Accuracy of Citrate Anticoagulant Amount, Volume, and Concentration in Evacuated Blood Collection Tubes Evaluated with UV Molecular Absorption Spectrometry on a Purified Water Model
Abstract
1. Introduction
- -
- the accuracy of the anticoagulant amount added into the tubes by a producer;
- -
- the accuracy of the volume of anticoagulant solution in the tube at the instant of examination;
- -
- the anticoagulant concentrations corresponding to the nominal draw or draw volume.
2. Results
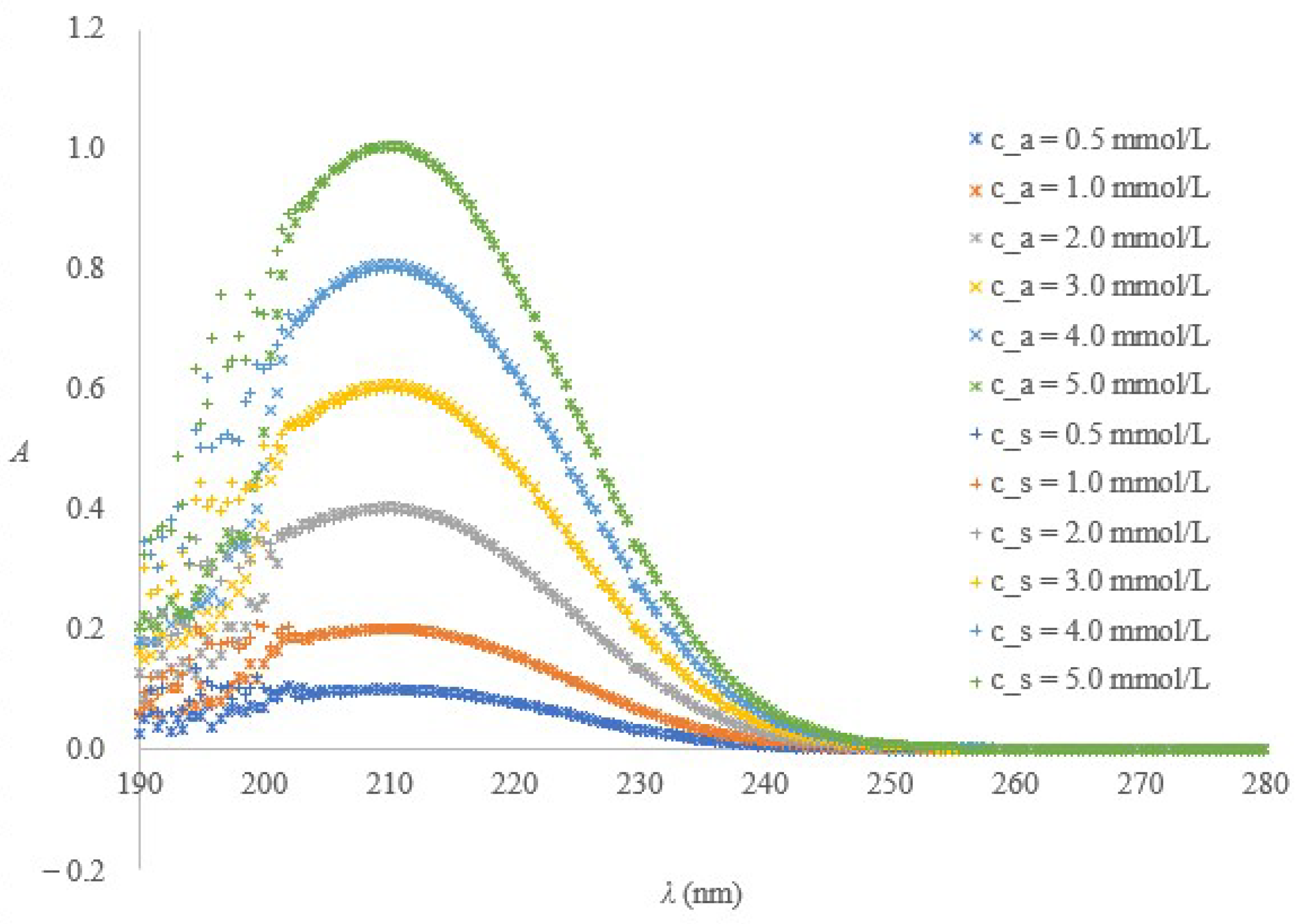
2.1. Spectrometric Method for Citrate or Buffered Citrate Determination
2.1.1. Method Performance
2.1.2. Fitness for Purpose
2.2. Evacuated Blood Collection Tubes for Coagulation Tests—Quality Evaluation
2.2.1. Water Loss during the Time
2.2.2. Draw Volume during the Time
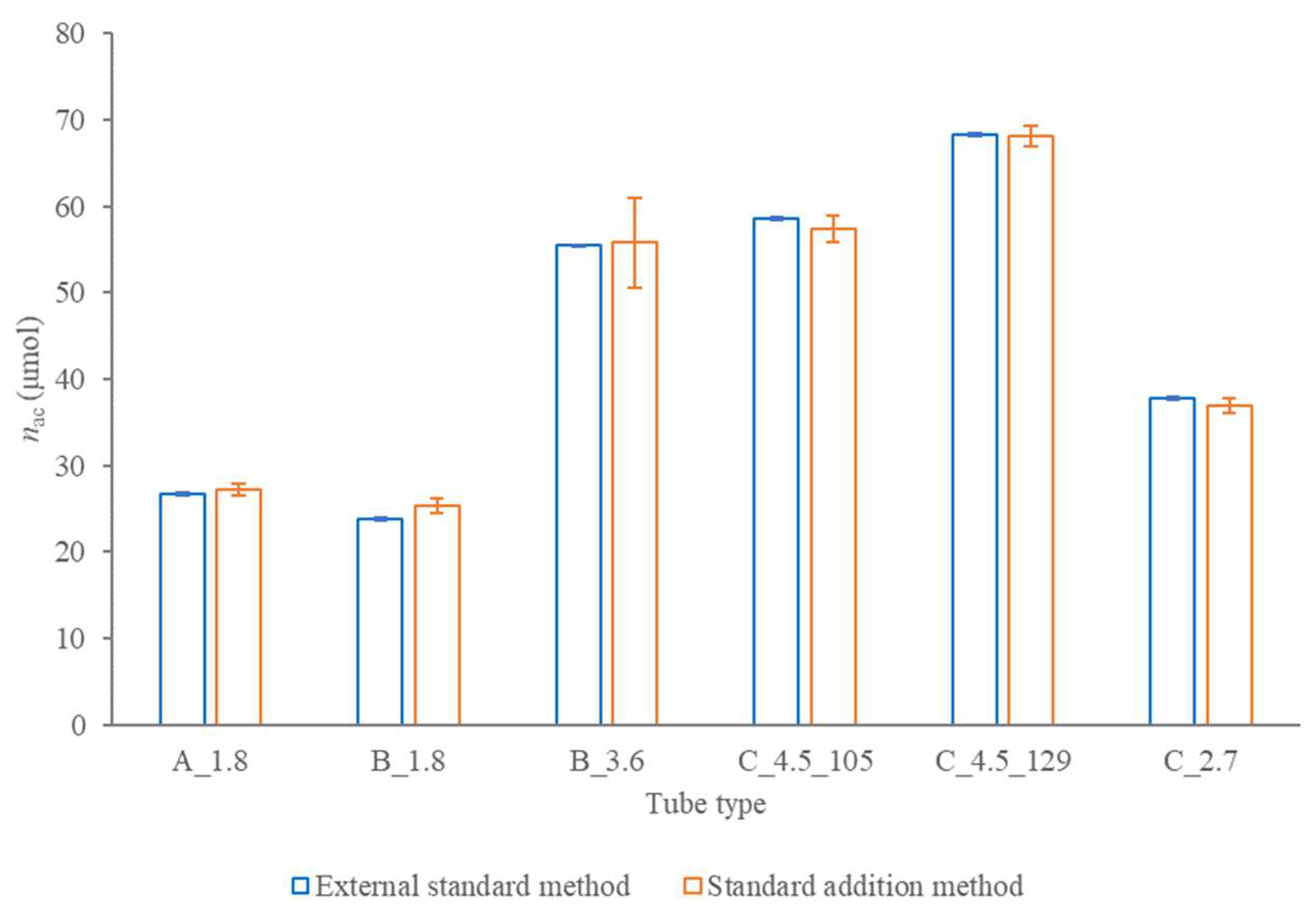
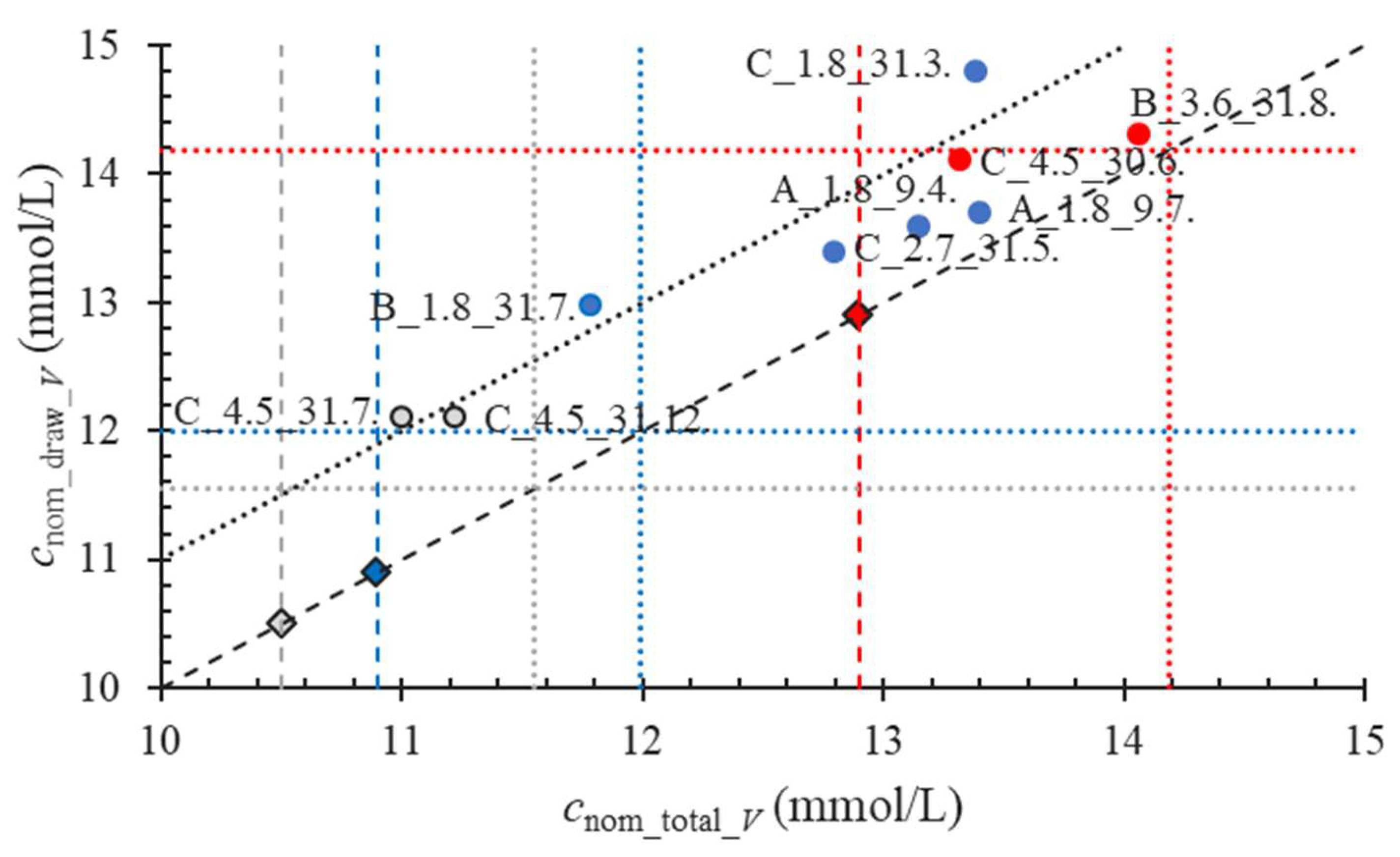
2.2.3. Accuracy of the Amount of the Anticoagulant Added into a Tube by a Producer
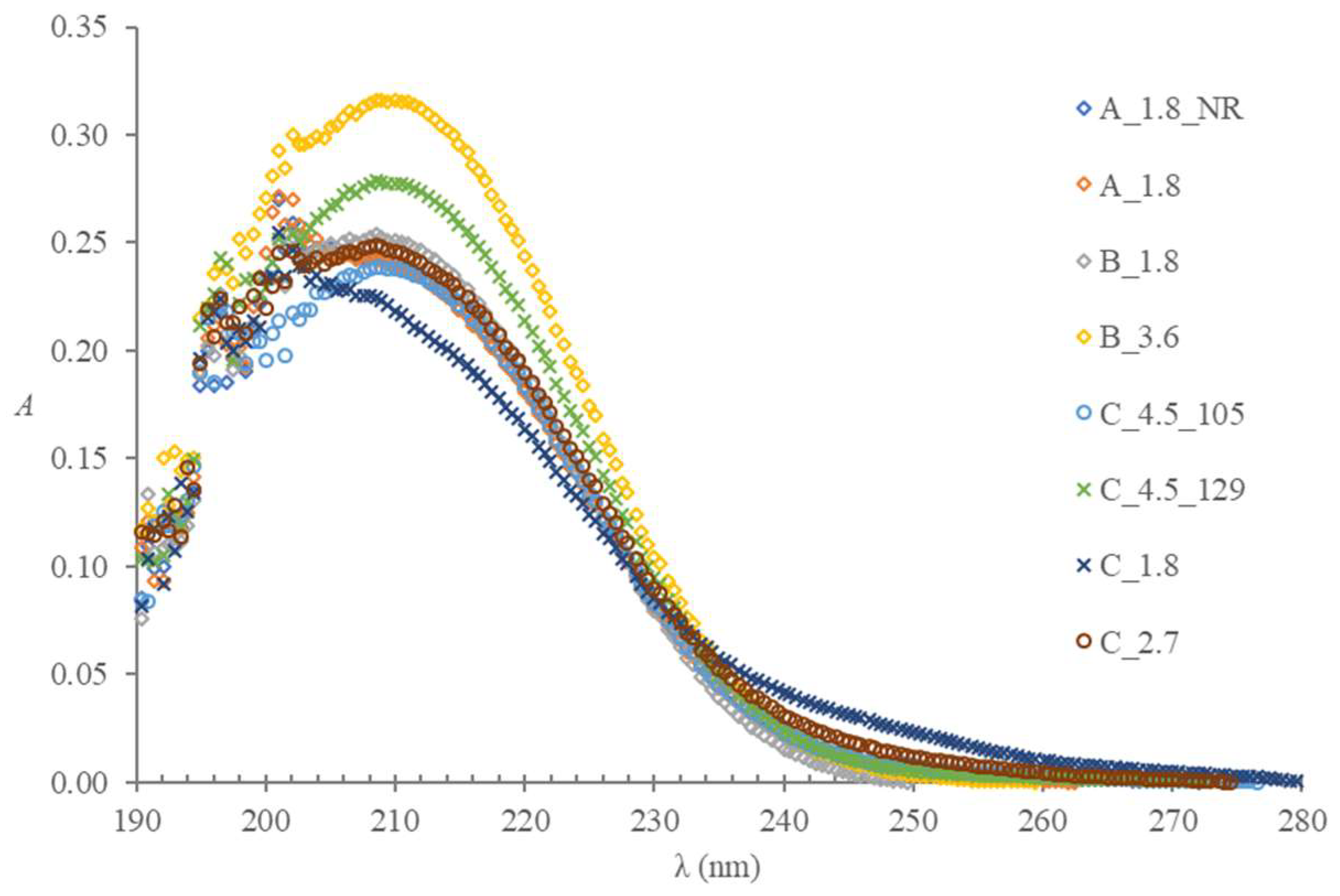
2.2.4. Accuracy of the Volume of the Anticoagulant Solution in a Tube at the Instant of the Examination
2.3. Contaminants or Potential Additives
3. Discussion
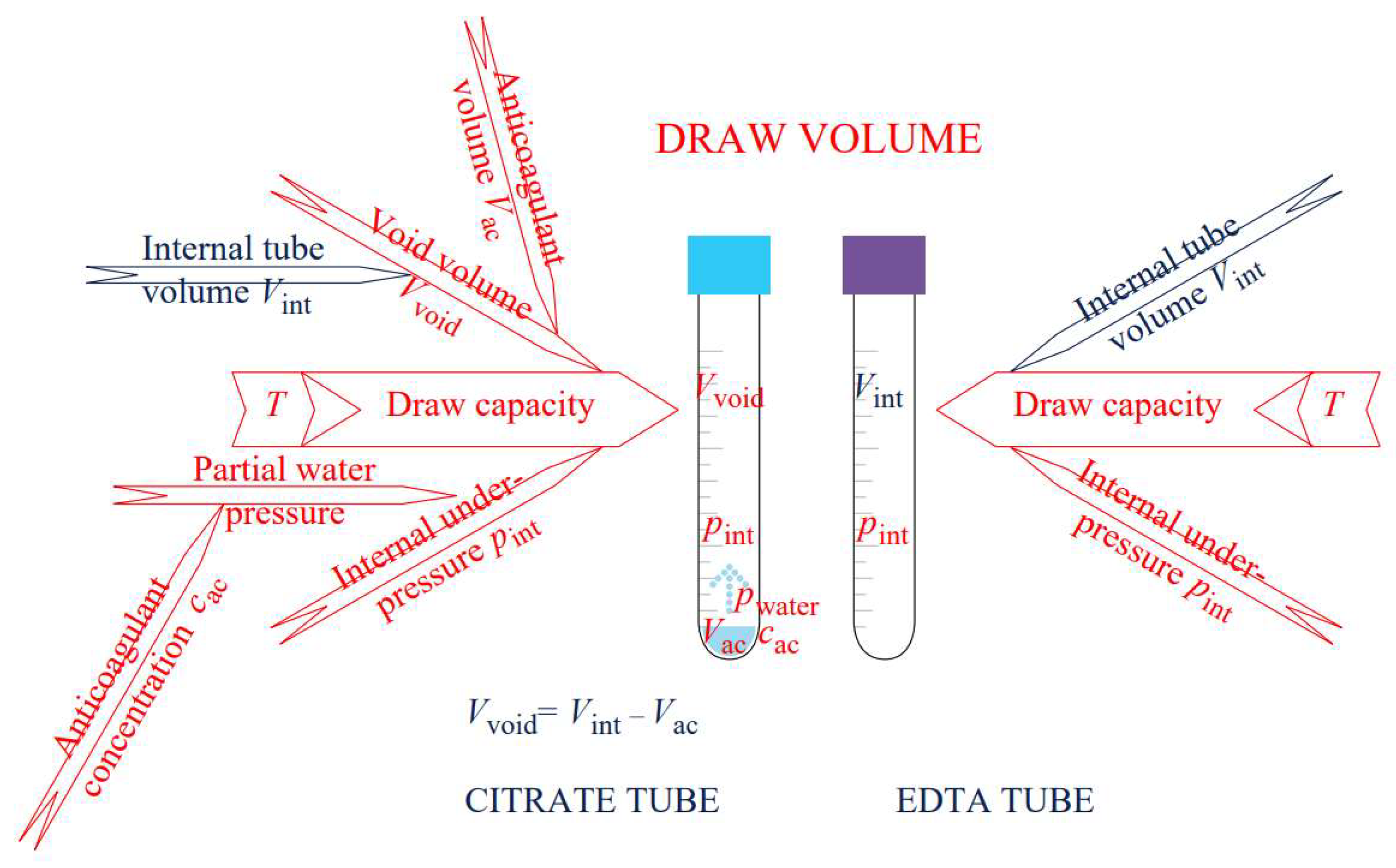
3.1. What Makes the Quality Evaluation of the Citrate Blood Collection Tubes More Challenging If Compared to the EDTA Tubes
- (a)
- Conductometry was confirmed as an adequate low cost easy-to-perform analytical method for the determination of EDTA anticoagulant concentration in tubes on a deionised or distilled water model.
- (b)
- A 5-mL-Bang burette-based method for the determination of draw volume on a model of deionised or distilled water in the laboratory setting at local experimental conditions was established.
- (c)
- A physical model was developed, which by correcting the measurements for the ambient temperature and the unreduced air pressure influence at the altitude of the experimental setting above sea level, was able to predict a draw volume and blood anticoagulant concentration for specimen collection under the assumption of no other phlebotomy-related adverse effect.
3.2. Quality of Evacuated Blood Collection Tubes for Coagulation Tests
3.3. Contaminants or Potential Additives
3.4. Implications and Limitations
4. Materials and Methods
4.1. Spectrometric Method for Citrate or Buffered Citrate Determination
4.2. Evacuated Blood Collection Tubes
4.2.1. Water Loss during a Time
4.2.2. Draw Volume during the Time
4.3. Contaminants or Potential Additives
4.3.1. Infrared Spectra
4.3.2. Atomic Absorption Spectrometry
5. Conclusions
- -
- the accuracy of the anticoagulant amount added into the tubes by a producer;
- -
- the accuracy of the volume of anticoagulant solution in the tube at the instant of examination;
- -
- the anticoagulant concentrations for a nominal draw or draw volume.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gosselin, R.C.; Marlar, R.A. Preanalytical Variables in Coagulation Testing: Setting the Stage for Accurate Results. Semin. Thromb. Hemost. 2019, 45, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.A.R.; Adcock, D.M. Blood collection tubes as medical devices: The potential to affect assays and proposed verification and validation processes for the clinical laboratory. Clin. Biochem. 2016, 49, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Validation and Verification of Tubes for Venous and Capillary Blood Specimen Collection; Approved Guideline; CLSI document GP34-A; CLSI: Wayne, PA, USA, 2010. [Google Scholar]
- Gosselin, R.C.; Bowyer, A.; Favaloro, E.J.; Johnsen, J.M.; Lippi, G.; Marlar, R.A.; Neeves, K.; Rollins-Raval, M.A. Guidance on the critical shortage of sodium citrate coagulation tubes for hemostasis testing. J. Thromb. Haemost. 2021, 19, 2857–2861. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Brocco, G.; Guidi, G.C. Preanalytical variability in laboratory testing: Influence of the blood drawing technique. Clin. Chem. Lab. Med. 2005, 43, 319–325. [Google Scholar] [CrossRef]
- Bowen, R.A.R.; Remaley, A.T. Interferences from blood collection tube components on clinical chemistry assays. Biochem. Med. 2014, 24, 31–44. [Google Scholar] [CrossRef]
- Bush, V.; Cohen, R. The evolution of evacuated blood collection tubes. Lab. Med. 2003, 34, 304–310. [Google Scholar] [CrossRef]
- Gosselin, R.C.; Janatpour, K.; Larkin, E.C.; Lee, Y.P.; Owings, J.T. Comparison of samples obtained from 3.2% sodium citrate glass and two 3.2% sodium citrate plastic blood collection tubes used in coagulation testing. Am. J. Clin. Pathol. 2004, 122, 843–848. [Google Scholar] [CrossRef]
- Flanders, M.M.; Crist, R.; Rodgers, G.M. A comparison of blood collection in glass versus plastic vacutainers on results of esoteric coagulation assays. Lab. Med. 2003, 34, 732–735. [Google Scholar] [CrossRef]
- van den Besselaar, A.; Chantarangkul, V.; Tripodi, A. A comparison of two sodium citrate concentrations in two evacuated blood collection systems for prothrombin time and ISI determination. Thromb. Haemost. 2000, 84, 664–667. [Google Scholar]
- Yavas, S.; Ayaz, S.; Kose, S.K.; Ulus, F.; Ulus, A.T. Influence of Blood Collection Systems on Coagulation Tests. Turk. J. Hematol. 2012, 29, 367–375. [Google Scholar] [CrossRef]
- Tripodi, A.; Chantarangkul, V.; Bressi, C.; Mannucci, P.M. How to evaluate the influence of blood collection systems on the international sensitivity index. Protocol applied to two new evacuated tubes and eight coagulometer/thromboplastin combinations. Thromb. Res. 2002, 108, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Germanovich, K.; Femia, E.A.; Cheng, C.Y.; Dovlatova, N.; Cattaneo, M. Effects of pH and concentration of sodium citrate anticoagulant on platelet aggregation measured by light transmission aggregometry induced by adenosine diphosphate. Platelets 2018, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Elst, K.V.; Vermeiren, S.; Schouwers, S.; Callebaut, V.; Thomson, W.; Weekx, S. Validation of the minimal citrate tube fill volume for routine coagulation tests on ACL TOP 500 CTS (R). Int. J. Lab. Hematol. 2013, 35, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.M.; Casey, C.R.; Duncan, B.M.; Lloyd, J.V. Effect of concentration of trisodium citrate anticoagulant on calculation of the international normalized ratio and the international sensitivity index of thromboplastin. Thromb. Haemost. 1994, 72, 84–88. [Google Scholar]
- Reneke, J.; Etzell, J.; Leslie, S.; Ng, V.L.; Gottfried, E.L. Prolonged prothrombin time and activated partial thromboplastin time due to underfilled specimen tubes with 109 mmol/L (3.2%) citrate anticoagulant. Am. J. Clin. Pathol. 1998, 109, 754–757. [Google Scholar] [CrossRef][Green Version]
- Marlar, R.A.; Potts, R.M.; Marlar, A.A. Effect on routine and special coagulation testing values of citrate anticoagulant adjustment in patients with high hematocrit values. Am. J. Clin. Pathol. 2006, 126, 400–405. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Demonte, D.; Gelati, M.; Poli, G.; Favaloro, E.J.; Lippi, G. Thrombin generation in different commercial sodium citrate blood tubes. J. Med. Biochem. 2020, 39, 19–24. [Google Scholar] [CrossRef]
- CLSI. Tubes and Additives for Venous and Capillary Blood Specimen Collection, 6th ed.; CLSI document GP39-A6; CLSI: Wayne, PA, USA, 2010. [Google Scholar]
- NCCLS. Tubes and Additives for Venous Blood Specimen Collection, 5th ed.; NCCLS document H1-A5; NCCLS: Albany, NY, USA, 2003. [Google Scholar]
- Gros, N. Evacuated blood-collection tubes for haematological tests-a quality evaluation prior to their intended use for specimen collection. Clin. Chem. Lab. Med. 2013, 51, 1043–1051. [Google Scholar] [CrossRef]
- Gros, N. Pre-Analytical Within-Laboratory Evacuated Blood-Collection Tube Quality Evaluation. In Biochemical Testing: Clinical Correlation and Diagnosis; Varaprasad, B.E., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Lito, M.; Camoes, M.; Covington, A.K. Effect of citrate impurities on the reference pH value of potassium dihydrogen buffer solution. Anal. Chim. Acta 2003, 482, 137–146. [Google Scholar] [CrossRef]
- Bates, R.G.; Pinching, G.D. Resolution of the dissociation constants of citric acid at 0-degrees to 50-degrees, and determination of certain related thermodynamic functions. J. Am. Chem. Soc. 1949, 71, 1274–1283. [Google Scholar] [CrossRef]
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Biljak, V.R.; Kralj, A.K.; Kuktic, I.; Gelati, M.; Simundic, A.M. Filling accuracy and imprecision of commercial evacuated sodium citrate coagulation tubes. Scand. J. Clin. Lab. Investig. 2019, 79, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Weikart, C.M.; Breeland, A.P.; Wills, M.S.; Baltazar-Lopez, M.E. Hybrid Blood Collection Tubes: Combining the Best Attributes of Glass and Plastic for Safety and Shelf life. SLAS Technol. Transl. Life Sci. Innov. 2020, 25, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Lima-Oliveira, G.; Salvagno, G.L.; Danese, E.; Favaloro, E.J.; Guidi, G.C.; Lippi, G. Sodium citrate blood contamination by K-2-ethylenediaminetetraacetic acid (EDTA): Impact on routine coagulation testing. Int. J. Lab. Hematol. 2015, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- van den Besselaar, A.; van Dam, W.; Sturk, A.; Bertina, R.M. Prothrombin time ratio is reduced by magnesium contamination in evacuated blood collection tubes. Thromb. Haemost. 2001, 85, 647–650. [Google Scholar]
- van den Besselaar, A.; van Zanten, A.P.; Brantjes, H.M.; Elisen, M.; van der Meer, F.J.M.; Poland, D.C.W.; Sturk, A.; Leyte, A.; Castel, A. Comparative Study of Blood Collection Tubes and Thromboplastin Reagents for Correction of INR Discrepancies A Proposal for Maximum Allowable Magnesium Contamination in Sodium Citrate Anticoagulant Solutions. Am. J. Clin. Pathol. 2012, 138, 248–254. [Google Scholar] [CrossRef]
- van den Besselaar, A.; van Vlodrop, I.J.H.; Berendes, P.B.; Cobbaert, C.M. A comparative study of conventional versus new, magnesium-poor Vacutainer (R) Sodium Citrate blood collection tubes for determination of prothrombin time and INR. Thromb. Res. 2014, 134, 187–191. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| A 210 nm | 0.28716 | 0.28839 | 0.28825 | 0.28799 | 0.28860 | 0.28827 | 0.28647 | 0.28802 | 0.28620 | 0.28868 |
| c (mmol/L) | 1.4118 | 1.4178 | 1.4171 | 1.4159 | 1.4189 | 1.4173 | 1.4085 | 1.4160 | 1.4071 | 1.4192 |
| Date | a | sa | b | sb | sy/x | R2 |
|---|---|---|---|---|---|---|
| 21 February 2022 | 0.1992 | 0.0041 | 0.0146 | 0.0091 | 0.0135 | 0.99835 |
| 25 February 2022 | 0.2032 | 0.0010 | 0.0002 | 0.0021 | 0.0032 | 0.99991 |
| 28 February 2022 | 0.2039 | 0.0010 | 0.0006 | 0.0023 | 0.0034 | 0.99990 |
| 11 March 2022 | 0.2021 | 0.0006 | −0.0004 | 0.0013 | 0.0019 | 0.99997 |
| 6 April 2022 | 0.2027 | 0.0013 | 0.0022 | 0.0029 | 0.0043 | 0.99984 |
| 5 May 2022 | 0.2032 | 0.0028 | 0.0055 | 0.0064 | 0.0094 | 0.99922 |
| 16 June 2022 | 0.2038 | 0.0008 | −0.0009 | 0.0017 | 0.0026 | 0.99994 |
| 7 July 2022 | 0.2021 | 0.0005 | 0.0018 | 0.0011 | 0.0016 | 0.99998 |
| 17 August 2022 | 0.2050 | 0.0008 | −0.0047 | 0.0018 | 0.0027 | 0.99994 |
| (mmol/L) | 17 August 2022 | 7 July 2022 | 16 June 2022 | 5 May 2022 | 6 April 2022 | 11 March 2022 | 28 February 2022 | 25 February 2022 | 21 February 2022 |
|---|---|---|---|---|---|---|---|---|---|
| cmin | 11.9 | 11.7 | 11.6 | 11.6 | 11.6 | 11.7 | 11.5 | 13.7 | 14.1 |
| uc | ±0.2 | ±0.1 | ±0.2 | ±0.5 | ±0.3 | ±0.1 | ±0.2 | ±0.2 | ±0.7 |
| cmax | 17.7 | 15.8 | 16.3 | 16.4 | 16.0 | 15.7 | 14.7 | 15.9 | 17.4 |
| uc | ±0.2 | ±0.2 | ±0.2 | ±0.5 | ±0.3 | ±0.2 | ±0.2 | ±0.2 | ±0.7 |
| B_1.8_31.7. | Count | Sum | Average | Variance | Differences | ||||
| 25.02. | 10 | 64.5799 | 6.45799 | 0.000517959 | * | ||||
| 11.03. | 10 | 64.3702 | 6.43702 | 0.000437162 | * | ||||
| 06.04. | 10 | 64.1965 | 6.41965 | 0.000752418 | * | ||||
| 5.05. | 10 | 63.8514 | 6.38514 | 0.000254645 | * | ||||
| 16.06. | 10 | 63.4715 | 6.34715 | 0.000271512 | * | ||||
| 7.07. | 10 | 63.4062 | 6.34062 | 0.000507422 | * | ||||
| B_3.6_31.8. | Count | Sum | Average | Variance | Differences | ||||
| 25.02. | 10 | 66.8741 | 6.68741 | 0.000588828 | * | ||||
| 11.03. | 10 | 66.6654 | 6.66654 | 0.000599349 | * | ||||
| 06.04. | 10 | 66.3714 | 6.63714 | 0.000796518 | * | ||||
| 5.05. | 10 | 66.1818 | 6.61818 | 0.000428091 | * | ||||
| 16.06. | 10 | 65.8877 | 6.58877 | 0.00035316 | * | ||||
| 7.07. | 10 | 65.5968 | 6.55968 | 0.000334517 | * | ||||
| Tubes | cac_nom (mmol/L) | nac_expt (μmol) | nac_dtmn (μmol) | nac Relative Bias (%) | c_V_total_nom (mmol/L) |
|---|---|---|---|---|---|
| A_1.8_9.4. | 109 | 21.8 | 26.3 | 20.6 | 13.2 |
| A_1.8_9.7. | 109 | 21.8 | 26.8 | 23.0 | 13.4 |
| B_1.8_31.7. | 109 | 21.8 | 23.6 | 8.2 | 11.8 |
| C_1.8_31.3. | 109 | 21.8 | 26.8 | 22.8 * | 13.4 |
| C_2.7_31.5. | 109 | 32.7 | 38.4 | 17.4 | 12.8 |
| B_3.6_31.8. | 129 | 51.6 | 56.3 | 9.0 | 14.1 |
| C_4.5_31.7. | 105 | 52.5 | 55.0 | 4.8 | 11.0 |
| C_4.5_31.12. | 105 | 52.5 | 56.1 | 6.9 | 11.2 |
| C_4.5_30.6. | 129 | 64.5 | 66.6 | 3.2 | 13.3 |
| Tubes 1 | V_draw_nom (mL) | cac_expt (mmol/L) | cac_dtmn (μmol) | cac Relative Bias (%) | Vac_nom (mL) | Vac (mL) | Vac Relative Bias (%) |
|---|---|---|---|---|---|---|---|
| A_1.8_9.4. | 1.8 | 10.9 | 13.6 | 24.8 | 0.2 | 0.134 | −33.1 |
| A_1.8_9.7. | 1.8 | 10.9 | 13.7 | 25.7 | 0.2 | 0.157 | −21.6 |
| B_1.8_31.7. | 1.8 | 10.9 | 13.0 | 19.0 | 0.2 | 0.018 | −91.1 |
| C_1.8_31.3. | 1.8 | 10.9 | 14.8 | 35.8 * | 0.2 | 0.008 | −95.9 |
| C_2.7_31.5. | 2.7 | 10.9 | 13.4 | 22.9 | 0.3 | 0.164 | −45.3 |
| B_3.6_31.8. | 3.6 | 12.9 | 14.3 | 10.9 | 0.4 | 0.334 | −16.6 |
| C_4.5_31.7. | 4.5 | 10.5 | 12.1 | 15.2 | 0.5 | 0.048 | −90.4 |
| C_4.5_31.12. | 4.5 | 10.5 | 12.1 | 15.2 | 0.5 | 0.137 | −72.6 |
| C_4.5_30.6. | 4.5 | 12.9 | 14.1 | 9.3 | 0.5 | 0.223 | −55.5 |
| Tubes | cac_nom (mmol/L) | Expiration Date | V_draw_nom (mL) | Vac_nom (mL) | c (K+) (μmol/L) | s (n = 3) | c (Mg2+) (μmol/L) | s (n = 3) |
|---|---|---|---|---|---|---|---|---|
| A_1.8_NR | 109 | 3 March 2022 | 1.8 | 0.2 | / | / | 209.1 | ±1.8 |
| A_1.8 | 109 | 9 April 2022 | 1.8 | 0.2 | / | / | 187.0 | ±1.9 |
| B_1.8 | 109 | 31 December 2021 | 1.8 | 0.2 | / | / | 208.3 | ±1.4 |
| B_3.6_129 | 129 | 28 February 2022 | 3.6 | 0.4 | / | / | 46.4 | ±0.1 |
| C_1.8 | 109 | 30 September 2021 | 1.8 | 0.2 | 139.3 | ±2.6 | / | / |
| C_2.7 | 109 | 30 November 2021 | 2.7 | 0.3 | 146.8 | ±1.9 | / | / |
| C_4.5_105 | 105 | 31 July 2022 | 4.5 | 0.5 | 170.2 | ±0.7 | 167.5 | ±0.4 |
| C_4.5_129 | 129 | 30 June 2022 | 4.5 | 0.5 | 168.4 | ±3.8 | 164.1 | ±0.2 |
| Abbreviation | Anticoagulant c (mmol/L) | Expiration Date | Draw Volume (mL) |
|---|---|---|---|
| B_1.8 | 109 | 31 December 2021 | 1.8 |
| C_1.8 | 109 * | 30 September 2021 | 1.8 |
| C_2.7 | 109 * | 30 November 2021 | 2.7 |
| A_1.8_9.4. | 109 | 9 April 2022 | 1.8 |
| A_1.8_3.3._NR | 109 | 3 March 2022 | 1.8 |
| A_1.8_14.4._NR | 109 | 14 April 2022 | 1.8 |
| A_1.8_9.7. | 109 | 9 July 2022 | 1.8 |
| B_1.8_31.7. | 109 | 31 July 2022 | 1.8 |
| B_3.6_31.8. | 129 | 31 August 2022 | 3.6 |
| C_1.8_31.3. | 109 * | 31 March 2022 | 1.8 |
| C-2.7_31.5. | 109 * | 31 May 2022 | 2.7 |
| C_4.5_30.6. | 129 * | 30 June 2022 | 4.5 |
| C_4.5_31.7. | 105 * | 31 July 2022 | 4.5 |
| C_4.5_31.12 | 105 * | 31 December 2022 | 4.5 |
| Metal Ion | K+ | Mg2+ |
|---|---|---|
| Wavelength (nm) | 766.5 | 285.2 |
| Slot width (nm) | 1 | 0.5 |
| Concentration range (mg/L) | 0.25 to 1 | 0.05 to 05 |
| Medium | 1% HCl | 1% HCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gros, N.; Klobučar, T.; Gaber, K. Accuracy of Citrate Anticoagulant Amount, Volume, and Concentration in Evacuated Blood Collection Tubes Evaluated with UV Molecular Absorption Spectrometry on a Purified Water Model. Molecules 2023, 28, 486. https://doi.org/10.3390/molecules28020486
Gros N, Klobučar T, Gaber K. Accuracy of Citrate Anticoagulant Amount, Volume, and Concentration in Evacuated Blood Collection Tubes Evaluated with UV Molecular Absorption Spectrometry on a Purified Water Model. Molecules. 2023; 28(2):486. https://doi.org/10.3390/molecules28020486
Chicago/Turabian StyleGros, Nataša, Tadej Klobučar, and Klara Gaber. 2023. "Accuracy of Citrate Anticoagulant Amount, Volume, and Concentration in Evacuated Blood Collection Tubes Evaluated with UV Molecular Absorption Spectrometry on a Purified Water Model" Molecules 28, no. 2: 486. https://doi.org/10.3390/molecules28020486
APA StyleGros, N., Klobučar, T., & Gaber, K. (2023). Accuracy of Citrate Anticoagulant Amount, Volume, and Concentration in Evacuated Blood Collection Tubes Evaluated with UV Molecular Absorption Spectrometry on a Purified Water Model. Molecules, 28(2), 486. https://doi.org/10.3390/molecules28020486







