Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications
Abstract
1. Introduction
2. Poly Lactic Acid and Its Synthesis
2.1. Copolymerization of PLA
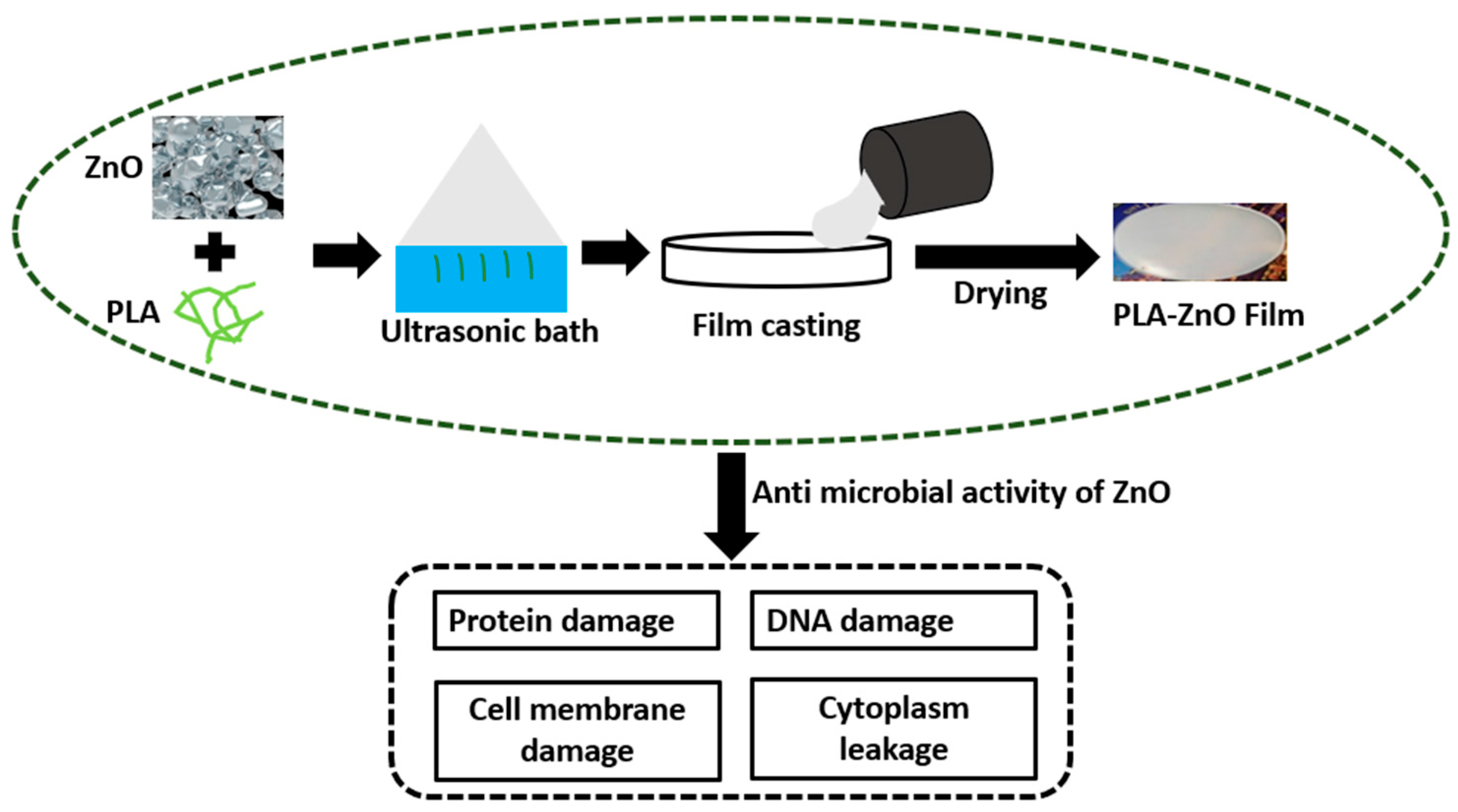
2.2. Fabrication of PLA Composites for Biomedical Applications
3. Scope of PLA in Biomedical Applications
3.1. Polylactic Acid in Drug Delivery
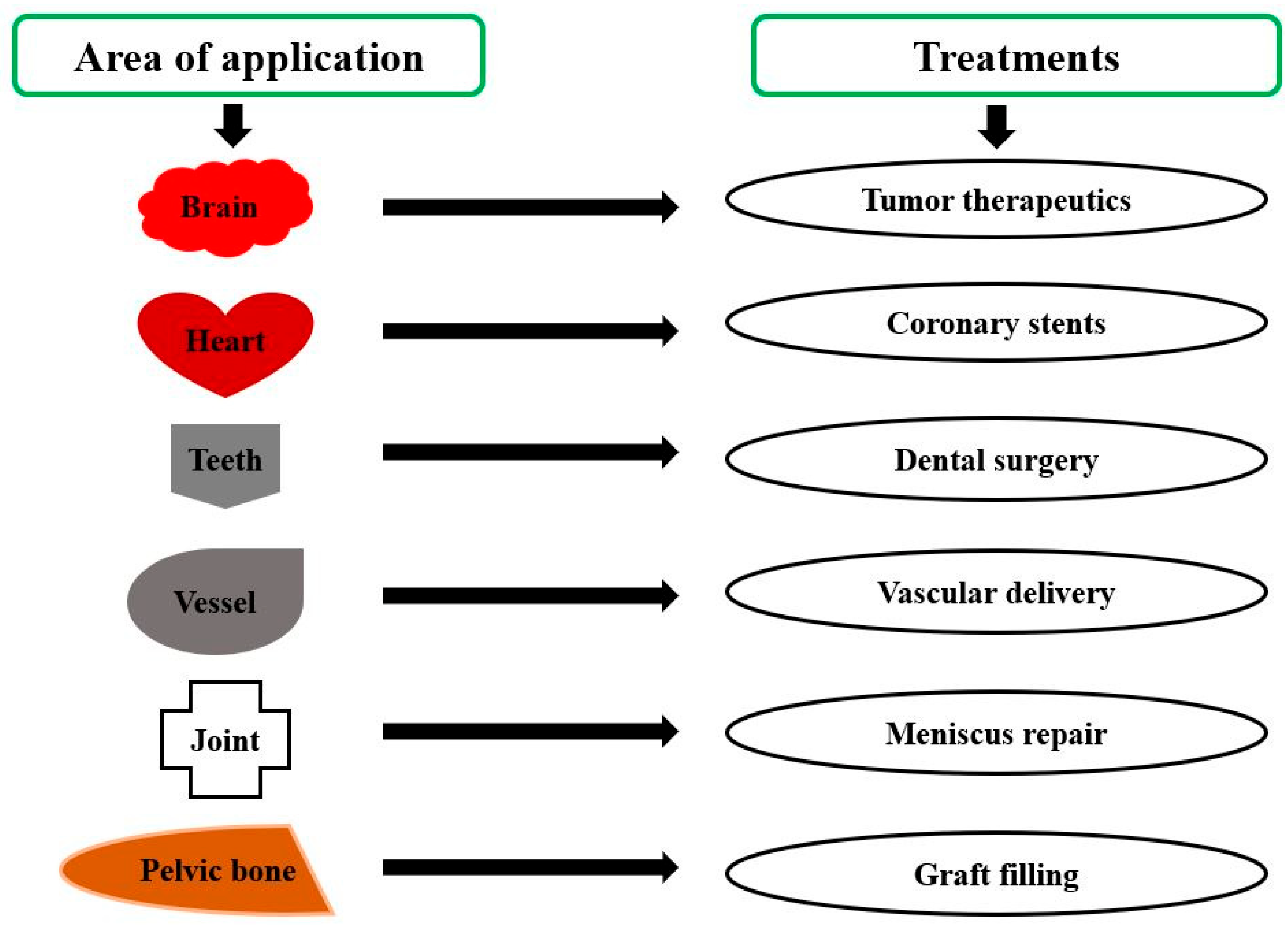
3.2. Polylactic Acid in Implants
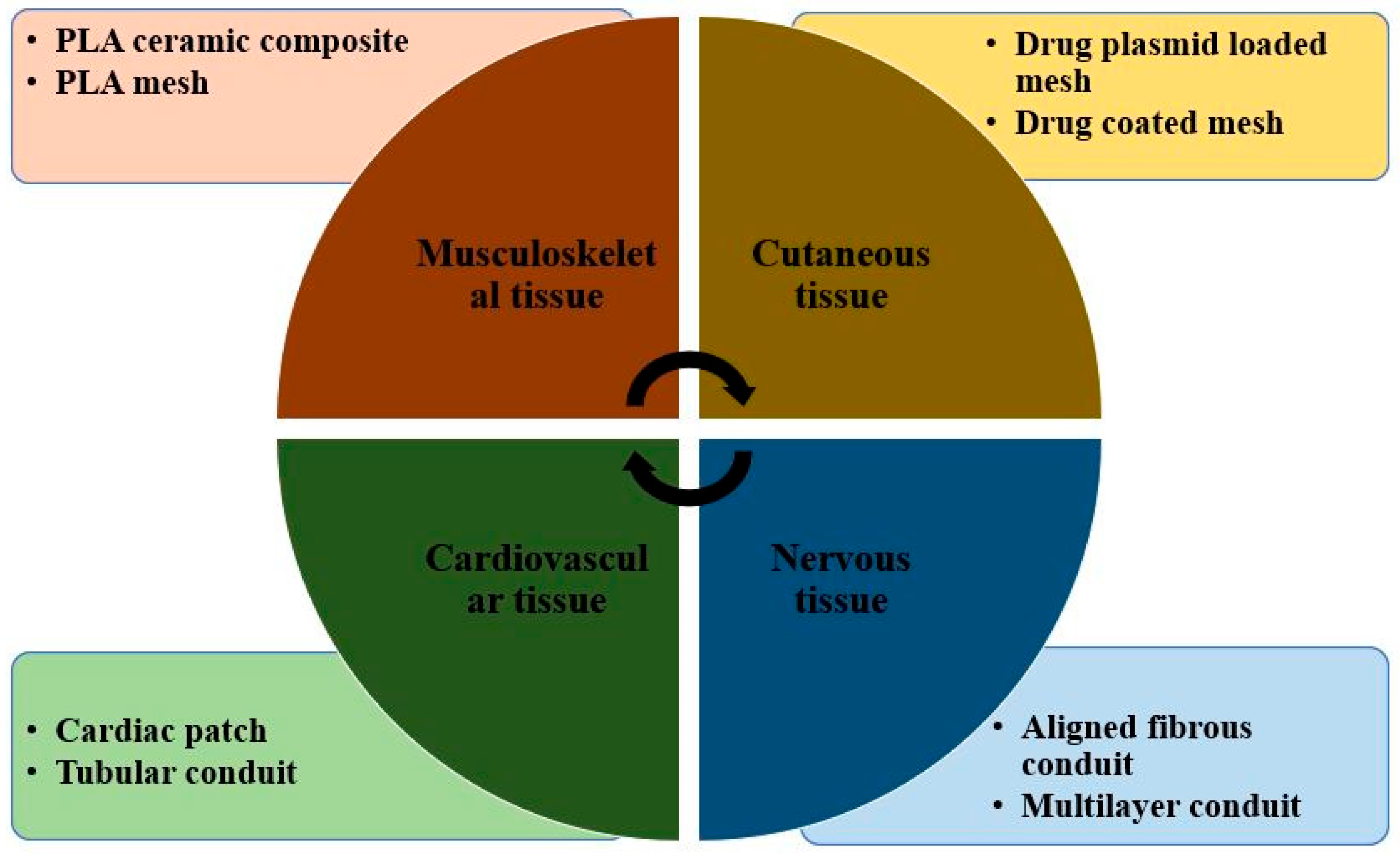
3.3. Polylactic Acid in Tissue Engineering
4. Food and Drug Administration Approved PLA Formulations Used in Medicine
5. Current Limitations
6. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotrocz, L.; Bakonyi, P. Pinpoint Loading Examinations of Poly(lactic acid) Biopolymers. Acta Tech. Jaurinensis 2018, 11, 206–217. [Google Scholar] [CrossRef][Green Version]
- Tábi, T.; Hajba, S. Investigation of long cellulose fibre reinforced and injection moulded poly (lactic acid) biocomposites. Acta Tech. Jaurinensis 2018, 11, 150–164. [Google Scholar]
- Mishra, S.K.; Dahiya, S.; Gangil, B.; Ranakoti, L.; Agrawal, N. Mechanical properties of fibre/filler based poly(Lactic Acid) (Pla) composites: A brief review. Acta Innov. 2021, 41, 5–18. [Google Scholar] [CrossRef]
- Lendvai, L.; Brenn, D. Mechanical, morphological and thermal characterization of compatibilized poly (lactic acid)/thermoplastic starch blends. Acta Tech. Jaurinensis 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Lendvai, L.; Omastova, M.; Patnaik, A.; Dogossy, G.; Singh, T. Valorization of Waste Wood Flour and Rice Husk in Poly(Lactic Acid)-Based Hybrid Biocomposites. J. Polym. Environ. 2022, 1–11. [Google Scholar] [CrossRef]
- Singh, T.; Pattnaik, P.; Kumar, S.R.; Fekete, G.; Dogossy, G.; Lendvai, L. Optimization on physicomechanical and wear properties of wood waste filled poly (lactic acid) biocomposites using integrated entropy-simple additive weighting approach. S. Afr. J. Chem. Eng. 2022, 41, 193–202. [Google Scholar] [CrossRef]
- Fekete, I.; Ronkay, F.; Lendvai, L. Highly toughened blends of poly(lactic acid) (PLA) and natural rubber (NR) for FDM-based 3D printing applications: The effect of composition and infill pattern. Polym. Test. 2021, 99, 107205. [Google Scholar] [CrossRef]
- Lalit, R.; Mayank, P.; Ankur, K. Natural fibers and biopolymers characterization: A future potential composite material. Stroj. Časopis-J. Mech. Eng. 2018, 68, 33–50. [Google Scholar] [CrossRef]
- Ranakoti, L.; Rakesh, P.K.; Gangil, B. Effect of Tasar Silk Waste on the Mechanical Properties of Jute/Grewia Optiva Fibers Reinforced Epoxy Laminates. J. Nat. Fibers 2021, 19, 10462–10474. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical recycling of post-consumer PLA waste for sustainable production of ethyl lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Cuker, B.E. Instead of Eating Fish: The Health Consequences of Eating Seafood from the Chesapeake Bay Compared to Other Choices. In Diet for a Sustainable Ecosystem; Springer: Cham, Switzerland, 2021; pp. 247–268. [Google Scholar] [CrossRef]
- Pramod, C.V.; Fauziah, R.; Seshan, K.; Lange, J.P. Bio-based acrylic acid from sugar via propylene glycol and allyl alcohol. Catal. Sci. Technol. 2018, 8, 289–296. [Google Scholar] [CrossRef]
- James, R.; Manoukian, O.S.; Kumbar, S.G. Poly(lactic acid) for delivery of bioactive macromolecules. Adv. Drug Deliv. Rev. 2016, 107, 277–288. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Lozano, C.C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Saari, N.; Hussin, A.S.M. Review on the Biological Detoxification of Mycotoxins Using Lactic Acid Bacteria to Enhance the Sustainability of Foods Supply. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Byers, J.A.; Biernesser, A.B.; Chiaie, K.R.D.; Kaur, A.; Kehl, J.A. Catalytic Systems for the Production of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Springer: Cham, Switzerland, 2017; pp. 67–118. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Applications of Poly(lactic Acid); William Andrew Publishing: Oxford, UK, 2013; pp. 301–327. [Google Scholar] [CrossRef]
- Mustățea, G.; Ungureanu, E.L.; Belc, N. Polylactic acid (pla) for food packaging applications—A short overview. Ann. Food Sci. Technol. 2019, 20, 9–14. [Google Scholar]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly developed techniques on polycondensation, ringopening polymerization and polymer modification: Focus on poly (lactic acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef]
- Li, J.; Stayshich, R.M.; Meyer, T.Y. Exploiting Sequence To Control the Hydrolysis Behavior of Biodegradable PLGA Copolymers. J. Am. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Marszałek-Harych, A.; Jędrzkiewicz, D.; Ejfler, J. Bio- and chemocatalysis cascades as a bridge between biology and chemistry for green polymer synthesis. Cell. Mol. Biol. Lett. 2017, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-R.; Liang, Y.; Li, Z.-L. Precision Aliphatic Polyesters via Segmer Assembly Polymerization. Molecules 2018, 23, 452. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2010, 40, 1761–1776. [Google Scholar] [CrossRef]
- Johnson, R.M.; Fraser, C.L. Metalloinitiation Routes to Biocompatible Poly(lactic acid) and Poly(acrylic acid) Stars with Luminescent Ruthenium Tris(bipyridine) Cores. Biomacromolecules 2004, 5, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Barón, M.; Hellwich, K.-H.; Hess, M.; Horie, K.; Jenkins, A.D.; Jones, R.G.; Kahovec, J.; Kratochvíl, P.; Metanomski, W.V.; Mormann, W.; et al. Glossary of class names of polymers based on chemical structure and molecular architecture (IUPAC Recommendations 2009). Pure Appl. Chem. 2009, 81, 1131–1186. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Dijkstra, P.J.; Feijen, J. In Situ Forming Poly (ethylene glycol)-Poly (l-lactide) Hydrogels via Michael Addition: Mechanical Properties, Degradation, and Protein Release. Macromol. Chem. Phys. 2012, 213, 766–775. [Google Scholar] [CrossRef]
- Velthoen, I.W.; Tijsma, E.J.; Dijkstra, P.J.; Feijen, J. Thermo-Responsive Hydrogels Based on Branched Poly(L-lactide)-poly(ethylene glycol) Copolymers. Macromol. Symp. 2008, 272, 13–27. [Google Scholar] [CrossRef]
- Salaam, L.E.; Dean, D.; Bray, T.L. In vitro degradation behaviour of biodegradable 4-star micelles. Polymer 2006, 47, 310–318. [Google Scholar] [CrossRef]
- Liu, M.-J.; Chen, S.-C.; Yang, K.-K.; Wang, Y.-Z. Biodegradable polylactide based materials with improved crystallinity, mechanical properties and rheological behaviour by introducing a long-chain branched copolymer. RSC Adv. 2015, 5, 42162–42173. [Google Scholar] [CrossRef]
- Song, C.X.; Feng, X.D. Synthesis of ABA triblock copolymers of iε-caprolactone and DL-lactide. Macromolecules 1984, 17, 2764–2767. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.S.; Lee, Y.M.; Ihn, K.J.; Kim, S.H.; Kim, Y.H. Morphology of elastic poly (L-lactide-co-ε-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaf-folds. BioMacromolecules 2004, 5, 1303–1309. [Google Scholar] [CrossRef]
- Rozga-Wijas, K.; Stanczyk, W.A.; Kurjata, J.; Kazmierski, S. Star-shaped and linear POSS-polylactide hybrid copolymers. Materials 2015, 8, 4400–4420. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ding, J.; Li, J.; Liu, Y.; Hao, J. Blends of poly (d, l-lactide) with polyhedral oligomeric silsesquioxanes-based biodegradable polyester: Synthesis, morphology, miscibility, and mechanical property. J. Appl. Polym. Sci. 2014, 131, 40776. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Li, X.; Yuan, J.; Wang, S. Organic/inorganic hybrid star-shaped block copolymers of poly(l-lactide) and poly(N-isopropylacrylamide) with a polyhedral oligomeric silsesquioxane core: Synthesis and self-assembly. Eur. Polym. J. 2012, 48, 720–729. [Google Scholar] [CrossRef]
- Breitenbach, A.; Kissel, T. Biodegradable comb polyesters: Part 1 synthesis, characterization and structural analysis of poly (lactide) and poly (lactide-coglycolide) grafted onto water-soluble poly (vinyl alcohol) as backbone. Polymer 1998, 39, 3261–3271. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Cai, C.; Rytting, E.; Steele, T.; Kissel, T. Biodegradable branched polyesters poly (vinyl sulfonatecovinyl alcohol)-graft poly (D, L-lactic-coglycolic acid) as a negatively charged polyelectrolyte platform for drug delivery: Synthesis and characterization. Macromolecules 2008, 41, 2791–2799. [Google Scholar] [CrossRef]
- Bednarek, M. Branched aliphatic polyesters by ring-opening (co)polymerization. Prog. Polym. Sci. 2016, 58, 27–58. [Google Scholar] [CrossRef]
- Basko, M.; Bednarek, M.; Kubisa, P. Cationic copolymerization of L, L-lactide with hydroxyl substituted cyclic ethers. Polym. Adv. Technol. 2015, 26, 804–813. [Google Scholar] [CrossRef]
- Kubisa, P.; Penczek, S. Cationic activated monomer polymerization of heterocyclic monomers. Prog. Polym. Sci. 1999, 24, 1409–1437. [Google Scholar] [CrossRef]
- Tasaka, F.; Ohya, Y.; Ouchi, T. Synthesis of Novel Comb-Type Polylactide and Its Biodegradability. Macromolecules 2001, 34, 5494–5500. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby Jr, W.C.; Bondoc, C.C.; Jung, W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981, 194, 413. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mikos, A.G. The Importance of New Processing Techniques in Tissue Engineering. MRS Bull. 1996, 21, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-drying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Napiwocki, B.N.; Li, Z.-T.; Turng, L.-S.; Huang, H.-X. Fabrication of fibrous silica sponges by self-assembly electrospinning and their application in tissue engineering for three-dimensional tissue regeneration. Chem. Eng. J. 2018, 331, 652–662. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and bio-composite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Tsai, W.C.; Wang, Y. Progress of supercritical fluid technology in polymerization and its applications in biomedical engineering. Prog. Polym. Sci. 2019, 98, 101161. [Google Scholar] [CrossRef]
- Salerno, A.; Pascual, C.D. Bio-based polymers, supercritical fluids and tissue engineering. Process. Biochem. 2015, 50, 826–838. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Supercritical fluids in biomedical and tissue engineering applications: A review. Int. Mater. Rev. 2009, 54, 214–222. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2018, 96, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Khan, T.; Hussain, Z.; Ullah, H. Nanocomposite scaffolds for tissue engineering; properties, preparation and applications. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Sawston, Cambridge, 2018; pp. 701–735. [Google Scholar]
- Huang, R.; Zhu, X.; Tu, H.; Wan, A. The crystallization behavior of porous poly(lactic acid) prepared by modified solvent casting/particulate leaching technique for potential use of tissue engineering scaffold. Mater. Lett. 2014, 136, 126–129. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Chou, M.L.; Chew, C.H.; Noll, J.; Burnouf, T. Intelligent micro-/nanorobots as drug and cell carrier devices for biomedical therapeutic advancement: Promising development opportunities and translational challenges. Biomaterials 2020, 260, 120163. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Ravi Kumar, M.N.V. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Nahar, M.; Dutta, T.; Murugesan, S.; Asthana, A.; Mishra, D.; Rajkumar, V.; Tare, M.; Saraf, S.; Jain, N.K. Functional Polymeric Nanoparticles: An Efficient and Promising Tool for Active Delivery of Bioactives. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 259–318. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer International Publishing: Cham, Switzerlands, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Wu, W.; Tony To, S.S.; Zhao, H.; Wang, J. Advances in lipid-based drug delivery: Enhancing efficiency for hydrophobic drugs. Expert Opin. Drug Deliv. 2015, 12, 1475–1499. [Google Scholar] [CrossRef]
- Siegel, R.A. Stimuli sensitive polymers and self regulated drug delivery systems: A very partial review. J. Control. Release 2014, 190, 337–351. [Google Scholar] [CrossRef]
- Blasi, P. Poly (lactic acid)/poly (lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering Immunomodulatory Biomaterials to Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef]
- Goor, O.J.; Hendrikse, S.I.; Dankers, P.Y.; Meijer, E.W. From supramolecular polymers to multi-component biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Ivirico, J.L.E.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2018, 5, 128–154. [Google Scholar] [CrossRef]
- Srivastava, P.; Hira, S.K.; Paladhi, A.; Singh, R.; Gupta, U.; Srivastava, D.N.; Manna, P.P. Studies on interaction po-tency model based on drug synergy and therapeutic potential of triple stimuli-responsive delivery of doxorubicin and 5-fluoro-2-deoxyuridine against lymphoma using disulfide-bridged cysteine over mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 1411–1421. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Paritosh, K.; Pareek, N.; Vivekanand, V. Composites based on bioderived polymers: Potential role in tissue engineering: Vol VI: Resorbable polymer fibers. Mater. Biomed. Eng. 2019, 259–296. [Google Scholar] [CrossRef]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2020, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2020, 31, 2005941. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of nanomaterials for bone-targeted drug delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Acero, E.H.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(L-lactic acid) for drug delivery applications. Process. Biochem. 2017, 59, 77–83. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Shahraki, B.T.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Tavakolizadeh, M.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Pourjavadi, A.; et al. Burgeoning Polymer Nano Blends for Improved Controlled Drug Release: A Review. Int. J. Nanomed. 2020, 15, 4363–4392. [Google Scholar] [CrossRef]
- Prasad, M.; Ghosh, M.; Kumar, R.; Brar, B.; Surjith, K.P.; Lambe, U.P.; Ranjan, K.; Banerjee, S.; Prasad, G.; Khurana, S.K.; et al. The Importance of Nanomedicine in Prophylactic and Theranostic Intervention of Bacterial Zoonoses and Reverse Zoonoses in the Era of Microbial Resistance. J. Nanosci. Nanotechnol. 2021, 21, 3404–3452. [Google Scholar] [CrossRef]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Transl. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Kurt, I.; Gozutok, K.; Ozkan, B.; Ozkan, B.; Isildak, I. Polymer-Based Biocomposites. Biomater. Tissue Eng. 2017, 4, 49–89. [Google Scholar]
- Lim, K.S.; Park, J.K.; Jeong, M.H.; Bae, I.H.; Nah, J.W.; Park, D.S.; Kang, J.C. Effect of stents coated with a combination of sirolimus and alpha-lipoic acid in a porcine coronary restenosis model. J. Mater. Sci. Mater. Med. 2016, 27, 66. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Dong, Y.; Lau, K.T. Tetracycline hydrochloride (TCH)-loaded drug carrier based on PLA: PCL nan-ofibre mats: Experimental characterisation and release kinetics modelling. J. Mater. Sci. 2014, 49, 6270–6281. [Google Scholar] [CrossRef]
- Son, J.S.; Hwang, E.J.; Kwon, L.S.; Ahn, Y.G.; Moon, B.K.; Kim, J.; Lee, S.Y. Antibacterial Activity of Propo-lis-Embedded Zeolite Nanocomposites for Implant Application. Materials 2021, 14, 1193. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.K.; Panikar, D.; Nair, S.V.; et al. Theranostic 3-Dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Sci. Rep. 2017, 7, srep43271. [Google Scholar] [CrossRef]
- Nagam Hanumantharao, S.; Rao, S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Krlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible polymer materials with antimicrobial properties for preparation of stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-Based Delivery of Nucleic Acid Therapeutics for Enhanced Bone and Cartilage Repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef]
- Thompson, E.M.; Matsiko, A.; Kelly, D.J.; Gleeson, J.P.; O’Brien, F.J. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part A 2016, 22, 556–567. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Complications of a poly-l-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology 2018, 106, 360–368. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhai, T.; Wang, X.; Dan, Y.; Turng, L.-S. The surface grafting of graphene oxide with poly(ethylene glycol) as a reinforcement for poly(lactic acid) nanocomposite scaffolds for potential tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 403–413. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hussein, K.H.; Casettari, L.; Khalil, K.A.; Hamdy, A.S. Fabrication of novel high performance ductile poly(lactic acid) nanofiber scaffold coated with poly(vinyl alcohol) for tissue engineering applications. Mater. Sci. Eng. C 2016, 60, 143–150. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of poly (ethylene glycol) grafted cellulose nanocrystals in poly (lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue en-gineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kucinska-Lipka, J.; Gubańska, I.C.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Salatin, S.; Saadat, Y.R.; Vahed, S.Z.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef]
- Shim, I.K.; Jung, M.R.; Kim, K.H.; Seol, Y.J.; Park, Y.J.; Park, W.H.; Lee, S.J. Novel three-dimensional scaffolds of poly(L-lactic acid) microfibers using electrospinning and mechanical expansion: Fabrication and bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Cheon, K.H.; Park, C.; Jang, T.S.; Kim, H.E.; Jung, H.D. Fabrication of poly (lactic acid)/Ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (FFF)–based 3D printing. Addit. Manuf. 2019, 30, 100883. [Google Scholar] [CrossRef]
- Nabilla, F.; Widiyanti, P.; Hikmawati, D. Fabrication and Characterizations of Gelatin/Chitosan with Aloe Vera and Achatina Fulica sp Mucus as Scaffold for Skin Tissue Engineering. J. Teknol. 2019, 81, 51–57. [Google Scholar] [CrossRef]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A New Bone Substitute Developed from 3D-Prints of Polylactide (PLA) Loaded with Collagen I: An In Vitro Study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Li, Q. Three dimensional printed macroporous polylactic ac-id/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Zhao, D.; Jiang, W.; Du, Z.; Li, Q.; Han, D. Three dimensional printed polylactic ac-id-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: An in vivo bioreactor model. Sci. Rep. 2017, 7, 15255. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Yeon, Y.K.; Park, H.S.; Lee, J.M.; Lee, J.S.; Lee, Y.J.; Sultan, M.T.; Park, C.H. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J. Biomater. Sci. Polym. Ed. 2018, 29, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2009, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Jung, H.M.; Woo, K.M.; Kim, H.J. Electrospun Silk Fibroin Scaffolds with Macropores for Bone Regeneration: An In Vitro and In Vivo Study. Tissue Eng. Part A 2010, 16, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. Excli J. 2021, 20, 454. [Google Scholar]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef]
- Boro, U.; Kashyap, N.; Moholkar, V.S. Sonochemical Synthesis of Poly(lactic acid) Nanocomposites with ZnO Nanoflowers: Effect of Nanofiller Morphology on Physical Properties. ACS Eng. Au 2021, 2, 46–60. [Google Scholar] [CrossRef]
- Fattahi, F.S.; Khoddami, A.; Avinc, O. Poly (lactic acid) (PLA) nanofibers for bone tissue engineering. J. Text. Polym. 2019, 7, 47–64. [Google Scholar]
- Fattahi, F.S. Poly (lactic acid) nano-structures for cartilage regeneration and joint repair: Strategies and ideas. Recent Trends Nanosci. Technol. 2020, 1, 1–19. [Google Scholar]
- Cao, H.; Chen, M.-M.; Liu, Y.; Liu, Y.-Y.; Huang, Y.-Q.; Wang, J.-H.; Chen, J.-D.; Zhang, Q.-Q. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 1098–1106. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic Acid Based Nanocomposites: Promising Safe and Biodegradable Materials in Biomedical Field. Int. J. Polym. Sci. 2016, 2016, 6869154. [Google Scholar] [CrossRef]
- Erten, E.; Arslan, Y.E. The Great Harmony in Translational Medicine: Biomaterials and Stem Cells. Adv. Exp. Med. Biol. 2018, 1119, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent functionalized carbon quantum dots loaded electroactive Silk fibroin/PLA nanofibrous bioactive scaffolds for cardiac tissue engineering. J. Photochem. Photobiol. B Biol. 2019, 202, 111680. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 65, 24. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, X.; Qiu, X.; Gao, T.; Yu, W.; Zhang, M.; Song, L.; Liu, D.; Dong, J.; Jiang, Z. Bioactive composite Janus nanofibrous membranes loading Ciprofloxacin and Astaxanthin for enhanced healing of full-thickness skin defect wounds. Appl. Surf. Sci. 2023, 610, 155290. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L. Current advances in sustained-release systems for parenteral drug delivery. Expert Opin. Drug Deliv. 2005, 2, 1039–1058. [Google Scholar] [CrossRef]
- Sun, S.-J.; Deng, P.; Peng, C.-E.; Ji, H.-Y.; Mao, L.-F.; Peng, L.-Z. Extraction, Structure and Immunoregulatory Activity of Low Molecular Weight Polysaccharide from Dendrobium officinale. Polymers 2022, 14, 2899. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Zhong, H.-J.; Huang, D.-N.; Wu, L.-H.; He, X.-X. Beneficial Effects of Repeated Washed Microbiota Transplantation in Children With Autism. Front. Pediatr. 2022, 10, 928785. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Li, B.; Yang, X.; Zeng, R.; Liu, Y.; Li, T.; Ho, R.J.; Shao, J. PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment. J. Colloid Interface Sci. 2017, 490, 542–552. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Hu, J.; Chen, X.; Jing, X. Enantiomeric PLA–PEG block copolymers and their stereocomplex micelles used as rifampin delivery. J. Nanopart. Res. 2006, 9, 777–785. [Google Scholar] [CrossRef]
- Chen, S.; Singh, J. In vitro release of levonorgestrel from phase sensitive and thermosensitive smart polymer delivery systems. Pharm. Dev. Technol. 2005, 10, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. PLA-PEG-PLA copolymer-based polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs: Preparation and evaluation with atorvastatin and lisinopril. Drug Dev. Ind. Pharm. 2014, 40, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Gross, A.L.; Martin, D.R.; E Byrne, M. Polyethylene glycol-b-poly(lactic acid) polymersomes as vehicles for enzyme replacement therapy. Nanomedicine 2017, 12, 2591–2606. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izák, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
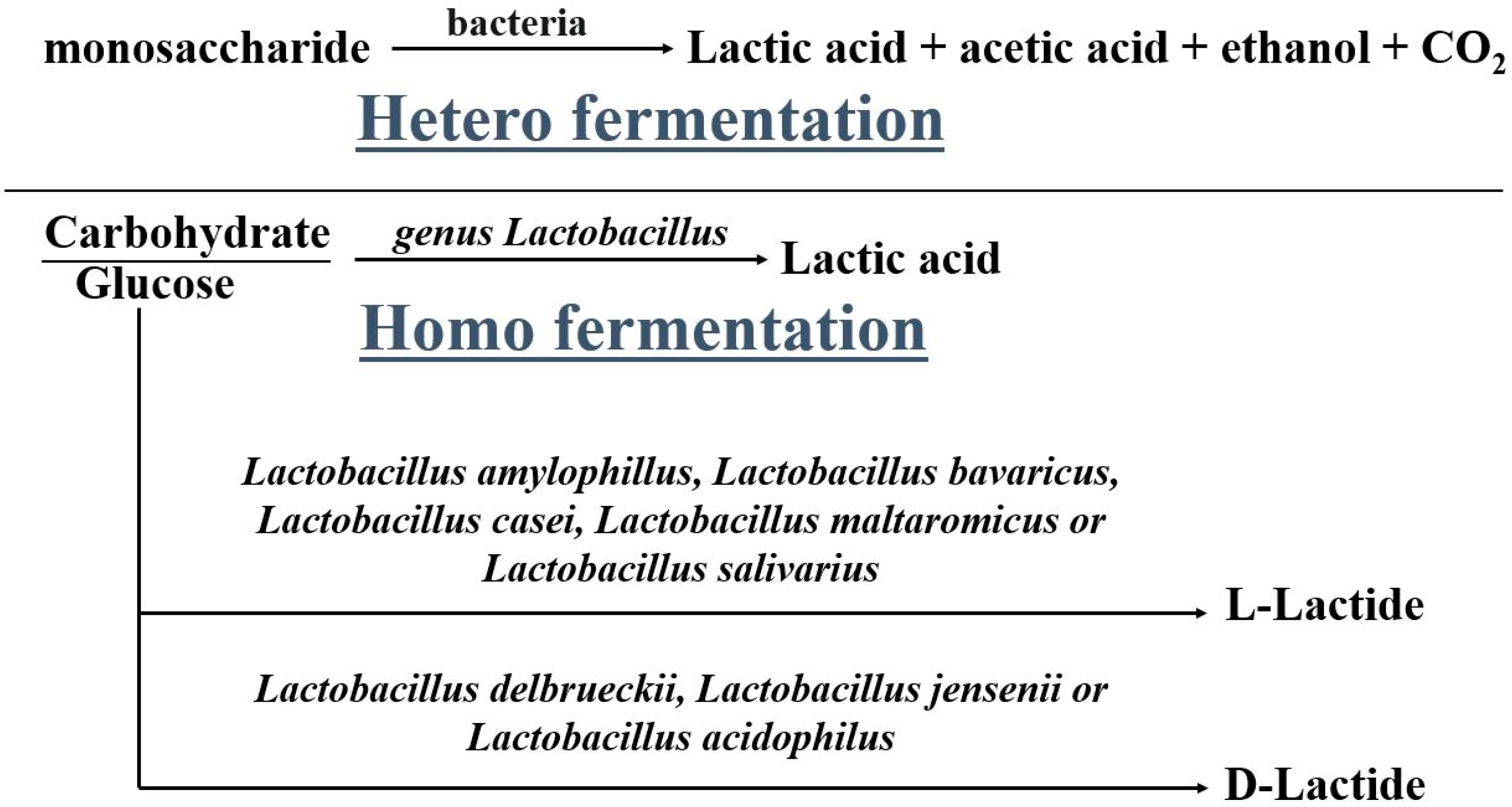

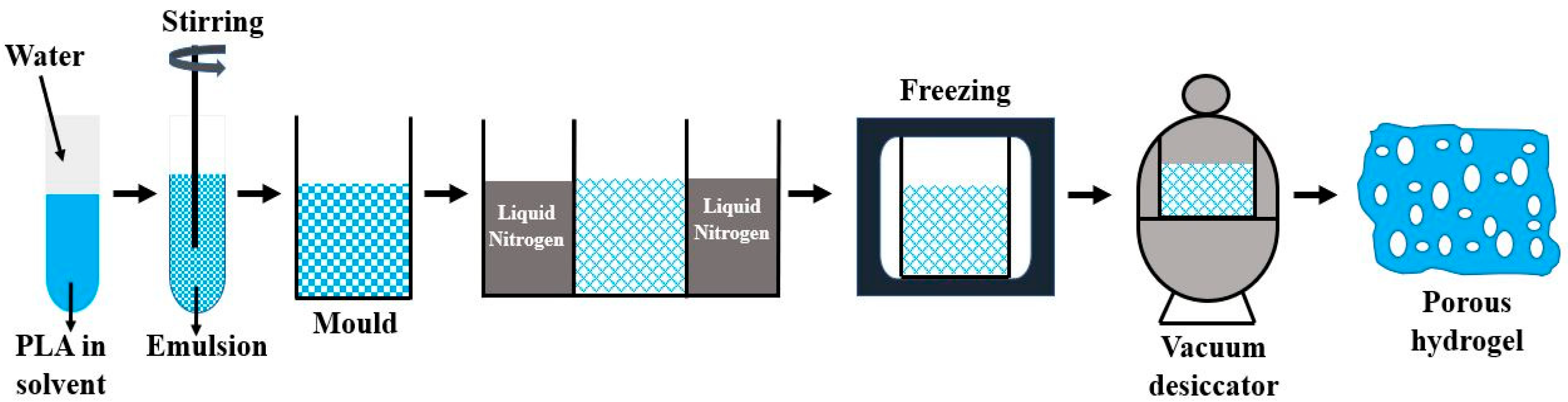
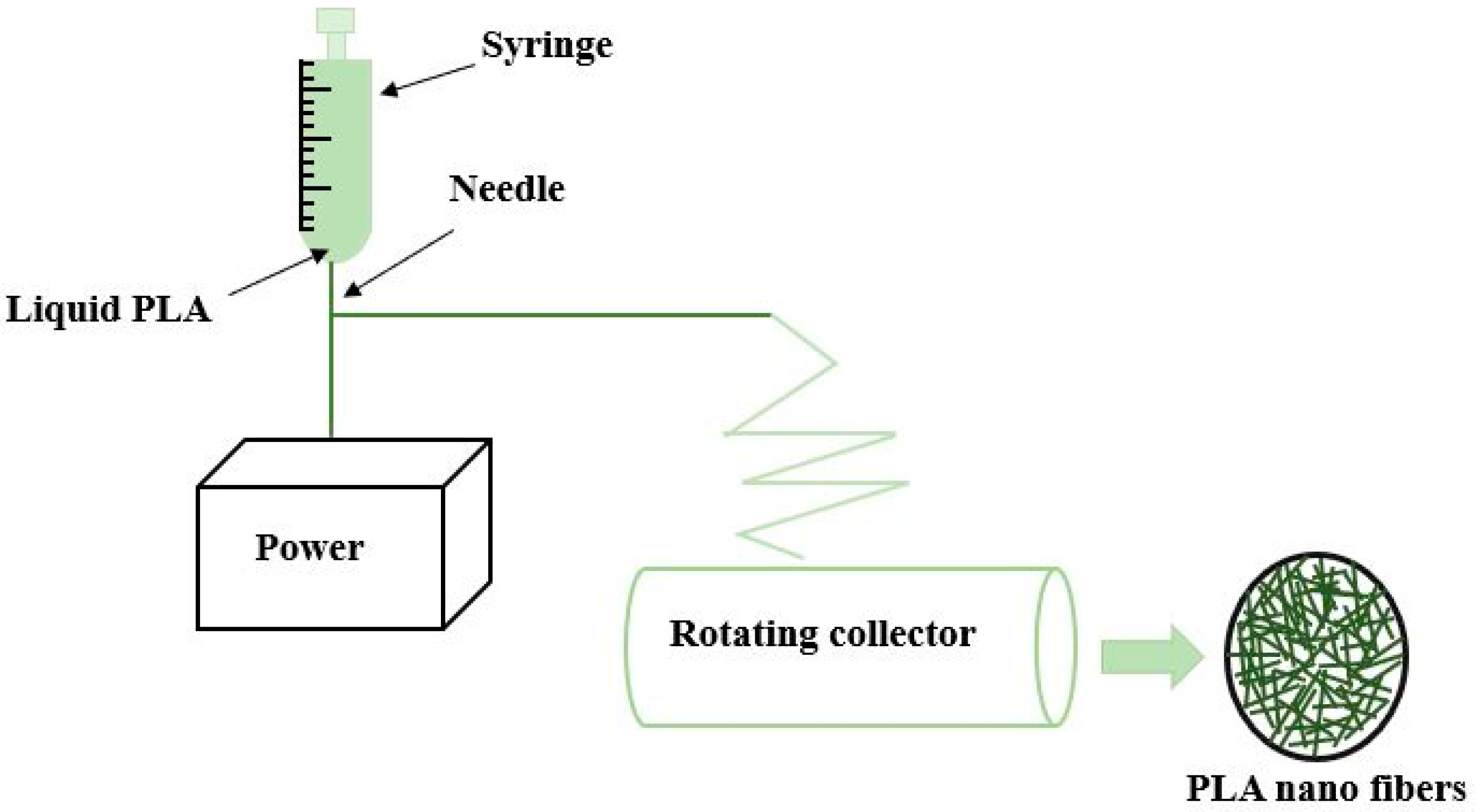
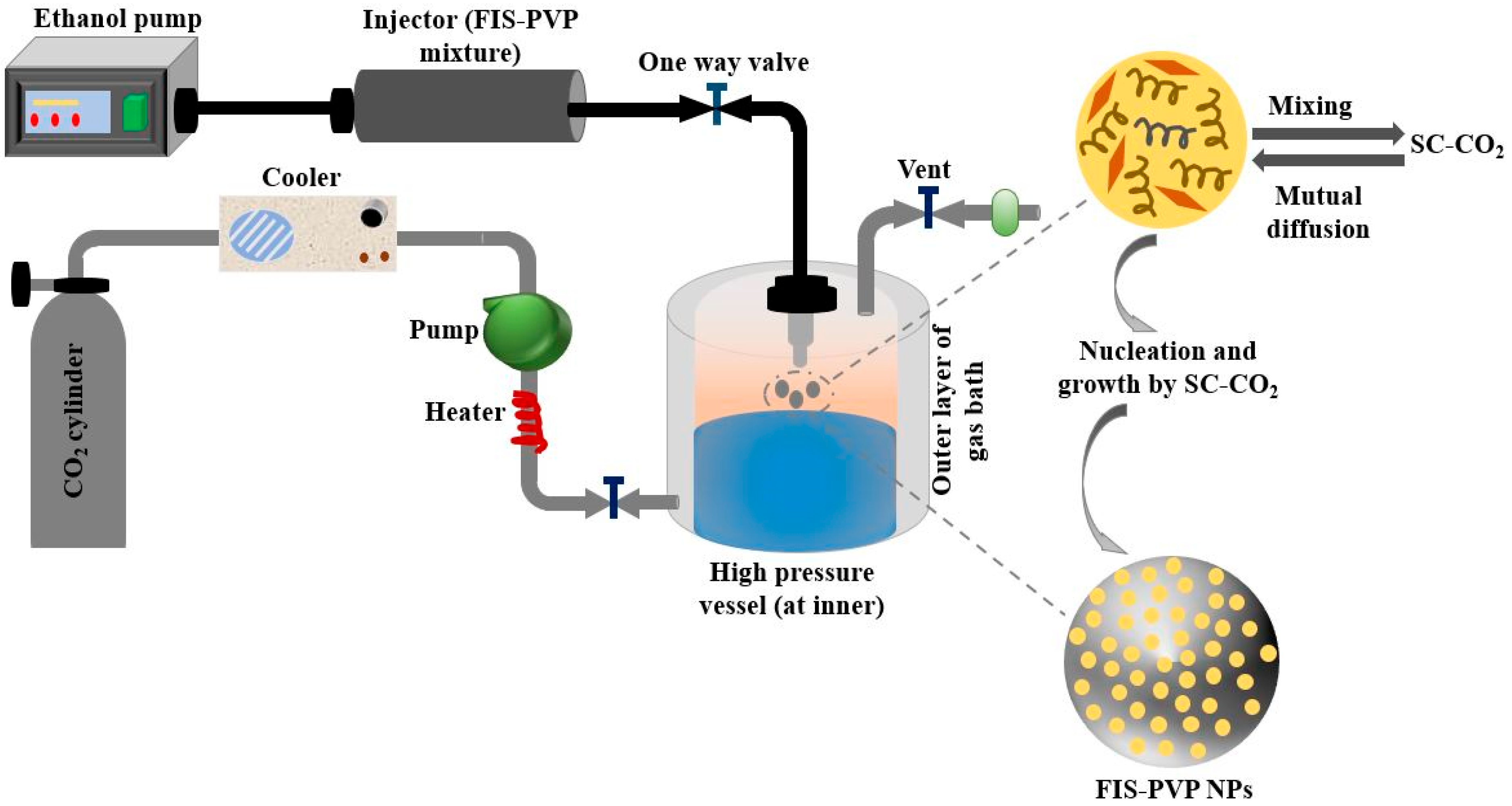


| Copolymer | Architecture | Copolymerization Technique | Enhanced Properties | Applications | References |
|---|---|---|---|---|---|
| Poly(D,L-lactide-co-glycolide) (PLGA) | Linear polymer | Solution poly condensation, ROP, segmer assembly polymerization | Shortens the degradation time, higher molecular weight of copolymer | Drug delivery system | [24,25,26] |
| Metal-Centered Star-Shaped PLA (Co)Polymers | Star polymer | atom transfer radical polymerization | Hydrophobicity at core and hydrophilicity at corona | Drug delivery of optical imaging, biomedicine | [27,28,29] |
| Poly ethylene glycol-PLA | Star polymer | Michael-type addition reaction | Lower degradation time from few days to month, enhanced mechanical strength, and imparts thermal responsive behavior | Scaffolds, tissue engineering, biocompatible hydrogels, and drug delivery | [30,31,32] |
| Polycarbonate-PLA | Linear polymer | Formed by the addition of hexamethylene diisocyanate in the chemical reaction | Improve crystallinity, rheological behavior, mechanical properties, and higher elongation at break | Medicine, tough membrane for stimuli drug delivery system, and tubular scaffolds | [33,34,35] |
| Polyhedral oligomeric silsesquioxane-PLA | Star polymer | ROP, solution casting, reversible addition fragmentation transfer (RAFT) polymerization | Enhanced ductility, improved toughness, and elongation at break | Nerve engineering, serve as basis for collagen | [36,37,38] |
| Poly vinyl alcohol-g-PLA | Graft polymer | Graft polymerization | Crystallinity and biodegradability enhance, melting point increases and glass transition temperature improves | Agriculture and food packaging and drug delivery | [39,40] |
| PLA-Glycidol | Branch polymer | Terminal ring opening polymerization | Thermal behavior improved; hydrophilicity enhanced. | Biomedical and industrial applications | [41,42,43] |
| PLA-Co-Polyesters | Graft comb polymer | Graft polymerization | Increase in hydrophilic characteristics and biodegradability | Scaffolding and tissue engineering | [44] |
| Manufacturing Technique | Biopolymer Composition | Influencing Properties | Domain of Application | References |
|---|---|---|---|---|
| Electrospinning | Tricalcium phosphate-Poly(L-lactic-co-glycoside) | Excellent moldability and absorbance ability are enhanced. | Repairs bone defects | [123,124,125,126] |
| Gelatin-PLLA | New calcified bone formed within 84 days of induction, improves cell proliferation and adhesion. | |||
| Silk fiber-PLLA | Enables uniform distribution of cells in the matrix and improves cell adhesion. | Engineer cartilage tissues | ||
| Collagen-PLL-co-glycolide) | Matrix becomes hydrophobic and enables easy induction of myogenesis. | Regenerate skeletal tissues | ||
| Collagen1-PDLLA | Hydrophobicity increase, higher rate of cell proliferation, and improves stability. | Bone- reconstruction | ||
| Collagen-PLLA | Plays significant role in invitro osteogenesis, large bone aggregates are obtained due to the even distribution of minerals, the expressions of osteoblastic genes obtained are comparable and higher. | Bone -regeneration | ||
| Freeze drying | Gelatin-PLA | Decreases inflammation, cell proliferation, and attachment enhanced. | Repair cartilage | [123,124] |
| SFC | Demineralized bone matrix-PLA | Mechanical strength improves | Repair bone defects | [126] |
| 3D bio printing | PC-poly(L-lactide-co-glycoside)-triphosphate | Capable of forming new bone around implant, Osseo-integration | Bone-reconstruction | [127] |
| Electrochemical | Silk fibroin-PLA | Cardiomyocytes functionality improves, better swelling characteristics and comprehensive modulus | Application of nursing and regeneration of cardiac tissue | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranakoti, L.; Gangil, B.; Bhandari, P.; Singh, T.; Sharma, S.; Singh, J.; Singh, S. Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules 2023, 28, 485. https://doi.org/10.3390/molecules28020485
Ranakoti L, Gangil B, Bhandari P, Singh T, Sharma S, Singh J, Singh S. Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules. 2023; 28(2):485. https://doi.org/10.3390/molecules28020485
Chicago/Turabian StyleRanakoti, Lalit, Brijesh Gangil, Prabhakar Bhandari, Tej Singh, Shubham Sharma, Jujhar Singh, and Sunpreet Singh. 2023. "Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications" Molecules 28, no. 2: 485. https://doi.org/10.3390/molecules28020485
APA StyleRanakoti, L., Gangil, B., Bhandari, P., Singh, T., Sharma, S., Singh, J., & Singh, S. (2023). Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules, 28(2), 485. https://doi.org/10.3390/molecules28020485








