C).

Table 1.
1H (400 MHz) and 13C NMR (100 MHz) spectroscopic data of 1–4 in C6D6.
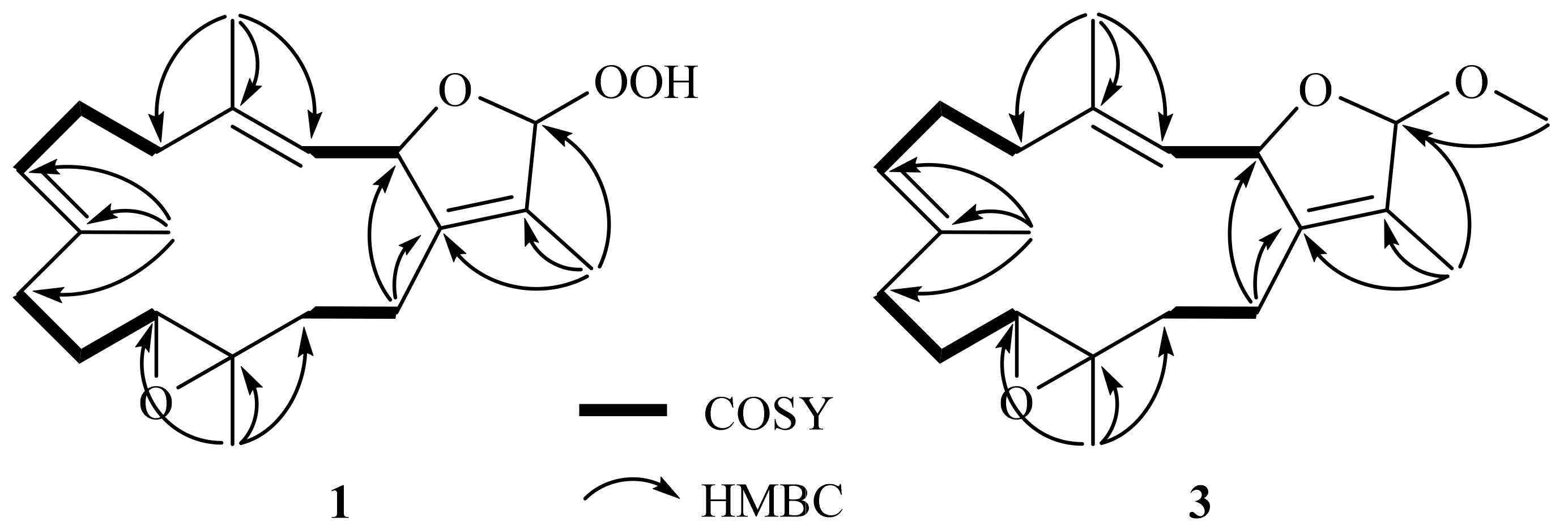
The gross structure of 1 was established by 2D NMR spectra, including correlation spectroscopy (COSY) and heteronuclear multiple bond correlation (HMBC) spectroscopic data (Figure 2). Analysis of COSY correlations suggested four partial structures from H-2 to H-3, H2-5 to H-7, H2-9 to H-11, and H2-13 to H2-14 (Figure 2). These moieties were connected by HMBC correlations of H3-17 to C-1, C-15, and C-16; H3-18 to C-3, C-4, and C-5; H3-19 to C-7, C-8, and C-9; H3-20 to C-11, C-12, and C-13; and H2-14 to C-1 (Figure 2). Although the HMBC correlation of H-16 (or H-2) to C-2 (or C-16) was not observed, the downfield shifts of C-2 and C-16 suggested an ether linkage between C-2 and C-16 to generate a 3-methyl-2,5-dihydrofuran moiety (Figure 2) [17]. Furthermore, the hydroperoxy group with the proton resonance appearing at δH 8.36 was assigned to attach at the acetal carbon C-16 due to its downfield chemical shifts.
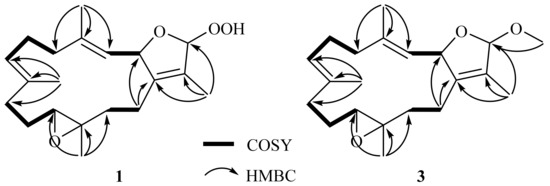
Figure 2.
COSY and selective HMBC correlations of 1 and 3.
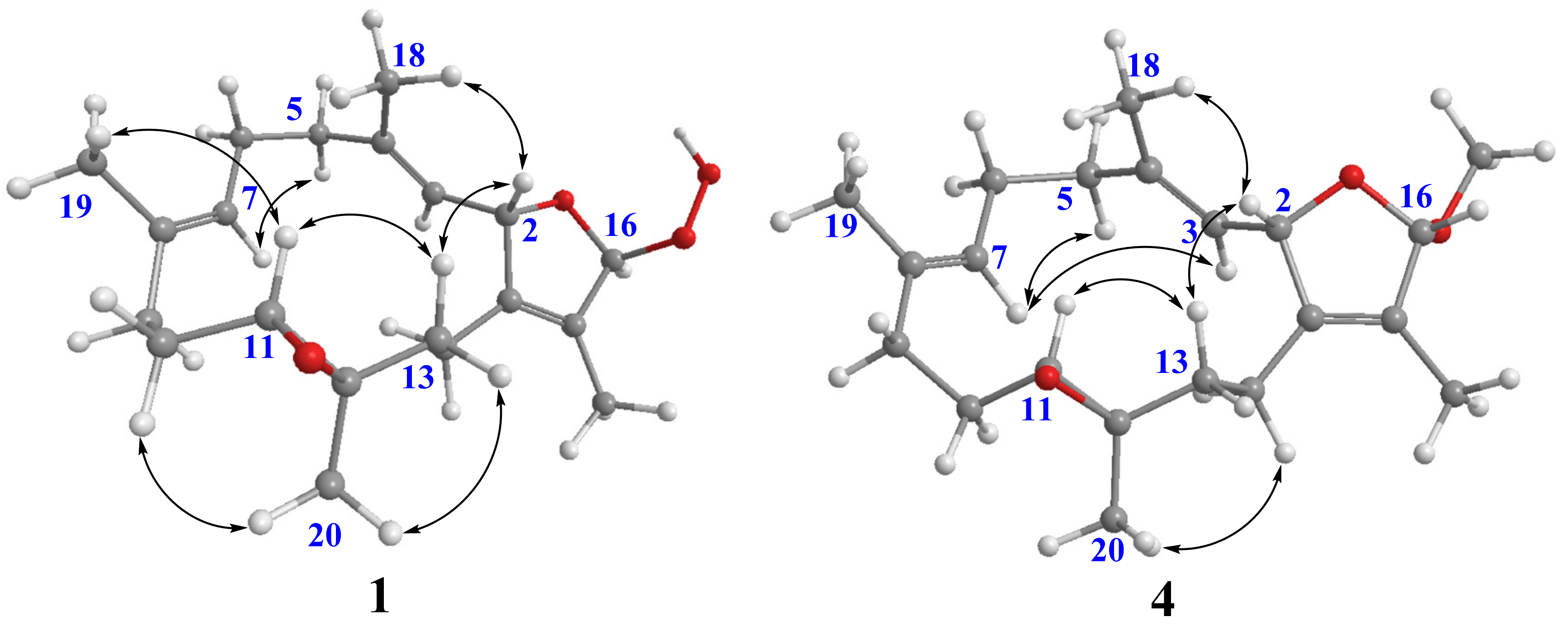
16β-Hydroperoxyisosarcophytoxide (2) was also isolated as a white amorphous powder. It was found to have the same molecular formula as that of 1. A comparison of the NMR data of 2 with those of 1 suggested that they are structurally related and should be epimeric at C-16. The NOE correlations of H-2/H-13β and H-11/H-13β were observed for both compounds, which were consistent with the related analogues possessing the same 2S*,11R*,12R*-configuration (Figure 3) [14]. The upfield chemical shifts for both C-18 and C-19 (δC 14.90 and δC 14.8, respectively, in compound 1; δC 14.8 and δC 14.9, respectively, in compound 2) indicated the E geometry of C-3/C-4 and C-7/C-8 double bonds. A comparison of their NMR data coupled with the above analysis supported 2 to be a C-16 epimer of 1. However, due to the lack of NOE correlations of H-16 with other protons in both compounds, the C-16 configurations of 1 and 2 were unable to be determined.
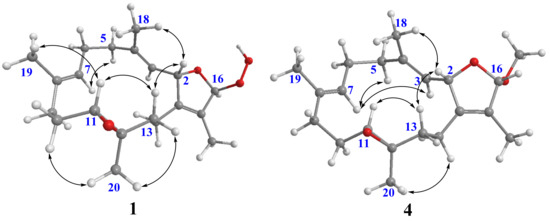
Figure 3.
Selective NOE correlations of 1 and 4.
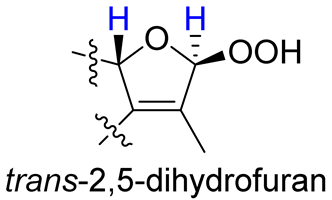
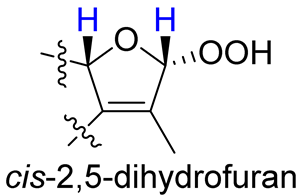
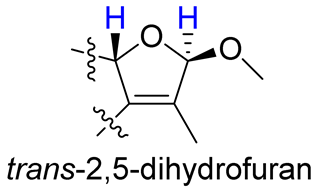
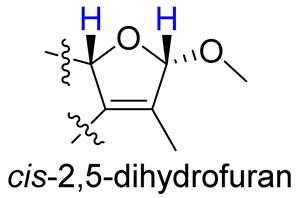
As compounds 1 and 2 exhibited very similar chemical shifts, the result of DP4+ probability analysis might become occasionally erratic and lead to a wrong assignment [26]. A comparison of their COSY spectra revealed that they showed distinct differences at cross peaks between H-2 and H-16 (Figures S7 and S23), in which the COSY cross peak of H-2/H-16 was observed only for compound 1. This implied that 1 has a larger long-range proton-proton coupling constant (4J2,16) than 2, which is also consistent with the experimental data (Table 1). In view of their differences observed in the COSY spectra and coupling patterns, a solution to the stereochemistry assignment was performed using the reported quantum chemical calculations for J-value based on the DU8 basis set [27]. The 4J2,16 coupling constants of 16α-H and 16β-H epimers (i.e., trans-2,5-dihydrofuran and cis-2,5-dihydrofuran analogues, Table 2) were calculated at B3LYP/DU8//B3LYP-D3(BJ)/6-31+G(d,p) level of theory, and calibrated by both empirical scaling parameters and the NBO hybridization coefficients. The resulting data were weighted by Boltzmann distribution using energies calculated at M06-2X/6-31+G(d,p)//B3LYP-D3(BJ)/6-31+G(d,p) level. The result revealed that the structure with trans-2,5-dihydrofuran moiety was found to have larger 4J2,16 coupling constants than that of the cis analogue (Table 2). Moreover, the calculated result also showed good agreement with the analysis of cis and trans 2,5-dihydrofuran analogues reported by Barfield et al. [28].

Table 2.
The calculated and experimental 4JH,H coupling constants, as well as experimental COSY correlations between H-2 and H-16 of compounds 1–4.
16β-Methoxyisosarcophytoxide (3), obtained as a white amorphous powder, with a sodiated ion peak at m/z 355.2245 (calculated 355.2244, C21H32O3Na) in the HRESIMS spectrum suggested a molecular formula of C21H32O3. The 13C NMR (Table 1) and DEPT spectra showed 21 signals, including five methyl groups, six methylenes, five methines, and five quaternary carbons. In the NMR spectral data (Table 1), the chemical shifts at δH 3.35 and δC 53.8 suggested the presence of a methoxy group. A detailed analysis of the NMR data of 3 suggested its close similarity with those of 1. Furthermore, the HMBC correlation of OMe/C-16 (δC 111.8) also demonstrated the attachment of the methoxy group to C-16 (Figure 2).
16α-Methoxyisosarcophytoxide (4), a white amorphous powder, exhibited the same molecular formula as that of 3, based on the analysis of its HRESIMS spectrum (found: m/z 355.2241, calculated: m/z 355.2244, [M + Na]+, C21H32O3Na). The detailed analysis of the 1D and 2D NMR spectra revealed the same planar structure for both 3 and 4.
The H-2 of compound 3, similar to the cases of compounds 1 and 2, also showed a COSY cross peak with H-16 but this was not found in compound 4 (Table 2), implying that 3 may have the same C-16 configuration as 1, while 4 shared the same C-16 configuration with 2. This was further confirmed by computational calculation for 4J2,16 coupling constants of both trans and cis isomers (Table 2), which showed a consistent result with the experimental and literature data [28].
In 1990, Kusumi et al. isolated isosarcophine from the Okinawa soft coral, Sinularia mayi, and demonstrated its cytotoxicity against the human colorectal carcinoma (HCT-116) cell line [24]. In 1992, Wu et al. also isolated the same metabolite from Formosna soft coral, Sarcophyton trocheliophorum, and reported its cytotoxicity toward cancer cells [29]. A related analogue, isosarcophytoxide, was also reported to exhibit significant cytotoxic and moderate anti-inflammatory properties [30]. Up to date, more related derivatives have been discovered from different soft corals, and diversified biological activities of some cembranoids remain unveiled [3]. In the present study, compounds 1–5 were also assayed for their cytotoxicity toward human small cell lung cancer (H1688) cells, and the result showed that 3 possessed a moderate cytotoxicity (IC50 = 27.5 ± 6.4 μM) and 4 exhibited weak activity (IC50 = 85.8 μM) toward the H1688 cell line, while the other compounds were nontoxic with IC50 values over 100 μM. In addition, the isolates were evaluated for their immunosuppressive effect by measuring TNF-α expression in LPS-stimulated murine dendritic cells (DCs). However, none of the isolates could reduce the TNF-α expression (IC50 > 100 μM) in LPS-stimulated DCs.
3. Materials and Methods
3.1. General Experimental Procedures
The NMR experiments with reference to residue signals of C6D6 (δH 7.16; δC 128.4) were carried out on a Varian Unity INOVA 500 FT-NMR (Varian Inc., Palo Alto, CA, USA). Specific optical rotations were measured in MeOH on the Jasco P-1020 polarimeter (JASCO Corporation, Tokyo, Japan). The IR spectra were recorded on an FT/IR-4100 infrared spectrophotometer (JASCO Corporation, Tokyo, Japan). Measurements of circular dichroism spectra were performed on Jasco J-715 CD spectrometer (JASCO Corporation, Tokyo, Japan). HRESIMS were measured on the Impact HD Q-TOF (Bruker, Bremen, Germany) mass spectrometer. The ESI mode (spray potential 4.0 kV) was used. Data analysis was controlled by Bruker DataAnalysis. Full scan spectra were acquired in the ion peak centroid or profile modes over the mass/charge range of 50–1000. The mass spectra were obtained by direct infusion method. Thin-layer chromatography (TLC) analysis was performed on pre-coated silica gel plates (Silica gel 60 F254, 100 μm, Merck, Darmstadt, Germany) or C18 gel plates (Silica gel 60 RP-18 F254s, 100 μm, Merck, Darmstadt, Germany). The open column chromatography was performed on a glass column using adsorbents of silica gel (40–63 μm, Merck, Billerica, USA) or reversed-phase silica gel (RP-18, 40–63 μm, Merck, Darmstadt, Germany). The Hitachi L-2455 HPLC apparatus (Hitachi, Tokyo, Japan) with a Supelco C18 column (250 × 21.2 mm, 5 μm, Supelco, Bellefonte, PA, USA) were used for HPLC purification.
3.2. Animal Material
Soft coral S. cinereum, collected at a depth of 5–10 m by hand using scuba off the coast of the Xiao Liuqiu islands of Taiwan in 2012, was stored in a freezer until extraction. The species was identified based on its colony shape, the distribution of autozooids and siphonozooids on capitulum, and the morphology of sclerites in capitulum and stalk. These morphological features fit well with those described in literature [31]. A sample was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The freeze-dried soft coral S. cinereum (wet weight: 281.5 g; dry weight: 107.4 g) was minced and subjected for extraction using EtOAc. An oily residue (14.6 g) obtained by vacuum evaporation of the EtOAc extract was chromatographed by a silica gel column with the gradient elution of EtOAc in n-hexane (0–100%), acetone in ethyl acetate (0–100%), methanol in acetone (0–100%) to afford 22 fractions. Fraction 7 (1.0 g), eluted by n-hexane-EtOAc (8:1), was further purified over silica gel (elution: n-hexane-acetone = 6.5:1) to obtain five subfractions (7A–7E). Subfraction 7D was purified by reversed-phase HPLC with the elution of methanol-H2O (3:1) to afford 1 (8.8 mg), 2 (7.0 mg), 3 (2.0 mg), and 4 (3.3 mg). Subfraction 7E was purified by reversed-phase HPLC with the elution of methanol-H2O (1.3:1) to afford 5 (1.0 mg).
16α-Hydroperoxyisosarcophytoxide (1): white amorphous powder, + 89.8 (c 0.4, MeOH), IR (KBr) vmax 3463, 2922, 1595, 1444, 1380, 1012, 958 cm−1; for 13C and 1H data see Table 1; electrospray ionization mass spectrometry (ESIMS) m/z 357; HRESIMS m/z 357.2039 [M + Na]+ (calculated for C20H30O4Na: 357.2037).
16β-Hydroperoxyisosarcophytoxide (2): white amorphous powder, + 23.2 (c 0.33, MeOH), IR (KBr) vmax 3391, 2921, 2927, 1445, 1384, 1226, 1000 cm−1; for 13C and 1H data see Table 1; ESIMS m/z 357; HRESIMS m/z 357.2036 [M + Na]+ (calculated for C20H30O4Na: 357.2037).
16α-Methoxyisosarcophytoxide (3): white amorphous powder, + 121.7 (c 0.3, MeOH), IR (KBr) vmax 2923, 1661, 1593, 1445, 1378, 1090, 1012 cm−1; for 13C and 1H data (see Table 1); ESIMS m/z 355; HRESIMS 355.2245 m/z [M + Na]+ (calculated for C21H32O3Na: 355.2244).
16β-Methoxyisosarcophytoxide (4): white amorphous powder, + 21.5 (c 0.26, MeOH), IR (KBr) vmax 2923, 1661, 1593, 1443, 1377, 1090, 1011 cm−1; for 13C and 1H data (see Table 1); ESIMS m/z 355; HRESIMS m/z 355.2241 [M + Na]+ (calculated for C21H32O3Na: 355.2244).
3.4. Proton-Proton Coupling Constant Calculation
The conformers within the 10 Kcal/mol energy window were computed at the MMFF94 force field with the aid of a GMMX package implemented in Gaussian 16 [32]. After the removal of duplicates, the conformers of candidate structures were subjected to geometry optimizations at the B3LYP/6-31G(d) level in gas phase with Natural Bond Orbital (NBO) analysis. Further optimization and frequency calculations were performed at B3LYP-D3(BJ)/6-31+G(d,p) level using the polarizable continuum model (PCM) in benzene. The proton-proton coupling constants were calculated at B3LYP/DU8 level and the resulting data were scaled using both empirical scaling parameters for 4J(1,3)-type and the NBO hybridization coefficients [27], and the data were weighted according to Boltzmann populations using SCF energies refined at M06-2X/6-31+G(d,p)//B3LYP-D3(BJ)/6-31+G(d,p) level in benzene with solvation model based on density (SMD).
3.5. Cytotoxicity Assay
The cytotoxicities of compounds were analyzed by using the human small cell lung cancer H1688 cell line and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) assay [33,34] at 37 °C for 24 h. (3R*,4S*,5E,7Z)-7-dichlorothyl-3,4,8-trichloro-3-methylocta-1,5,7-triene was used as a positive control with IC50’s of 4.2 ± 2.1 μM [35].
3.6. Immunosupressive Activity
3.6.1. Cell Viability of Dendritic Cells (DCs)
The DCs (1 × 106) were treated with tested compounds (100 μg/mL) and cultured at 37 °C for 24 h. Cytotoxicity was examined using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s protocol. The absorbance was recorded on a microplate reader (Tecan Group Ltd., Männedorf, Switzerland) at a wavelength of 450 nm [36,37]. Quercetin (50 μM) was used as a positive control, which showed cytotoxicity against DCs with IC50’s of 78.8 ± 7.3 μM.
3.6.2. Measurement of TNF-α Expression by LPS-Induced DCs
The TNF-α expressions of LPS-induced DCs were measured by enzyme-link immunosorbent assay (ELISA), in the same way as the previously reported method [38,39]. The DCs were treated with 100 ng/mL lipopolysaccharide (LPS) and the isolated compounds for 24 h. The optical density of the production of TNF-α was measured at 450 nm by the ELISA reader. Quercetin (50 μM) was used as a positive control, which inhibited TNF-α expressions on LPS-induced DCs with IC50’s of 23.1 ± 5.2 μM.
4. Conclusions
Our present chemical investigation on the soft coral S. cinereum resulted in the isolation of four new isosarcophytoxide derivatives (1–4) and one previous metabolite, lobocrasol (5), in which compounds 3 and 4 were found to show moderate and weak cytotoxicity, respectively, against H1688 cells. The results of this investigation, along with our previous studies [6,9], further evidenced that the soft corals of Sarcophyton genus are important sources of structurally diversified cembranoid derivatives. Structurally, the lack of NOE correlations for C-16 substituted 2,5-dihydrofuran analogues 1–4 could hinder the elucidation of the relative configuration. Our present work successfully resolved this problem using quantum chemical calculations, which might provide a useful strategy for the determination of relative configurations of other similar cases in the future.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020641/s1, Figures S1–S32: HRESIMS and NMR spectra of compounds 1–4, Tables S1–S4: Cartesian coordinates and Boltzmann populations of compounds 1–4.
Author Contributions
J.-H.S. conceptualized and guided the experiment; C.-H.C., Y.-J.W. and T.-Y.H. analyzed and elucidated the structures of compounds and prepared the manuscript; C.-H.C. and H.-C.H. contributed to computational calculation and related software; C.-J.T., C.-C.L. and C.-Y.H. performed data acquisition and bioassays; Y.-J.C. contributed to part of purification; C.-F.D. identified the species of soft coral. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Technology (MOST 104-2113-M-110-006, MOST 107-2320-B-110-001-MY3, and MOST 111-2320-B-110-010) of Taiwan.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of National Chung Hsing University (IACUC no. 110-047).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data of the present study are available in the article and Supplementary Materials. Samples of the compounds are not available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Cerri, F.; Saliu, F.; Maggioni, D.; Montano, S.; Seveso, D.; Lavorano, S.; Zoia, L.; Gosetti, F.; Lasagni, M.; Orlandi, M.; et al. Cytotoxic compounds from Alcyoniidae: An overview of the last 30 Years. Mar. Drugs 2022, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.N.; Botts, R.T.; Davis, T.S.; Okada, B.K.; Maloney, E.M.; Leber, C.A.; Alvarado, O.; Brayton, C.; Caraballo-Rodríguez, A.M.; Chari, J.V.; et al. Cryptic species account for the seemingly idiosyncratic secondary metabolism of Sarcophyton glaucum specimens collected in Palau. J. Nat. Prod. 2020, 83, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Lin, K.-H.; Huang, C.-Y.; Hwang, T.-L.; Dai, C.-F.; Huang, H.-C.; Sheu, J.-H. Computationally assisted structural elucidation of cembranoids from the soft coral Sarcophyton tortuosum. Mar. Drugs 2022, 20, 297. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chen, Y.-J.; Huang, C.-Y.; Chang, F.-R.; Dai, C.-F.; Sheu, J.-H. Cembranolides and related constituents from the soft coral Sarcophyton cinereum. Molecules 2022, 27, 1760. [Google Scholar] [CrossRef]
- Bilasy, S.E.S.; Khalifa, S.I.; Saleh, S.M.; Abou El-Ela, S.H. HPLC method for the quantitative determination of sarcophine, a source of cembranoids with cancer chemopreventive activity. J. Pharm. Biomed. Anal. 2008, 46, 784–787. [Google Scholar] [CrossRef]
- Koh, M.; Iwanaga, T.; Hatanaks, M.; Nakano, A.; Morihara, K.; Takemura, K. Distribution of sarcophytol A in soft coral of the Sarcophyton genus. Biosci. Biotechnol. Biochem. 2000, 64, 858–861. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chao, C.-H.; Huang, C.-Y.; Hwang, T.-L.; Chang, F.-R.; Dai, C.-F.; Sheu, J.-H. An unprecedented cembranoid with a novel tricyclo [9.3.0.02,12]tetradecane skeleton and related diterpenes from the soft coral Sarcophyton cinereum. Bull. Chem. Soc. Jpn. 2022, 95, 374–379. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, Y.-C.; Huang, C.-Y.; Tseng, Y.-J.; Chen, S.-R.; Cheng, Y.-B.; Hwang, T.-L.; Wang, S.-Y.; Chen, H.-Y.; Dai, C.-F.; et al. Cembranoid-related diterpenes, novel secoditerpenes, and an unusual bisditerpene from a Formosan soft coral Sarcophyton tortuosum. Bull. Chem. Soc. Jpn. 2021, 94, 2774–2783. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Huang, C.-Y.; Chen, S.-R.; Weng, J.-R.; Tu, T.-H.; Cheng, Y.-B.; Wu, S.-H.; Sheu, J.-H. New hydroquinone monoterpenoid and cembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum. Mar. Drugs 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Huang, C.-Y.; Chao, C.-H.; Lin, C.-C.; Dai, C.-F.; Su, J.-H.; Sung, P.-J.; Wu, S.-H.; Sheu, J.-H. New biscembranoids sardigitolides A–D and known cembranoid-related compounds from Sarcophyton digitatum: Isolation, structure elucidation, and bioactivities. Mar. Drugs 2020, 18, 452. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, Y.-K.; Lin, S.-C.; Su, J.-H.; Chao, Y.-H.; Tang, K.-T. Crassolide suppresses dendritic cell maturation and attenuates eperimental antiphospholipid syndrome. Molecules 2021, 26, 2492. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Sheu, J.-H. Anti-inflammatory cembranoids from a Formosa soft coral Sarcophyton cherbonnieri. Mar. Drugs 2020, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Chen, Y.-W.; Huang, C.-Y.; Tseng, Y.-J.; Lin, C.-C.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Isolation and structure elucidation of cembranoids from a Dongsha Atoll soft coral Sarcophyton stellatum. Mar. Drugs 2018, 16, 210. [Google Scholar] [CrossRef]
- Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef]
- Shen, S.-M.; Li, W.-S.; Ding, X.; Luo, H.; Zhang, H.-Y.; Guo, Y.-W. Ximaoglaucumins A–F, new cembranoids with anti-inflammatory activities from the South China Sea soft coral Sarcophyton glaucum. Bioorg. Med. Chem. 2021, 38, 116139. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Ren, L.; Leng, X.; Li, T.; Liu, J.; Wang, W.; Yan, X.; Yan, X.; He, S. Four new cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Nat. Prod. Res. 2021, 37, 39–46. [Google Scholar] [CrossRef]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Lan, L.-F.; Guo, Y.-W. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Atia, M.A.M.; Mohamed, T.A.; Moustafa, M.F.; Hakami, A.R.; Khalifa, S.A.M.; Alhumaydhi, F.A.; Alrumaihi, F.; Abidi, S.H.; et al. Blue biotechnology: Computational screening of Sarcophyton cembranoid diterpenes for SARS-CoV-2 main protease inhibition. Mar. Drugs 2021, 19, 391. [Google Scholar] [CrossRef]
- Yin, F.-Z.; Yao, L.-G.; Zhang, Z.-Y.; Wang, J.-R.; Wang, H.; Guo, Y.-W. Polyoxygenated cembranoids from the Hainan soft coral Lobophytum crassum. Tetrahedron 2021, 90, 132204. [Google Scholar] [CrossRef]
- Kusumi, T.; Yamada, K.; Ishitsuka, M.O.; Fujita, Y.; Kakisawa, H. New cembranoids from the Okinawan soft coral Sinularia mayi. Chem. Lett. 1990, 19, 1315–1318. [Google Scholar] [CrossRef]
- Chao, C.-H.; Li, W.-L.; Huang, C.-Y.; Ahmed, A.-F.; Dai, C.-F.; Wu, Y.-C.; Lu, M.-C.; Liaw, C.-C.; Sheu, J.-H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Marcarino, M.O.; Cicetti, S.; Zanardi, M.M.; Sarotti, A.M. A critical review on the use of DP4+ in the structural elucidation of natural products: The good, the bad and the ugly. A practical guide. Nat. Prod. Rep. 2022, 39, 58. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, A.G.; Mukhina, O.A. Minimalist relativistic force field: Prediction of proton−proton coupling constants in 1H NMR spectra is perfected with NBO hybridization parameters. J. Org. Chem. 2015, 80, 5218–5225. [Google Scholar] [CrossRef]
- Barfield, M.; Spear, R.J.; Sternhell, S. Interproton spin-spin coupling across a sual path in 2,5-dihydrofurans and phthalans. J. Am. Chem. Soc. 1975, 97, 5160–5167. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Hsieh, P.-W.; Duh, C.-Y.; Wang, S.-K.; Soong, K.; Fang, L.-S. Studies on the Formosan soft corals I-Cytotoxic cembrane diterpenes from Sarcophyton trocheliophorum. J. Chin. Chem. Soc. 1992, 39, 355–357. [Google Scholar] [CrossRef]
- Li, S.; Ye, F.; Zhu, Z.; Huang, H.; Mao, S.; Guo, Y. Cembrane-type diterpenoids from the South China Sea soft coral Sarcophyton mililatensis. Acta Pharm. Sin. B 2018, 8, 944–955. [Google Scholar] [CrossRef]
- Verseveldt, J. A revision of the genus Sarcophyton Lesson (Octocorallia, Alcyonacea). In Zoologische Verhandelingen; Brill: Leiden, The Netherlands, 1982; Volume 192, pp. 1–91. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Antunes, E.M.; Afolayan, A.F.; Chiwakata, M.T.; Fakee, J.; Knott, M.G.; Whibley, C.E.; Hendricks, D.T.; Bolton, J.J.; Beukes, D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenated monoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry 2011, 72, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Li, Y.-R.; Huang, W.-Y.; Su, J.-H.; Chan, H.-L.; Lin, S.-H.; Liu, C.-S.; Lin, S.-C.; Lin, C.-C.; Lin, C.-H. Sinulariolide suppresses LPS-induced phenotypic and functional maturation of dendritic cells. Mol. Med. Rep. 2017, 16, 6992–7000. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Su, J.-H.; Lin, S.-C.; Chang, C.-C.; Hsia, T.-C.; Tung, Y.-T.; Lin, C.-C. A Soft Coral-Derived Compound, 11-Dehydrosinulariolide, Induces G2/M Cell Cycle Arrest and Apoptosis in Small Cell Lung Cancer. Mar. Drugs 2018, 16, 479. [Google Scholar] [CrossRef]
- Lin, M.-K.; Yu, Y.-L.; Chen, K.-C.; Chang, W.-T.; Lee, M.-S.; Yang, M.-J.; Cheng, H.-C.; Liu, C.-H.; Chen, D.-C.; Chu, C.-L. Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef]
- Lai, K.-H.; You, W.-J.; Lin, C.-C.; El-Shazly, M.; Liao, Z.-J.; Su, J.-H. Anti-inflammatory cembranoids from the soft coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).