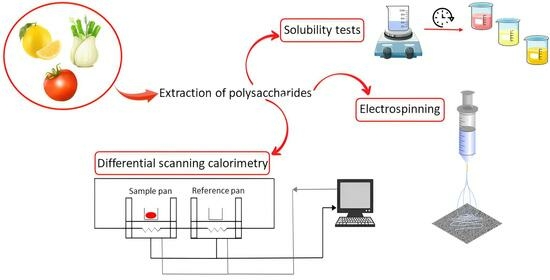
Physico-Chemical Properties and Valorization of Biopolymers Derived from Food Processing Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Polysaccharides from Fennel, Lemon and Tomato Waste
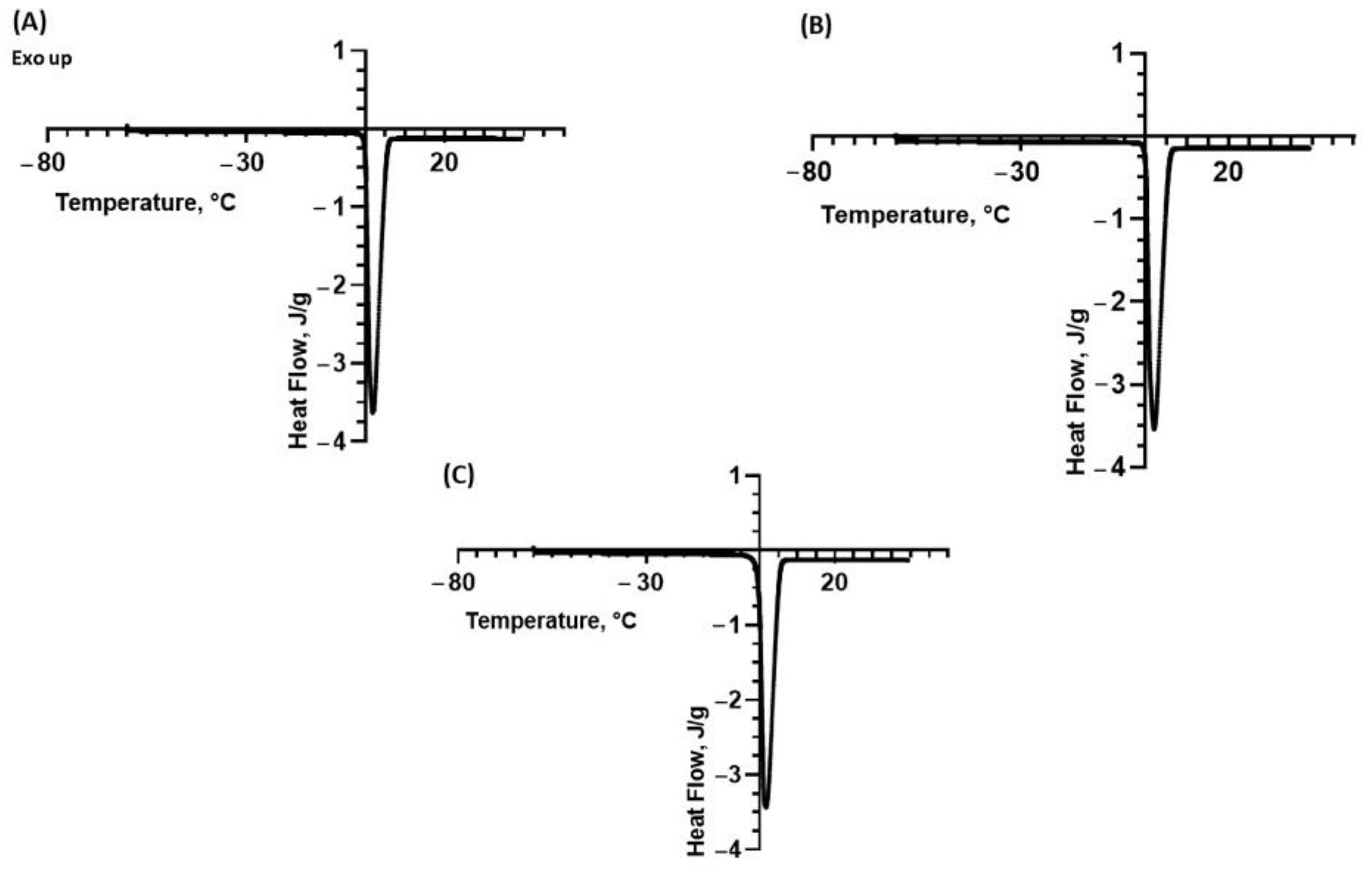
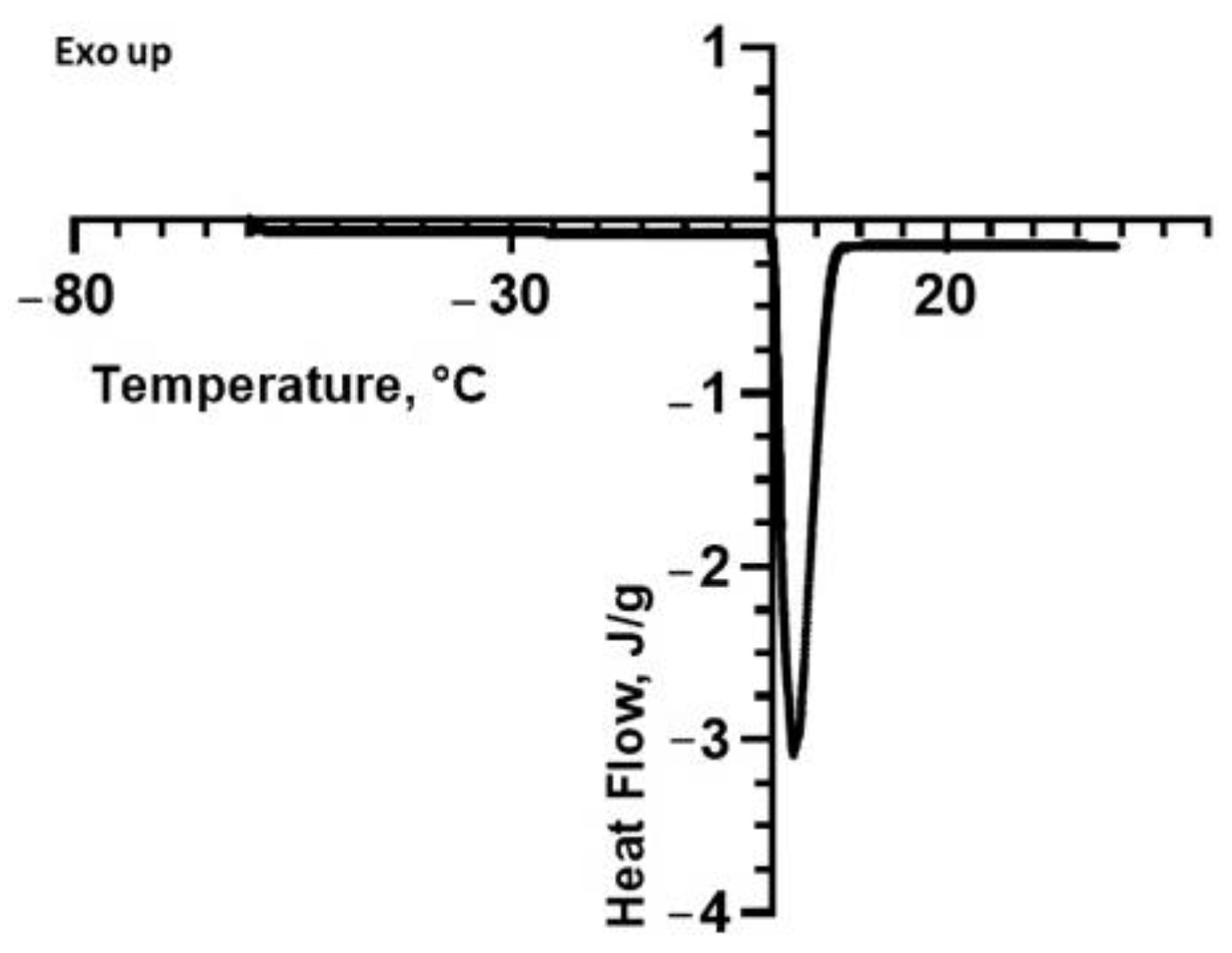
2.2. Differential Scanning Calorimetry (DSC)
2.3. Solubility Test
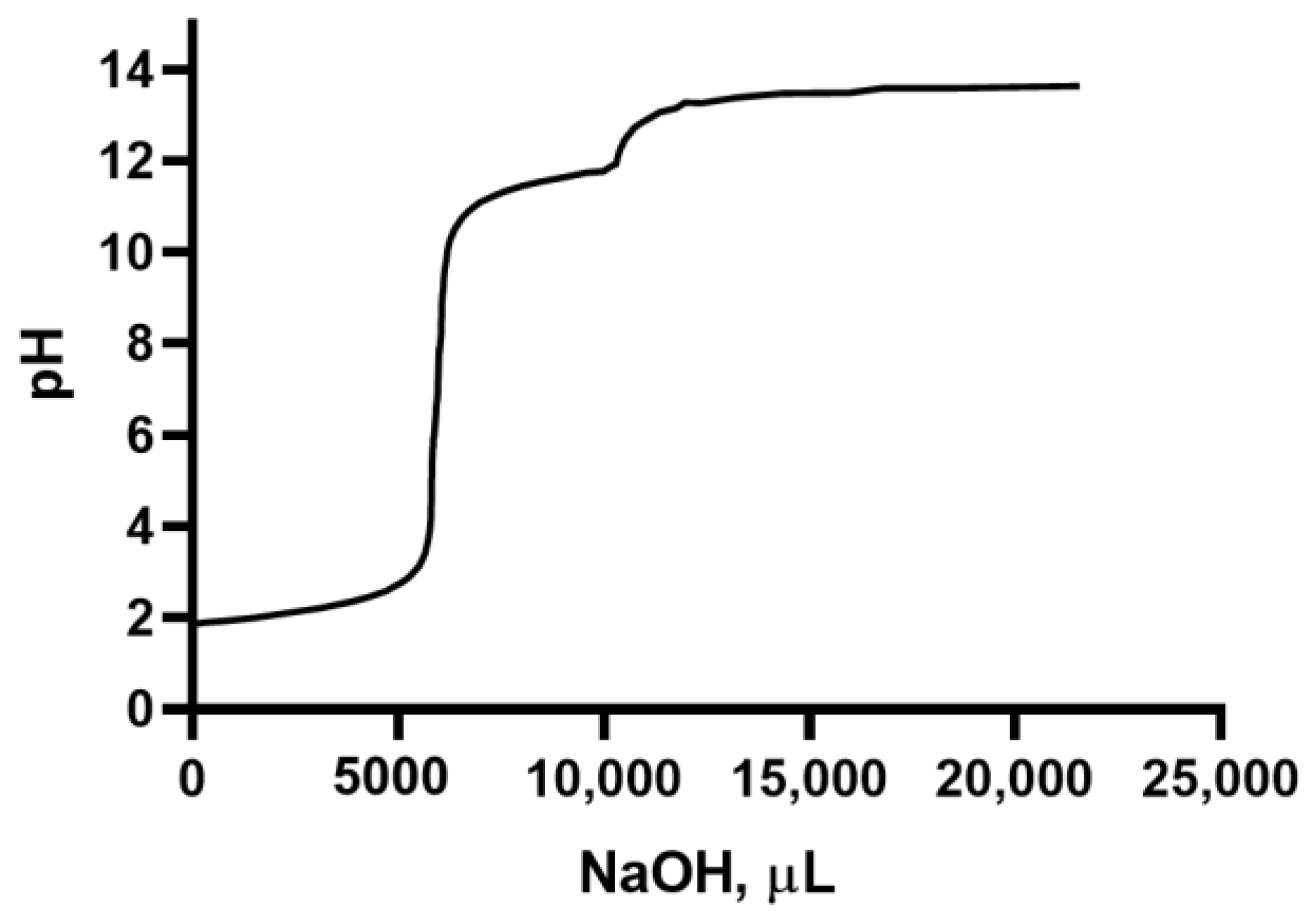
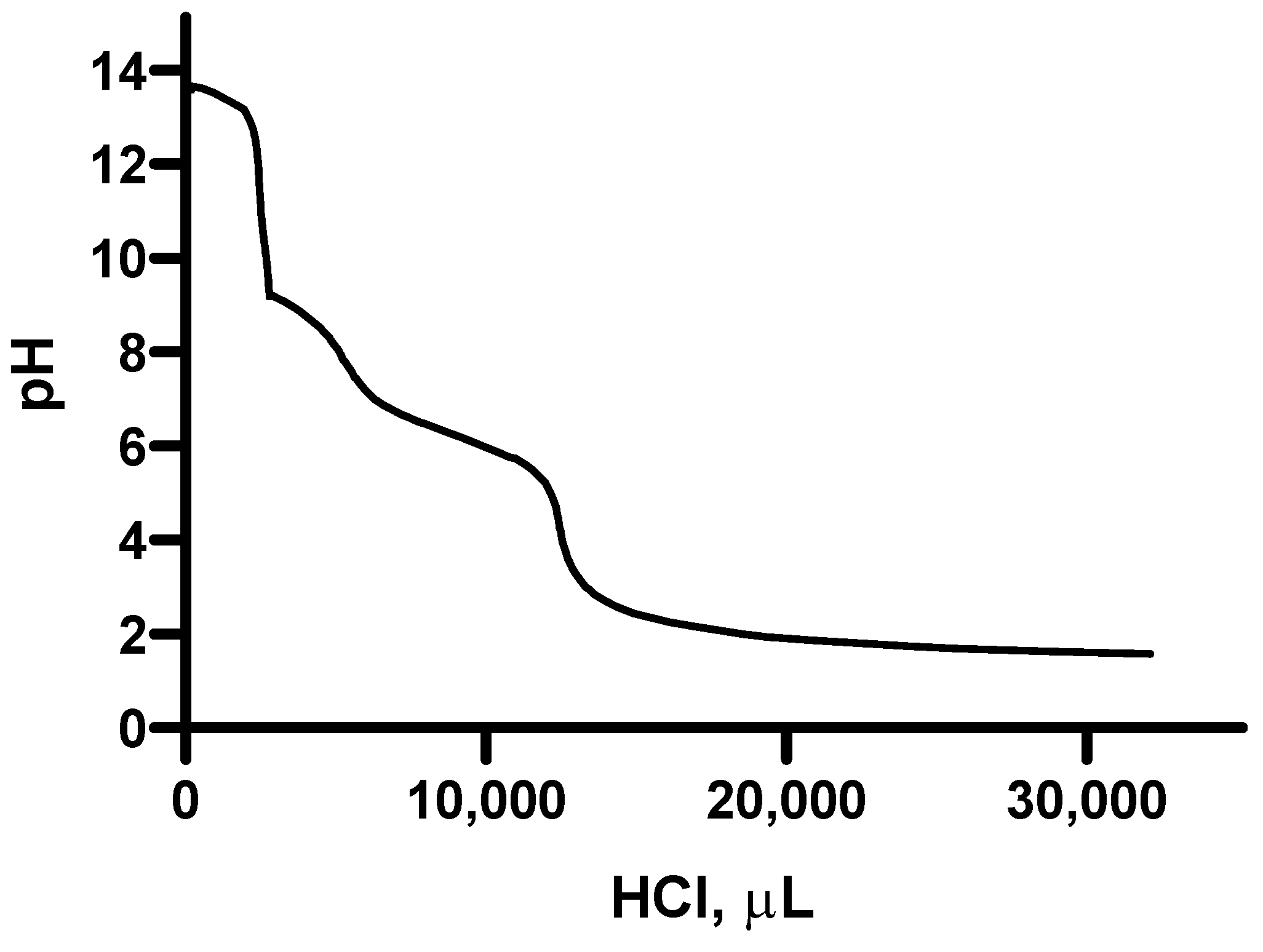
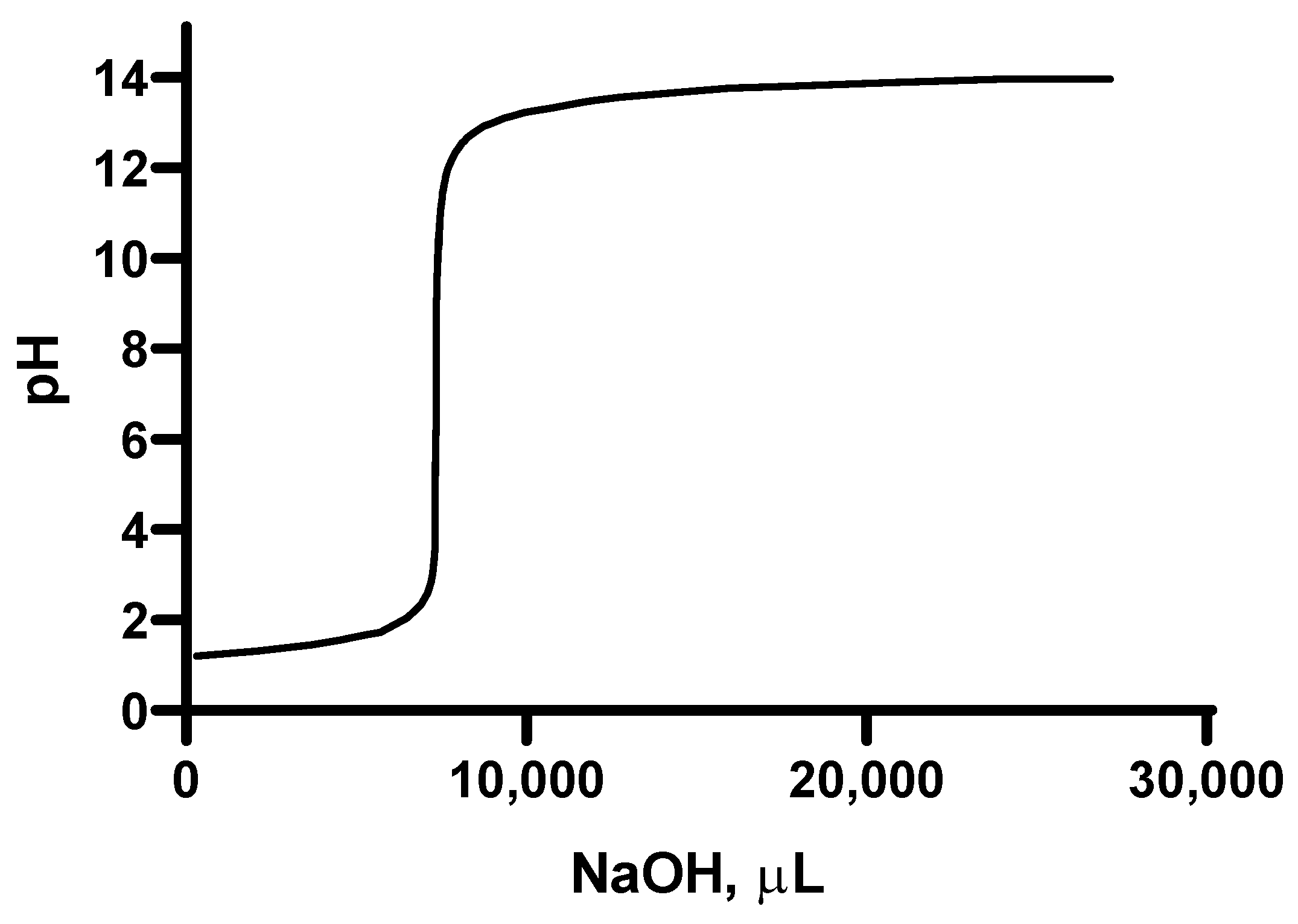
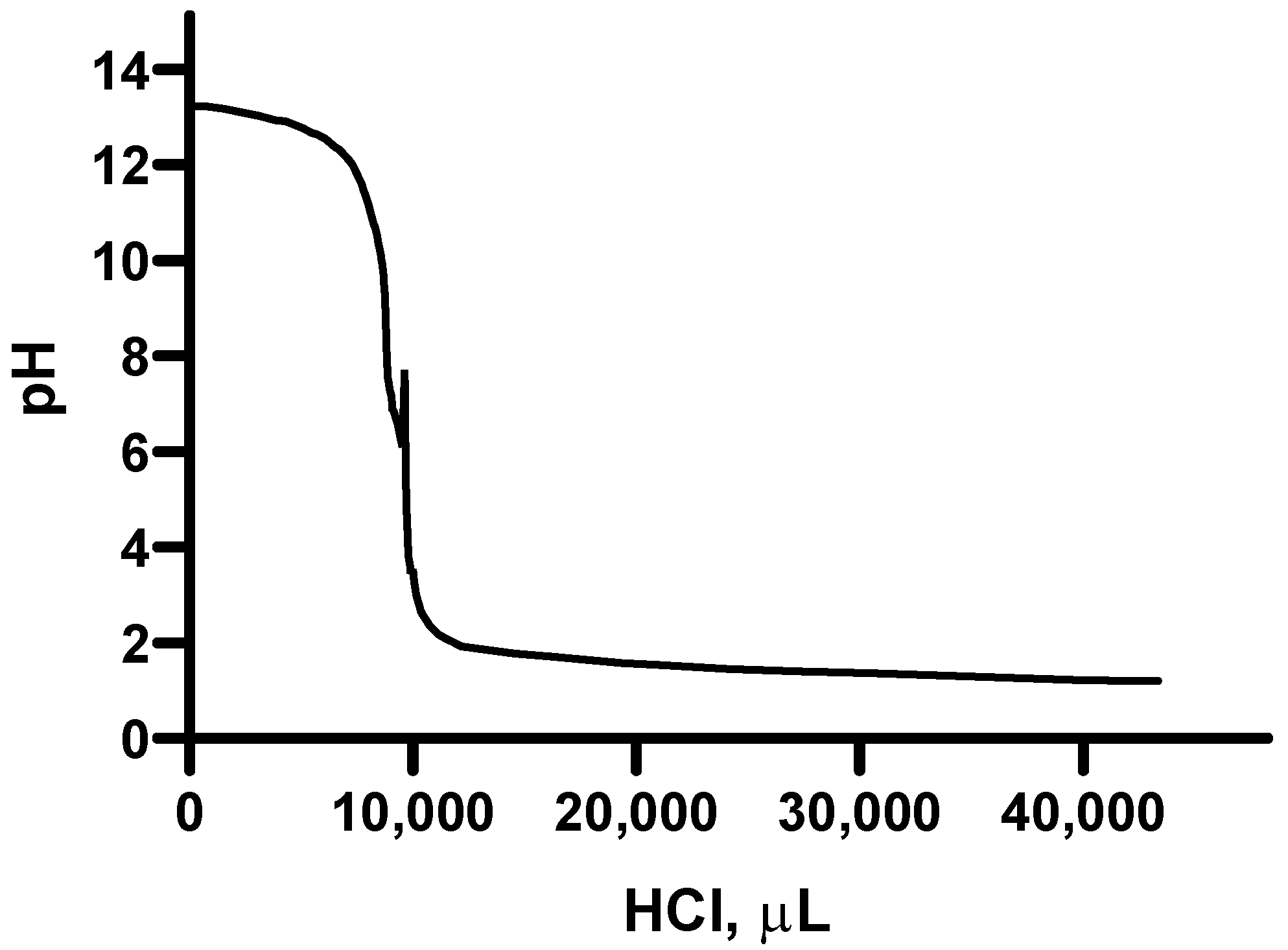
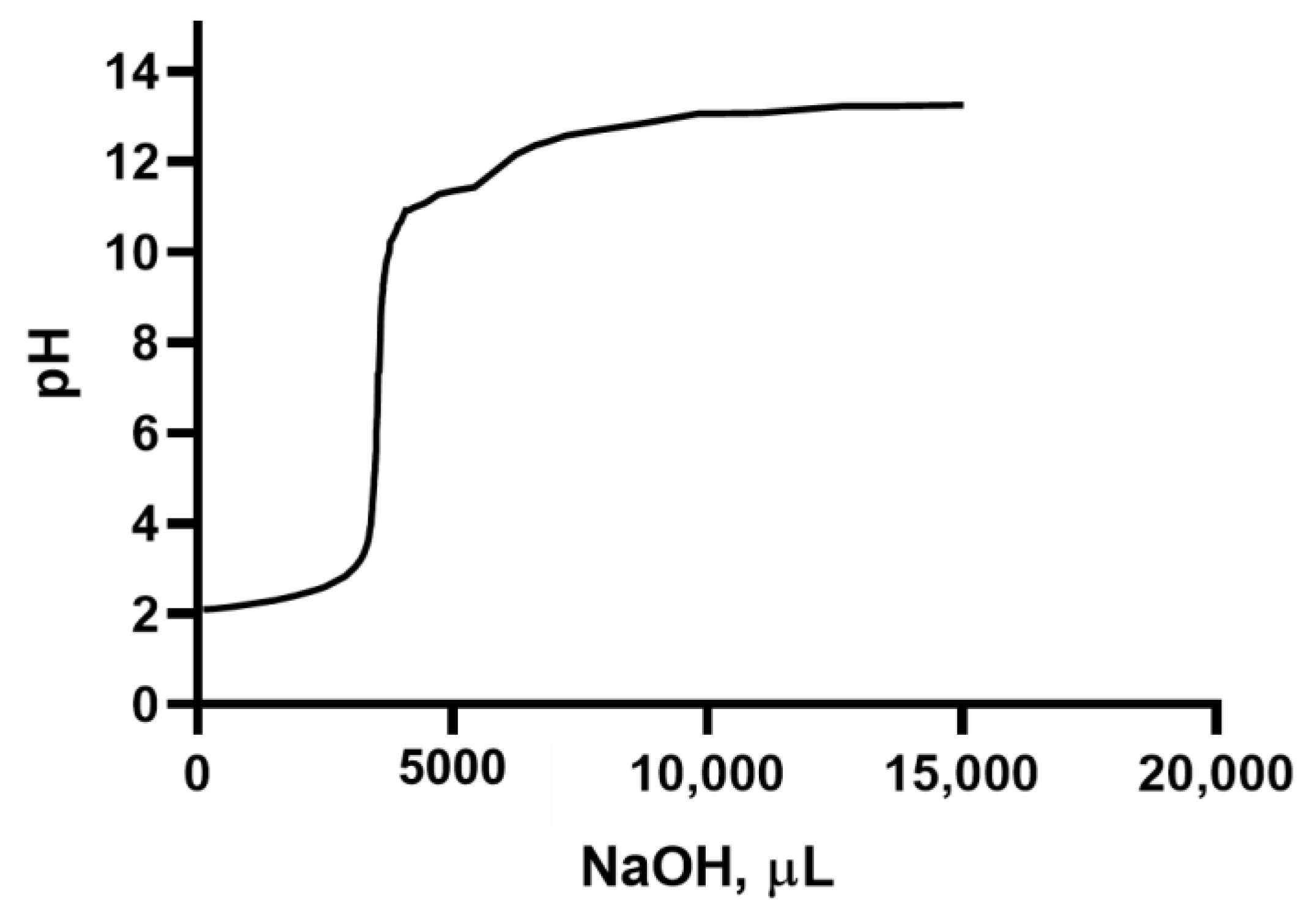
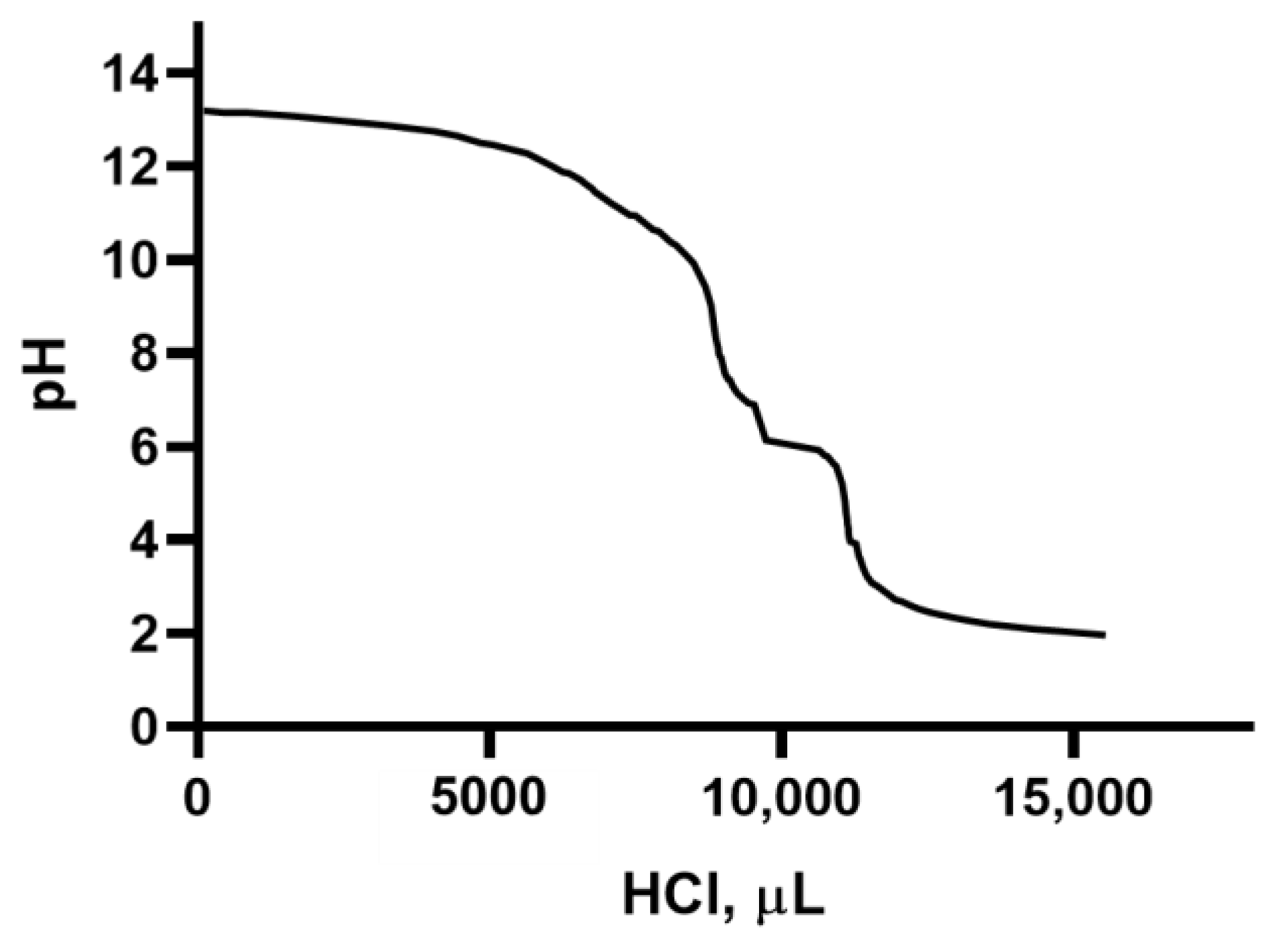
2.4. Titration
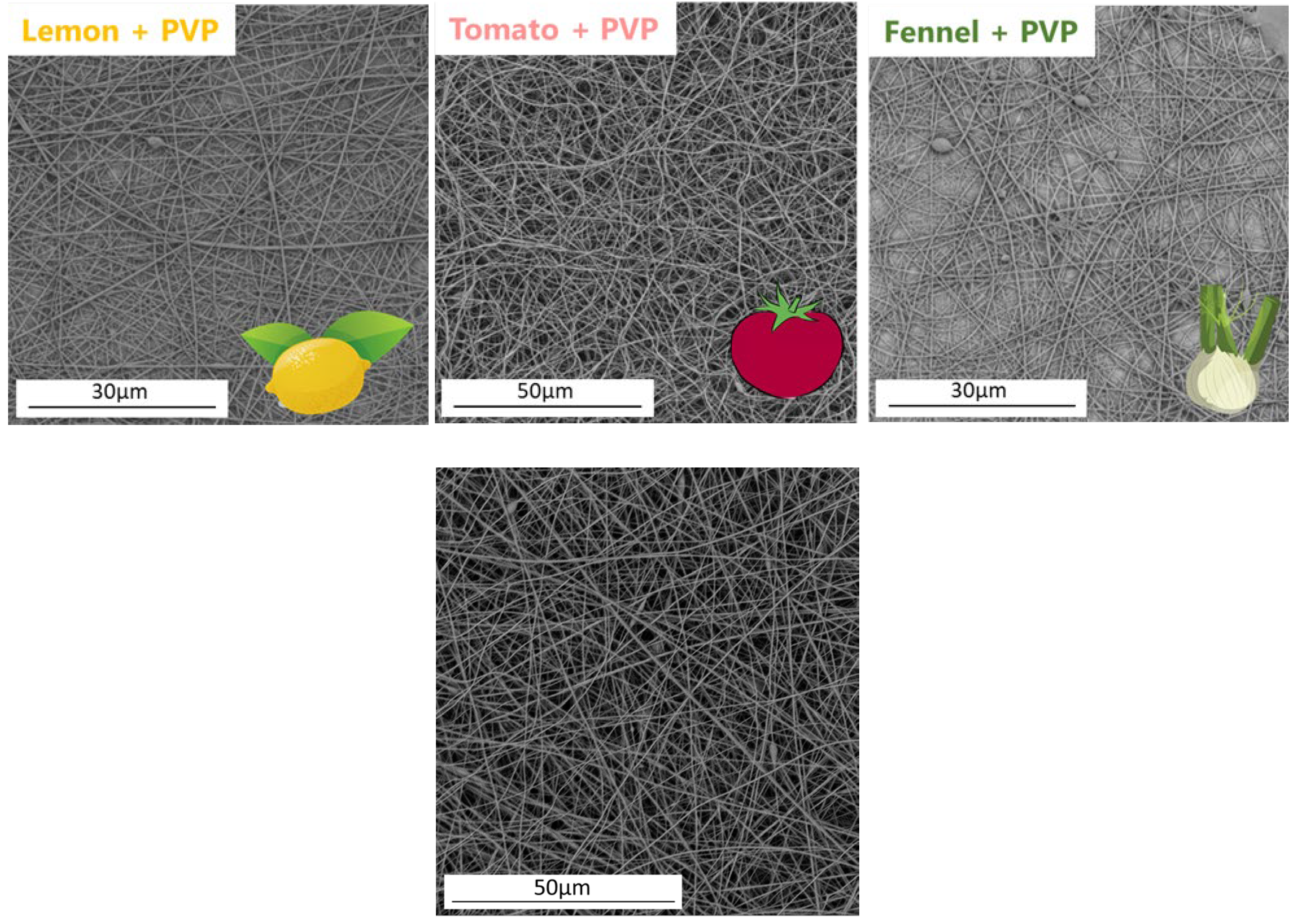
2.5. Fiber Formation
3. Materials and Methods
3.1. Materials
3.2. Polysaccharide Extraction and Gross Chemical Composition
3.3. Differential Scanning Calorimetry (DSC)
3.4. Solubility Tests
3.5. Titration
3.6. Fiber Realization
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Epps, T.H.; Korley, L.T.J.; Yan, T.; Beers, K.L.; Burt, T.M. Sustainability of Synthetic Plastics: Considerations in Materials Life-Cycle Management. JACS Au 2022, 2, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Zhou, Z.; Shen, L.; He, X.; Shen, J. Macroporous Hydrogel Scaffolds with Tunable Physicochemical Properties for Tissue Engineering Constructed Using Renewable Polysaccharides. ACS Appl. Mater. Interfaces 2020, 12, 13256–13264. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ahmed, S.A.; Tabish, M.; Hasnain, M.S. Natural Polysaccharides in Tissue Engineering Applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Koyyada, A.; Orsu, P. Natural Gum Polysaccharides as Efficient Tissue Engineering and Drug Delivery Biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Salah Eldin, R.E.; Abu Serea, E.S.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in Nanotechnology and Antibacterial Properties of Biodegradable Food Packaging Materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Noreen, S.; Ma, J.X.; Saeed, M.; Pervaiz, F.; Hanif, M.F.; Ahmed, B.; Farooq, M.I.; Akram, F.; Safdar, M.; Madni, A.; et al. Natural Polysaccharide-Based Biodegradable Polymeric Platforms for Transdermal Drug Delivery System: A Critical Analysis. Drug Deliv. Transl. Res. 2022, 12, 2649–2666. [Google Scholar] [CrossRef]
- Morozkina, S.; Strekalovskaya, U.; Vanina, A.; Snetkov, P.; Krasichkov, A.; Polyakova, V.; Uspenskaya, M. The Fabrication of Alginate–Carboxymethyl Cellulose-Based Composites and Drug Release Profiles. Polymers 2022, 14, 3604. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Quesada, M.R.; Astúa, S.V.; Zamora, J.P.M.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef]
- Gowe, C. Review on Potential Use of Fruit and Vegetables By-Products as A Valuable Source of Natural Food Additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- Chianese, E.; Galletti, A.; Giunta, G.; Landi, T.C.; Marcellino, L.; Montella, R.; Riccio, A. Spatiotemporally Resolved Ambient Particulate Matter Concentration by Fusing Observational Data and Ensemble Chemical Transport Model Simulations. Ecol. Modell. 2018, 385, 173–181. [Google Scholar] [CrossRef]
- Ansah, F.A.; Amodio, M.L.; De Chiara, M.L.V.; Colelli, G. Effects of Equipments and Processing Conditions on Quality of Fresh-Cut Produce. J. Agric. Eng. 2018, 49, 139–150. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Lan, T.; Bao, S.; Zhao, Q.; Sun, X.; Liu, X. How to Comprehensively Improve Juice Quality: A Review of the Impacts of Sterilization Technology on the Overall Quality of Fruit and Vegetable Juices in 2010–2021, an Updated Overview and Current Issues. In Critical Reviews in Food Science and Nutrition; Taylor Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Aнан’єва, В.В.; Kричкoвська, B.B.; Bаранкіна, O.O.; Бєлінська, A.П.; Якушкo, B.C. Development of Complex Acidifier for Emulsion Foodstuffs for Wellness Purposes. Technol. Audit Prod. Reserv. 2016, 5, 53–58. [Google Scholar] [CrossRef]
- Pereira, P.; Cebola, M.J.; Oliveira, M.C.; Bernardo Gil, M.G. Antioxidant Capacity and Identification of Bioactive Compounds of Myrtus Communis L. Extract Obtained by Ultrasound-Assisted Extraction. J. Food Sci. Technol. 2017, 54, 4362–4369. [Google Scholar] [CrossRef]
- Mrvčić, J.; Posavec, S.; Kazazić, S.; Stanzer, D.; Peša, A.; Stehlik-Tomas, V. Spirit Drinks: A Source of Dietary Polyphenols. Croat. J. food Sci. Technol. 2012, 4, 102–111. [Google Scholar]
- Debernardi-Vazquez, T.d.J.; Aguilar-Rivera, N.; Núñez-Pastrana, R. Composting of Byproducts from the Orange (Citrus sinensis (l.) Osbeck) and Sugarcane (Saccharum Spp. Hybrids) Agroindustries. Ing. Investig. 2020, 40, 81–88. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental Challenges Impeding the Composting of Biodegradable Municipal Solid Waste: A Critical Review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- De Feo, G.; De Gisi, S. Using MCDA and GIS for Hazardous Waste Landfill Siting Considering Land Scarcity for Waste Disposal. Waste Manag. 2014, 34, 2225–2238. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Waking Up from Four Decades’ Long Dream of Valorizing Agro-Food Byproducts: Toward Practical Applications of the Gained Knowledge. J. Agric. Food Chem. 2018, 66, 3069–3073. [Google Scholar] [CrossRef]
- Zuluaga, A.M.; Mena-García, A.; Chito-Trujillo, D.; Rada-Mendoza, M.; Sanz, M.L.; Ruiz-Matute, A.I. Development of a Microwave-Assisted Extraction Method for the Recovery of Bioactive Inositols from Lettuce (Lactuca sativa) Byproducts. Electrophoresis 2020, 41, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Lourenço-Lopes, C.; Otero, P.; Rodriguez, M.C.; Pereira, A.G.; Echave, J.; Soria-Lopez, A.; Chamorro, F.; Prieto, M.A.; Simal-Gandara, J. Application of Green Extraction Techniques for Natural Additives Production. In Natural Food Additives; BoD–Books on Demand: Norderstedt, Germany, 2022. [Google Scholar]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual-Teresa, S.; Poli, A.; Nicolaus, B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valoriz. 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Biobased Technologies for the Efficient Extraction of Biopolymers from Waste Biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible Coating and Films as Promising Packaging: A Mini Review. J. Food Meas. Charact. 2021, 15, 4205–4214. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Khatib, M.; Giuliani, C.; Rossi, F.; Adessi, A.; Al-Tamimi, A.; Mazzola, G.; Di Gioia, D.; Innocenti, M.; Mulinacci, N. Polysaccharides from By-Products of the Wonderful and Laffan Pomegranate Varieties: New Insight into Extraction and Characterization. Food Chem. 2017, 235, 58–66. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus Alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef]
- Thu Dao, T.A.; Webb, H.K.; Malherbe, F. Optimization of Pectin Extraction from Fruit Peels by Response Surface Method: Conventional versus Microwave-Assisted Heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, X.; Chen, H. Optimization of Enzyme Assisted Extraction of Fructus Mori Polysaccharides and Its Activities on Antioxidant and Alcohol Dehydrogenase. Carbohydr. Polym. 2014, 111, 775–782. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Application of Celluclast 1.5 L in Apple Pectin Extraction. Carbohydr. Polym. 2015, 134, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-C.; Chu, D.; Zhang, J.X.; Zheng, Y.F.; Li, Y. Microwave-Assisted Extraction, Partial Purification and Biological Activity in Vitro of Polysaccharides from Bladder-Wrack (Fucus Vesiculosus) by Using Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Poli, A.; Gomez d’Ayala, G.; Nicolaus, B.; Malinconinco, M.; Santagata, G. Vegetable Wastes Derived Polysaccharides as Natural Eco-Friendly Plasticizers of Sodium Alginate. Carbohydr. Polym. 2020, 229, 115427. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.B.; Fajardo, A.R.; Gerola, A.P.; Rodrigues, J.H.S.; Nakamura, C.V.; Muniz, E.C.; Hsieh, Y. Lo First Report of Electrospun Cellulose Acetate Nanofibers Mats with Chitin and Chitosan Nanowhiskers: Fabrication, Characterization, and Antibacterial Activity. Carbohydr. Polym. 2020, 250, 116954. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Extraction Method | Average Yield (% w/w) | Ref. |
|---|---|---|
| Hot water | 2.6–12.0 | [30,31] |
| Ultrasound assisted | 11.0–18.00 | [30] |
| Microwave assisted | 13.18–15.07 | [30,32] |
| Enzyme assisted | 15.0–19.0 | [33,34] |
| Supercritical fluids | 17.6–18.5 | [30] |
| Eutectic solvents | 8.97–11.5 | [35] |
| Sample | Yield (mg/g Dry Waste) | Carbohydrates (% w/w Dry Extract) | Proteins (% w/w Dry Extract) |
|---|---|---|---|
| Lemon | 42.9 ± 0.9 | 97.9 ± 0.8 | 2.1 ± 0.5 |
| Fennel | 65.3 ± 2.2 | 90.1 ± 3.7 | 2.3 ± 0.3 |
| Tomato | 44.1 ± 0.6 | 87.3 ± 4.1 | 1.1 ± 0.5 |
| Sample | Peak Temperature, Tpeak (°C) | Onset Temperature (°C) | Enthalpy, ΔH (J/g) |
|---|---|---|---|
| Water Fennel | 2.38 ± 0.15 3.60 ± 1.19 | 0.11 ± 0.03 0.20 ± 0.18 | 352 ± 5 315 ± 13 |
| Lemon | 2.68 ± 0.01 | 0.15 ± 0.05 | 341 ± 8 |
| Tomato | 1.07 ± 1.39 | 0.12 ± 0.01 | 349 ± 3 |
| Sample | Peak Temperature, Tpeak (°C) | Onset Temperature (°C) | Enthalpy, ΔH (J/g) |
|---|---|---|---|
| Water Fennel | 2.38 ± 0.15 2.34 ± 0.47 | 0.11 ± 0.03 0.63 ± 1.05 | 352 ± 5 342 ± 8 |
| Lemon | 2.31 ± 0.39 | 0.05 ± 0.07 | 343 ± 7 |
| Tomato | 1.82 ± 0.04 | 0.14 ± 0.04 | 349 ± 15 |
| Sample | Solvent | Insoluble | Poorly Soluble | Soluble |
|---|---|---|---|---|
| Lemon | DMSO | X | ||
| Acetone | X | |||
| Ethanol | X | |||
| DCM | X | |||
| Fennel | DMSO | X | ||
| Acetone | X | |||
| Ethanol | X | |||
| DCM | X | |||
| Tomato | DMSO | X | ||
| Acetone | X | |||
| Ethanol | X | |||
| DCM | X |
| Sample | pH | Insoluble | Poorly Soluble | Soluble |
|---|---|---|---|---|
| Lemon | 12 | X | ||
| 2 | X | |||
| Fennel | 12 | X | ||
| 2 | X | |||
| Tomato | 12 | X | ||
| 2 | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestri, T.; Di Donato, P.; Bonadies, I.; Poli, A.; Frigione, M.; Biondi, M.; Mayol, L. Physico-Chemical Properties and Valorization of Biopolymers Derived from Food Processing Waste. Molecules 2023, 28, 6894. https://doi.org/10.3390/molecules28196894
Silvestri T, Di Donato P, Bonadies I, Poli A, Frigione M, Biondi M, Mayol L. Physico-Chemical Properties and Valorization of Biopolymers Derived from Food Processing Waste. Molecules. 2023; 28(19):6894. https://doi.org/10.3390/molecules28196894
Chicago/Turabian StyleSilvestri, Teresa, Paola Di Donato, Irene Bonadies, Annarita Poli, Mariaenrica Frigione, Marco Biondi, and Laura Mayol. 2023. "Physico-Chemical Properties and Valorization of Biopolymers Derived from Food Processing Waste" Molecules 28, no. 19: 6894. https://doi.org/10.3390/molecules28196894
APA StyleSilvestri, T., Di Donato, P., Bonadies, I., Poli, A., Frigione, M., Biondi, M., & Mayol, L. (2023). Physico-Chemical Properties and Valorization of Biopolymers Derived from Food Processing Waste. Molecules, 28(19), 6894. https://doi.org/10.3390/molecules28196894