Abstract
Albendazole is the preferred deworming drug and has strong insecticidal effects on human and animal helminth parasites, showing remarkable activity against hepatocellular carcinoma and colorectal cancer cells. However, it is classified as being in class II in the Biopharmaceutics Classification System due to its poor water solubility (0.2 mg/L) and high permeability, which make the clinical application of albendazole impractical. Through complexation with methyl-β-cyclodextrin, as the best result so far, albendazole’s water solubility was increased by 150,000 times, and albendazole could be 90% released during the first 10 min. In an in vivo pharmacokinetic study, the Cmax and Tmax of the active metabolized sulfoxide were changed from 2.81 µg/mL at 3 h to 10.2 µg/mL at 6 h and the AUC0–48 was increased from 50.72 h⁎μg/mL to 119.95 h⁎μg/mL, indicating that the inclusion complex obtained can be used as a new oral therapeutic anti-anthelmintic and anti-tumor agent formulation.
1. Introduction
Albendazole has a broad anthelmintic spectrum, strong insecticidal effect on human and animal helminth parasites, low toxicity, and good safety, and was recommended by the World Health Organization as the preferred deworming drug [1]. Furthermore, albendazole is a microtubule depolymerizing agent with remarkable activity against hepatocellular carcinoma and colorectal cancer cells in vitro and in vivo [2].
Albendazole belongs to class II in the Biopharmaceutics Classification System due to its poor water solubility (0.2 mg/L) and high permeability [3]. Albendazole’s low intestinal absorption rate, low blood concentration in the body, and low bioavailability required a high dosage for treating systemic helminth infections; the current clinical cure rate for non-intestinal parasitic diseases is only about 30% [4]. The clinical application of the drug in cancer using IP or IV routes may require a much higher aqueous solubility, usually 5–10 mg/mL. This is because low concentrations of drugs in solution would require very large volumes to reach the required therapeutic dose, which made the application of albendazole impractical, and its clinical usefulness as an anti-cancer agent is severely hampered [5].
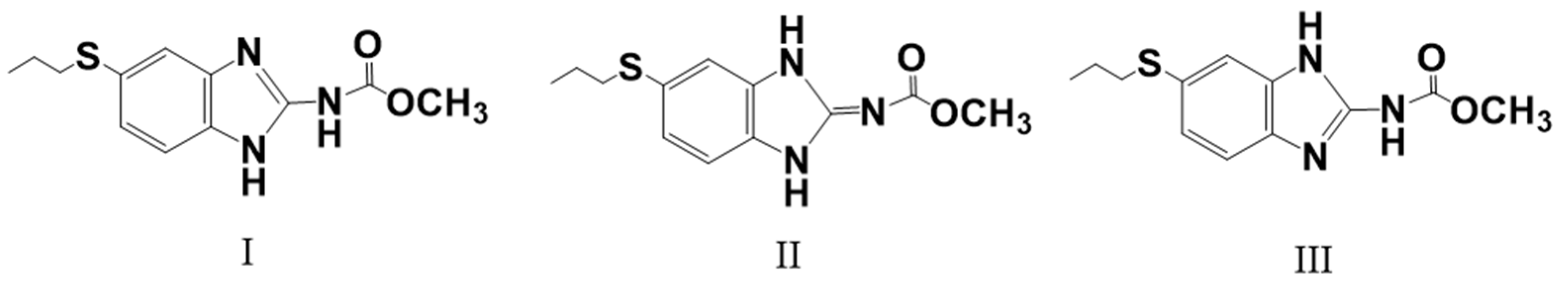
Since 1991, numerous attempts have been made to improve albendazole’s solubility and thereby enhance its efficacy, such as the use of soybean oil emulsion [6], surfactants [7], liposomes [8], polyvinylpyrrolidone [9], ionization in acids [10], and complexations with cyclodextrins. Among these methods, complexation with cyclodextrins is better than the others, since albendazole presents three tautomeric forms, I, II, and III, as shown in Figure 1 [11]. Cyclodextrins can form inclusion complexes with them to prevent their interconversion. Forms I and II are enantiomerically related forms [12], and the commercially available form I is metastable at room temperature while form II is stable. Therefore, complexation with cyclodextrins can not only increase solubility, but also stabilize the compounds [13].
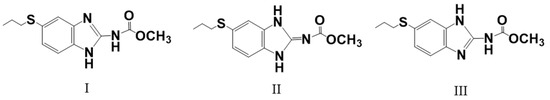
Figure 1.
Three tautomeric forms of albendazole.
A β-Cyclodextrin inclusion complex has been reported to stabilize albendazole and, consequently, improve its water solubility and dissolution rate. However, this complexation only increased the solubility 53.4 times due to β-cyclodextrin’s low aqueous solubility (18 mg/mL). The solid product of the complexation was analyzed using solid NMR spectroscopy, however, signals from albendazole in the complex were not observed in the spectrum [14,15].
The solubility of albendazole in a complex with HP-β-cyclodextrin was increased from 2 to 10,000 fold to reach a solubility as high as 1.9 mg/mL [16,17,18,19,20,21]. Adding citric acid, ascorbic acid, hydrochloric acid, or acetic acid to the aqueous solution of HP-β-cyclodextrin could increase the solubility of albendazole from 0.2 µm/mL to 1.5 mg/mL [22,23,24]. When albendazole was stirred with 40% of sulfobutylether-β-cyclodextrin in water at 25 °C with a pH of 2.3 for 3 days, its water solubility reached 8 mg/mL. Under the same conditions, HP-β-cyclodextrin could make its water solubility reach 6.4 mg/mL [25]. However, as albendazole is soluble in a 0.1 HCl solution (0.4 mg/mL), it was hard to say whether the 8 mg/mL solubility was due to the acidic condition or due to cyclodextrin’s solubilization capability [26].
A variety of techniques such as FTIR, DSC, TAG, X-ray, and SEM have been used to confirm the formation of complexes in the solid state [27]. However, an increased water solubility can only be confirmed by its proton NMR spectrum in D2O.
When albendazole was treated with 40% of HP-β-cyclodextrin, sulfobutylether-β-cyclodextrin, or methyl-β-cyclodextrin in water, its aqueous solubility was remarkably increased from 0.2 μg/mL to 0.79 mg/mL, 1.17 mg/mL, and 1.52 mg/mL, respectively, and these increased water solubilities were confirmed by proton NMR spectra in D2O [28,29,30].
Citrate-β-cyclodextrin, itaconyl-β-cyclodextrin, and succinyl-β-cyclodextrin were synthesized and complexed with albendazole via spray drying. The drug dissolution rate and water solubility were improved slightly, and their proton NMR spectra in 0.1N DCl in D2O were recorded. Since albendazole is soluble in a 0.1 HCl solution (0.4 mg/mL), the chemical shifts from albendazole in the complex in the NMR spectrum could not fully support the drug’s water solubility [31,32].
A trace amount of water-soluble polymer such as polyvinylpyrrolidone K30 was added to the cyclodextrin complex to increase the drug’s solubility [33,34,35].
Recently, the co-precipitation of β-cyclodextrin and albendazole using a supercritical antisolvent technique in a miscible solution of DMSO, acetone, and CO2 produced the precipitate as microparticles. After characterization using spectroscopic, thermal, and crystallographic analyses, this new solid form was found to contain tautomer II of albendazole. The dissolution rate was increased about four times in comparison to that of albendazole alone due to the formation of the inclusion complex and a synergetic effect between both components. However, the water solubility increase was not measured and confirmed [36]. It was reported that the cyclodextrin’s solubilization capability in a complexation with albendazole can be enhanced by adding bile salt [37].
Overall, even with great efforts towards preparing albendazole/cyclodextrin complexes, the water solubility has still not been significantly improved and is far away from the 5–10 mg/mL that is required for a cancer therapeutic dose. In the continuation of an interest in drug inclusion complexes [38,39,40,41,42,43,44,45,46,47], there was a further study on improving the water solubility of albendazole through the formation of inclusion complexes with cyclodextrins. In this paper, new results are reported on albendazole’s water solubility through complexation with methyl β-cyclodextrin, where the water solubility of albendazole was increased from 0.2 μg/mL to 30 mg/mL.
2. Results and Discussion
UV spectra of the solutions of albendazole in the mixture of acetic acid and ethanol (1:19, 0.1 mg/mL) and cyclodextrins in water (1 mg/mL) were recorded from 200 nm to 400 nm, and the maximum absorption of albendazole at 295 nm was observed. Cyclodextrins have no UV absorption around this wavelength, and 295 nm was selected for a HPLC analysis of albendazole. A series of albendazole solutions in methanol with concentrations of 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1 mg/mL were analyzed using HPLC, and, based on the concentrations and peak areas, the regression equation was obtained as Y = 15598X + 10.71 (R2 = 0.9991, Y = concentration, and X = peak area).
The HPLC standard curves and a sample analysis including plasma samples were performed in the same HPLC instrument with the same C-18 column over a short period of time to avoid systematic errors, before and after the HPLC analysis, and the standard solution of ketoconazole was checked using HPLC to confirm the stability of the HPLC analysis conditions.
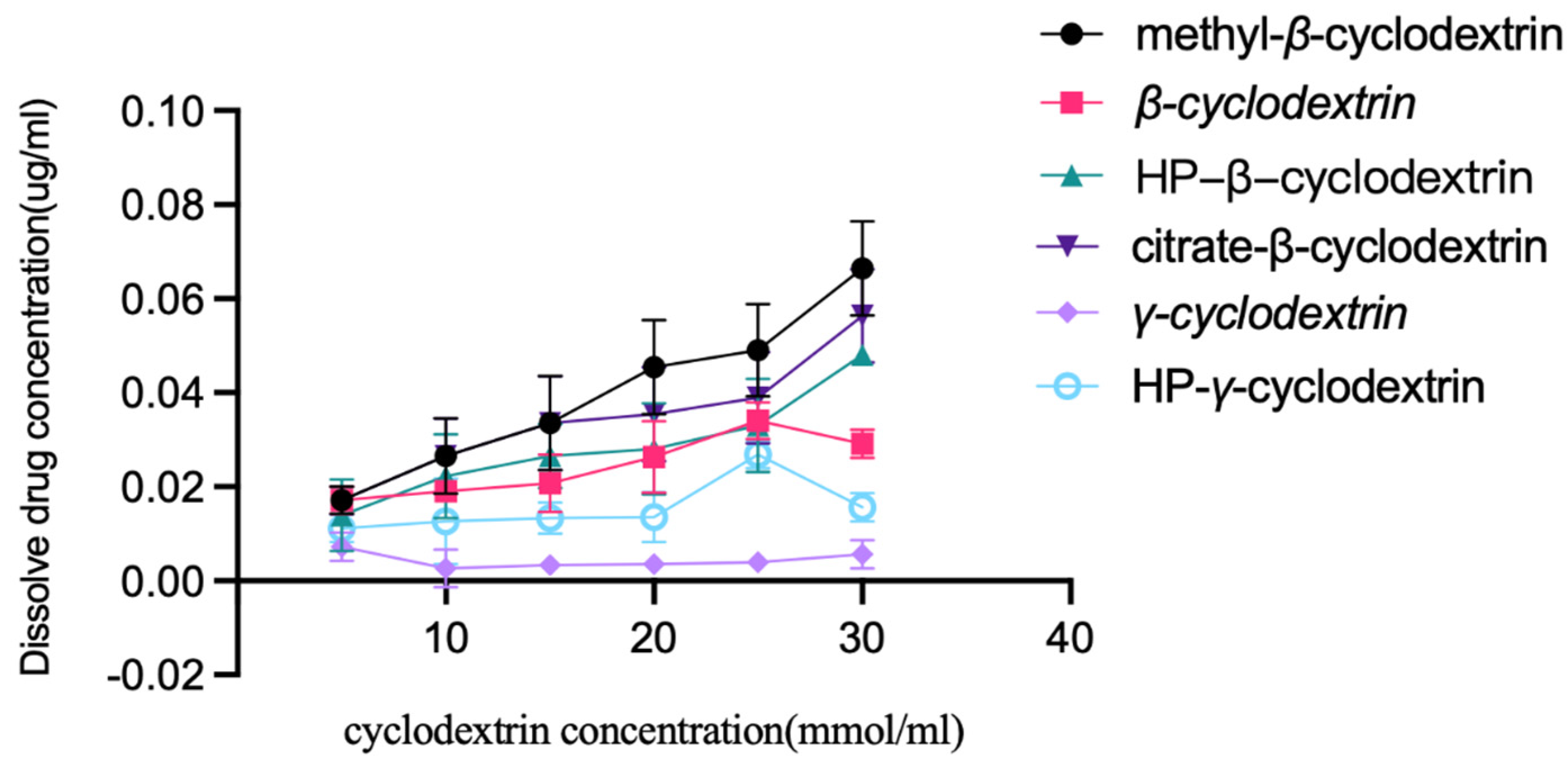
Extra albendazole was added to the solutions of β-cyclodextrin, HP-β-cyclodextrin, methyl β-cyclodextrin, HP-γ-cyclodextrin γ-cyclodextrin, and citrate-β-cyclodextrin in water with different concentrations (5, 10, 15, 20, 25, and 30 mmol/mL). After stirring at room temperature for 48 h, each mixture was filtered, diluted with water, and checked using HPLC; the results are summarized in Figure 2. It was found that methyl-β-cyclodextrin had the best solubilization effect on albendazole, and the linear relationship between albendazole and methyl-β-cyclodextrin was classified as AL type according to Higuchi and Connors’s description [48].
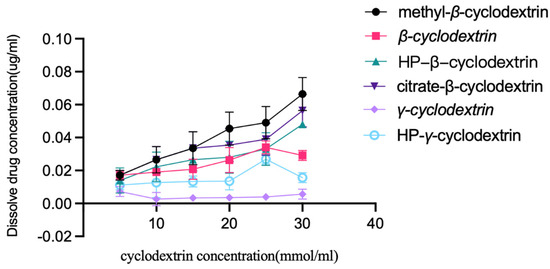
Figure 2.
Solubility of albendazole in water solutions of β-cyclodextrin, HP-β-cyclodextrin, methyl-β-cyclodextrin, citrate-β-cyclodextrin, γ-cyclodextrin and HP-γ-cyclodextrin with different concentrations.
By using the ultrasonic method, aqueous solution stirring method, hydrothermal reactor, microwave method, and CO2 supercritical method [49], methyl-β-cyclodextrin was complexed with albendazole. The resulting solutions were analyzed using HPLC, and it was found that the ultrasonic method could provide the complex with the largest increase in water solubility.
The reaction temperature, reaction time, ratio of albendazole and methyl-β-cyclodextrin, and ultrasonic power affected the complexation. Through single-factor and orthogonal strategies based on the Design of Experiments approach [50], many complexation conditions were designed to form the complexes with methyl β-cyclodextrin based on changing the ratio, ultrasonic power, reaction temperature, and reaction time. After the HPLC analysis, the best condition for increased solubility was found as follows: the ratio should be 1:3, the reaction time should be 45 min (every 5 s reaction with 10 s intervals), the ultrasonic power should be 80%, and the reaction temperature should be 25 °C with occasional ice cooling. The best solubility was found as high as 27 mg/mL.
Through self-association, cyclodextrins and their complexes can form aggregates or micelle-like structures to solubilize water-insoluble drugs by non-inclusion complexation [51], and water-soluble polymers can enhance the solubilizing effect of cyclodextrins [52]. Small amounts (0.25%) of ten water-soluble polymers or organic salts were added to albendazole’s complexation reaction, respectively. After workup and HPLC analysis, it was found that complexation with the addition of sodium dodecyl sulfate could increase albendazole’s water solubility to as high as 30 mg/mL, which is 150,000 times better than that of the drug alone and the best result so far. This complex has a 16% inclusion rate and 86% inclusion yield, and was used for complexation confirmation in in vitro and in vivo pharmacokinetic studies.
Only a trace amount of sodium dodecyl sulfate (0.25% based on the amount of albendazole) existed in the complex; the FTIR, DSC, and NMR spectra of the complex could not exhibit its characteristic signals.
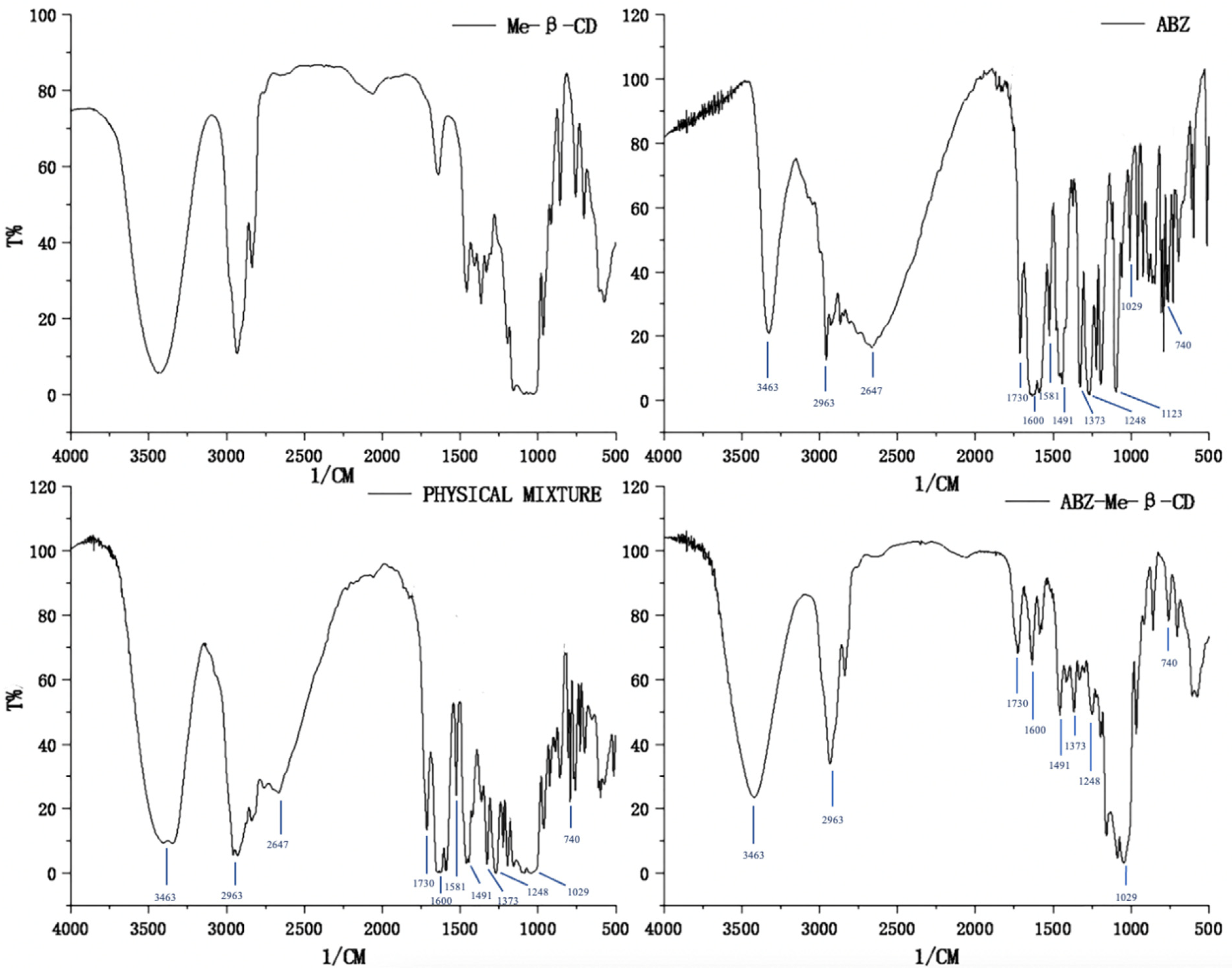
FTIR spectra were used to confirm the complex of albendazole with random methyl-β-cyclodextrin. The FTIR spectra of methyl-β-cyclodextrin, albendazole, the physical mixture of albendazole and methyl-β-cyclodextrin, and their inclusion complex are shown in Figure 3. Compared to the spectrum of methyl-β-cyclodextrin, the characteristic peaks of albendazole appeared at 3463 cm−1 (N-H stretching in carbamate), 2963 cm−1 (C-H aliphatic stretching vibration), 2647 cm−1 (-NH intramolecular in imidazole), 1730 cm−1 (bending vibration of C=O bond in carbamate), 1600 cm−1 (C=C aromatic and N-H out of the plane bending in benzimidazole), 1581 cm−1 (stretching vibration of C=N group), 1491 cm−1 and 1373 cm−1 (C-N and C-O stretching vibrations), 1248 cm−1, 1123 cm−1, and 1029 cm−1 (CH and NH in plane bending vibrations and CH deformation), and 1000–600 cm−1 (skeletal vibrations, CH out of plane bending, NH2 rocking, and C-S stretching vibrations), and they are obviously seen in the physical mixture of albendazole and methyl-β-cyclodextrin, but are slightly reduced in the inclusion complex. These changes in the characteristic peaks of albendazole confirmed that the albendazole was complexed with cyclodextrin.
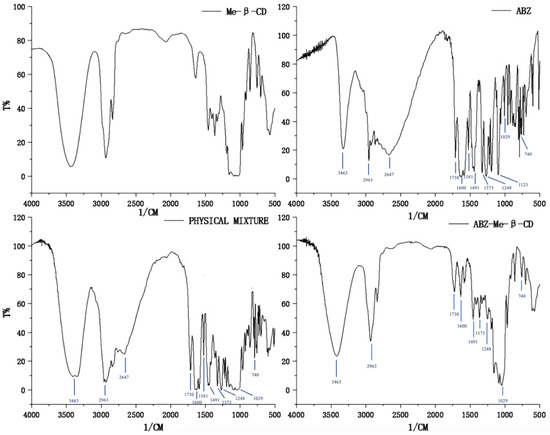
Figure 3.
Fourier-transform infrared spectra of methyl-β-cyclodextrin, albendazole, the physical mixture of methyl-β-cyclodextrin and albendazole, and their inclusion complex.
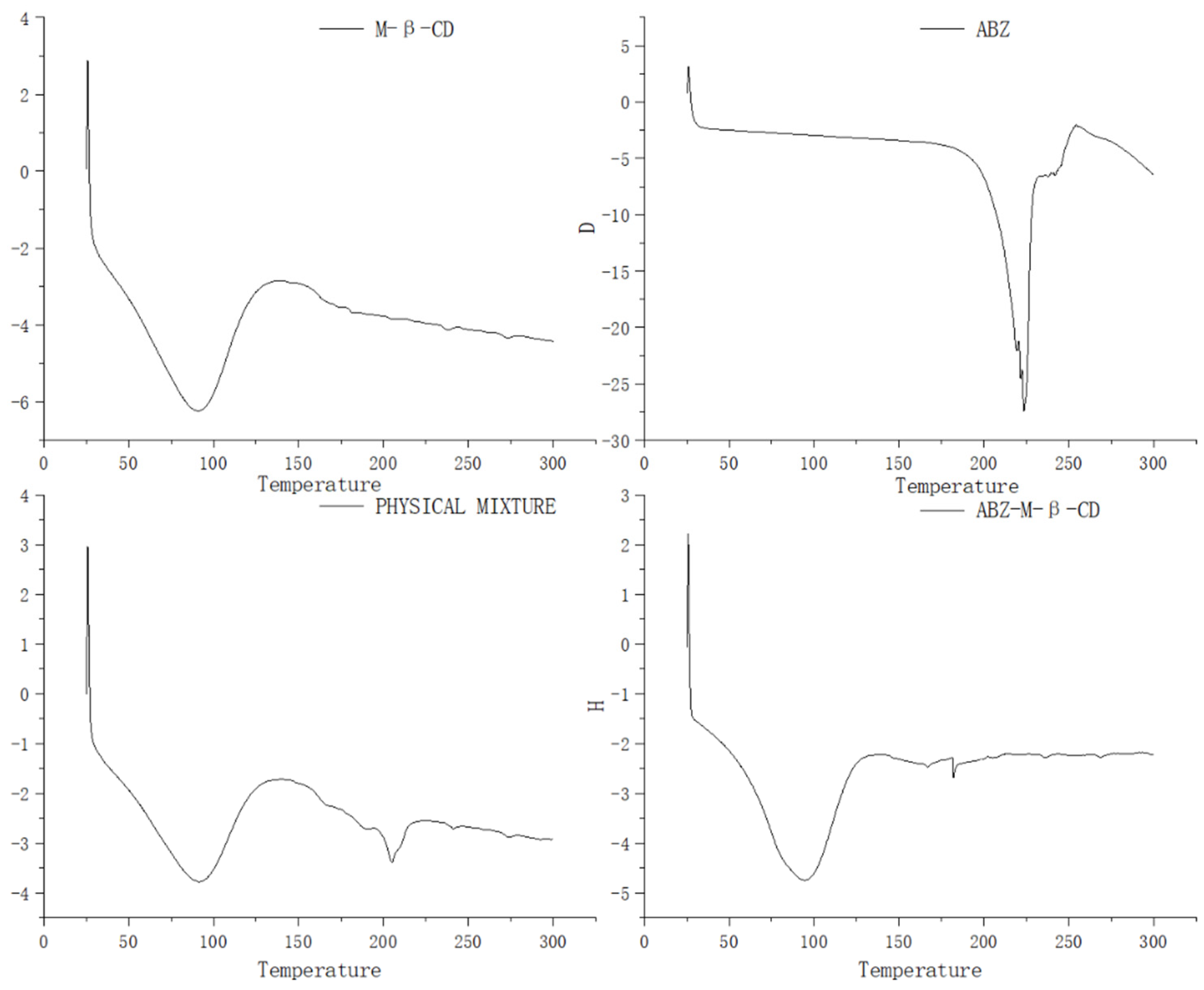
The differential scanning calorimetry curves of albendazole, methyl-β-cyclodextrin, their physical mixture, and inclusion complex were recorded and are shown in Figure 4. The endothermic event in curve for methyl-β-cyclodextrin corresponding to dehydration from the cavity was seen in the range of 50–125 °C, in curve for albendazole, the endothermic event at 225 °C was seen due to its melting effect, in curve for the physical mixture of methyl-β-cyclodextrin and albendazole, the endothermic event in the range of 50–125 °C was seen for the dehydration of methyl-β-cyclodextrin, and the absence of the endothermic event at 225 °C corresponding to albendazole’s melting and the presence of an endothermic event at 205 °C suggested an interaction between albendazole and methyl-β-cyclodextrin in the physical mixture. In the curve of the inclusion complex, the same thermal profile as that of methyl-β-cyclodextrin was presented, probably due to the extra methyl-β-cyclodextrin in the complex. The characteristic endothermic peak of albendazole at 225 °C disappeared due to the loss of the crystalline structure caused by encapsulation, which confirmed the interaction between albendazole and methyl-β-cyclodextrin and the formation of a new phase.
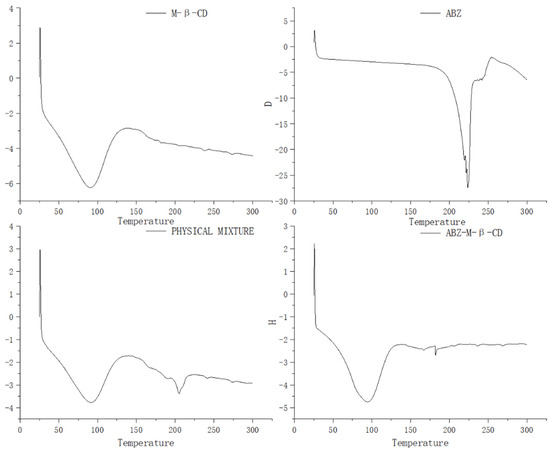
Figure 4.
Differential scanning calorimetry curves of methyl-β-cyclodextrin, albendazole, the physical mixture of methyl-β-cyclodextrin and albendazole, and the inclusion complex.
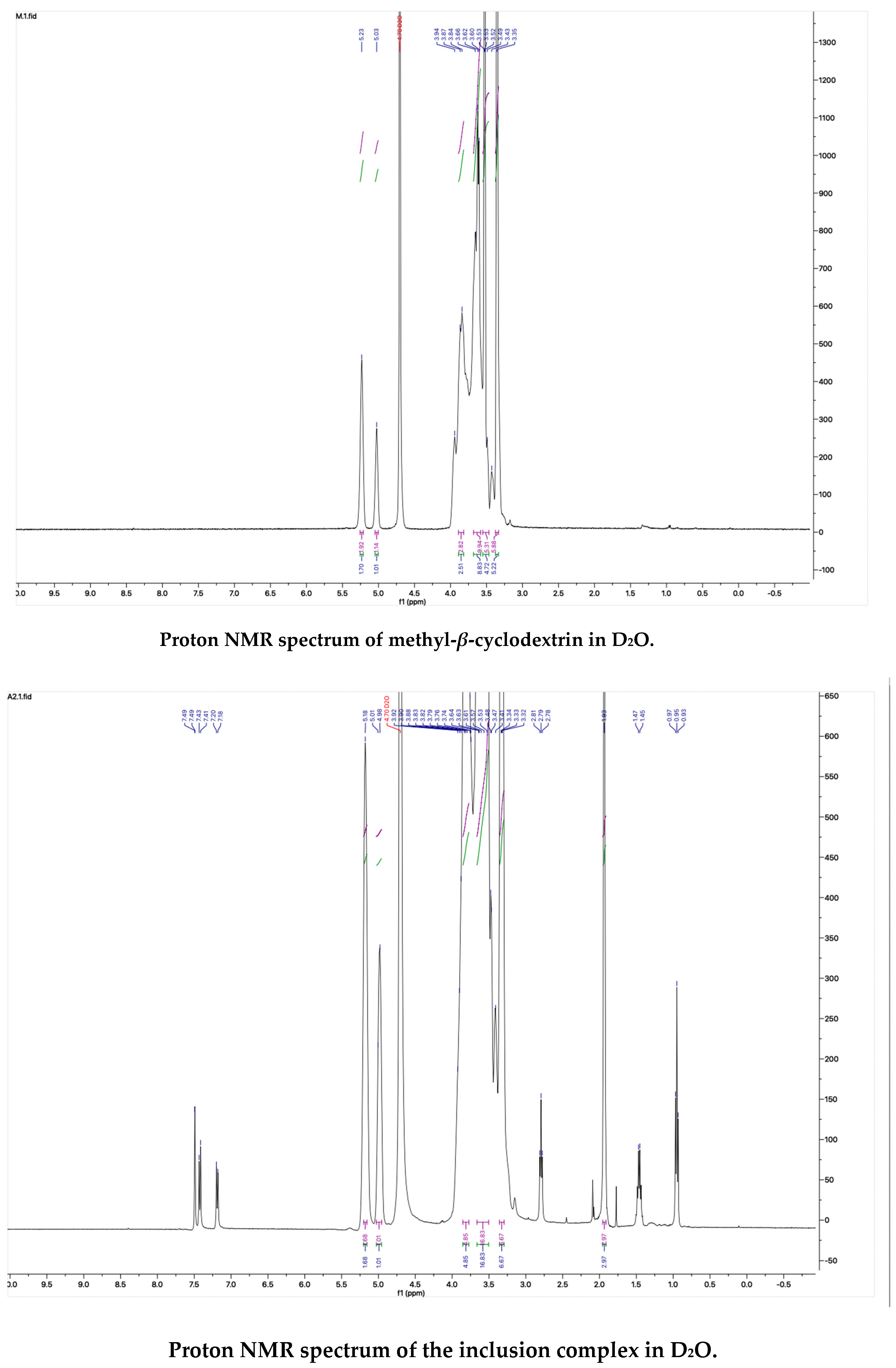
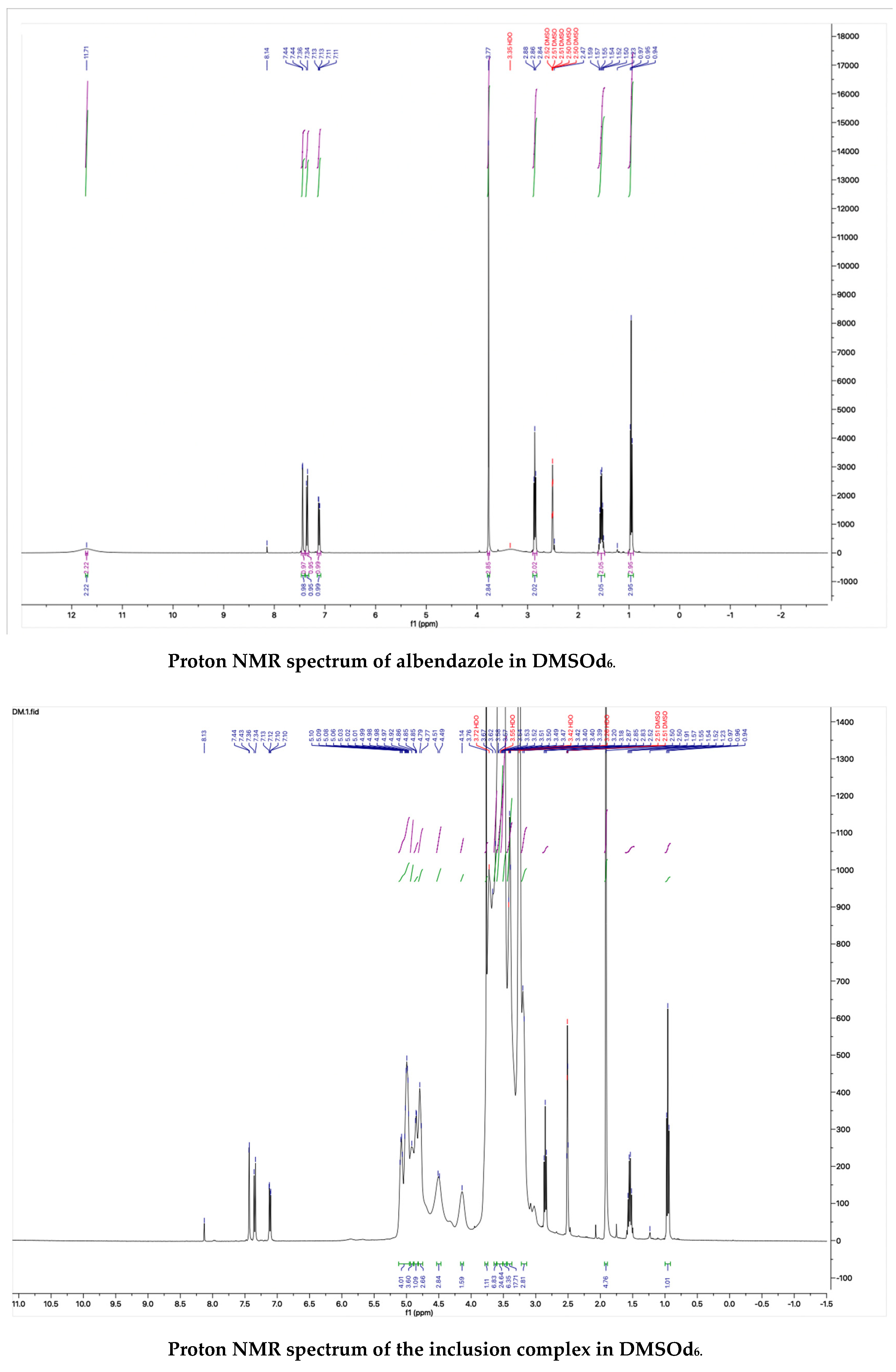
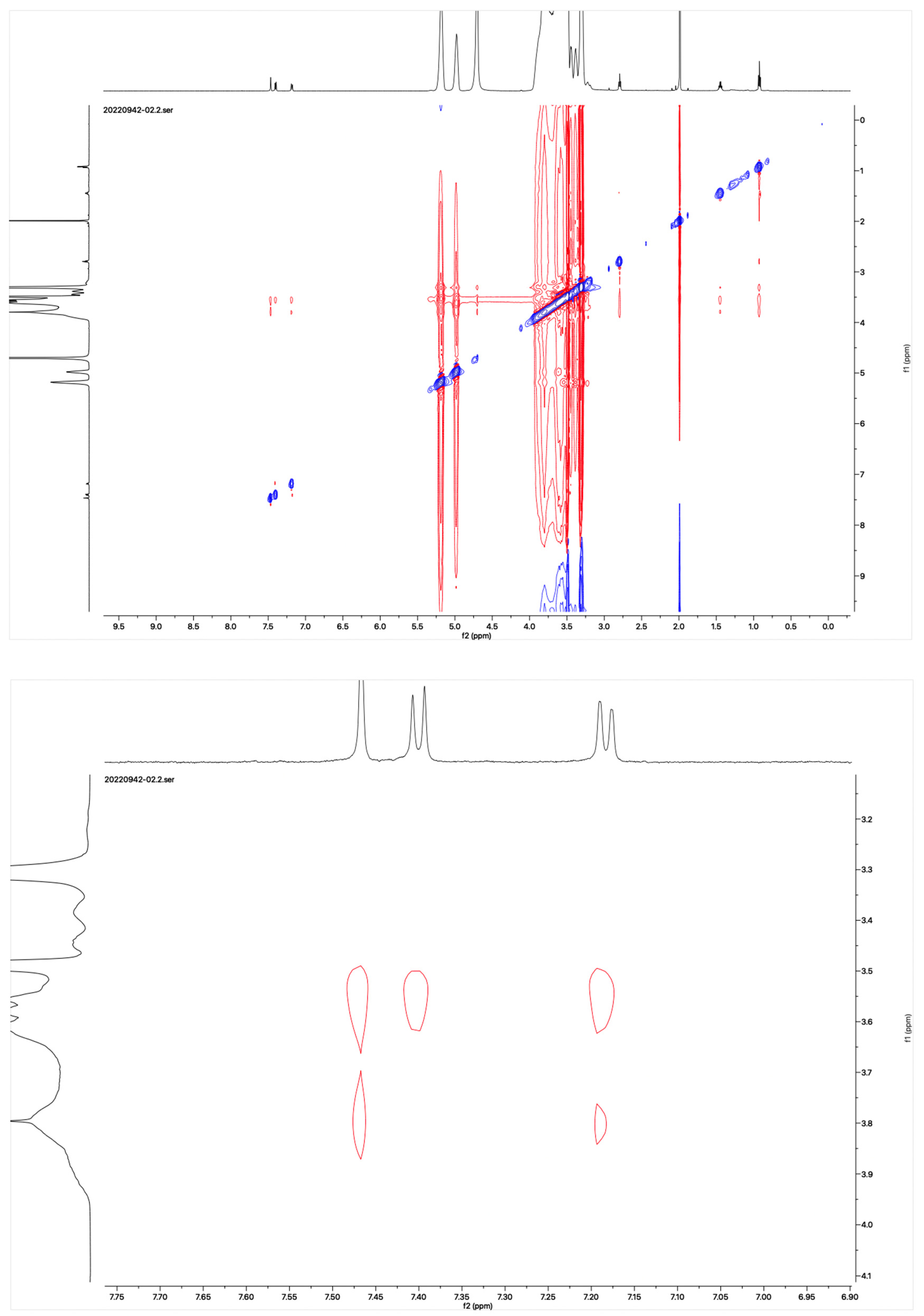
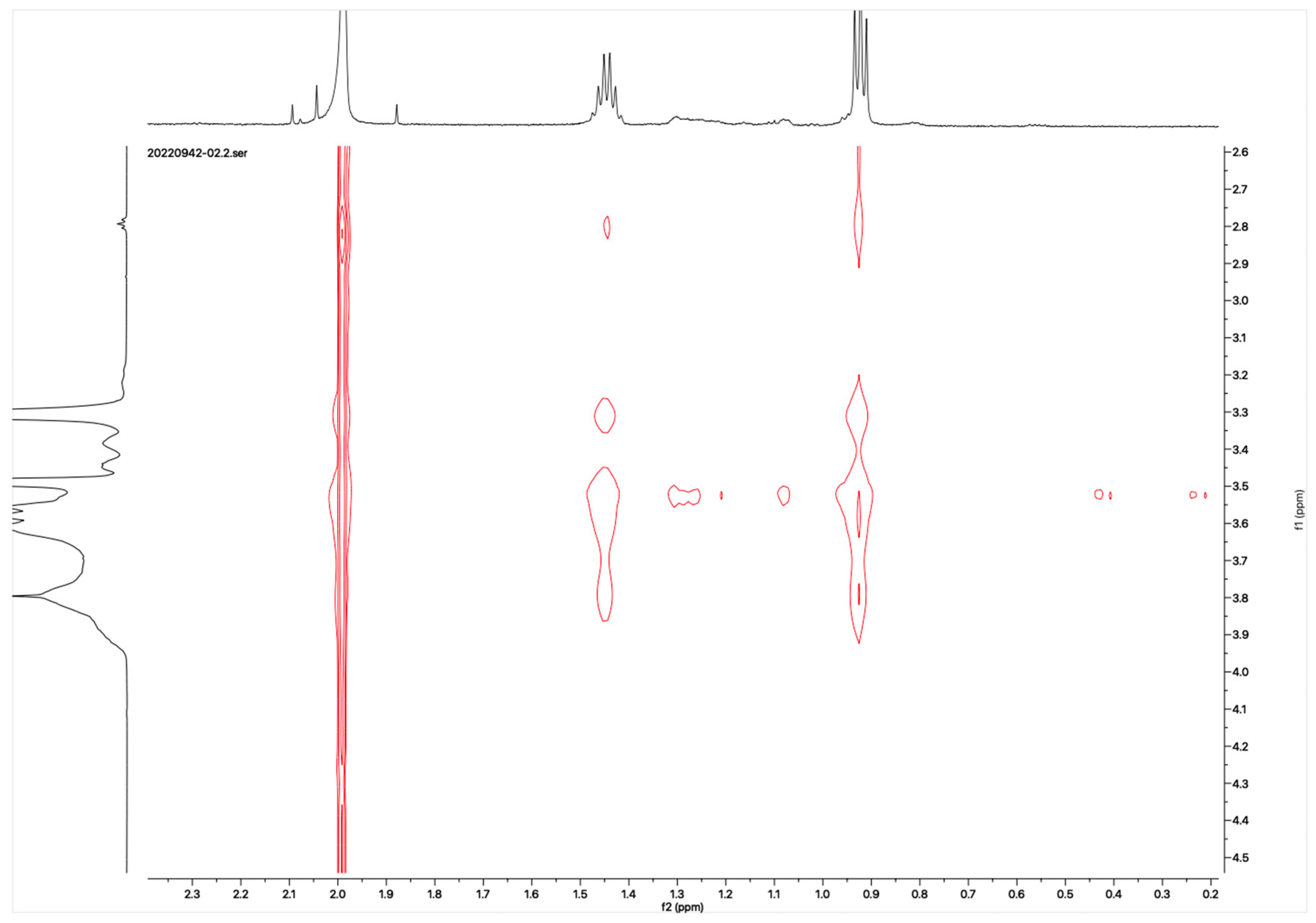
The proton NMR spectra of albendazole in DMSOd6, methyl-β-cyclodextrin in D2O, and the inclusion complex in D2O and DMSOd6 are shown in Figure 5. The proton NMR spectrum of the complex in D2O exhibited the signals from albendazole at 7.61 (1H, s), 7.56 (1H, d, J1 = 8.0 Hz), 7.33 (1H, d, J1 = 8.0 Hz), 2.91 (2H, t, J1 = 8.0 Hz), 1.54 (2H, m), and 1.06 (3H, t, J1 = 8.0). The signals of OCH3 overlapped with the signals from methyl-β-cyclodextrin in the range from 4.00 ppm to 3.50 ppm. The proton NMR spectrum of albendazole in DMSOd6 exhibited signals at 7.46 (1H, s), 7.34 (1H, d, J1 = 8.0 Hz), 7.11 (1H, d, J1 = 8.0 Hz), 3.76 (3H, s, OCH3), 2.85 (2H, d, J1 = 8.0 Hz), 1.53 (2H, d, J1 = 8.0 Hz), and 0.95 (3H, d, J1 = 8.0 Hz); the proton NMR data of albendazole in the complex in DMSOd6 exhibited signals at 7.42 (1H, s), 7.34 (2H, d, J1 = 8.0 Hz), 7.10 (2H, d, J1= 8.0 Hz), 2.65 (2H, d, J1 = 8.0 Hz), 1.54 (2H, d, J1 = 8.0 Hz), and 0.95 (3H, d, J1 = 8.0 Hz). The chemical shift difference in the signals of albendazole in DMSOd6 between the complex and the drug alone could not support the formation of the inclusion complex. The Roesy spectrum of the inclusion complex in D2O was recorded (Figure 6). The signals from the three aromatic protons, two methylene, and one methyl protons showed the interactions with methyl-β-cyclodextrin, which confirmed the formation of the inclusion complex.
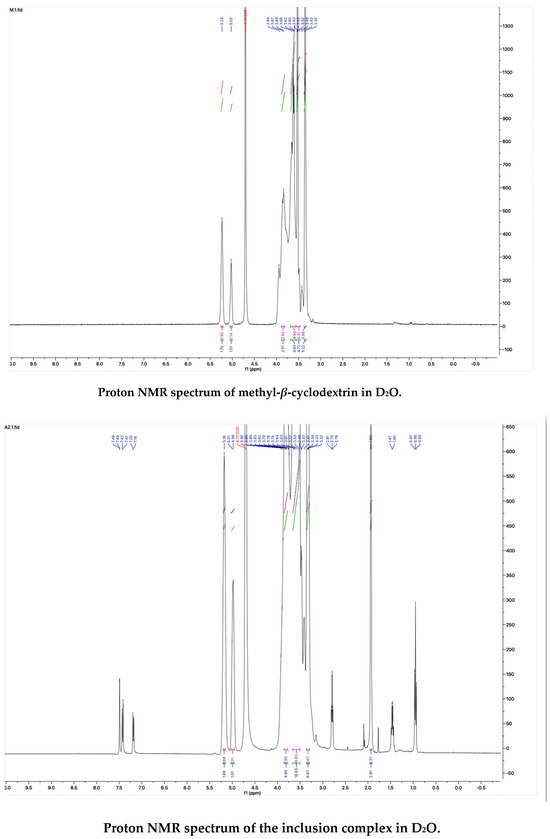
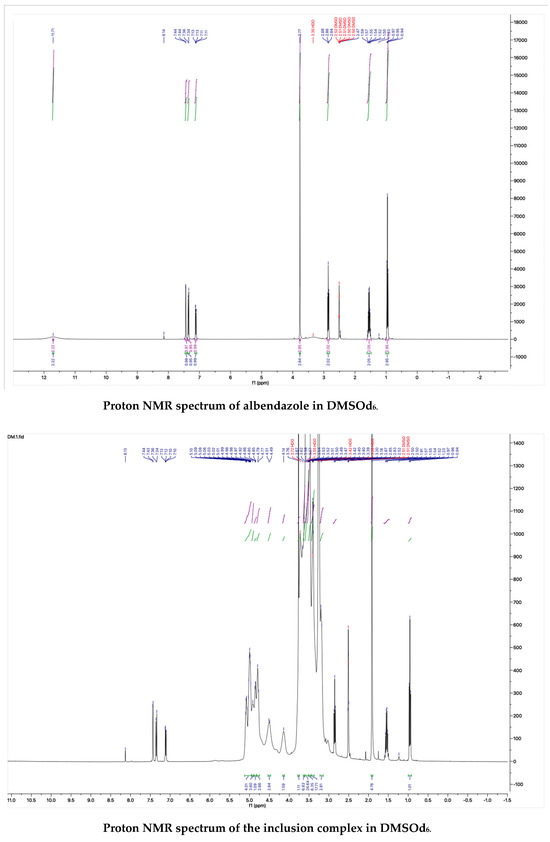
Figure 5.
The proton NMR spectra of methyl-β-cyclodextrin and the inclusion complex in D2O, and the proton NMR spectra of albendazole and the inclusion complex in DMSOd6.
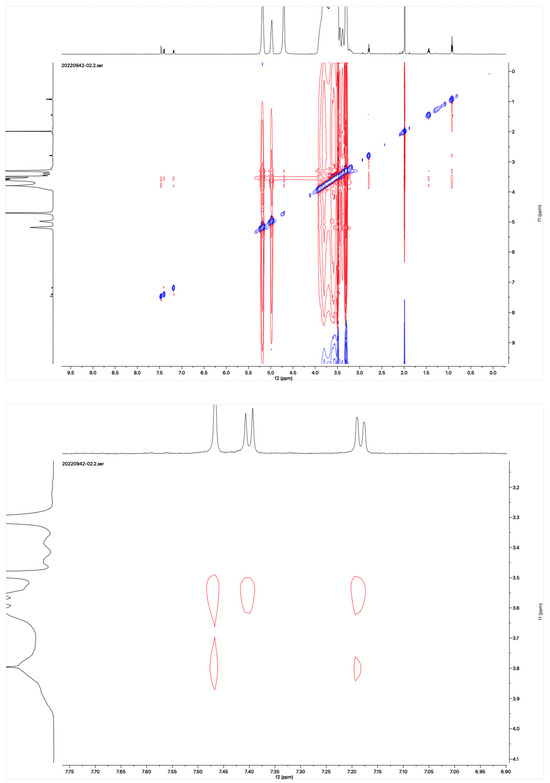
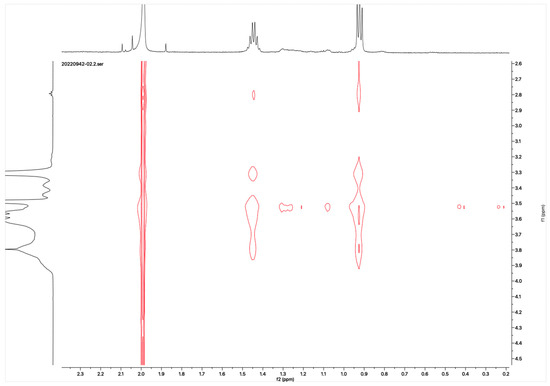
Figure 6.
Roesy spectrum of the albendazole/methyl-β-cyclodextrin inclusion complex in D2O.
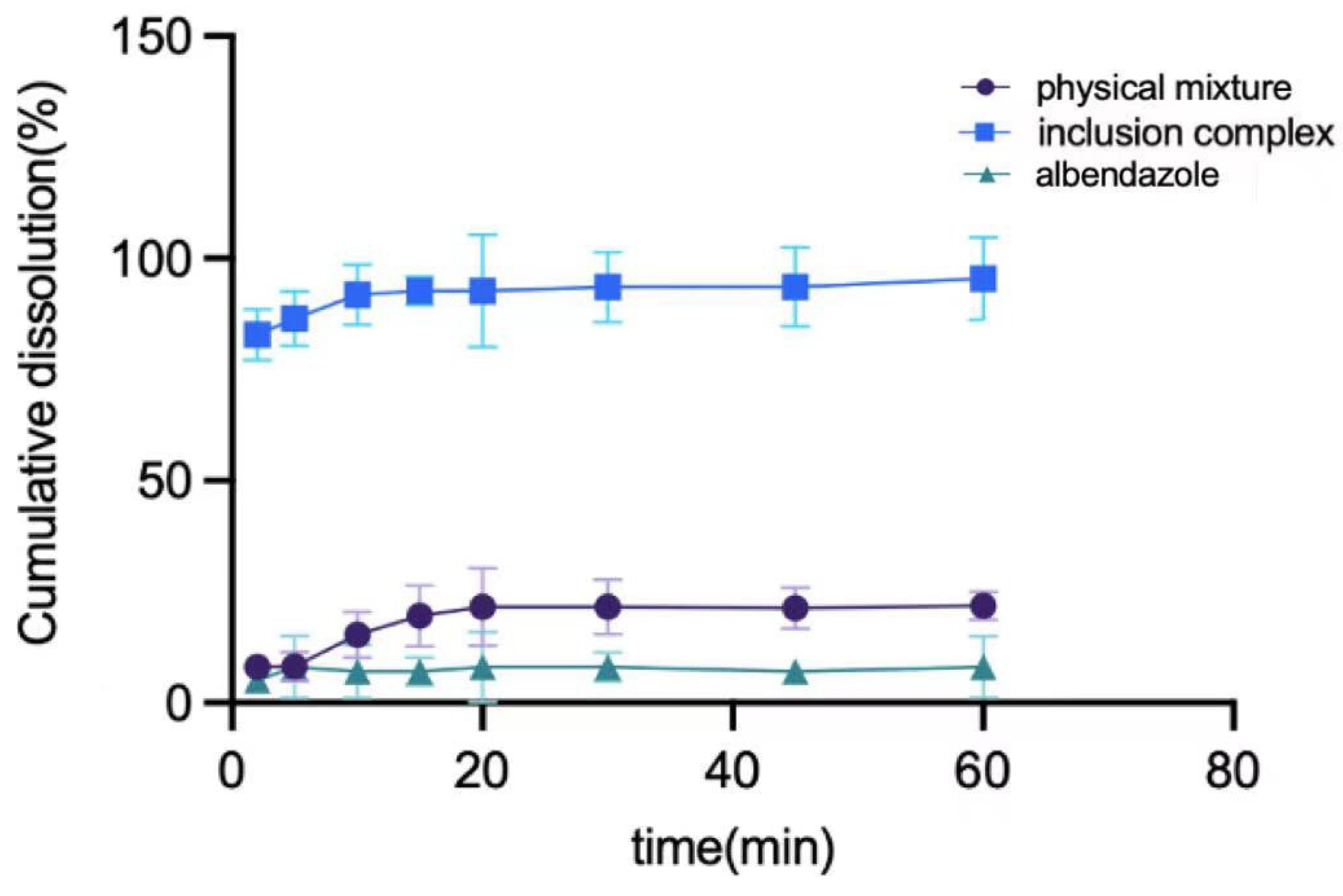
The in vitro PK study of albendazole, the physical mixture of albendazole and methyl-β-cyclodextrin, and the complex are summarized in Figure 7. The albendazole in the complex can be 90% released in 10 min, which is 14 times better than that of albendazole alone and 5 times better than that of the physical mixture.
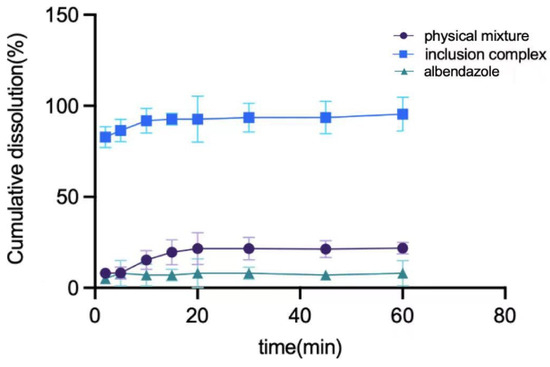
Figure 7.
The dissolution curves of albendazole, the physical mixture of albendazole and methyl-β-cyclodextrin, and their inclusion complex.
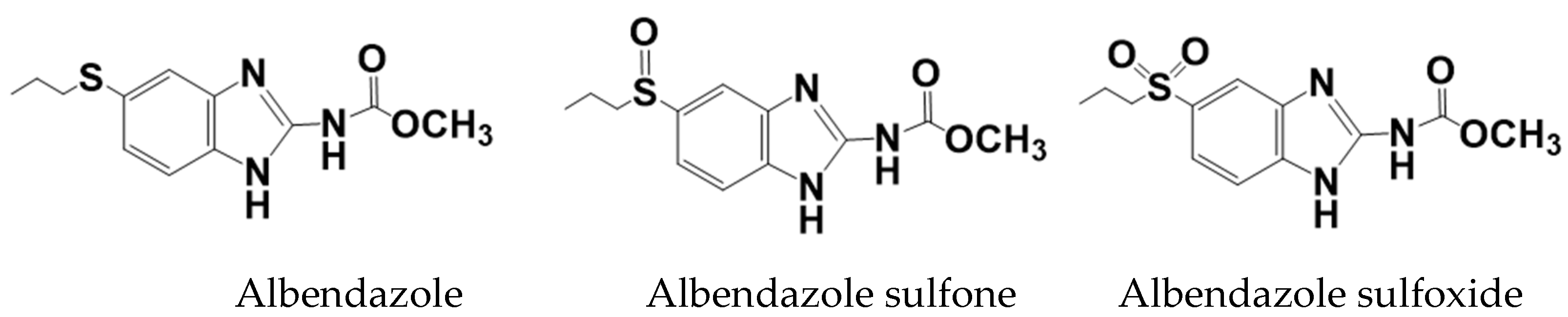
Albendazole is rapidly metabolized in the liver to albendazole sulfoxide and albendazole sulfone [53], and their structures are shown in Figure 8. Albendazole sulfoxide has excellent anthelmintic activity [54]; therefore, the sulfoxide is mostly found in the blood, and hence has been primarily characterized in the PK studies conducted to date [55].
Figure 8.
Molecular structures of albendazole, albendazole sulfone, and albendazole sulfoxide.
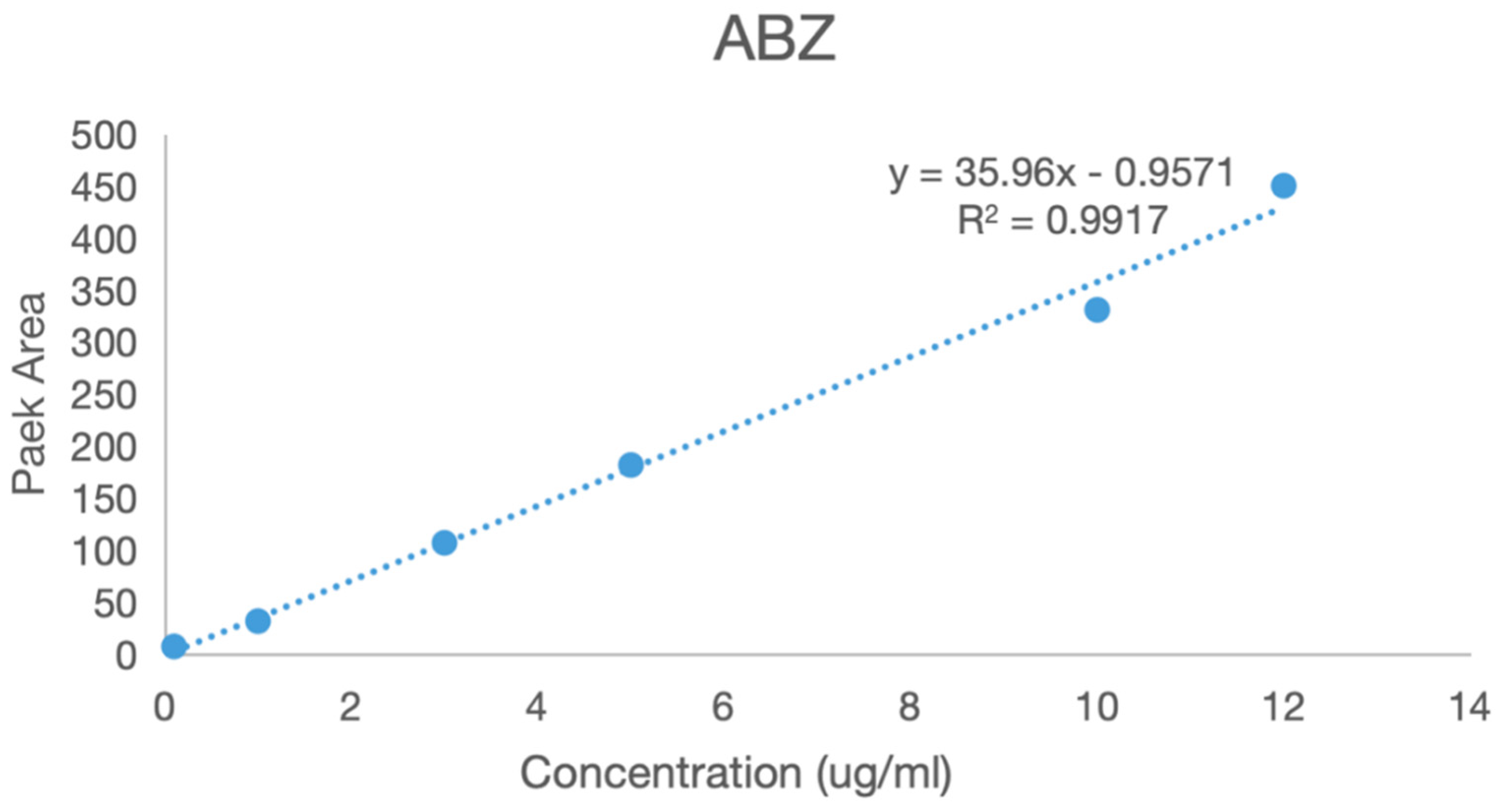
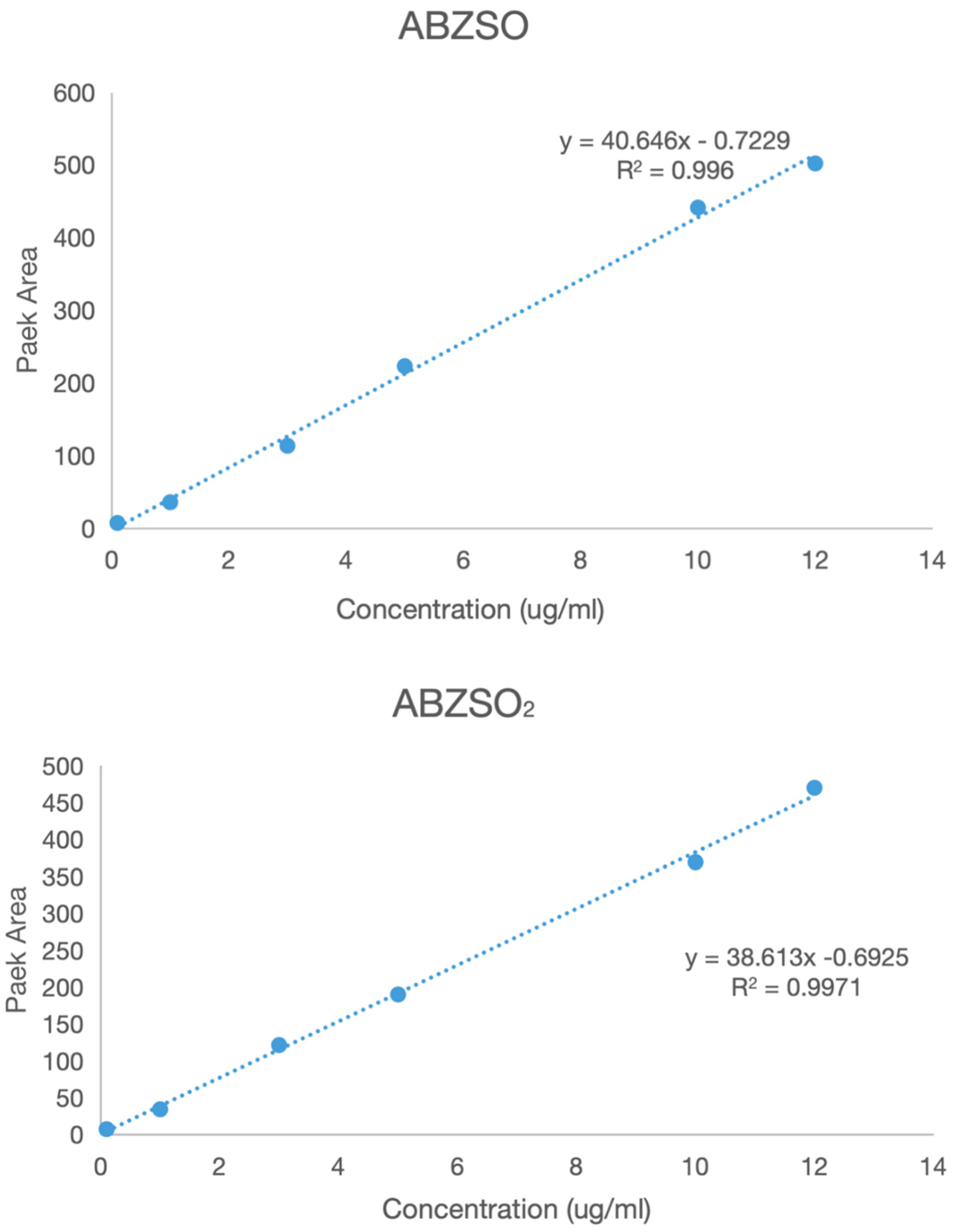
A series of solutions of albendazole, albendazole sulfone, and albendazole sulfoxide in blank plasma with different concentrations were analyzed using HPLC in the range from 0.1 μg/mL to 5 μg/mL. The peak areas in HPLC and the concentrations of albendazole in the plasma solutions had a linear relationship, and the standard curve equations were obtained for albendazole as: Y = 35.96X − 0.9571 (coefficient of determination R2 = 0.9917, Y = peak area, X = concentration, S/N ≥ 10, LLOD = 0.054 μg/mL, LLOQ = 0.165 μg/mL), for albendazole sulfoxide as: Y = 40.464X − 0.7299 (coefficient of determination R2 = 0.996, Y = peak area, X = concentration, S/N ≥ 10: LLOD = 0.043 μg/mL, LLOQ = 0.141 μg/mL), and for albendazole sulfone as: Y = 38.613X − 0.6925 (coefficient of determination R2 = 0.9971, Y = peak area, X = concentration, S/N ≥ 10: LLOD = 0.044 μg/mL, LLOQ = 0.147 μg/mL). Their standard curves are shown in Figure 9.
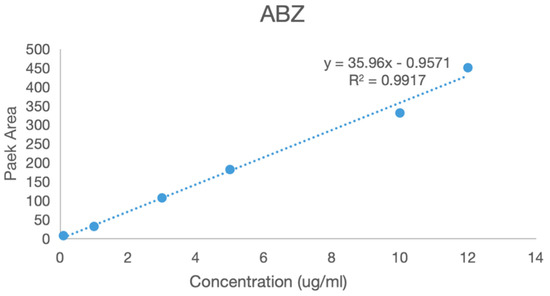
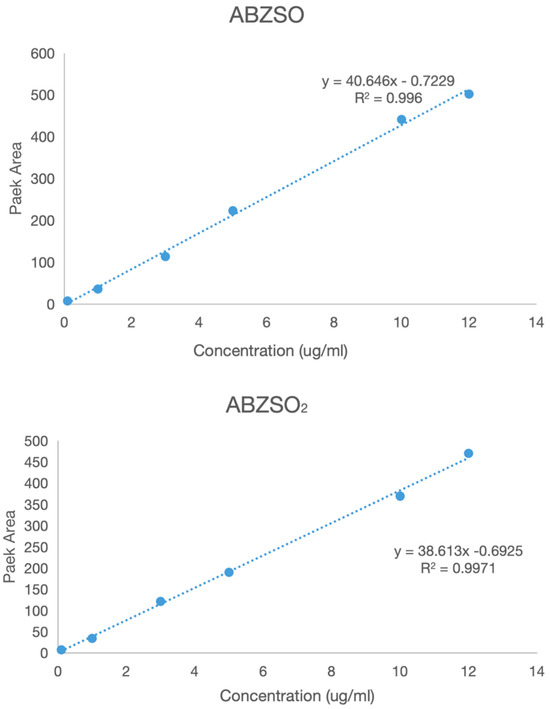
Figure 9.
The standard curves of albendazole, albendazole sulfone, and albendazole sulfoxide in blank dog plasma.
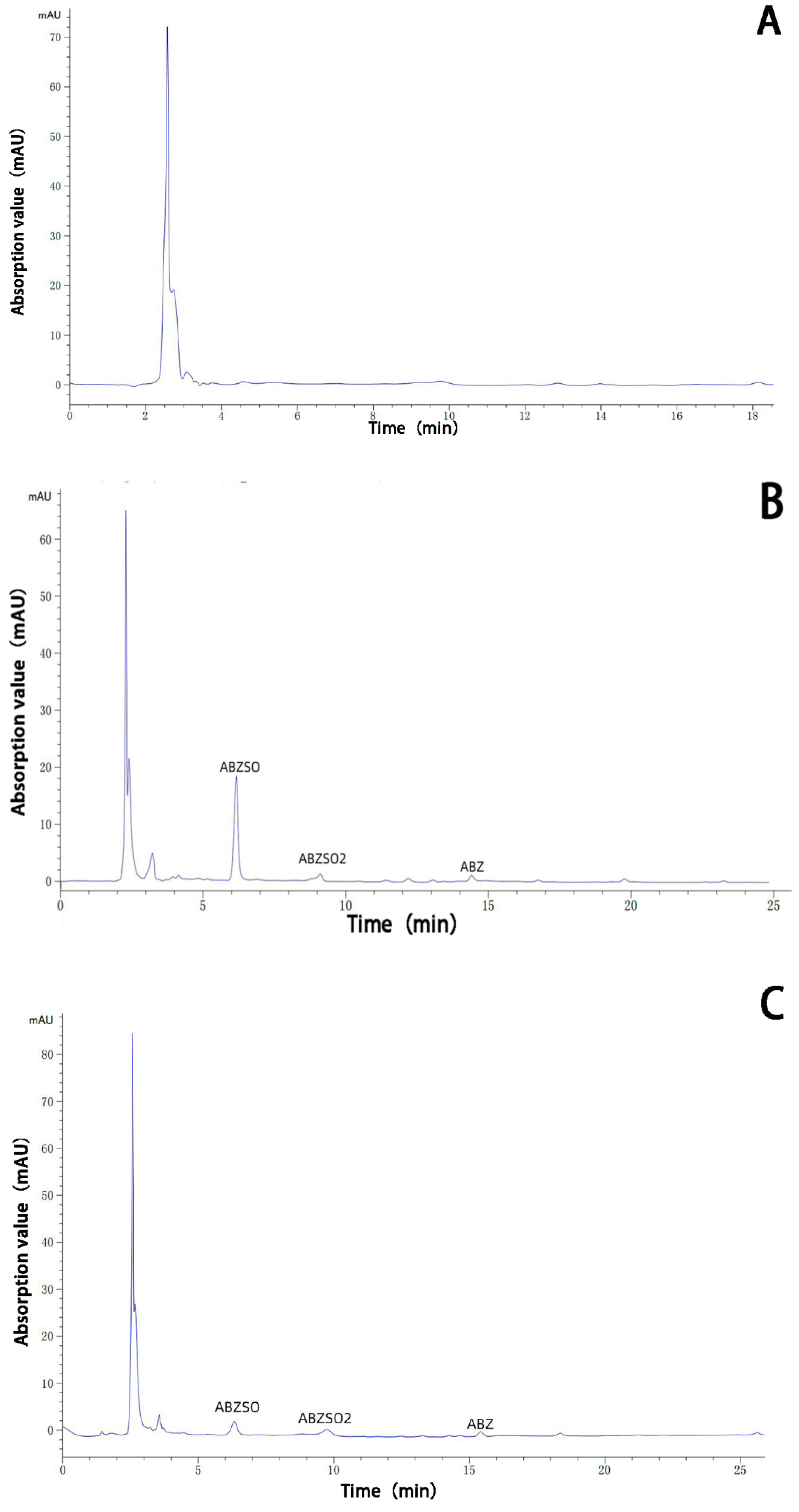
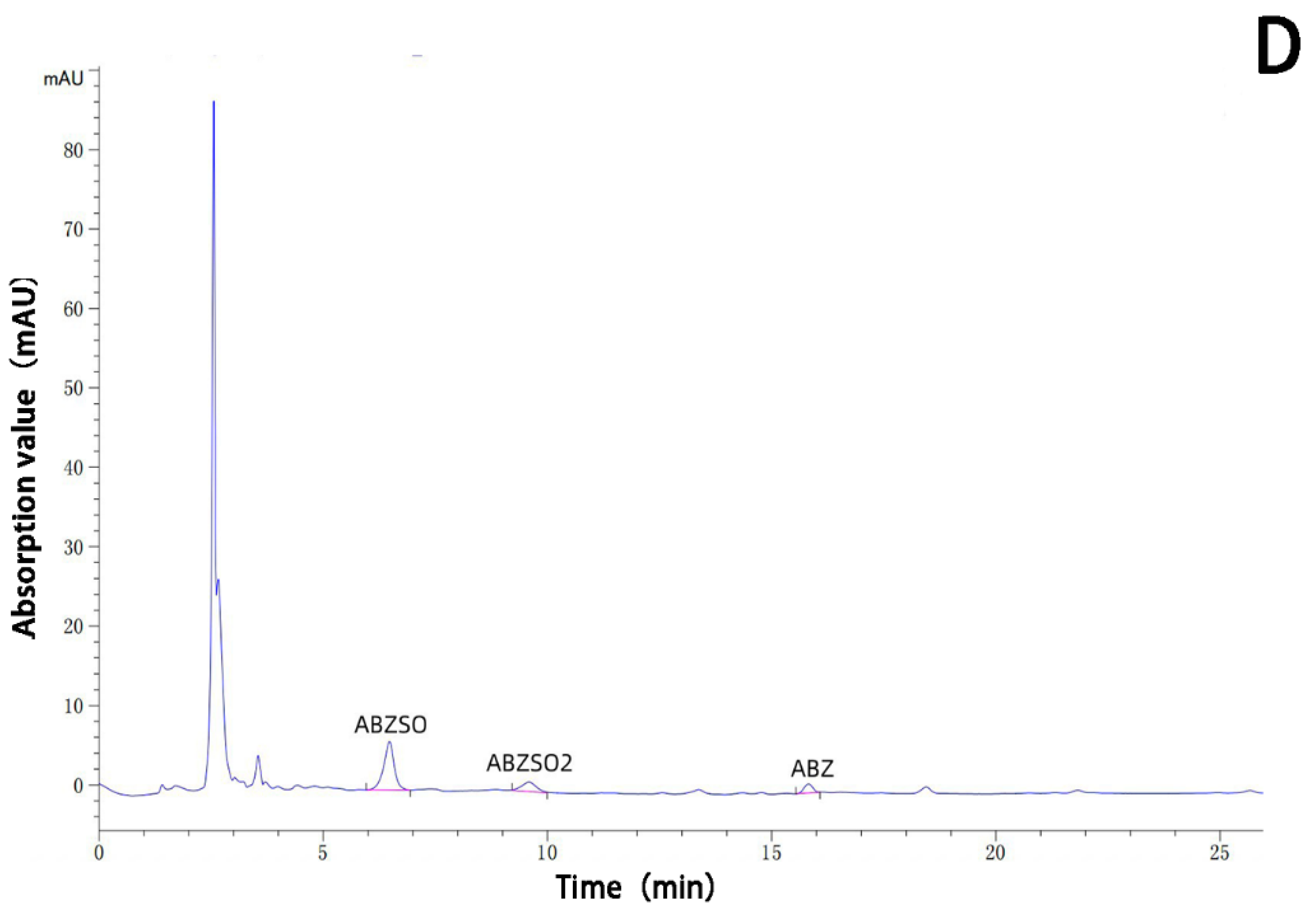
The albendazole, albendazole sulfoxide, and albendazole sulfone in blank dog plasma and the blank dog plasma were analyzed using HPLC and are shown in Figure 10. Under the HPLC conditions we used, the blank dog plasma did not interfere with the detection of albendazole, albendazole sulfoxide, and albendazole sulfone in the dog’s plasma.
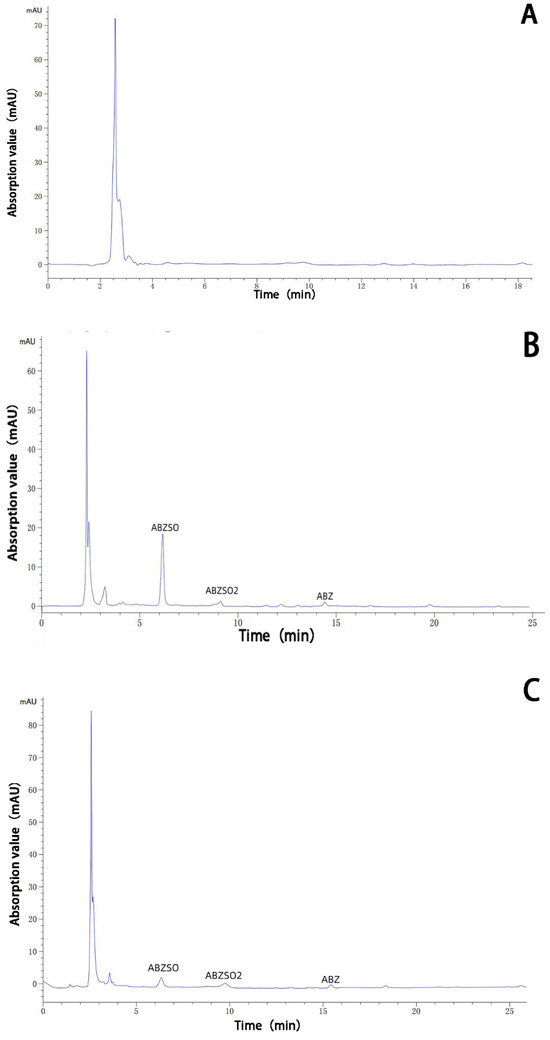
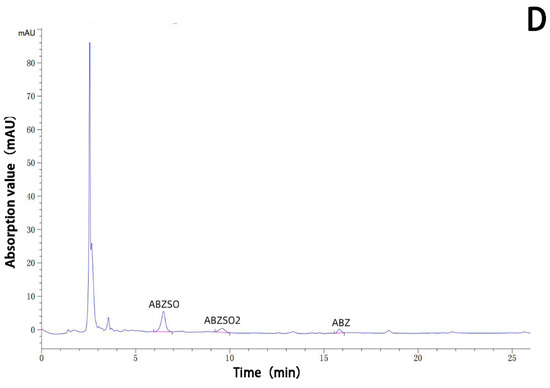
Figure 10.
HPLC analysis of the sample of the blank dog plasma (A), the dog plasma containing albendazole, albendazole sulfoxide, and albendazole sulfone (B), the plasma from dogs dosed with albendazole (C), and the plasma from dogs dosed with the complex (D).
The recovery rate and intra-assay coefficient of variation of the variations in albendazole, albendazole sulfoxide, and albendazole sulfone in the blank plasma with concentrations of 2 μg/mL, 5 μg/mL, and 10 μg/mL were determined and summarized as follows:
| recovery rate | intra-assay coefficient of variation | |
| Albendazole | 90.2 ± 6.1% to 109.4 ± 4.8% | 2.2% to 3.62% |
| Albendazole sulfoxide | 89.8 ± 3.6% to 117.2 ± 2.2% | 1.81% to 2.24% |
| Albendazole sulfone | 93.9 ± 4.1% to 103.2 ± 3.1% | 2.78% to 5.49% |
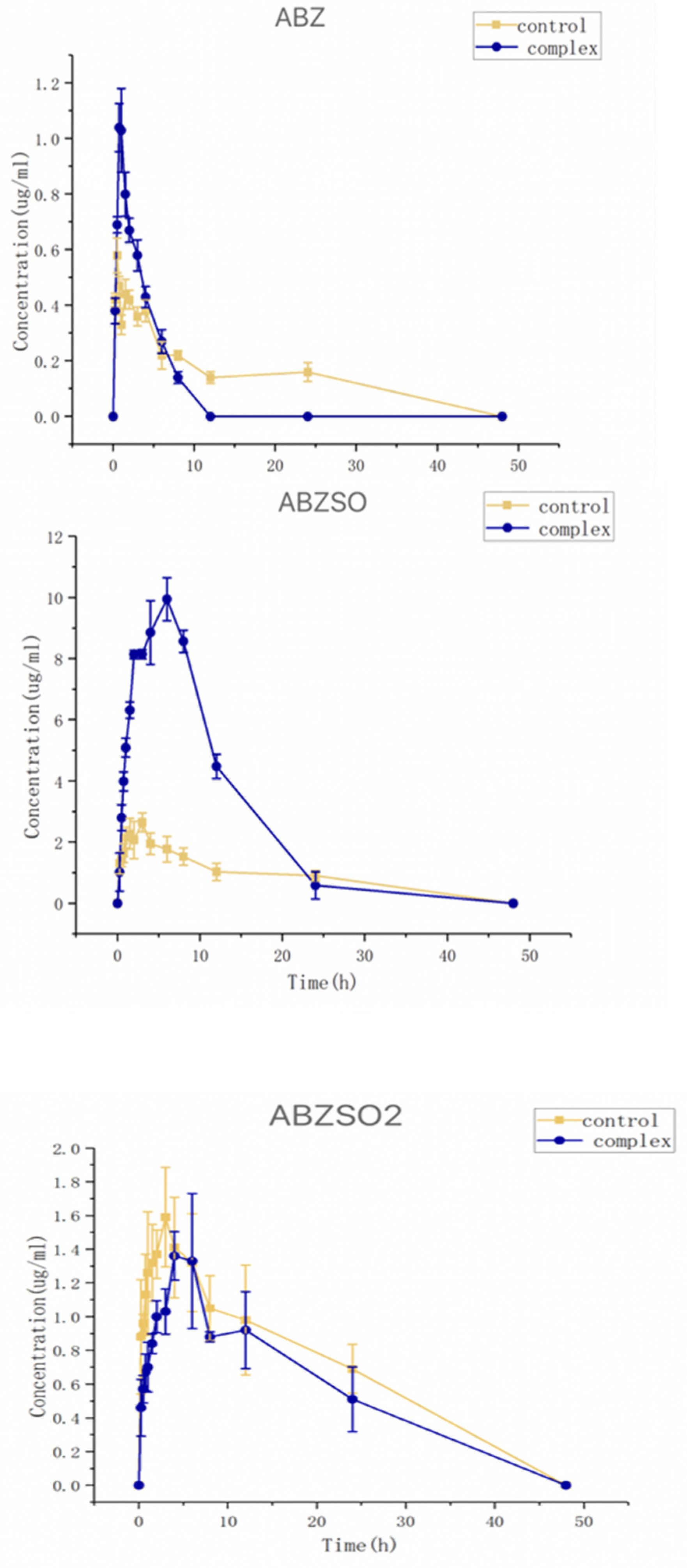
The collected plasma samples from dogs dosed with albendazole or its complex were analyzed using HPLC. The relationships between the concentrations of albendazole, albendazole sulfoxide, or albendazole sulfone in the dog plasma and time are shown in Figure 11. The pharmacokinetic parameters (mean ± SD) were calculated by using a noncompartmental analysis by Phoenix WinNonlin software (version 8.3) and are summarized in Table 1.
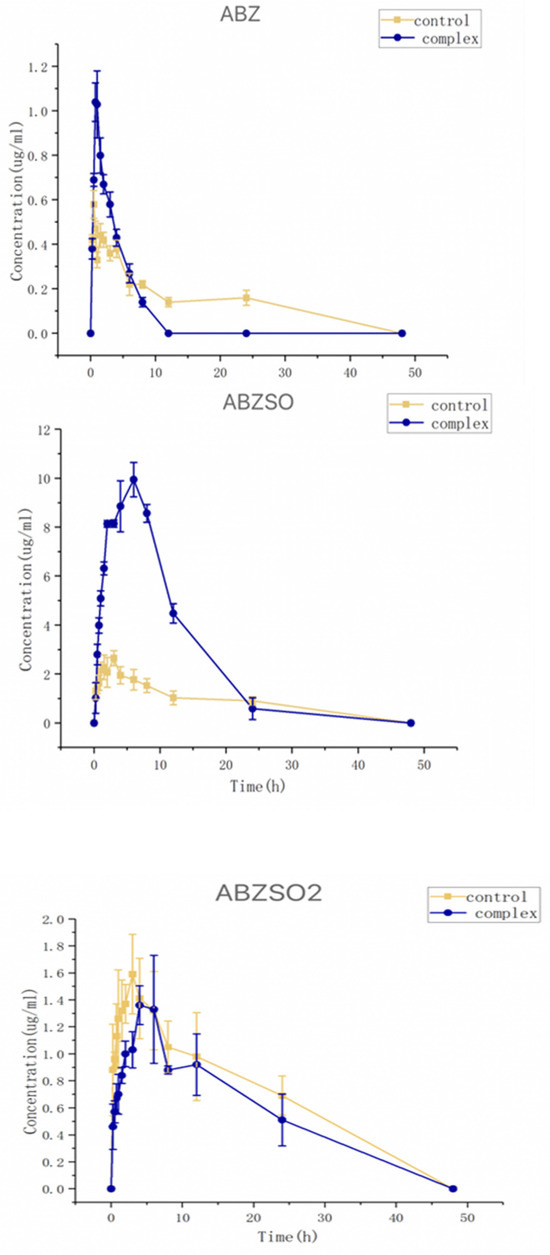
Figure 11.
Curves of concentrations of albendazole, albendazole sulfone, and albendazole sulfoxide in plasma from dogs administered with albendazole or its methyl-β-cyclodextrin complex.

Table 1.
Pharmacokinetic parameters of albendazole, albendazole sulfoxide, and albendazole sulfone (mean ± SD) in albendazole group and complex group.
After oral administration, the albendazole was partially metabolized to sulfoxide and albendazole sulfone, and the albendazole sulfoxide was the anthelmintic active substance. The Cmax and Tmax of the albendazole sulfoxide in the dog plasma were found to be 10.18 μg/mL at 6.0 h for the complex group and 2.81 μg/mL at 3.0 h for the albendazole group. Through complexation, the Cmax of albendazole sulfoxide was increased by 3.62 times, and the delayed Tmax could require less frequent dosing within the clinically effective therapeutic range. The AUC0–48 of albendazole sulfoxide changed from 0.72 h⁎μg/mL in the albendazole group to 119.95 h⁎μg/mL in the complex group to make the relative bioavailability as high as 236%; the Cmax of albendazole was increased by 2.11 times (from 0.51 μg/mL at 0.5 h to 1.08 μg/mL at 0.75 h) and the AUC0–48 of albendazole was decreased from 9.92 h⁎μg/mL to 3.92 h⁎μg/mL; and the Cmax of albendazole sulfone was decreased from 1.63 μg/mL at 4 h to 1.16 μg/mL at 4 h and the AUC0–48 of albendazole sulfone was decreased from 34.66 h⁎μg/mL to 22.50 h⁎μg/mL.
Through complexation with methyl-β-cyclodextrin, the AUC0–48 of the active substance albendazole sulfoxide in vivo was doubled, the Cmax was more than tripled, and the Tmax was doubled, indicating that the inclusion complex of albendazole with methyl-β-cyclodextrin is the best formulation so far of albendazole as a potential anti-anthelmintic and anti-tumor agent.
3. Methods and Materials
Methyl-β-cyclodextrin (average molecular weight: 1303), hydroxypropyl-γ-cyclodextrin, albendazole, albendazole sulfoxide standard (98%), and albendazole sulfone (98%) were obtained from Shanghai Macklin, Shanghai, P. R. China; citric acid, HP-β-cyclodextrin, and β-cyclodextrin were obtained from Sa’en Chemistry Technology, Guangzhou, Guangdong, P. R. China; anhydrous sodium acetate was purchased from Xilong Science Co., Ltd., Shantou, Guangdong, P. R. China; dogs were obtained from Guangdong Medical Laboratory Animal Center, Guangzhou, Guangdong, P. R. China; blank dog plasma was obtained from Guangzhou Rui-Te Co., Ltd., Guangzhou, Guangdong, P. R. China; low-fat dog foods were obtained from Shanghai Jibai Chong Industrial Co., Ltd., Shanghai, P. R. China.
3.1. Albendazole’s UV Maximum Absorbance Wavelength Determination
The solution of albendazole (10 mg) in acetic acid (5 mL) was diluted with ethanol (95%, 95 mL), 5 mL of the solution was diluted with ethanol (95%, 45 mL), and the resulting solution was used for UV (ultraviolet visible spectrophotometer, J51903001 from Shanghai Jinghua Technology Instrument Co., Ltd., Shanghai, P. R. China) scanning in the range of 200–400 nm.
3.2. Establishment of Albendazole HPLC Standard Curve
A series of methanolic solutions of albendazole at concentrations of 0.01 mg/mL, 0.02 mg/mL, 0.04 mg/mL, 0.06 mg/mL, 0.08 mg/mL, and 0.1 mg/mL were analyzed using HPLC (LC-15C high-performance liquid chromatograph from Shimadzu Enterprise Management Co., Ltd., Kyoto, Japan) with a UV detector (295 nm) on a Shim-pack VP-ODS C18 column (250 mm × 4.6 mm) using acetonitrile/water (30:70, v/v) as a mobile phase with a 1.0 mL/min flow rate. Based on the peak areas in the HPLC peaks and the concentrations, the linear regression equation and HPLC standard curve were obtained.
3.3. Preparation of Citrate-β-cyclodextrin
Citrate-β-cyclodextrin was prepared by using the procedure reported in the literature [56].
3.4. Solubility of Albendazole in Different Cyclodextrin Solutions
Excess albendazole was added to the solutions of β-cyclodextrin, HP-β-cyclodextrin, methyl-β-cyclodextrin, citrate-β-cyclodextrin, γ-cyclodextrin, or HP-γ-cyclodextrin in water at 5, 10, 15, 20, 25, and 30 mmol/mL concentrations. Each solution was stirred at room temperature for 48 h, filtered, diluted with water, and analyzed using HPLC.
3.5. Preparation of the Inclusion Complexes of Albendazole with Methyl-β-cyclodextrin
Method using sonication: the solution of albendazole (150 mg) in acetic acid (5 mL) was added to the solution of methyl-β-cyclodextrin (750 mg) in water (10 mL) with stirring uing a magnetic stirrer (RCT digital was obtained from IKA). The resulting solution was ultrasonicated in an ultrasonic reactor (JY92-IIN from Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo, Zhejiang, China) at 25 °C and 520 W for 1 h with occasional iced water cooling, refrigerated at 4 °C for a few hours, evaporated, dissolved in water (15 mL), filtered through a 0.22 µm filter membrane. and freeze-dried at around −45 °C under a pressure of 32 pa for 48–72 h to give a clathrate solid powder.
Solution method: the solution of albendazole (150 mg) in acetic acid (5 mL) was added to the solution of methyl-β-cyclodextrin (750 mg) in water (10 mL). The mixture was stirred at 50 °C and 800 rpm for 6 h, refrigerated at 4 °C for several hours, evaporated, dissolved in water (15 mL), filtered through a membrane (0.22 µm), and freeze-dried at around −45 °C under a pressure of 32 pa for 48–72 h to give the clathrate solid powder.
Using an autoclave: the mixture of albendazole (150 mg) and methyl-β-cyclodextrin (750 mg) in water and acetic acid (30 mL, 67:33) was stirred at 120 °C and 800 rpm for 8 h in a high-temperature and pressure reaction kettle (BZ-100ML/SC-L from Shanghai Baikal Technology Group Co., Ltd., Shanghai, China), evaporated, added to water, filtered, and freeze-dried at around −45 °C under a pressure of 32 pa for 48–72 h to give the product.
Using a microwave reactor: the solution of albendazole (150 mg) in acetic acid (5 mL) was mixed with the solution of methyl-β-cyclodextrin (750 mg) in water (10 mL), and the resulting mixture was stirred at 300 rpm in a microcomputer microwave chemical reactor (WBFY-201 from Gongyi Yuhua Instrument Co., Ltd., Xinxiang, Henan, China) for 1 h. During the reaction, every 10 min of heating was followed by 5 min. of no heating. After refrigeration at 4 °C for several hours and evaporation, the residue was dissolved in water (15 mL), filtered (0.22 µm), and freeze-dried at around −45 °C under a pressure of 32 pa for 48–72 h to provide the solid powder.
Using supercritical CO2: the solution of albendazole (150 mg) in glacial acetic acid (5 mL) was mixed with the solution of methyl-β-cyclodextrin (750 mg) in water (10 mL), pumped with liquid CO2 in the reactor (BZ-100ML/S0-L from Baikal Shanghai Intelligent Technology Co., Ltd., Shanghai, China), and stirred at 25 °C with a pressure of 6.5 Mpa for 12 h. After the pressure was slowly released, the solution was kept in a refrigerator at 4 °C for a few hours, the solvent was removed via rotary evaporation, the residue was dissolved in water (15 mL), and, after filtration through a 0.22 μm filter membrane and freeze-drying at around −45 °C under a pressure of 32 pa for 48–72 h, the product was obtained as a solid powder.
3.6. The Preparation of the Physical Mixture of Albendazole and Methyl-β-cyclodextrin
The grounded albendazole and methyl-β-cyclodextrin were mixed (1:3 molar ratio) and passed through the 80-mesh sieve to provide the physical mixture.
3.7. Preparation of the Inclusion Complexes with Addition of Trace Amount of Water-Soluble Polymer or Organic Salt
The solution of albendazole (150 mg) in acetic acid (5 mL) was added to the solution of methyl-β-cyclodextrin (750 mg) in water (10 mL), 5.6 mg of water-soluble polymer or organic salts (0.25% of albendazole) was added, and after stirring for 30 min, the resulting solution was ultrasonicated at 25 °C and 520 W for 40 min with occasional ice cooling, refrigerated at 4 °C for a few hours, evaporated, dissolved in water (15 mL), filtered through a 0.22 µm filter membrane, and freeze-dried at around −45 °C under a pressure of 32 pa for 48–72 h to give a clathrate solid powder. The inclusion complex from the ultrasonic method with the addition of a trace amount of sodium dodecyl sulfate was used for the FTIR, DSC, NMR, and in vitro and in vivo PK study.
3.8. Fourier-Transform Infrared Spectroscopy Study
The dried samples of albendazole, methyl-β-cyclodextrin, their physical mixture, and inclusion complex were ground in an agate mortar, respectively, on a VERTEX 70 Fourier transform infrared spectrometer (From Bruker company, Leipzig, Germany). Each of them was mixed with spectrally pure potassium bromide under an infrared lamp, poured into a compression mold, and pressed to provide the KBr pellets for the Fourier-transform infrared spectroscopy analysis in the range of 400–4000 cm−1.
3.9. Differential Scanning Calorimetry Study
The differential scanning calorimetry curves of albendazole, methyl-β-cyclodextrin, their mixture, and inclusion compound were recorded on a DSC 214 differential scanning calorimeter (from Netzsch, Bobingen, Germany) by placing them in an aluminum crucible, heating at a rate of 10 °C/min in the scan range from 25 to 300 °C under a nitrogen atmosphere (150 mL/min). Al2O3 was used as a reference.
3.10. Proton NMR Study
The proton NMR spectra of the solutions of methyl-β-cyclodextrin, its inclusion complex with albendazole in D2O, the solutions of albendazole, and its inclusion complex in DMSOd6 were recorded on a Bruker spectrometer (400 MHz). The Roesy spectrum of the complex recorded in D2O was recorded on a Bruker spectrometer (600 MHz).
3.11. Determination of the Albendazole Content in the Complex
The inclusion complex (100 mg) was dissolved in water (1 mL) with stirring for 2 h, and the solution was diluted with water to the range of 10 μg/mL–100 μg/mL (the albendazole HPLC standard curve was established in this range). Based on the HPLC analysis, the albendazole content in the complex was calculated using the regression equation.
3.12. Determination of Water Solubility of Albendazole in the Complex
An excess amount of the inclusion complex was dissolved in water (1 mL) for 2 h to obtain a saturated aqueous solution; after filtration, the sample was ready for HPLC analysis.
3.13. Inclusion Rate and Inclusion Yield Determination
The inclusion rate and yield of the inclusion compound were calculated by using the methods shown below:
Inclusion yield (%) = [albendazole inclusion complex (mg)/added albendazole (mg) + methyl-β-cyclodextrin (mg)] × 100%
Inclusion ratio (%) = [albendazole in inclusion complex (mg)/added albendazole (mg)] × 100%.
3.14. Dissolution Rate Determination
The paddle method in Chinese Veterinary Pharmacopoeia 2010 Edition I was adopted, albendazole (50 mg), the albendazole inclusion compound (containing 50 mg of albendazole), and the physical mixture of albendazole and methyl-β-cyclodextrin (containing 50 mg of albendazole) in degassed 0.1 mol/L hydrochloric acid (900 mL) were stirred at 50 r/min at 37 ± 0.5 °C, respectively, on an RC-3 dissolution meter (basket USP Apparatus 1 from Tianjin Xintianguang Analytical Instrument Technology Co., Ltd., Tianjin, China). In total, 5 mL was taken from each of the mixtures at 1, 3, 5, 10, 15, 30, 45, 60, and 75 min, followed by the addition of 5 mL of the same dissolution medium at the same times. After filtration through a 0.22 μm microporous membrane for 30 s, the sample was analyzed using HPLC at a wavelength of 295 nm, and, based on the HPLC data, the dissolution rate curves were obtained.
3.15. In Vivo Pharmacokinetic Studies
Wenzhou-kean university approved the study and the ID of the ethics approval is issued as WKULAEC2023-00.
Twelve healthy adult dogs (half males and half females, 5 ± 0.1 kg) were grouped as the albendazole group and the complex group, weighed, numbered, fed with low-fat food and normal drinking water in a warm and ventilated environment for 1 week, fasted for one day, and orally dosed with albendazole or albendazole complex in water (25 mg/kg for albendazole), respectively.
Blood samples (2 mL) were collected through the forearm vein after the administration of the drugs at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 48 h, transferred immediately to a heparinized test tube, centrifuged at 2000 rpm for 10 min, and stored at −20 °C in a freezer.
The thawed plasma sample (0.5 mL) was pipetted into a centrifuge tube (2 mL), added with sodium thiosulfate (200 μg) and ethyl acetate (1 mL), vortexed for 5 min, centrifuged (13,000 rpm) for 10 min, and transferred to a test tube. After repeated vortexing and centrifugation, the supernatant in the test tube was blown at 40 °C in a nitrogen blower to dryness, added with methanol (0.5 mL), vortexed for 5 min, centrifuged (13,000 r/min) for 10 min, and filtered with a 0.22 μm microporous membrane for HPLC analysis.
3.16. Standard Curves for Albendazole, Albendazole Sulfoxide, and Albendazole Sulfone in Plasma
The blank plasma (0.5 mL) in a plastic centrifuge tube was added to the solutions of albendazole, albendazole sulfoxide, or albendazole sulfone at concentrations of 5 μg/mL, 1 μg/mL, 0.5 μg/mL, 0.1 μg/mL, 0.05 μg/mL, and 0.025 μg/mL, vortexed and vibrated, extracted with ethyl acetate or dichloromethane, blown with nitrogen, dissolved in methanol (1 mL), and analyzed using high-performance liquid chromatography at 295 nm using a Shim-packVP-ODS C18 chromatographic column (250 L × 4.6 mm) to provide the standard curves.
3.17. Statistical Analysis
Each experiment was performed in triplicate, and the results were expressed as the mean ± standard deviation. The statistical analysis was performed through a one-way analysis of variance followed by Tukey’s honestly significant difference test using DPS software. Statistical significance was defined as p < 0.05.
4. Conclusions
The inclusion complex of albendazole with methyl-β-cyclodextrin prepared by using the ultrasonic method with the addition of a trace amount of sodium dodecyl sulfate could increase the water solubility of albendazole by around 150,000 times compared to albendazole alone. In its in vivo pk study, the Cmax and Tmax of the active metabolized substance sulfoxide was changed from 2.81 μg/mL at 3 h to 10.2 μg/mL at 6 h, and the AUC0–48 was increased from 50.72 h⁎μg/mL to 119.95 h⁎μg/mL, indicating that this inclusion complex can be used as a new potent oral therapeutic anti-anthelmintic and anti-tumor agent.
Author Contributions
Z.Z., C.D., S.X. and Z.X. did the research work, Z.X., C.D. and Y.D. were working on manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
The research project was funded by the “Innovation and Entrepreneurship Team Project” of the Blue Ocean Talents Program in Shishan Town, Nanhai District, Foshan City, Guangdong Province, for a period of three years (2019–2021, the ninth batch, funding number: 201804300016).
Institutional Review Board Statement
Wenzhou-Kean University approved the study. The ID of the ethics approval is WKULAEC2023-00.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Horton, J. Albendazole: A broad spectrum anthelminthic for treatment of individuals and populations. Curr. Opin. Infect. Dis. 2002, 15, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ehteda, A.; Galettis, P.; Pillai, K.; Morris, D.L. Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice. BMC Cancer 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Medina, L.; Garcia, L.; Fuentes, I.; Esparza, M.R. Absorption studies of albendazole and some physicochemical properties of the drug and its metabolite albendazole sulphoxide. J. Pharm. Pharmacol. 1998, 50, 43–48. [Google Scholar] [CrossRef]
- Chai, J.Y.; Jung, B.K.; Hong, S.J. Albendazole and mebendazole as anti-parasitic and anti-cancer agents: An update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Martin, J.H.; Mclachlan, A.J.; Boddy, A.V. Precision dosing of targeted anticancer drugs—Challenges in the real world. Transl. Cancer Res. 2017, 6 (Suppl. S10), S1500–S1511. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, S.; Chai, J.; Liang, B.; Fu, C.; Shen, W.; Peter, H. Albendazole-soybean oil emulsion for the treatment of human cystic echinococcosis: Evaluation of bioavailability and bioequivalence. Acta Tropica 2002, 83, 177–181. [Google Scholar]
- Del Estal, J.L.; Alvarez, A.I.; Villaverde, C.; Coronel, P.; Fabra, S.; Prieto, J.G. Effect of surfactants on Albendazole absorption. J. Pharm. Biomed. Anal. 1991, 9, 1161–1164. [Google Scholar] [CrossRef]
- Maqbool, F.; Moyle, P.M.; Tan, M.S.A.; Thurecht, K.J.; Falconer, J.R. Preparation of albendazole-loaded liposomes by supercritical carbon dioxide processing. Artif. Cells Nanomed. Biotechnol. Int. J. 2018, 46, S1186–S1192. [Google Scholar] [CrossRef]
- Lopez, M.L.; Torrado, S.; Martínez, A.R.; Bolás, F. Improvement of Albendazole Efficacy against Enteral, but Not against Parenteral Stages of Trichinella spiralis by Preparing Solid Dispersions in Polyvinylpyrrolidone. Chemotherapy 1997, 43, 430–435. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, J.; Zhang, H.; Wang, J.; Ma, Y. Determination of Equilibrium solubility of albendazole and its Apparent Oil-Water partition coefficient. China Pharm. 2015, 12, 3092–3095. [Google Scholar]
- Kasetti, Y.; Bharatam, P.V. Tautomerism in drugs with benzimidazole carbamate moiety: An electronic structure analysis. Theor. Chem. Acc. 2012, 131, 1160. [Google Scholar] [CrossRef]
- Pranzo, M.B.; Cruickshank, D.; Coruzzi, M.; Caira, M.R.; Bettini, R. Enantiotropically related albendazole polymorphs. J. Pharm. Sci. 2010, 99, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Priotti, J.; García, A.; Leonardi, D.; Ferreira, M.J.; Lamas, M.C.; Nunes, T.G. Succinyl-β-cyclodextrin: Influence of the substitution degree on albendazole inclusion complexes probed by NMR. Mater. Sci. Eng. C 2018, 92, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Moriwaki, C.; Costa, G.L.; Ferracini, C.N.; de Moraes, F.F.; Zanin, G.M.; Pineda, E.A.G.; Matioli, G. Enhancement of solubility of albendazole by complexation with β-cyclodextrin. Braz. J. Chem. Eng. 2008, 25, 255–267. [Google Scholar] [CrossRef]
- Patel, V.P.; Parikh, R.K.; Gohel, M.C.; Desai, T.R.; Bhimani, D.R.; Tirgar, P.R. In Vitro Dissolution enhancement of albendazole by preparation of inclusion complex with HP-β-Cyclodextrin. Pharma Sci. Monit. Int. J. Pharm. Sci. 2011, 2, 161–173. [Google Scholar]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–557. [Google Scholar] [CrossRef]
- Bassani, V.L.; Krieger, D.; Duchene, D.; Wouessidjewe, D.; Szejtli, J.; Szente, L. Proceedings of the Eighth International Symposium on Cyclodextrons; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 321–324. [Google Scholar]
- Castillo, J.A.; Palomo-Canales, J.; Garcia, J.J.; Lastres, J.L.; Bolas, F. Preparation and characterization of albendazole β-cyclodextrin complexes. Drug Dev. Ind. Pharm. 1999, 25, 1241–1248. [Google Scholar] [CrossRef]
- Anjana, M.N.; Jipnomon, J.; Sreeja, C.N. Solubility and bioavailability enhancement of albendazole by complexing with hydroxy propyl β cyclodextrin. J. Chem. Pharm. Res. 2015, 7, 1131–1141. [Google Scholar]
- Stepniak, A.; Buczkowski, A.; Zavodnik, L.; Belica-Pacha, S.; Palecz, B. Study of the interaction of β-cyclodextrin with albendazole in aqueous solutions. J. Mol. Liq. 2017, 248, 19–23. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Wangoo, K.T.; Morris, D.L. Albendazole-cyclodextrin complex: Enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 2008, 28, 2775–2780. [Google Scholar]
- Evrard, B.; Chiap, P.; DeTullio, P.; Ghalmi, F.; Piel, G.; Van Hees, T.; Crommen, J.; Losson, B.; Delattre, L. Oral bioavailability in sheep of albendazole from a suspension and from a solution containing hydroxypropyl-β-cyclodextrin. J. Control. Release 2002, 85, 45–50. [Google Scholar] [CrossRef]
- Ehteda, A.; Galettis, P.; Chu, S.W.L.; Pillai, K.; Morris, D.L. Complexation of albendazole with hydroxypropyl-β-cyclodextrin significantly improves its pharmacokinetic profile, cell cytotoxicity and antitumor efficacy in nude mice. Anticancer Res. 2012, 32, 3659–3666. [Google Scholar]
- Pillai, K.; Akhter, J.; Morris, D.L. Super aqueous solubility of albendazole in β-cyclodextrin for parenteral application in cancer therapy. J. Cancer 2017, 8, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Anjana, M.N. Formulation, in vitro and in vivo analysis of cyclodextrin complexed albendazole composites for enhanced solubility. Der Pharma Chem. 2018, 10, 41–50. [Google Scholar]
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Pradines, B.; Gallard, J.; Iorga, B.I.; Gueutin, C.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. Investigation of the complexation of albendazole with cyclodextrins for the design of new antiparasitic formulations. Carbohydr. Res. 2014, 398, 50–55. [Google Scholar] [CrossRef]
- Garcı’a, A.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Characterization of albendazole-randomly methylated β-cyclodextrin inclusion complex and in vivo evaluation of its antihelmitic activity in a murine model of trichinellosis. PLoS ONE 2014, 9, e113296. [Google Scholar]
- Ferreira, M.J.G.; García, A.; Leonardi, D.; Salomon, C.J.; Lamas, M.C.; Nunes, T.G. 13C and 15N solid-state NMR studies on albendazole and cyclodextrin albendazole complexes. Carbohydr. Polym. 2015, 123, 130–135. [Google Scholar] [CrossRef]
- Garcı, A.; Leonardi, D.; Salazar, M.O.; Lamas, M.C. Modified β-cyclodextrin inclusion complex to improve the physicochemical properties of albendazole complete in vitro evaluation and characterization. PLoS ONE 2014, 9, 88234. [Google Scholar]
- García, A.; Priotti, J.; Codina, A.V.; Vasconi, M.D.; Quiroga, A.D.; Hinrichsen, L.I.; Leonardi, D.; Lamas, M.C. Synthesis and characterization of a new cyclodextrin derivative with improved properties to design oral dosage forms. Drug Deliv. Transl. Res. 2019, 9, 273–283. [Google Scholar] [CrossRef]
- Rodrigues, L.N.C.; Tavares, A.C.M.; Ferreira, B.T.; Reis, A.K.C.A.; Katiki, L.M. Inclusion complexes and self-assembled cyclodextrin aggregates for increasing the solubility of benzimidazoles. Braz. J. Pharm. Sci. 2019, 55, 1–11. [Google Scholar] [CrossRef]
- Trandafirescu, C.; Ledeti, I.; Soica, C.; Ledeti, A.; Vlase, G.; Borcan, F.; Dehelean, C.; Coricovac, D.; Racoviceanu, R.; Aigner, Z. Albendazole-cyclodextrins binary systems. J. Therm. Anal. Calorim. 2019, 138, 3039–3054. [Google Scholar] [CrossRef]
- Pacheco, P.A.; Rodrigues, L.N.C.; Ferreira, J.F.S.; Gomes, A.C.P.; Veríssimo, C.J.; Louvandini, H.; Costa, R.L.D.; Katiki, L.M. Inclusion complex and nanoclusters of cyclodextrin to increase the solubility and efficacy of albendazole. Parasitol. Res. 2018, 117, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Rosas, M.D.; Piqueras, C.M.; Piva, G.K.; Veronica, M.; Cardozo, R.L.; Bucalá, F.V. Simultaneous formation of inclusion complex and microparticles containing albendazole and β-cyclodextrin by supercritical antisolvent co-precipitation. J. CO Util. 2021, 47, 101505. [Google Scholar] [CrossRef]
- Eriksen, J.B.; Christensen, S.B.; Bauer-Brandl, A.; Brandl, M. Dissolution/permeation of albendazole in the presence of cyclodextrin and bile salts: A mechanistic in vitro study into factors governing oral bioavailability. J. Pharm. Sci. 2022, 111, 1667–1673. [Google Scholar] [CrossRef]
- Ding, Y.; Prasad, C.V.N.S.V.; Ding, C.; Wang, B. Synthesis of carbohydrate conjugated 6A,6D-bifunctionalized β-cyclodextrin derivatives as potential liver cancer drug carriers. Carbohydr. Poly. 2018, 181, 957–963. [Google Scholar] [CrossRef]
- Ding, Y.; Pang, Y.; Prasad, C.V.N.S.V.; Wang, B. Formation of inclusion complex of enrofloxacin with 2-hydroxypropyl-β-cyclodextrin. Drug Deliv. 2020, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cui, W.; Pang, Y.; Prasad, C.V.N.S.V.; Wang, B. Preparation of inclusion complex of praziquantel with 2-hydroxypropyl-β-cyclodextrin and pharmacokinetic property improvement. Arab. J. Chem. 2021, 14, 103307. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, B.; Zhang, J.; Ding, C.; Zhang, Z.; Xu, S.; Li, L.; Yu, H. Tilmicosin/γ-cyclodextrin complexation through supercritical carbon dioxide assistance and its pharmacokinetic and anti-bacterial study. Euro. J. Pharm. Biopharm. 2022, 181, 104–112. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, B.; Zhou, S.; Ding, C.; Zhang, Z.; Xu, S.; Xu, Z. Improvement of solubility and pharmacokinetic profile of hepatoprotector icariin through complexation with HP-γ-cyclodextrin. Front. Pharmacol. 2023, 14, 1138686. [Google Scholar] [CrossRef]
- De Marco, F.I. Preparation of non-steroidal anti-inflammatory drug/β-cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Util. 2021, 44, 101397. [Google Scholar]
- Wang, C.; Yan, T.; Yan, T.; Wang, Z. Fabrication of hesperetin/hydroxypropyl-β-cyclodextrin complex nanoparticles for enhancement of bioactivity using supercritical antisolvent technology. J. Mol. Struct. 2023, 1279, 134947. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Marathe, S.A.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Formulating ternary inclusion complex of sorafenib tosylate using β-cyclodextrin and hydrophilic polymers: Physicochemical characterization and in vitro assessment. AAPS PharmSciTech 2022, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Mohandoss, S.; Velu, K.S.; Stalin, T.; Ahmad, N.; Alomar, S.Y.; Lee, Y.R. Tenofovir antiviral drug solubility enhancement with β-cyclodextrin inclusion complex and in silico study of potential inhibitor against SARS-CoV-2 main protease (Mpro). J. Mol. Liq. 2023, 377, 121544. [Google Scholar] [CrossRef]
- Huang, Y.; Zu, Y.; Zhao, X.; Wu, M.; Feng, Z.; Deng, Y.; Zu, C.; Wang, L. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016, 511, 921–930. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Couto, A.R.S. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Dayan, A.D. Albendazole, mebendazole, and praziquantel: Review of nonclinical toxicity and pharmacokinetics. Acta Trop. 2003, 86, 141–159. [Google Scholar] [CrossRef]
- Arroyo, G.; Bustos, J.A.; Lescano, A.G.; Gonzales, I.; Saavedra, H.; Rodriguez, S.; Pretell, D.E.J.; Bonato, P.S.; Lanchote, V.L.; Takayanagui, O.M.; et al. Albendazole sulfoxide plasma levels and efficacy of antiparasitic treatment in patients with parenchymal neurocysticercosis. Clin. Infect. Dis. 2019, 69, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121, S113–S312. [Google Scholar] [CrossRef] [PubMed]
- Chaleawlert-umpon, S.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Ruktanonchai, U.R.; Sajomsang, W.; Pimpha, N. Effect of citrate spacer on mucoadhesive properties of a novel water-soluble cationic β-cyclodextrin-conjugated chitosan. Carbohydr. Poly. 2011, 84, 186–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).