Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes
Abstract
:1. Opening Remarks
2. Taste Transmission Mechanism in Humans
3. Taste-Masking Agents as Pharmaceutical Excipients
4. Cyclodextrins as Taste-Masking Agents
5. Taste Assessment Methods
6. Overview of APIs Complexed with CDs to Mask Their Taste
- A02: ranitidine hydrochloride (1), famotidine (3);
- A03: propantheline bromide (1), oxyphenonium bromide (1);
- A07: prednisolone (1), loperamide hydrochloride (1);
- A16: 4-phenylbutyrate (1);
- C01: lidocaine hydrochloride (1), Indomethacin (1);
- C03: furosemide (1);
- C05: diltiazem hydrochloride (1);
- C09: captopril (1);
- D04: promethazine hydrochloride (2);
- D08: triclosan (1);
- G04: vardenafil (1);
- J01: cefixime trihydrate (2), lomefloxacin hydrochloride (1), cefuroxime axetil (3);
- J05: oseltamivir phosphate (1);
- M01: meloxicam (4), lornoxicam (1), ibuprofen (1), aclofenac (1);
- N02: rizatriptan benzoate (1), sumatriptan succinate (1);
- N03: lamotrigine (1), gabapentin (1);
- N05: aripiprazole (1), hydroxyzine (1);
- N06: fluoxetine (1), donepezil (1), paroxetine hydrochloride (1), atomoxetine hydrochloride (1);
- P01: primaquine phosphate (1), artemether (1);
- R05: dextromethorphan hydrobromide (1);
- R06: cetirizine hydrochloride (3), cetirizine dihydrochloride (4), levocetirizine dihydrochloride (4), diphenhydramine epinastine (1), DL-chlorpheniramine (1);
- Others: bromelain hydrolysate (1), chitosan (1), allicin (1), arundic acid (1), bitterness suppressants of berberine hydrochloride (1).
7. Detailed Review of the Selected Examples
7.1. Alimentary Tract and Metabolism Drugs (A)
7.2. Cardiovascular System Drugs (C)
7.3. Dermatological Drugs (D)
7.4. Genito-Urinary System and Sex Hormone Drugs (G)
7.5. Anti-Infectives for Systemic Use Drugs (J)
7.6. Musculo-Skeletal System Drugs (M)
7.7. Nervous System Drugs (N)
7.8. Antiparasitic Products, Insecticides, and Repellents (P)
7.9. Respiratory System (R)
7.10. Others
8. Is Application of CDs as Taste-Masking Agents Successful?
9. Methodology
9.1. Study Design and Search Strategy
9.2. Study Selection and Criteria
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Bonferoni, M.C.; Giunchedi, P.; Dalpiaz, A. Versatile Nasal Application of Cyclodextrins: Excipients and/or Actives? Pharmaceutics 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Nagy, P.; Panyi, G.; Szente, L.; Varga, Z.; Zakany, F. Cyclodextrins: Only Pharmaceutical Excipients or Full-Fledged Drug Candidates? Pharmaceutics 2022, 14, 2559. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, X.; Zhang, S.; Quan, D. An Overview of Taste-Masking Technologies: Approaches, Application, and Assessment Methods. AAPS PharmSciTech. 2023, 24, 67. [Google Scholar] [CrossRef]
- Taruno, A.; Nomura, K.; Kusakizako, T.; Ma, Z.; Nureki, O.; Foskett, J.K. Taste transduction and channel synapses in taste buds. Pflugers Arch. 2021, 473, 3–13. [Google Scholar] [CrossRef]
- Roper, S.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef]
- Fábián, T.K.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular Mechanisms of Taste Recognition: Considerations about the Role of Saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef]
- Coupland, J.N.; Hayes, J.E. Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals. Pharm. Res. 2014, 31, 2921–2939. [Google Scholar] [CrossRef]
- Banerjee, S.; Joshi, U.; Singh, A.; Saharan, V.A. Lipids for Taste masking and Taste assessment in pharmaceutical formulations. Chem. Phys. Lipids 2020, 235, 105031. [Google Scholar] [CrossRef]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for children—Taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child. 2011, 97, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Temussi, P.A. Sweet, bitter and umami receptors: A complex relationship. Trends Biochem. Sci. 2009, 34, 296–302. [Google Scholar] [CrossRef]
- Pockle, R.D.; Masareddy, R.S.; Patil, A.S.; Patil, P.D. A comprehensive review on pharmaceutical excipients. Ther. Deliv. 2023, 14, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Santoveña-Estévez, A.; Suárez-González, J.; Vera, M.; González-Martín, C.; Soriano, M.; Fariña, J.B. Effectiveness of Antimicrobial Preservation of Extemporaneous Diluted Simple Syrup Vehicles for Pediatrics. J. Pediatr. Pharmacol. Ther. 2018, 23, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.A.; Varshosaz, J.; Arabian, S. Formulation development and evaluation of metformin chewing gum with bitter taste masking. Adv. Biomed. Res. 2014, 3, 92. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- French, D. The schardinger dextrins. In Adv. Carbohydr. Chem.; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1957; Volume 12, pp. 189–260. [Google Scholar]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Lewandowska, I.; Zielińska-Pisklak, M.; Szeleszczuk, Ł.; Pisklak, D.M.; Sobczak, M. CYKLODEKSTRYNY—ZASTOSOWANIE W PRZEMYŚLE FARMACEUTYCZNYM. Prospect. Pharm. Sci. 2020, 18, 19–26. [Google Scholar] [CrossRef]
- Aree, T. Advancing insights on β-cyclodextrin inclusion complexes with SSRIs through lens of X-ray diffraction and DFT calculation. Int. J. Pharm. 2021, 609, 121113. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł. A Review of Applications of Solid-State Nuclear Magnetic Resonance (ssNMR) for the Analysis of Cyclodextrin-Including Systems. Int. J. Mol. Sci. 2023, 24, 3648. [Google Scholar] [CrossRef]
- Napiórkowska, E. Overview of cyclodextrins and medicinal products containing cyclodextrins currently registered in Poland. Prospect. Pharm. Sci. 2023, 21, 30–37. [Google Scholar] [CrossRef]
- Devi, K.N.; Rao, N.; Priyanka, K.; Deepika, K.; Mounika, G. Elimination of bitter, disgusting taste of Levocetrizine di hydrochloride by HP β -Cyclodextrin. Int. J. PharmTech Res. 2015, 8, 470–473. [Google Scholar] [CrossRef]
- Krishnan, S.; Chockalingam, V. Oral Disintegrating Tablets of Analgesic Drugs alone and in Combination for Pain Management. Asian J. Pharm. 2015, 9, 243. [Google Scholar]
- Cho, S.; Moazzem, M.S. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
- Wasilewski, T.; Migoń, D.; Gębicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, M.; Low, S.S.; Xu, N.; Chen, Z.; Lv, C.; Cui, Y.; Shi, Y.; Men, H. Fuzzy Evaluation Output of Taste Information for Liquor Using Electronic Tongue Based on Cloud Model. Sensors 2020, 20, 686. [Google Scholar] [CrossRef]
- Preis, M.; Grother, L.; Axe, P.; Breitkreutz, J. In-vitro and in-vivo evaluation of taste-masked cetirizine hydrochloride formulated in oral lyophilisates. Int. J. Pharm. 2015, 491, 8–16. [Google Scholar] [CrossRef]
- Chay, S.K.; Keating, A.; James, C.; Aliev, A.E.; Haider, S.; Craig, D.Q. Evaluation of the taste-masking effects of (2-hydroxypropyl)-β-cyclodextrin on ranitidine hydrochloride; a combined biosensor, spectroscopic and molecular modelling assessment. RSC Adv. 2018, 8, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Vavia, P.R. Preparation and Evaluation of Taste Masked Famotidine Formulation Using Drug/β-cyclodextrin/Polymer Ternary Complexation Approach. AAPS PharmSciTech 2008, 9, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Abou-Taleb, A.E.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Ishiguro, T.; Hirayama, F.; Uekama, K.; Otagiri, M. Enhancement of the aqueous solubility and masking the bitter taste of famotidine using drug/SBE-beta-CyD/povidone K30 complexation approach. J. Pharm. Sci. 2010, 99, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Aboutaleb, A.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Taguchi, K.; Anraku, M.; Hirayama, F.; Uekama, K.; Otagiri, M. Evaluation of carboxymethyl-β-cyclodextrin with acid function: Improvement of chemical stability, oral bioavailability and bitter taste of famotidine. Int. J. Pharm. 2010, 397, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Funasaki, N.; Uratsuji, I.; Okuno, T.; Hirota, S.; Neya, S. Masking Mechanisms of Bitter Taste of Drugs Studied with Ion Selective Electrodes. Chem. Pharm. Bull. 2006, 54, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Aviya, K.R.; Bhattacharya, A. Development and characterization of mouth dissolving tablets of prednisolone. J. Pharm. Investig. 2014, 44, 79–102. [Google Scholar] [CrossRef]
- Preis, M.; Eckert, C.; Häusler, O.; Breitkreutz, J. A comparative study on solubilizing and taste-masking capacities of hydroxypropyl-β-cyclodextrin and maltodextrins with high amylose content. Sens. Actuators B—Chem. 2014, 193, 442–450. [Google Scholar] [CrossRef]
- Commey, K.; Nakatake, A.; Enaka, A.; Nishi, K.; Tsukigawa, K.; Yamaguchi, K.; Ikeda, H.; Iohara, D.; Hirayama, F.; Otagiri, M.; et al. Study of the inclusion complexes formed between 4-phenylbutyrate and α-, β- and γ-cyclodextrin in solution and evaluation on their taste-masking properties. J. Pharm. Pharmacol. 2022, 75, 236–244. [Google Scholar] [CrossRef]
- Wei, Y.; Nedley, M.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H.S. Masking the bitter taste of injectable lidocaine HCL formulation for dental procedures. AAPS PharmSciTech 2014, 16, 455–465. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Göbel, A.; Breitkreutz, J.; Sande, S.A.; Tho, I. Investigation of hydroxypropyl-β-cyclodextrin inclusion complexation of two poorly soluble model drugs and their taste-sensation—Effect of electrolytes, freeze-drying and incorporation into oral film formulations. J. Drug Deliv. Sci. Technol. 2021, 61, 102245. [Google Scholar] [CrossRef]
- Lopalco, A.; Manni, A.; Keeley, A.; Haider, S.; Li, W.; Lopedota, A.; Altomare, C.D.; Denora, N.; Tuleu, C. In Vivo Investigation of (2-Hydroxypropyl)- β-cyclodextrin-Based Formulation of Spironolactone in Aqueous Solution for Paediatric Use. Pharmaceutics 2022, 14, 780. [Google Scholar] [CrossRef]
- Jagdale, S.; Gawali, V.U.; Kuchekar, B.S.; Chabukswar, A.R. Formulation and in vitro evaluation of taste-masked oro-dispersible dosage form of diltiazem hydrochloride. Braz. J. Pharm. Sci. 2011, 47, 907–916. [Google Scholar] [CrossRef]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Popa, V.T.; Sarbu, I.; Mircioiu, C.; Abdalrb, G.A.; Mitu, M.A.; Ozon, E.A. Development and characterization of orally disintegrating tablets containing a Captopril-Cyclodextrin complex. Pharmaceutics 2020, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.N.; Shah, K.N.; Mehta, T.A. Hydroxy propyl β-cyclodextrin complexation of promethazine hydrochloride for the formulation of fast dissolving sublingual film: In vitro and in vivo evaluation. J. Pharm. Investig. 2015, 45, 91–99. [Google Scholar] [CrossRef]
- Ganguly, I.; Abraham, S.; Srinivasan, B.; Madhavan, V. Development of fast dissolving sublingual wafers of promethazine hydrochloride. Iran. J. Pharm. Sci. 2014, 10, 71–92. Available online: http://www.ijps.ir/article_12241_5f8418854df02b60e286c9ca1234c785.pdf (accessed on 26 July 2023).
- Dinge, A.; Nagarsenker, M.S. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech 2008, 9, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Al-Gethmy, H.A.; Fahmy, U.A.; Alhakamy, N.A.; Ahmed, O.A.A.; El-Say, K.M. Optimization of the Factors Affecting the Absorption of Vardenafil from Oral Disintegrating Tablets: A Clinical Pharmacokinetic Investigation. Pharmaceutics 2019, 11, 11. [Google Scholar] [CrossRef]
- Khan, Q.-u.-a.; Siddique, M.I.; Rasool, F.; Naeem, M.; Usman, M.; Zaman, M. Development and characterization of orodispersible film containing cefixime trihydrate. Drug Dev. Ind. Pharm. 2020, 46, 2070–2080. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Nerli, G.; Rubia, J.; Casalone, E.; Melani, F.; Maestrelli, F.; Mura, P. Combined Use of Cyclodextrins and Amino Acids for the Development of Cefixime Oral Solutions for Pediatric Use. Pharmaceutics 2021, 13, 1923. [Google Scholar] [CrossRef]
- Nanjwade, V.; Manvi, F.V.; Nanjwade, B. Formulation and Evaluation of Dispersible Tablets of Lomefloxacin HCl. Int. J. Drug Dev. Res. 2013, 5, 103–113. [Google Scholar]
- Kaushik, S.; Verma, R.; Purohit, D.; Pandey, P.; Kumar, M.; Kumari, B.; Kaushik, D. Development of binary and ternary complex of cefuroxime axetil with cyclodextrin for improving pharmaceutical characteristics. Int. J. Appl. Pharm. 2020, 12, 107–111. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Harindran, J. Formulation and evaluation of taste masked oral suspension of cefuroxime axetil using hydroxypropyl-beta-cyclodextrin. Asian J. Pharm. Clin. Res. 2016, 9, 1–3. [Google Scholar]
- Prabhakaran, R.; Kunchithapatham, J.; Harindran, J. Improvement of bioavailability of taste masked cefuroxime axetil oral suspension using hydroxy propyl-betacyclodextrin. Pharm. Lett. 2015, 7, 227–233. [Google Scholar]
- Sevukarajan, M.; Bachala, T.; Rahul, N. Novel Inclusion Complexs of Oseltamivir Phosphate-With ß Cyclodextrin: Physico-Chemical Characterization. J. Pharm. Sci. Res. 2010, 2, 583–589. [Google Scholar]
- Samprasilt, W.; Rojanarata, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Opanasopit, P. Fabrication of Meloxicam taste-Masked oral fast disintegrating tablet by ion exchange resin and cyclodextrin. Thai J. Pharm. Sci. 2013, 38, 165–169. [Google Scholar]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Formulation and evaluation of meloxicam oral disintegrating tablet with dissolution enhanced by combination of cyclodextrin and ion exchange resins. Drug Dev. Ind. Pharm. 2015, 41, 1006–1016. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015, 487, 213–222. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Meloxicam Taste-Masked Oral Disintegrating Tablet with Dissolution Enhanced by Ion Exchange Resins and Cyclodextrin. AAPS PharmSciTech 2013, 14, 1118–1128. [Google Scholar] [CrossRef]
- Purnamasari, N.; Saputra, P. Evaluation of orally disintegrating tablet of ibuprofen-β-cyclodextrin inclusion complex. Int. J. Appl. Pharm. 2020, 12, 60–64. [Google Scholar] [CrossRef]
- Basalious, E.B.; Abdullah, A.; Ibrahim, M. Utility of Mannitol and Citric Acid for Enhancing the Solubilizing and Taste Masking Properties of β-Cyclodextrin: Development of Fast-Dissolving Tablets Containing Extremely Bitter Drug. J. Pharm. Innov. 2014, 9, 309–320. [Google Scholar] [CrossRef]
- Kasliwal, N.; Negi, J.S. Development, characterization and performance evaluation of oro-dispersible tablet containing aceclofenac hydroxypropyl-β-cyclodextrin binary system. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 215–224. [Google Scholar] [CrossRef]
- Dungarwal, U.N.; Patil, S.B. Development of orodispersible tablets of taste masked rizatriptan benzoate using hydroxypropyl β cyclodextrin. J. Pharm. Investig. 2016, 46, 537–545. [Google Scholar] [CrossRef]
- Prajapati, S.T.; Patel, P.B.; Patel, C.N. Formulation and evaluation of sublingual tablets containing Sumatriptan succinate. Int. J. Pharm. Investig. 2012, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kande, K.V.; Kotak, D.J.; Degani, M.S.; Kirsanov, D.; Legin, A.; Devarajan, P.V. Microwave-Assisted Development of Orally Disintegrating Tablets by Direct Compression. AAPS PharmSciTech 2017, 18, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Bhingole, R.C. Nanosponge-based pediatric-controlled release dry suspension of Gabapentin for reconstitution. Drug Dev. Ind. Pharm. 2015, 41, 2029–2036. [Google Scholar] [CrossRef]
- Gangotia, P.; Nehate, S.; Jain, H.; Meshram, D.B. Formulation and Evaluation of Fast Dissolving Tablets of Aripiprazole. Res. J. Pharm. Tech. 2019, 12, 1827–1831. [Google Scholar] [CrossRef]
- Ono, N.; Miyamoto, Y.; Ishiguro, T.; Motoyama, K.; Hirayama, F.; Iohara, D.; Seo, H.; Tsuruta, S.; Arima, H.; Uekama, K. Reduction of Bitterness of Antihistaminic Drugs by Complexation with β-Cyclodextrins. J. Pharm. Sci. 2011, 100, 1935–1943. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Osman, D.A.; Mohamed, O.S. In vitro and in vivo evaluation of taste-masked orodispersible tablets of fluoxetine hydrochloride for the treatment of depression. Drug Dev. Ind. Pharm. 2021, 47, 645–653. [Google Scholar] [CrossRef]
- Liu, T.; Wan, X.; Luo, Z.; Liu, C.; Quan, P.; Cun, D.; Fang, L. A donepezil/cyclodextrin complexation orodispersible film: Effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Asian J. Pharm. Sci. 2019, 14, 183–192. [Google Scholar] [CrossRef]
- Shinde, S.V.; Phatak, S.; Awale, G.; Nikam, S. Development of taste masked orodispersible film containing paroxetine hydrochloride. Indian J. Pharm. Educ. Res. 2020, 54, s98–s107. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Khan, A.R.; He, S.; Wang, Y.; Xu, J.; Zhai, G. Quantitative prediction of the bitterness of atomoxetine hydrochloride and taste-masked using hydroxypropyl-β-cyclodextrin: A biosensor evaluation and interaction study. Asian J. Pharm. Sci. 2020, 15, 492–505. [Google Scholar] [CrossRef]
- Shah, P.; Mashru, R. Formulation and evaluation of taste masked oral reconstitutable suspension of primaquine phosphate. AAPS PharmSciTech 2008, 9, 1025–1030. [Google Scholar] [CrossRef]
- Shah, P.; Mashru, R. Palatable reconstitutable dry suspension of artemether for flexible pediatric dosing using cyclodextrin inclusion complexation. Pharm. Dev. Technol. 2010, 15, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Amin, A. Optimization and characterization of rapidly dissolving films of Cetirizine hydrochloride using cyclodextrins for taste masking. Int. J. PharmTech Res. 2013, 5, 536–552. [Google Scholar]
- Lee, C.; Kim, S.; Youn, Y.; Widjojokusumo, E.; Lee, Y.; Kim, J.; Lee, Y.; Tjandrawinata, R.R. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 55, 348–357. [Google Scholar] [CrossRef]
- Katewongsa, P.; Lertsuphotvanit, N.; Phaechamud, T. Cetirizine Dihydrochloride, β-Cyclodextrin Inclusion Complex by Ethanol Kneading for Taste Masking. Indian J. Pharm. Sci. 2017, 79, 758–767. [Google Scholar] [CrossRef]
- Stojanov, M.; Larsen, K.L. Cetirizine release from cyclodextrin formulated compressed chewing gum. Drug Dev. Ind. Pharm. 2011, 38, 1061–1067. [Google Scholar] [CrossRef]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef]
- Kazsoki, A.; Palcsó, B.; Omer, S.; Kovacs, Z.; Zelkó, R. Formulation of Levocetirizine-Loaded Core–Shell type nanofibrous orally dissolving webs as a potential alternative for immediate release dosage forms. Pharmaceutics 2022, 14, 1442. [Google Scholar] [CrossRef]
- Labib, G.S. Novel levocetirizine HCl tablets with enhanced palatability: Synergistic effect of combining taste modifiers and effervescence technique. Drug Des. Dev. Ther. 2015, 2015, 5135–5146. [Google Scholar] [CrossRef]
- Mahesh, A.; Shastri, N.R.; Sadanandam, M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Curr. Drug Deliv. 2010, 7, 21–27. [Google Scholar] [CrossRef]
- Normah, I.; Nurul Fasihah, R. Evaluation of β-cyclodextrin masking effect on the bitterness of angelwing clam (Pholas orientalis) hydrolysate. Int. Food Res. J. 2017, 24, 1500–1506. [Google Scholar]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan–cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Fragr. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, J.; Peng, H.; Guo, T.; Xiao, J.; Zhu, W.; Qian, W.; Zhang, J.; Wu, L. Allicin inclusions with α-cyclodextrin effectively masking its odor: Preparation, characterization, and olfactory and gustatory evaluation. J. Food Sci. 2021, 86, 4026–4036. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Nakahara, M.; Motoyama, K.; Ishiguro, T.; Oda, Y.; Yamanoi, T.; Okamoto, I.; Yagi, A.; Nishimura, H.; Hirayama, F.; et al. Improvement of some physicochemical properties of arundic acid, (R)-(−)-2-propyloctanonic acid, by complexation with hydrophilic cyclodextrins. Int. J. Pharm. 2011, 413, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-X.; Gao, X.-J.; Wang, J.-M.; Dai, L.-P.; Kang, B.-Y.; Zhang, L.; Shi, J.-H.; Gui, X.-J.; Liu, P.; Li, X.-L. Traditional human taste panel and taste sensors methods for bitter taste masking research on combined bitterness suppressants of berberine hydrochloride. Sens. Mater. 2017, 29, 105. [Google Scholar] [CrossRef]
- Tran, C.T.H.; Nargotra, P.; Pham, H.T.C.; Lieu, D.M.; Huynh, P.K.; Wang, H.M.D.; Dong, C.-D.; Kuo, C.-H. The effect of carboxymethyl cellulose and β-cyclodextrin as debittering agents on bitterness and physicochemical properties of bitter gourd extract. J. Food Sci. Technol. 2023, 60, 1521–1529. [Google Scholar] [CrossRef]
- Monge Neto, A.A.; Ströher, R.; Assenha, H.B.R.; Scagion, V.P.; Correa, D.S.; Zanin, G.M. Interaction of peptides obtained from the enzymatic hydrolysis of soybean meal with cyclodextrins: An evaluation of bitterness reduction. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 59–69. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Cyclodextrins produced by cyclodextrin glucanotransferase mask beany off-flavors in plant-based meat analogs. PLoS ONE 2022, 17, e0269278. [Google Scholar] [CrossRef]
- Meier, M.M.; Drunkler, D.A.; Bordignon-Luiz, M.T.; Fett, R.; Szpoganicz, B. The influence of β-cyclodextrin on goaty flavour—Characterization of synthetic inclusion complexes with capric acid and caprylicacid. Br. Food J. 2001, 103, 281–290. [Google Scholar] [CrossRef]

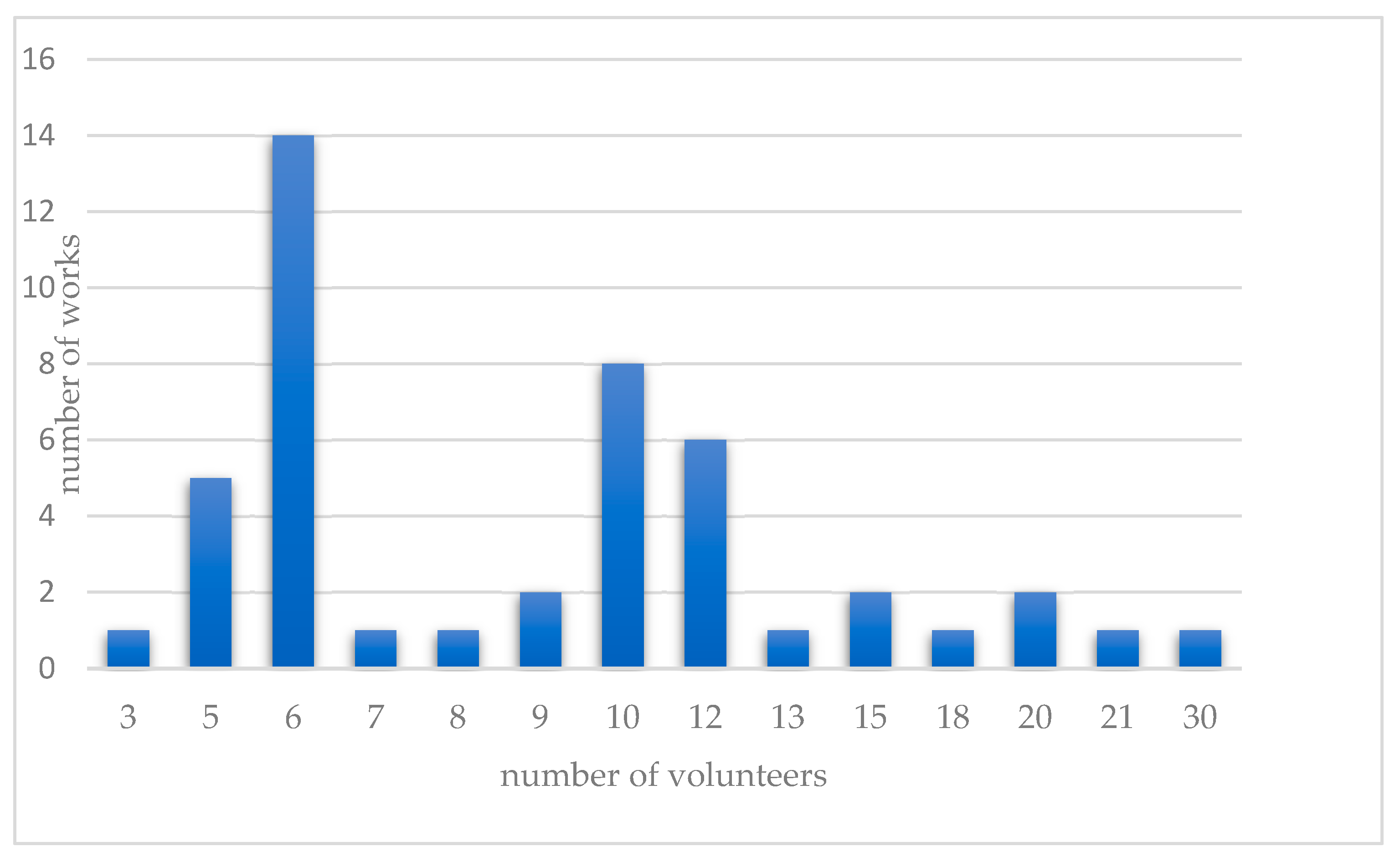


| CD | Number of Works |
|---|---|
| α-CD | 10 |
| β-CD | 36 |
| γ-CD | 9 |
| HP-β-CD | 28 |
| HP-γ-CD | 1 |
| HP-α-CD | 1 |
| 2,6-O-Dimethyl-β-CD | 1 |
| 6-Glucosyl-β-CD | 1 |
| 6-Maltosyl-β-CD | 1 |
| Sulfobutyl-ether-β-CD | 1 |
| Carboxymethyl-β-CD | 1 |
| Parameters | Score of Taste Evaluation | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Bitterness | Etremely bitter | Highly bitter | Acceptable | Very slightly bitter | Not at all bitter |
| Sweetness | Not at all sweet | Very slightly sweet | Acceptable | Highly sweet | Extremely sweet |
| Mouth feel | Very gritty | Gritty | Acceptable | Creamy | Very creamy |
| Flavour | Very unpleasant | Unpleasant | Acceptable | Pleasant | Very pleasant |
| Overall acceptability | Worst | Poor | Acceptable | Good | Very good |
| API | CD | Taste Evaluation Method | Other Methods | Ref. |
|---|---|---|---|---|
| Ranitidine hydrochloride | HP-β-CD | E-tongue: 0.2 mL of inner solution was placed within each of these sensors, compared to 0.4 mL inside the reference electrodes. | 1H NMR | [30] |
| Famotidine | β-cyclodextrin | Twelve healthy human volunteers were used in a study on taste perception. They were instructed to taste 2.5 mg of the items for 5 s after they were left in their mouths. The following statements were then presented to the volunteers: 1. “A bitter taste is present”. 2. “I sense something, but I can’t place the flavor”. 3. “I don’t taste anything”. | PXRD, DSC, FTIR, UV-VIS, PS, SEM, | [31] |
| Famotidine | β-cyclodextrin | Twelve male or female healthy human volunteers between the ages of 23 and 27 held about 3 mL of each sample in their mouths for 10 s. The degree of bitterness was measured after expectoration. Using a numerical scale, the following values were used: 0, tasteless; 1, mildly bitter; 2, moderately bitter; 4, moderately to strongly bitter; 5, very bitter; and 6, very strongly bitter. Testing of random samples was used to validate this numerical scale. To remove bias, the mouth cavity was washed three times with distilled water. The time between testing various samples was 10 min. | PS, 1H NMR, DSC, PXRD, UV-VIS | [32] |
| Famotidine | sulfobutyl-ether-β-cyclodextrin, carboxymethyl-β- cyclodextrin | Eight healthy human volunteers between the ages of 23 and 27 were instructed to hold the reference solutions in their mouths for 10 s and report on the concentrations and bitterness levels. The following values were utilized on a numerical scale: 0 = unappealing; 1 = mildly bitter; 2 = moderately bitter; 3 = bitter; and 4 = very bitter. Testing of random samples was used to validate this numerical scale. To remove bias, the mouth cavity was washed three times with distilled water. | PS, 13C NMR, HPLC | [33] |
| Propantheline bromide, oxyphenonium bromide | α-, β-, γ-cyclodextrin, 2,6-O-dimethyl-β-CD, HP-β-CD, 6-glucosyl-β-CD, 6-maltosyl-β-CD | The sensory test comprised five subjects. These panelists tasted 35 mL of an aqueous sodium bromide solution with 154 mM sodium content, OB or PB alone, or a combination of a masking agent and either 4 mM OB or 1.5 mM PB. The following scores were used to assess how bitter these solutions were: no bitter flavor at 0; a very slight bitter taste at 1; a minor bitter taste at 2; a noticeable bitter taste at 4; and an exceedingly bitter taste at 5. For subsequent investigation, the average bitter taste intensity among the five individuals was utilized. In most cases, the standard deviations of the bitter taste intensities were about 0.7. | [34] | |
| Prednisolone | β-cyclodextrin | Ten healthy human participants between the ages of 20 and 25 were chosen. Prednisolone solutions in pH 6.8 phosphate buffer at 10, 20, 30, 40, and 50 lg/mL concentrations were created. The volunteers judged the taste on a scale of 0 to 4 (0, no bitterness; 1, threshold bitterness; 2, bitter; 3, moderate bitterness; and 4, intense bitterness) after holding 10 mL of each solution in their mouths for 60 s. | FTIR, UV-VIS | [35] |
| Dextromethorphan hydrobromide, loperamide hydrochloride, cetirizine hydrochloride | HP-β-CD | Electronic taste assessment. | PS | [36] |
| 4-Phenylbutyrate | α-, β-, and γ-cyclodextrin | The TS-5000Z taste sensor device was used to evaluate flavor. First, it was discovered that PB concentration and sensor response are related. The sensors’ reaction to the CDs was then evaluated. The reference solution underwent a sensor measurement first, and then the sample was examined. | PS, HPLC, CD, 1H NMR | [37] |
| Lidocaine hydrochloride | HP-β-CD | Electronic tongue: Using a 48-position autosampler and 25 mL beaker samples, taste was evaluated using an Alpha MOS ASTREE e-tongue system that was equipped with Alpha M.O.S. sensor set no. 2. Periods of 120 and 180 s, respectively, were set as the acquisition and analysis times. The seven sensors (ZZ, AB, BA, BB, CA, DA, and JE) were used to measure the e-tongue signal of each solution at equilibrium. | PS, DSC, FTIR, PXRD, 1H NMR, SEM, HPLC | [38] |
| Indomethacin, furosemide | HP-β-CD | E-tongue: Each sample required 100 mL of liquid, and each sample was run five times, with the first two runs serving to prepare the membranes and the remaining results being discarded. The findings were presented as a change in the membrane potential measured in millivolts (mV) with respect to the corresponding reference solution corresponding to the particular sensor. | UV-VIS, PS | [39] |
| Spironolactone | HP-β-CD | A total of 24 healthy participants between the ages of 18 and 40 were randomly assigned 10 mL samples that were labeled with a special three-digit number. There were 11 females and 13 men in the group. Each sample was evaluated twice. The participants had to spit the sample into a container that was given after rinsing their mouths with the samples for 5 s to cover all oral surfaces. They were to score the taste using a computerized survey that contained a 100 mm horizontal visual analog scale (anchored from “not aver-sive” to “extremely aversive”) as soon as the sample was spat out. They were asked to offer free-form descriptions of taste and scent. Participants cleaned their mouths with mineral water before and after each sample, and they were allowed to eat unsalted crackers to acclimate their palates. An interval of up to 10 min was allowed between each sample such that the one before it was no longer noticeable. If necessary, participants might instantly taste each item again. | UV-VIS, 1H NMR, HPLC | [40] |
| Diltiazem hydrochloride | β-cyclodextrin | Five members of a panel completed a sensory evaluation of the drug-CD complex sample with regard to the bitter taste, categorizing the bitter taste into the following five groups. Class 5: extremely bitter flavor; Class 4: extremely bitter flavor; Class 3: slightly bitter flavor; Class 2: a little bitter flavor; Class 1: no sour flavor. The usual control medication, which had a mean bitter taste of 5.0, was employed. | PS, UV-VIS, IR, PXRD, DSC | [41] |
| Captopril | β-cyclodextrin | Six healthy volunteers were instructed to ingest one CAP pill for 20 s while recording their feelings. They were instructed to spit out the contents and rinse their mouths with water after 20 s. The following taste qualities were to be ranked from 1 to 5: bitter flavor, grittiness, other taste, lingering taste in mouth, acceptance, and sulfurous taste. After 30 min, they repeated the process with CAP-CD tablets. The primary aim was the determination of a bitter taste, with other metrics serving as supplementary endpoints. Taste was rated using a numerical scale with the following values: 0 for tasteless, 1 for pleasing, 2 for mildly sweet, 3 for slightly bitter, 4 for moderately bitter, and 5 for extremely bitter. | PXRD, FTIR, DSC, SEM | [42] |
| Promethazine hydrochloride | HP-β-CD | A taste panel (n = 6) evaluated the acceptability of the flavor by holding a film sample containing 25 mg of medication in the mouth until it disintegrated and then spitting it out and recording the amount of bitterness: the scale ranged from 0 (no bitterness) through 0.5 (threshold bitterness), 1, 2, and 3 (severe bitterness). | FTIR, PXRD, SEM | [43] |
| Promethazine hydrochloride | β-cyclodextrin | E-tongue: The samples were put in a beaker with the instrument electrodes attached, and 10 cc of distilled water was used to dissolve them. Before recording the outcome, the instrument was allowed to operate for 10 min. The outcomes were determined using a scale of 1 to 10. The scale was used to evaluate the e-tongue results. Taste scale: 1 very sweet; 2–3 sweet; 4–5 sweet; 10 acceptable, really bitter. | PXRD, 1H NMR, ESI-MS, FTIR, DSC, SEM, PS | [44] |
| Triclosan | HP-β-CD | Films of formulas F8 and F10 were tested for tongue feel, bitter taste masking, mouth freshening, and in vivo dissolution time in oral cavities of healthy human volunteers (n = 9; 7 males and 2 females) with their consent. The participants were instructed to put the film on the tongue. Later, the mobility of the tongue was not restricted for the volunteers. | DSC, FTIR, PXRD, 1H NMR | [45] |
| Vardenafil | β-cyclodextrin | On a scale of 0 to 3, six healthy human volunteers were asked to judge how bitter the improved recipe tasted. When the score was less than 1, the taste was tolerable, but when it was greater than 1, the tablet’s flavor was bitter and intolerable. | FTIR, HPLC | [46] |
| Cefixime trihydrate | β-cyclodextrin | Twelve healthy human volunteers cleaned their mouths with 200 mL of water before placing the film dosage form on their palates (the upper part of their mouths) with their tongues. They were to carefully avoid biting the film. They were then given a CFX ODF that had been taste-masked. The time–intensity approach was used to examine the film’s flavor. After the experiment, the volunteers washed their mouths with water without ingesting any of the film’s dissolved or disintegrating components. | PS, FTIR, UV-VIS, SEM | [47] |
| Cefixime trihydrate | HP-β-CD | In a study to gauge the smell of CEF formulations, ten (n= 10) healthy individuals with ages ranging from 18 to 28 (mean age = 22) were enrolled as adult sensory panelists. The “facial expressions scale” or Facial Affective Scale, FAS [47], which is typically used for taste evaluation but has been modified to assess olfactory perception, was used to rate each participant’s level of contentment. | PS, UV-VIS, 1H-NMR | [48] |
| Lomefloxacin hydrochloride | HP-β-CD | No detailed information provided. | XRD, UV-VIS, FTIR | [49] |
| Cefuroxime axetil | β-cyclodextrin | In vitro taste assessment study: A volumetric flask containing 10 mL of phosphate buffer was added with an optimal inclusion complex amount equal to 100 mg of cefuroxime axetil. The mixture was vortexed for 30 s, filtered, and subjected to a UV–Visible spectrophotometer analysis to determine the concentration of cefuroxime axetil at 281 nm, which was then compared to the threshold value. The amount of medication that is solubilized within 30 s should not be more than the medication’s threshold bitterness concentration in order to provide appropriate taste masking. | PS, FTIR, DSC, PXRD, UV-VIS | [50] |
| Cefuroxime axetil | HP-β-CD | Ten human volunteers were used to assess the oral suspensions’ flavors. The study employed the pure medicine cefuroxime axetil as the benchmark, and it also compared the formulations using a market sample. Four criteria were used to classify formulations: (1) no bitter taste or flavor that has been covered up; (2) slightly bitter; (3) bitter; and (4) very bitter. | [51] | |
| Cefuroxime axetil | HP-β-CD | Ten human volunteers were used to assess the oral suspensions’ flavors. The pure medicine, cefuroxime axetil, served as the study’s benchmark, and the commercial product was also utilized to compare with the preparations. Four criteria were used to classify formulations: (1) No bitterness or concealed bitterness. (2) Acceptable but a little bitter. (3) Acrid. (4) Extremely sour. To prevent carryover, volunteers were given enough water to drink and a one-hour rest in between each sample. | [52] | |
| Oseltamivir phosphate | β-cyclodextrin | Three groups of two participants each were formed from six healthy human volunteers. Pure medication (tasting score of 4) and placebo (taste score of 1) were used as positive and negative controls, respectively, in a random order for each group to prevent bias. By placing the provided sample on their tongues, tasting it for one minute, and then properly washing their mouths with water after each sample evaluation, subjects were able to rate the degree of bitterness. Each participant evaluated the flavor of the sample using a 5-point scale that varied from 0 to 4 (0 being highly bitter, 1 being tasteless, 2 being somewhat bitter, 3 being moderately bitter, and 4 being pleasant). | PS, DSC, FTIR, XRD, SEM | [53] |
| Meloxicam | HP-β-CD with molar substitution 0.6 | With six healthy human volunteers, in vivo disintegration time and taste evaluation was carried out. Every volunteer chose a pill at random and held it on their tongues. A numerical value was assigned to the taste, ranging from 0 (tasteless) to 3 (extremely bitter), with 1 denoting a mildly bitter flavor and 2 indicating a moderately bitter one. | UV-VIS | [54] |
| Meloxicam | HP-β-CD with molar substitution 0.6 | With six healthy human volunteers, in vivo disintegration time and taste evaluations were carried out. Each volunteer picked a pill at random and placed it on their tongue. The flavor was assessed and given a numerical rating, ranging from 0 (tasteless) to 1, 2, and 3, depending on how bitter it was. | SEM, XRD, DSC, PS | [55] |
| Meloxicam | β-cyclodextrin, HP-β-CD with molar substitution 0.6 | Six volunteers—two men (33%) and four women (67%) with ages ranging from 20 to 30—made up the research group. Each volunteer placed a nanofiber mat that had been loaded with MX on their tongue at random. The numerical values attributed to the flavor were 0 = tasteless, 1 = slight, 1.5 = slight–moderate, 2 = moderate, 2.5 = moderate–strong, 3 = powerful, and 3.5 = extremely strong bitterness. | PS, XRD, DSC, SEM | [56] |
| Meloxicam | HP-β-CD with molar substitution 0.6 | Six healthy human volunteers took one tablet each and kept it on their tongues. The taste was evaluated and assigned a numerical value, i.e., 0 = tasteless, 1 = slightly bitter, 2 = moderately bitter, and 3 = strongly bitter. | SEM, XRD, DSC | [57] |
| lornoxicam | β-cyclodextrin | Before the test, ten human participants were advised to rinse their mouths with a cup of water (200 mL) and move the dosage with their tongues against the top portions of their mouths without biting. When the dosage disintegrated, they were also told to spit the contents out. Participants were asked to score the formulations’ first taste, aftertaste, mouthfeel, flavor, and general acceptability. | FTIR, HPLC, DSC, PXRD | [25] |
| Ibuprofen | β-cyclodextrin | No detailed information provided. | FTIR, DSC, UV-VIS | [58] |
| Aceclofenac | β-cyclodextrin | A dose of ASTMGA equal to 100 mg of aceclofenac was applied to the tongues of three healthy male volunteers while not drinking any water. After 1 min, the participants were asked to record the amount of bitterness, which served as the basis for taste rating. After each measurement, the mouth was washed. Between each test sample, there was a 30 min washout interval. The following values were utilized on a numerical scale: 0 = unpalatable, 1 = mildly bitter, 2 = moderately bitter, 3 = bitter, and 4 = very bitter. | DSC, PS | [59] |
| Aceclofenac | HP-β-CD | Among nine healthy human participants, after initially obtaining their informed agreement, the time–intensity approach was used to assess taste. One ODT (containing 100 mg of aceclofenac) was kept in the mouth until it completely disintegrated, whereas the aceclofenac-HP-CD equivalent of 100 mg of aceclofenac was retained in the mouth for 10 s before being spat out. A scale from 0 to 3 was used to measure the intensity of the bitterness, with 0 denoting no bitterness, 0.5 denoting threshold bitterness, 1 denoting minor bitterness, 2 denoting moderate bitterness, and 3 denoting extreme bitterness. | PS, HPLC, FTIR, DSC, PXRD | [60] |
| Rizatriptan benzoate | HP-β-CD | Six healthy human participants between the ages of 22 and 26 were chosen from both sexes. For 30 s, each participant kept 2 mg of the rizatriptan benzoate combination in their mouths. The panel evaluated the taste of pure rizatriptan benzoate as a benchmark. After expectoration, the degree of bitterness was noted. A numerical scale was employed, with 0 denoting a very bitter flavor, (1) denoting a moderately bitter flavor, (2) denoting a mildly bitter flavor, and (3) denoting a bland flavor. For the validation of the aforementioned scale, random sampling was used. To prevent bias, the mouth cavity was cleaned twice with water. There was a 30 min gap between testing the various samples. | FTIR, DSC, UV-VIS | [61] |
| Sumatriptan succinate | β-cyclodextrin | With their written agreement, 12 healthy human participants were chosen to evaluate the degree of flavor masking of created taste-masked mixtures/granules. Separately applied mixture/granules were put on the posterior lobe of the tongue for 4–6 s before being spat out, and the mouth was washed with water. The flavor and gritty feel of the dispersion were then noted. | [62] | |
| Lamotrigine | β-cyclodextrin | Electronic tongue. | HPLC, PS, PXRD, FTIR, UV-VIS | [63] |
| Gabapentin | β-cyclodextrin | Ten healthy human volunteers between the ages of 22 and 27 were instructed to keep 10 mL of each solution in their mouths for 60 s while rating the flavor on a scale of 0 to 4 (0 denoting no bitterness, 1 denoting threshold bitterness, 2 denoting bitterness, 3 denoting moderate bitterness, and 4 denoting strong bitterness). | PS, UV-VIS, FTIR, DSC, PXRD, HPTLC | [64] |
| Aripiprazole | β-cyclodextrin | A volume of 25 mL of the appropriate, particle-free fluid was placed into each of the e-tongue test beakers. The tests were run six times in succession. The e-tongue BPM program detected and recorded the potentiometric difference produced between each individual sensor and the reference electrode. | [65] | |
| Diphenhydramine epinastine, hydroxyzine, cetirizine, dl-chlorpheniramine | α-, β-, γ-cyclodextrin, HP-β-CD | Antihistaminic medication solutions (1.0 or 5.0 mM) mixed in water in the presence of CDs (10, 20, and 30 mM) were tested using seven healthy volunteers who were asked to rate the severity of the bitterness. In order to express the effects of CDs on the bitterness of medication solutions (5.0 mM diphenhydramine, hydroxyzine, cetirizine, and chlorpheniramine and 1.0 mM epinastine), the relative bitter score (bitter score in the presence of CDs/bitter score in the absence of CDs) was utilized. All samples were maintained in the mouth for 10 s, and the sample size was 10.0 mL. Volunteers tasted one sample before thoroughly gargling and moving on to the next. | UV-VIS, 1H NMR, CD, HPLC | [66] |
| Fluoxetine | β-cyclodextrin | Participants: This study included six healthy human participants. Each subject received a sample of a specified weight of a pure drug or an equivalent quantity of the FLX-CD complex, which they were instructed to taste, evaluate how bitter it was, and respond to after ten seconds. The reaction was rated on a scale from 0 to 4. | UV-VIS, DSC, FTIR | [67] |
| Donepezil | HP-β-CD | E-tongue: Using relative sensor outputs, it was possible to determine the potential difference (Vs Vr) between the test sample and reference solution as well as the CPA values, which were calculated as the difference (Vr Vr) between the reference solution’s potentials prior to and following sample assessment (change in membrane potential brought on by sample adsorption). | PS, FTIR, DSC, PXRD | [68] |
| Paroxetine hydrochloride | HP-β-CD | A five-person panel performed a gustatory sensory evaluation of a sample of a drug-HP-CD complex (1:1 molar ratio), categorizing the bitter taste into the following five categories: Class 1: no bitter taste/tasteless; Class 5: very strongly bitter; Class 4: strongly bitter; Class 3: moderately bitter; Class 2: slightly bitter. | UV-VIS, FTIR, PXRD, DSC | [69] |
| Atomoxetine hydrochloride | HP-β-CD | Six healthy, trained volunteers—three men and three women, ranging in age from 26 to 38—took part in this study. Both a pure atomoxetine HCl solution and an atomoxetine HCl/HP-CyD solution, both of which contained around 1.028 mM atomoxetine HCl, were made. The subjects took all of the solutions at random and kept them in their mouths for 30 s before rating the amount of bitterness on a scale of 0 to 4, with 0 denoting no taste, 1 denoting threshold, 2 denoting mildly bitter, 3 denoting bitter, and 4 denoting extremely bitter. | DSC, PXRD, SEM, 1H NMR | [70] |
| Primaquine phosphate | β-cyclodextrin | For a quinine taste sensitivity test, 20 healthy human participants between the ages of 23 and 27 were chosen. Each volunteer held 1 cc of the dispersion in their mouth for 30 s immediately after its preparation. After expectoration, the degree of bitterness was noted. The following values were placed on a scale using numbers: taste thresholds ranged from 0 (tasteless) to 3+ (extremely strong), with 0.5 (very faintly bitter) being the mildest and 3+ (severely bitter) being the strongest. | FTIR, DSC, PXRD, UV-VIS | [71] |
| Artemether | β-cyclodextrin | The sensory test had twenty people. For 15 s, a physical mixture or kneaded system weighing 1 g was disseminated in 50 mL of water. Each subject held approximately 1mL of the dispersion in their mouth for 30s right after its preparation. The degree of bitterness was measured after expectoration. The control was the pure drug. The following values were placed on a scale using numbers: taste thresholds range from 0 (tasteless) to 3+ (extremely strong), with 0.5 (very faintly bitter) being the mildest and 3+ (severely bitter) being the strongest. The greatest number of volunteers who described the taste as bitter or mildly bitter was used to define the threshold of bitterness. | DSC, SEM, PXRD, FTIR, PS, UV-VIS | [72] |
| Cetirizine hydrochloride | HP-β-CD and HP-γ-CD | For the study, 30 participants between the ages of 18 and 63 (mean age: 38.7; 66.7% female) were enrolled. The participants were asked to score the product’s flavor, sweetness, and bitterness after 10 s in the first three questions. They next cleaned their mouths and were instructed to spit out any leftovers. The second questionnaire (Q4–Q13) covered topics such as general acceptability, intensity, flavor, sweetness, and bitterness, as well as mouthfeel and texture, disintegration time, and aftertaste. Five minutes later, the panelists were tasked with answering questions 14 and 15 on the aftertaste and how long it lasted and whether they liked or disliked the product. E-tongue: 100 mL of water was used to dilute pure cetirizine HCl and a variety of cetirizine HCl formulations, which were then analyzed using the Insent1. To simulate the amount of saliva that could be present in the mouth, the number of tablets put into the water was equal to one single dosage in 5 mL of water. Using the multivariate data analysis program Simca P+/−V12, principal component analysis (PCA) was carried out to depict the findings of all seven sensors in a two-dimensional format. | HPLC | [29] |
| Cetirizine hydrochloride | HP-β-CD | The amount of bitterness was assessed after a film sample containing 10 mg of medication was kept in the mouth for 5–10 s before being spat out by a taste panel of human volunteers (n = 6). Following the injection of the medication and the sample, the participants were instructed to gargle with distilled water. A scale of + = highly bitter, ++ = mild to bitter, +++ = somewhat bitter, ++++ = tasteless/taste-masked, and +++++ = great flavor masking was used to indicate the degree of taste masking. | FTIR, UV-VIS, DSC, PXRD | [73] |
| Cetirizine hydrochloride | β-cyclodextrin | In the 24- to 34-year-old age range, 15 human participants were selected. For five seconds, volunteers were instructed to taste items. The volunteers then recorded the amount of bitterness after the samples were spat out. The following numbers were used to represent the different levels of bitterness: 0 = tasteless, 0.5 = very little bitterness, 1 = slightly bitter, 1.5 = slightly to moderately bitter, 2 = moderately bitter, 2.5 = moderate to strong bitterness, 3 = strong bitterness, and 3+ = extremely strong. | 1H NMR, FTIR, PXRD, SEM, TD-GC-MSD | [74] |
| Cetirizine dihydrochloride | β-cyclodextrin | Six healthy human participants—of either sex—between the ages of 20 and 35 were chosen from a pool of twenty volunteers based on results of a taste sensitivity test. Six healthy participants were taught to identify the amount of bitterness in CTZ. To assess and identify the amount of bitterness in CTZ, caffeine was employed as the benchmark. To compare with an inclusion complex corresponding to 10 mg of CTZ and its PM, CTZ powder was put in the mouth for 15 s. The bitterness level was measured following expectoration, and the samples were then spat out. In this study, the bitterness was graded using an unstructured line scale with values ranging from 0 (no bitterness) to 12 (severe bitterness), as has been previously described. | FTIR, DSC, PXRD, 1H MR, | [75] |
| Cetirizine dihydrochloride | α-, β-, and γ-cyclodextrin | No detailed information provided. | FTIR, UV-VIS, HPLC | [76] |
| Cetirizine dihydrochloride | α-, β-, and γ-cyclodextrin | Thirteen healthy participants reported the bitterness tasted after having their mouths washed with 5 mL (1 mg/mL cetirizine) cetirizine-CD solutions for 10 s. There were 10 men and 3 women among the volunteers. The solutions were then expelled (spat out). The subjects carefully washed their mouths with water three minutes after tasting each sample. The levels of bitterness on the scale were as follows: 1 indicates no bitterness, 2 indicates mild bitterness, 3 indicates moderate bitterness, 4 indicates bitterness, and 5 indicates high bitterness. | UV-VIS, NMR | [77] |
| Levocetirizine dihydrochloride | HP-β-CD | E-tongue: Using a random sample sequence, e-tongue tests were conducted at constant ambient temperature (25 ± 0.5 °C). For the cleaning of the sensors in between measuring the samples, distilled water was utilized. Nine measurements of each sample were made under the following test conditions: 100 mL sample volume, 120 s sample collection time, and 20 s cleaning time. | SEM, UV-VIS | [78] |
| Levocetirizine dihydrochloride | HP-β-CD | The authors chose 10 human subjects. A complex made up of around 5 mg of drug equivalent was put on the tongue and tasted both immediately and after 20 s. By averaging the ratings of each volunteer, the overall assessment and bitterness level were reported. | UV-VIS | [24] |
| Levocetirizine dihydrochloride | HP-β-CD | Six healthy female volunteers between the ages of 35 and 48 were chosen to rate the drug’s level of bitterness on a scale of 0 to 5. A score of 0 indicated no bitterness, while a score of 5 suggested a highly bitter taste. The volunteers were instructed to keep a low dose of the medication (5 mg) in their mouths at the back of the tongue for 10 s while recording their results. | FTIR, DSC, UV-VIS | [79] |
| Levocetirizine dihydrochloride | HP-β-CD | Twelve healthy adult male human participants between the ages of 19 and 22 years old participated in taste assessment research (gustatory sensation test) to assess the mouthfeel, palatability, and in vivo disintegration time in the oral cavity for the F19 formulation. During the test, the subjects were instructed to limit their tongue movements. The reference product was first given to the participants and held in their mouths until it crumbled. After that, the participants spat out the dispersion and gargled with water. The participants assessed the test and reference formulations’ bitterness as 0—good; 1—not bitter; 2—bitter; or 3—very bitter. | [80] | |
| Bromelain hydrolysate | β-cyclodextrin | Quantitative descriptive analysis (QDA): Using caffeine as a reference solution, samples were assessed by fifteen panelists who had completed a three-week training program. The panelists were given caffeine solutions at six different concentrations (0 to 1000 ppm, spaced 200 ppm apart). These concentrations were to be ranked by each panelist from least to most bitter. At the conclusion of the training, the concentration with the mildest bitterness was determined and utilized to assess the hydrolysate samples. Panelists were positioned in individual booths for the duration of the evaluation session. Warm water and citrus water at 0.04% (w/v) were given to the participants. The degree of bitterness was measured and recorded on a 15 cm line scale that ranged from “none” to “very bitter.” | HPLC, GC-MS, FESEM | [81] |
| Chitosan | α-, β-, and γ-cyclodextrins | Twelve volunteers, six males and six females, 23 to 42 years old, were asked to score the bitterness intensity on a 5-point scale ranging from “like water” (0) to “exceedingly bitter” (5) and passing through “very faintly bitter” (1), “faintly bitter” (2), “definitely bitter” (3), and “strongly bitter” (4). | 1H NMR, FTIR, 13C NMR, ESI-MS | [82] |
| Allicin | α-cyclodextrin | The entirety of the planned research involved 18 participants, who were all between the ages of 20 and 45, in excellent health, and free of any discomfort or allergies and had the capacity to articulate and describe their sensations clearly. The volunteers were given equal portions of the physical mixture and allicin@-CD, which they were instructed to hold in their mouths for 10 s before exhaling. After this, the volunteers’ mouths were rinsed with plain water or made to feel better by chewing gum or using mouthwash (5–10 min later) while also sufficiently resting (by lying down in a ventilated area until the previous sample had no effect on tasting or sniffing) to prevent cross-contamination between samples. Volunteers were asked to rate the samples’ strength of taste and smell and respond to the following questions both during and after the evaluation: (i) Would you kindly outline each group’s preferences? (ii) Which group (within 5 s) had a greater garlic flavor at first taste? (iii) Which group exhibited a stronger garlic flavor in the latter stages (5–10 s)? | HPLC, FTIR, PXRD, 1H NMR, TGA | [83] |
| Arundic acid | α-, β-, γ-cyclodextrin, HP-α-CD and HP-β-CD | Five healthy participants participated in the gustatory sensation tests and were instructed to concentrate on the astringency and discomfort without being distracted by the fragrance. Quinine hydrochloride concentrations utilized as standards were 0.03, 0.1, 0.3, and 1 mM, and the associated bitterness and irritation ratings were 0, 1, 2, and 3, respectively. | UV-VIS, HPLC, 1H NMR, PXRD, PS | [84] |
| Bitterness suppressants of berberine hydrochloride | β-cyclodextrin | E-tongue: The solution’s total volume was 100 mL. The sensors were cleaned for 90 s in a purification bath before being submerged in a reference solution for a further 120 s of cleaning. The second reference solution was used after that for 120 s. After a 30 s equilibration period, the sensors were reset to zero. The testing started 30 s after establishing equilibrium. The sensors were briefly cleaned in each of the two reference solutions for three seconds before being put in a new reference solution to track aftertaste for thirty seconds. There were four iterations of this cycle. | [85] | |
| Bitter gourd extract (BGE) | β-cyclodextrin | The bitterness of samples was assessed by a trained panel (n = 10: 6 males and 4 females). Student panelists between the ages of 18 and 38 were present. Each panelist received 8 samples in total, including the extract without the bitterness-masking agent (the control sample) and the extract with the bitterness-masking agent (7 samples), as part of the evaluation process for bitterness. The participants were instructed to taste each sample and assess the level of bitterness on a 10 cm unstructured scale with “weak” and “strong” labels at the left and right extremes. | FTIR, 1HNMR, TGA | [86] |
| Soybean meal | α-, β-, and γ-cyclodextrin | Electronic tongue: Before being given to tasters, samples were assigned randomly generated numbers that they were instructed to sort in order of increasing bitterness. By designating note 1 for the least bitter sample and note 3 for the most bitter sample, the findings were examined. | SEM, TGA, DSC, 1H NMR, PXRD | [87] |
| Beany off-flavors in plant-based meat analogs | α-, β-, γ-cyclodextrin | Five Amano En-zyme Inc. staff members who had received sensory assessment training evaluated the flavor and aroma of plant-based patties. In taste testing, the items were judged on the basis of five characteristics: umami, bitterness, saltiness, and sweetness. The samples were assessed for two characteristics—odor and fragrance—in sniff tests. The samples were scored on a scale ranging from 1 (weak) to 5 (strong). | HPLC, HS-SPME-GC/MS, TPA | [88] |
| Goat’s milk | β-cyclodextrin | Trained panelists of both sexes (n = 21) were recruited. Three samples of goat’s milk were heated up to 50+/±18 C. One sample was designated as the standard (no β-cyclodextrin), and the other two had β-cyclodextrin incorporated. In order to determine whether the number of correct responses for the number tested was equal to or greater than the number indicated in the Table of the Critical Number (Minimum) of Correct Answers at 5, 1, and 0.1% levels of significance, the results were expressed as the number of correct responses (correctly identified) and the number of total responses. | DSC, 1H NMR | [89] |
| Formulation | Number of Volunteers Rating the Formulation as * | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Rizatriptan benzoate | 6 | - | - | - |
| Inclusion complex (1:1) | 6 | - | - | - |
| Inclusion complex (1:2) | - | 4 | 2 | - |
| Inclusion complex (1:3) | - | - | - | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. https://doi.org/10.3390/molecules28196964
Adamkiewicz L, Szeleszczuk Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules. 2023; 28(19):6964. https://doi.org/10.3390/molecules28196964
Chicago/Turabian StyleAdamkiewicz, Lena, and Łukasz Szeleszczuk. 2023. "Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes" Molecules 28, no. 19: 6964. https://doi.org/10.3390/molecules28196964
APA StyleAdamkiewicz, L., & Szeleszczuk, Ł. (2023). Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules, 28(19), 6964. https://doi.org/10.3390/molecules28196964






