Quantification and Validation of an HPLC Method for Low Concentrations of Apigenin-7-O-Glucuronide in Agrimonia pilosa Aqueous Ethanol Extract Topical Cream by Liquid–Liquid Extraction
Abstract
1. Introduction
2. Results
2.1. Optimization of the Sample Preparation
2.2. Summary of the Validation
2.3. System Suitability
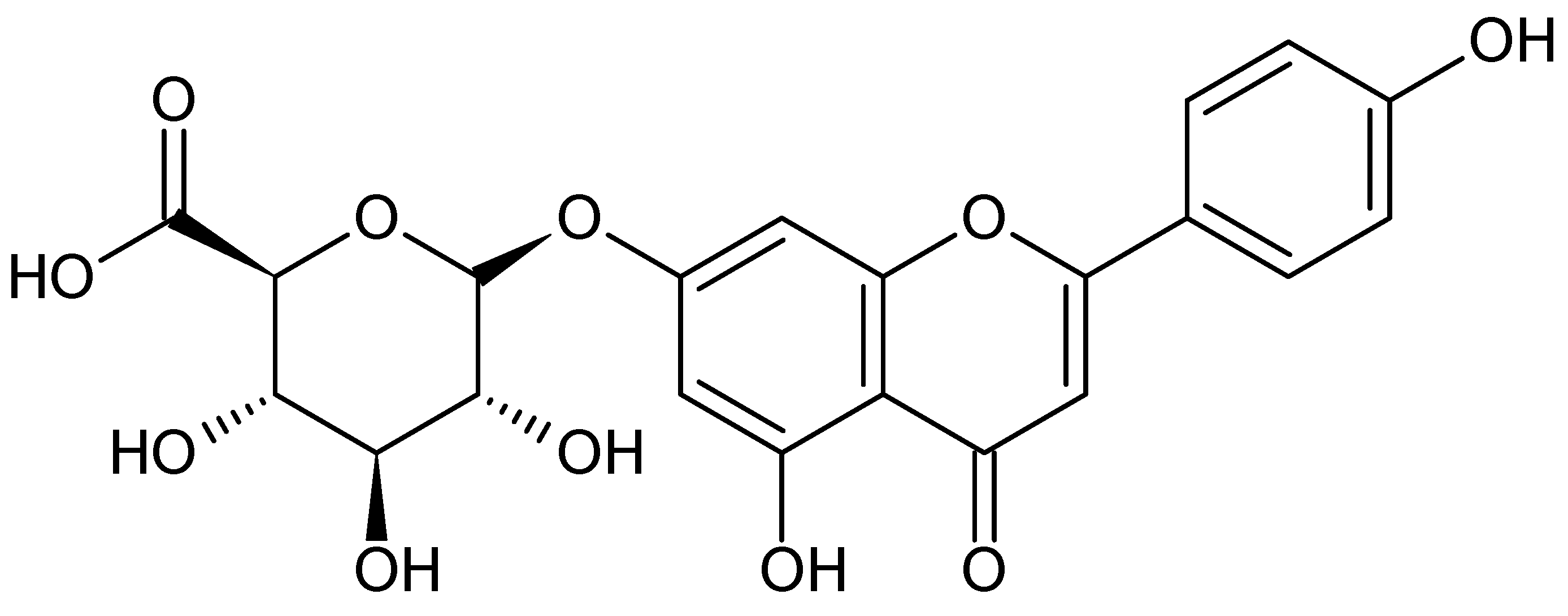
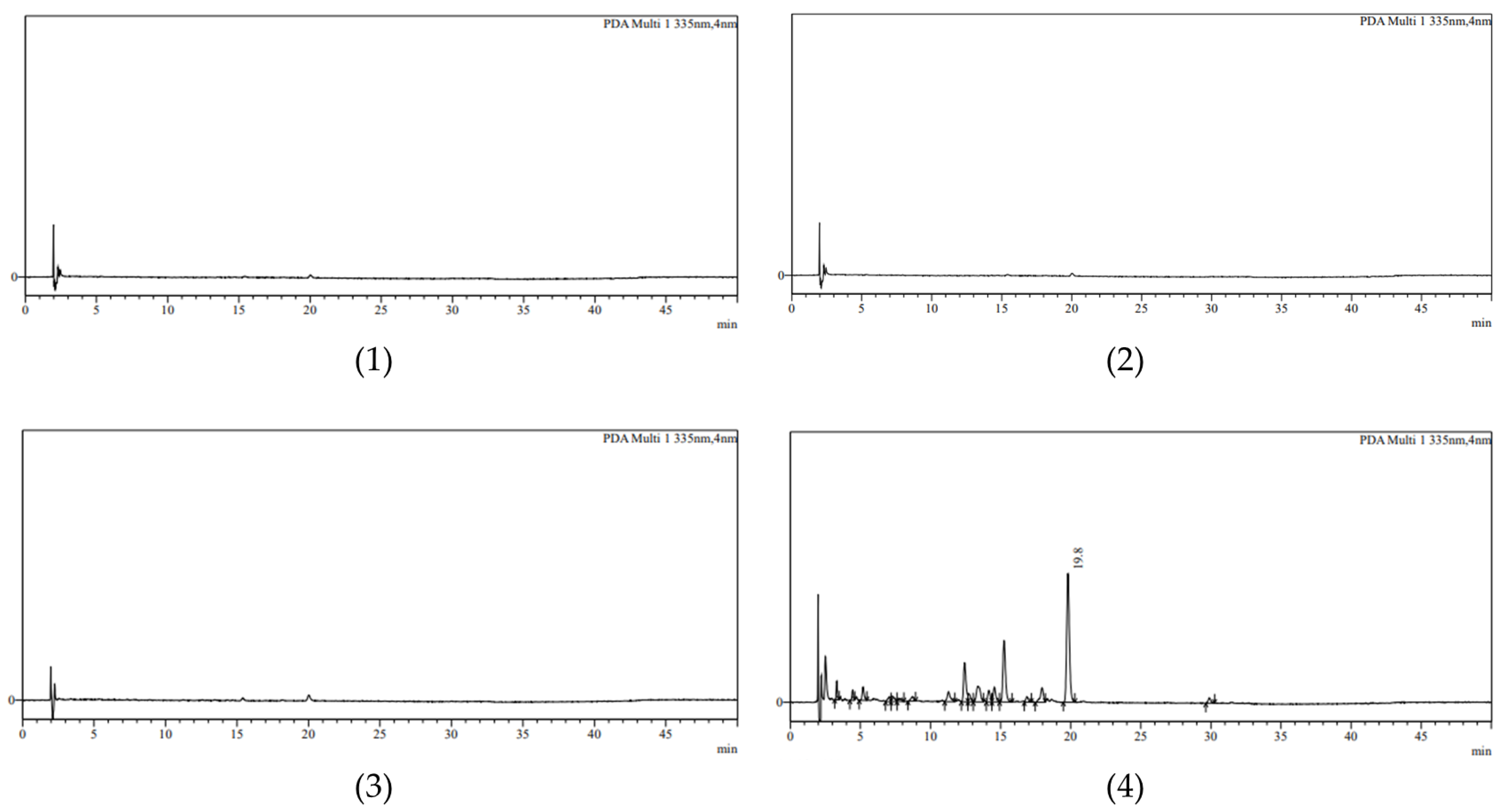
2.4. Specificity
2.5. Linearity, Limit of Detection (LOD), and Limit of Quantitation (LOQ)
2.6. Accuracy
2.7. Precision (Repeatability)
2.8. Intermediate Precision
2.9. Solution Stability
2.10. Quantification of the 0.1% AE Cream
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of the Agrimonia pilosa Extract
4.3. Preparation of 0.1% AE Cream
4.4. Instrument and Chromatographic Condition
4.5. Optimization of the Sample Preparation Method
4.6. Sample Preparation for the Validation
4.7. Validation
4.8. Quantification of the 0.1%AE Cream
- WI.S: Weight of the internal standard (i.e., baicalin);
- S.s: The concentration of A7OG in the standard solution (concentration correction required according to the purity of the standard product);
- Rc: The ratio (A7OG peak area/internal standard peak area) in the sample solution;
- Rs: The ratio (A7OG peak area/internal standard peak area) of the standard solution;
- S.I: The concentration of the internal standard in the standard solution (concentration correction required according to the purity of the standard product);
- W: Weight of the cream.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Le, Q.U.; Joshi, R.K.; Lay, H.L.; Wu, C.W. Agrimonia pilosa Ledeb: Phytochemistry, ethnopharmacology, pharmacology of an important traditional herbal medicine. Int. J. Pharmacogn. Phytochem. Res. 2018, 7, 3202–3211. [Google Scholar]
- Krasnopoļska, D.; Evarts-Bunders, P.; Svilāne, I. Contribution to knowledge of genus agrimonia L.(rosaceae) in Lavita. Acta Biol. Univ. Daugavp. 2020, 20, 47–53. [Google Scholar]
- Fei, X.; Yuan, W.; Jiang, L.; Wang, H. Opposite effects of Agrimonia pilosa Ledeb aqueous extracts on blood coagulation function. Ann. Transl. Med. 2017, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, X.; Wu, Y.; Yu, S.; Zhang, W.; Liu, D.; Yang, K.; Sun, J. Agrimonia pilosa Ledeb.: A review of its traditional uses, botany, phytochemistry, pharmacology, and toxicology. Heliyon 2022, 8, e09972. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Chi, L.; Ma, C. Agrimonia pilosa: A phytochemical and pharmacological review. Evid. Based Complement Alternat. Med. 2022, 2022, 3742208. [Google Scholar] [CrossRef]
- Kim, T.Y.; Koh, K.S.; Ju, J.M.; Kwak, Y.J.; Bae, S.K.; Jang, H.O.; Kim, D.S. Proteomics analysis of antitumor activity of Agrimonia pilosa Ledeb. In human oral squamous cell carcinoma cells. Curr. Issues Mol. Biol. 2022, 44, 3324–3334. [Google Scholar] [CrossRef]
- Kim, H.S. Physiological activities of Agrimonia pilosa extract. Korean J. Food Preserv. 2015, 22, 261–266. [Google Scholar] [CrossRef]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef]
- Kim, N.C. Need for pharmacopeial quality standards for botanical dietary supplements and herbal medicines. Food Suppl. Biomater. Health 2021, 1, e10. [Google Scholar] [CrossRef]
- Indrayanto, G. Recent development of quality control methods for herbal derived drug preparations. Nat. Prod. Commun. 2018, 13, 1599–1606. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality control of the traditional herbs and herbal products: A review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Rossi Forim, M.; Perlatti, B.; Soares Costa, E.; Facchini Magnani, R.; Donizetti de Souza, G. Concerns and considerations about the quality control of natural products using chromatographic methods. Curr. Chromatogr. 2015, 2, 20–31. [Google Scholar] [CrossRef]
- Länger, R.; Stöger, E.; Kubelka, W.; Helliwell, K. Quality standards for herbal drugs and herbal drug preparations–appropriate or improvements necessary? Planta Med. 2018, 84, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L. HPLC in natural product analysis: The detection issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Govindarajan, R.; Tejas, V.; Pushpangadan, P. High-performance liquid chromatography (HPLC) as a tool for standardization of complex herbal drug. J AOAC Int. 2019, 102, 986–992. [Google Scholar] [CrossRef]
- Chan, W.F.; Lin, C.W. High-pressure liquid chromatography: Quantitative analysis of Chinese herbal medicine. J. Chem. Educ. 2007, 84, 1982–1984. [Google Scholar] [CrossRef]
- Alqarni, M.H.; Alam, P. Highly Sensitive and Ecologically Sustainable Reversed-Phase HPTLC Method for the Determination of Hydroquinone in Commercial Whitening Creams. Procesess 2021, 9, 1631. [Google Scholar] [CrossRef]
- Lee, J.; Kumar, J. Liquid-Liquid extraction general principles A review. J. Korean Inst. Resour. Recycl. 2009, 18, 3–9. [Google Scholar]
- Raikos, N.; Spagou, K.; Vlachou, M.; Pouliopoulos, A.; Thessalonikeos, E.; Tsoukali, H. Development of a Liquid-Liquid Extraction Procedure for the Analysis of Amphetamine in Biological Specimens by GC-FID. Open Forensic. Sci. J. 2009, 2, 12–15. [Google Scholar]
- Yusiasih, R.; Marvalosha, R.; Suci, S.D.S.; Yuliani, E.; Pitoi, M.M. Low volume liquid-liquid extraction for the determination of benzene, toluene, and xylene in water by GC-FID and HPLC-UV. Environ. Earth Sci. 2019, 277, 012019. [Google Scholar] [CrossRef]
- Roque, L.R.; Morgado, G.P.; Nascimento, V.M.; Ienczak, J.L.; Rabelo, S.C. Liquid-liquid extraction: A promising alternative for inhibitors removing of pentoses fermentation. Fuel 2019, 242, 775–787. [Google Scholar] [CrossRef]
- Stone, J. Sample preparation techniques for mass spectrometry in the clinical laboratory. In Mass Spectrometry for the Clinical Laboratory; Academic Press: Cambridge, MA, USA, 2017; pp. 37–62. [Google Scholar]
- AOAC International. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; Available online: http://www.aoac.org/dietsupp6/Dietary-Supplement-web-site/slv_guidelines.pdf (accessed on 5 October 2021).


| Parameter | Result | ||
|---|---|---|---|
| System Suitability | %RSD of A7OG AREA | 0.4% | |
| %RSD of Baicalin AREA | 0.2% | ||
| Linearity (μg/mL) | 10.2–23.8 | ||
| Slope | 25,289 | ||
| Intercept | −9184 | ||
| Regression coefficient (R2) | 0.9999 | ||
| %Recovery | 60% | 97 ± 0.3 | |
| 100% | 99.8 ± 0.5 | ||
| 140% | 102 ± 0.4 | ||
| Precision (%RSD) | 0.20% | ||
| Intermediate Precision (%RSD) | 0.15% | ||
| LOD (μg/mL) | 0.28 | ||
| LOQ (μg/mL) | 0.87 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Nam, Y.R.; Kim, H.J.; Kim, W.K. Quantification and Validation of an HPLC Method for Low Concentrations of Apigenin-7-O-Glucuronide in Agrimonia pilosa Aqueous Ethanol Extract Topical Cream by Liquid–Liquid Extraction. Molecules 2023, 28, 713. https://doi.org/10.3390/molecules28020713
Lee JS, Nam YR, Kim HJ, Kim WK. Quantification and Validation of an HPLC Method for Low Concentrations of Apigenin-7-O-Glucuronide in Agrimonia pilosa Aqueous Ethanol Extract Topical Cream by Liquid–Liquid Extraction. Molecules. 2023; 28(2):713. https://doi.org/10.3390/molecules28020713
Chicago/Turabian StyleLee, Jin Seok, Yu Ran Nam, Hyun Jong Kim, and Woo Kyung Kim. 2023. "Quantification and Validation of an HPLC Method for Low Concentrations of Apigenin-7-O-Glucuronide in Agrimonia pilosa Aqueous Ethanol Extract Topical Cream by Liquid–Liquid Extraction" Molecules 28, no. 2: 713. https://doi.org/10.3390/molecules28020713
APA StyleLee, J. S., Nam, Y. R., Kim, H. J., & Kim, W. K. (2023). Quantification and Validation of an HPLC Method for Low Concentrations of Apigenin-7-O-Glucuronide in Agrimonia pilosa Aqueous Ethanol Extract Topical Cream by Liquid–Liquid Extraction. Molecules, 28(2), 713. https://doi.org/10.3390/molecules28020713







