Encapsulation and Biological Activity of Hesperetin Derivatives with HP-β-CD
Abstract
:1. Introduction
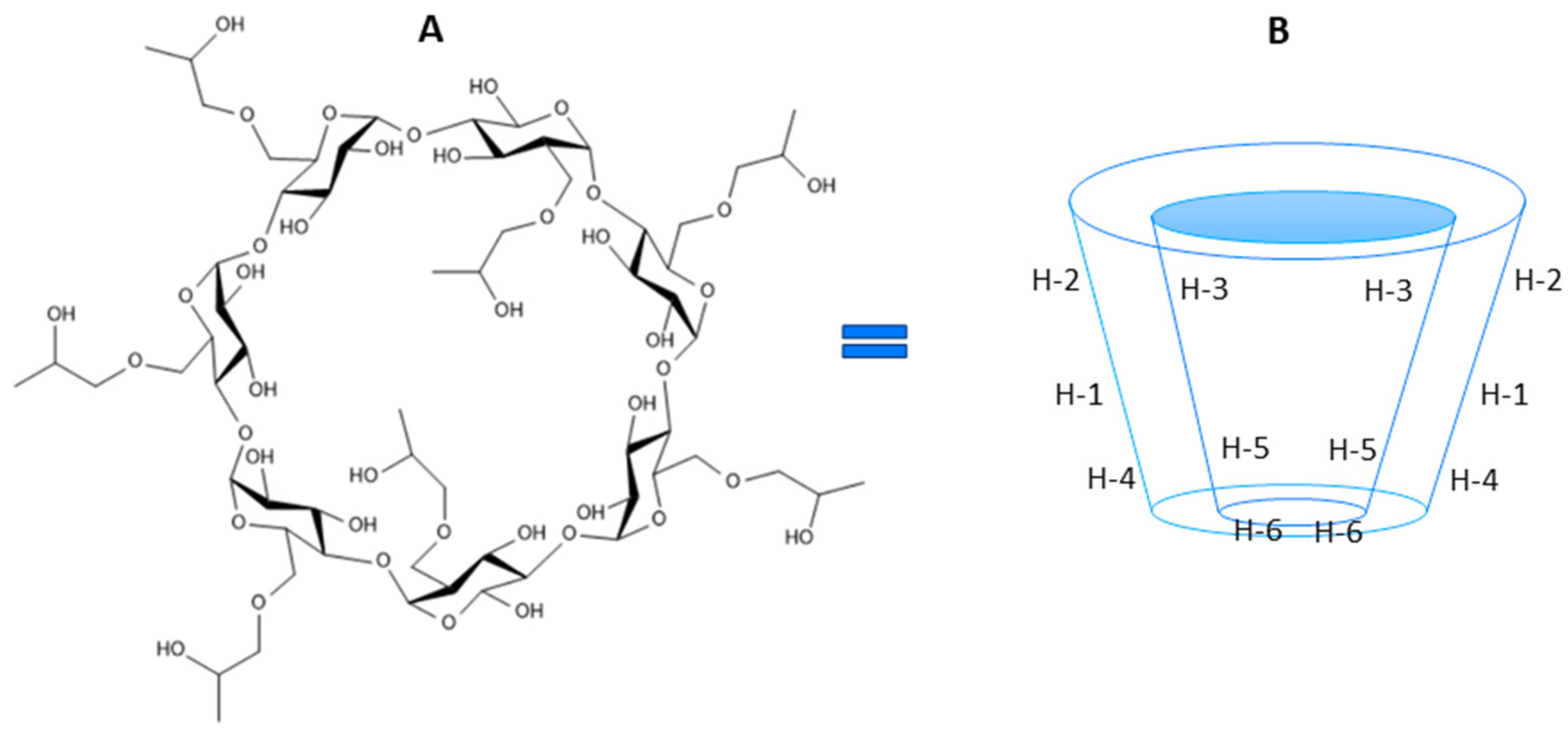
2. Results and Discussion
2.1. Encapsulation of Hesperetin Schiff Bases by HP-β-CD
2.2. Identification of Solid Systems of Hesperetin and Its Derivatives with HP-β-CD
2.2.1. Fourier Transform Infrared (FT-IR) Spectroscopy
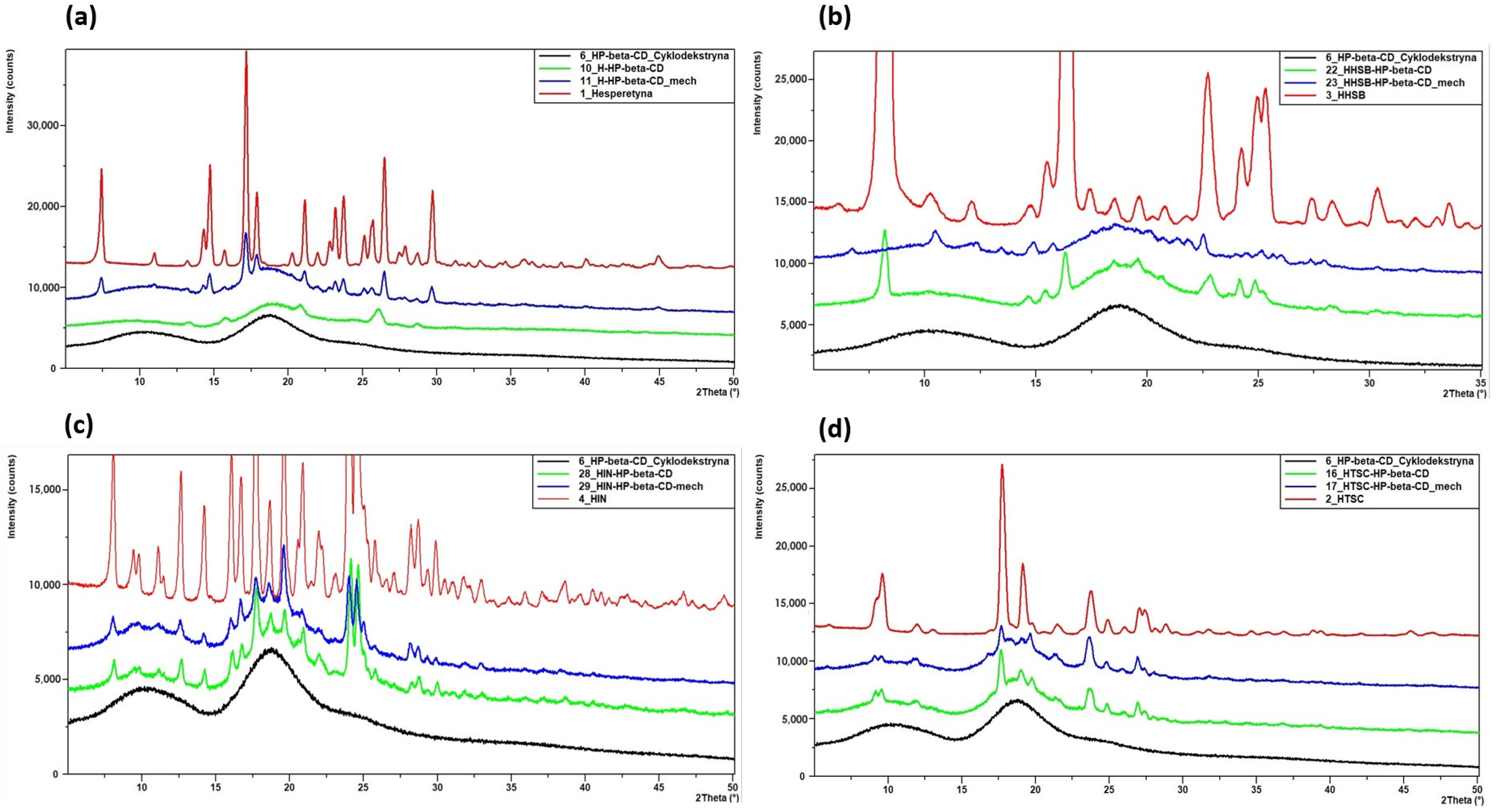
2.2.2. Powder X-ray Diffraction (PXRD) Studies
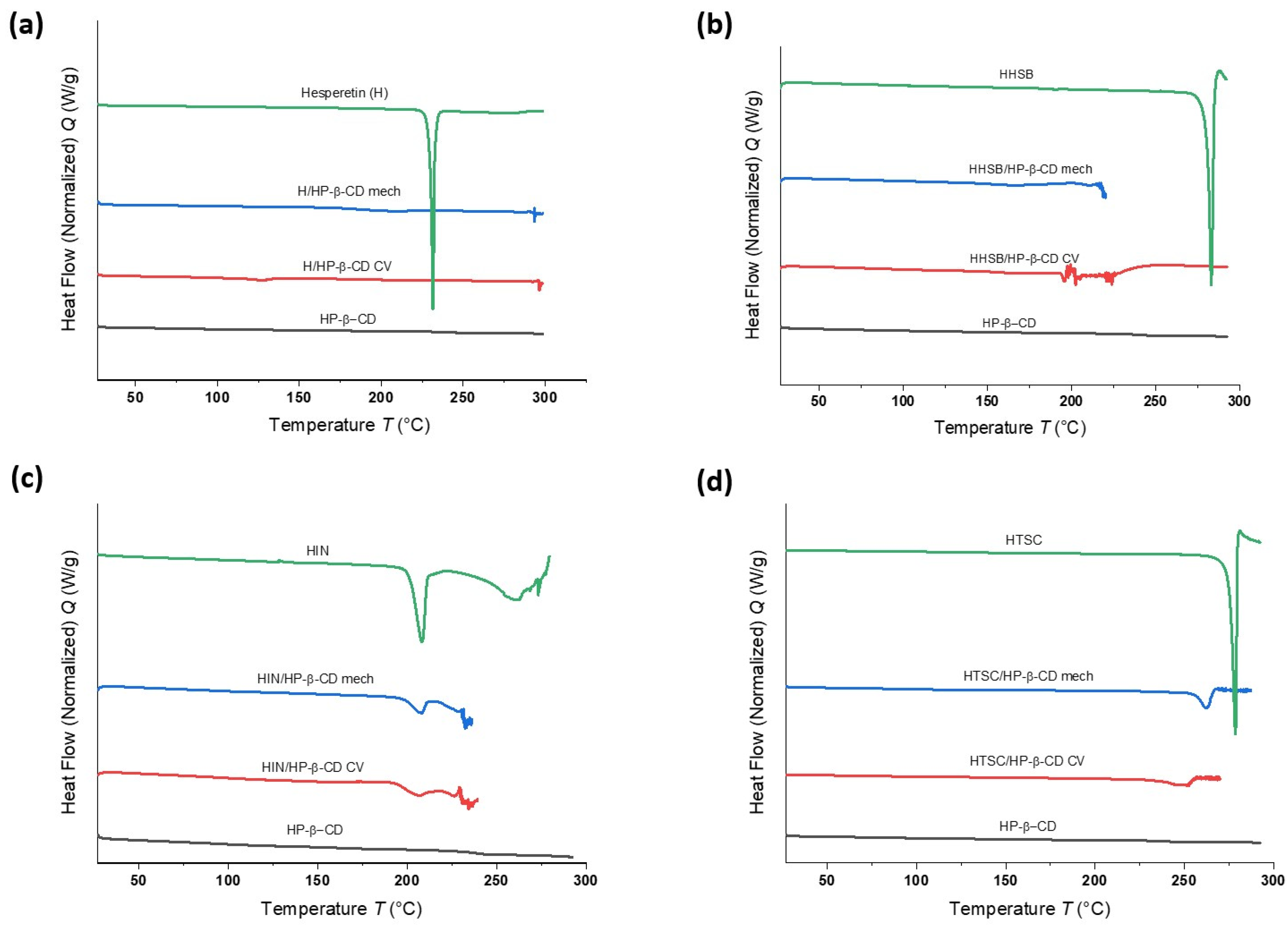
2.2.3. Differential Scanning Calorimetry (DSC) Studies
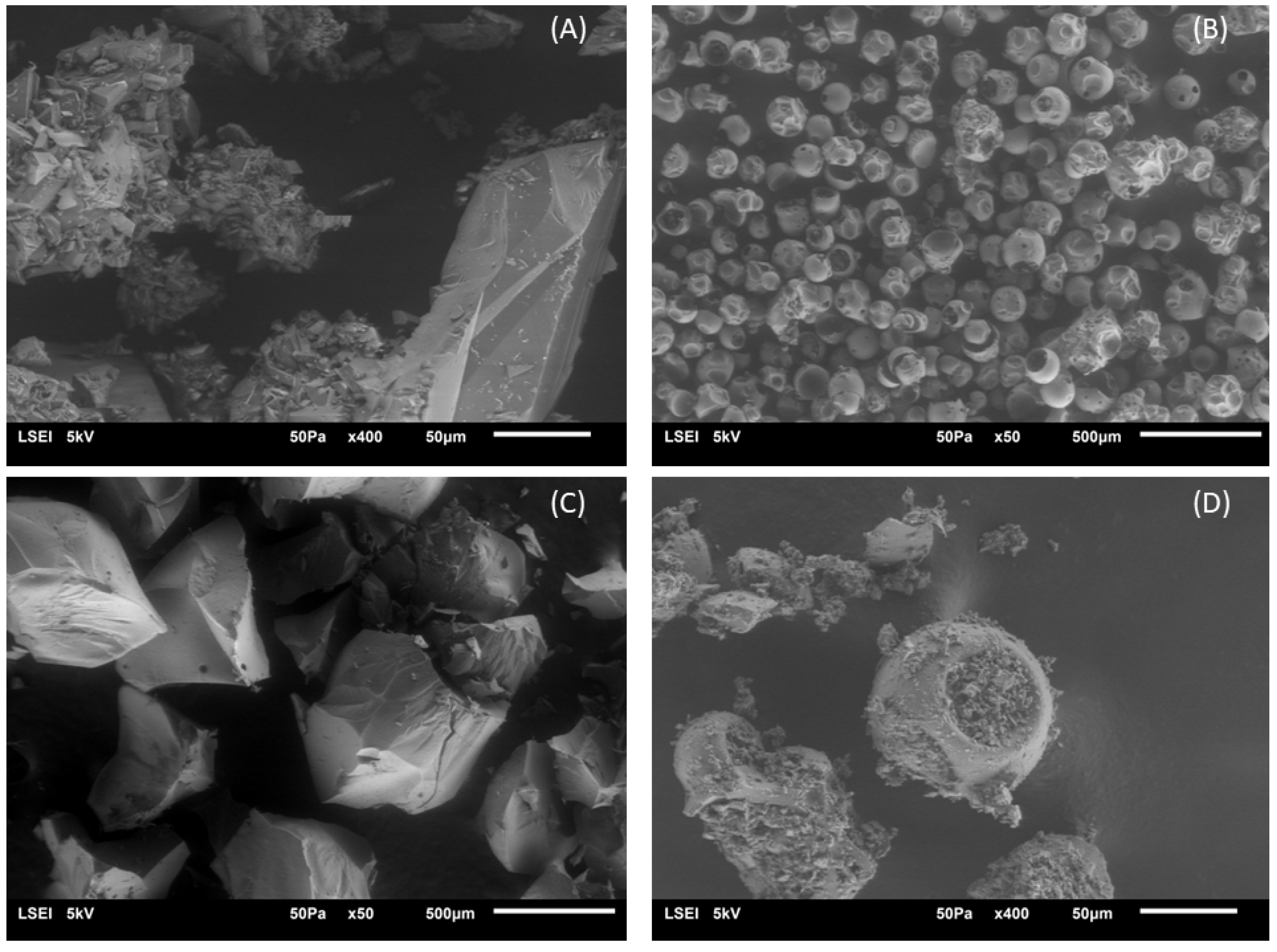
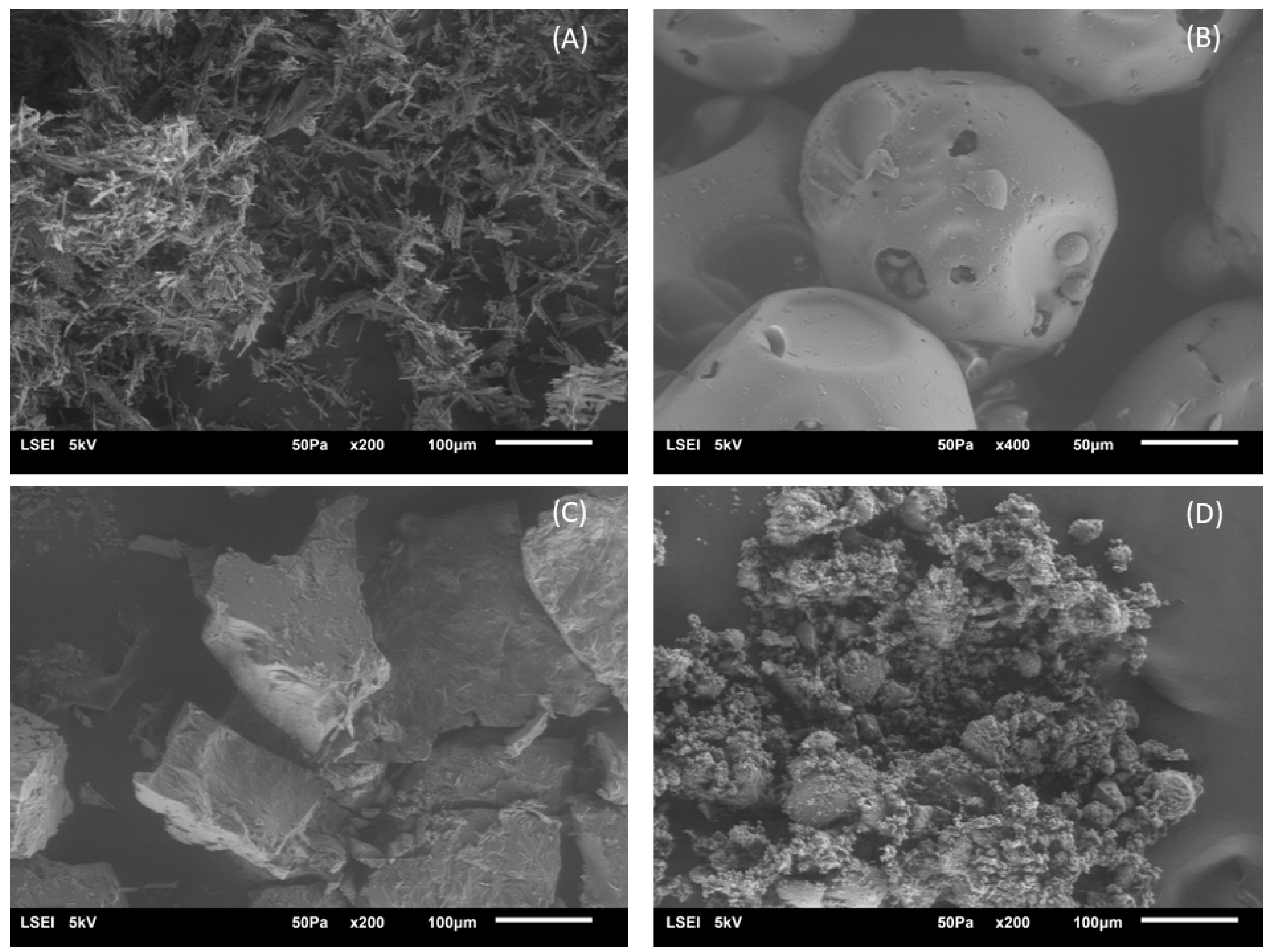
2.2.4. SEM Analysis
2.3. Identification of Systems of Hesperetin and Its Derivatives with HP-β-CD in Solutions
2.3.1. Nuclear Magnetic Resonance (NMR) Studies
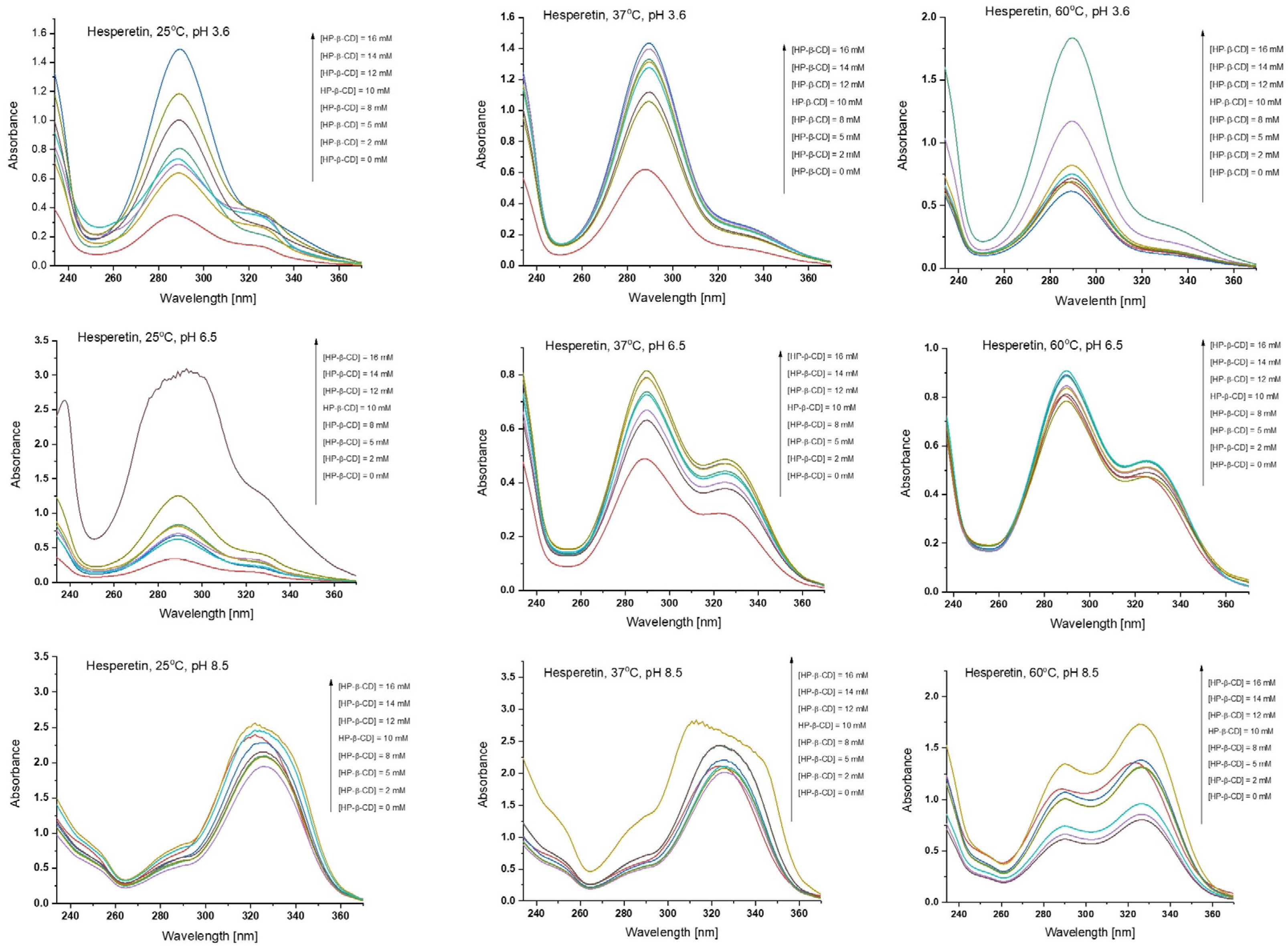
2.3.2. UV-Vis Spectroscopy Studies
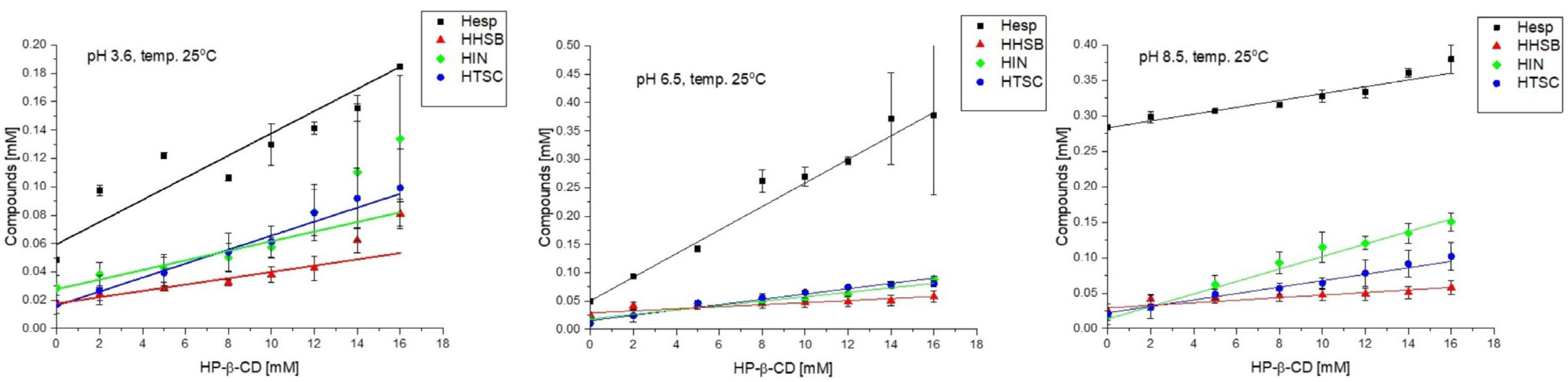
2.3.3. Phase Solubility Studies
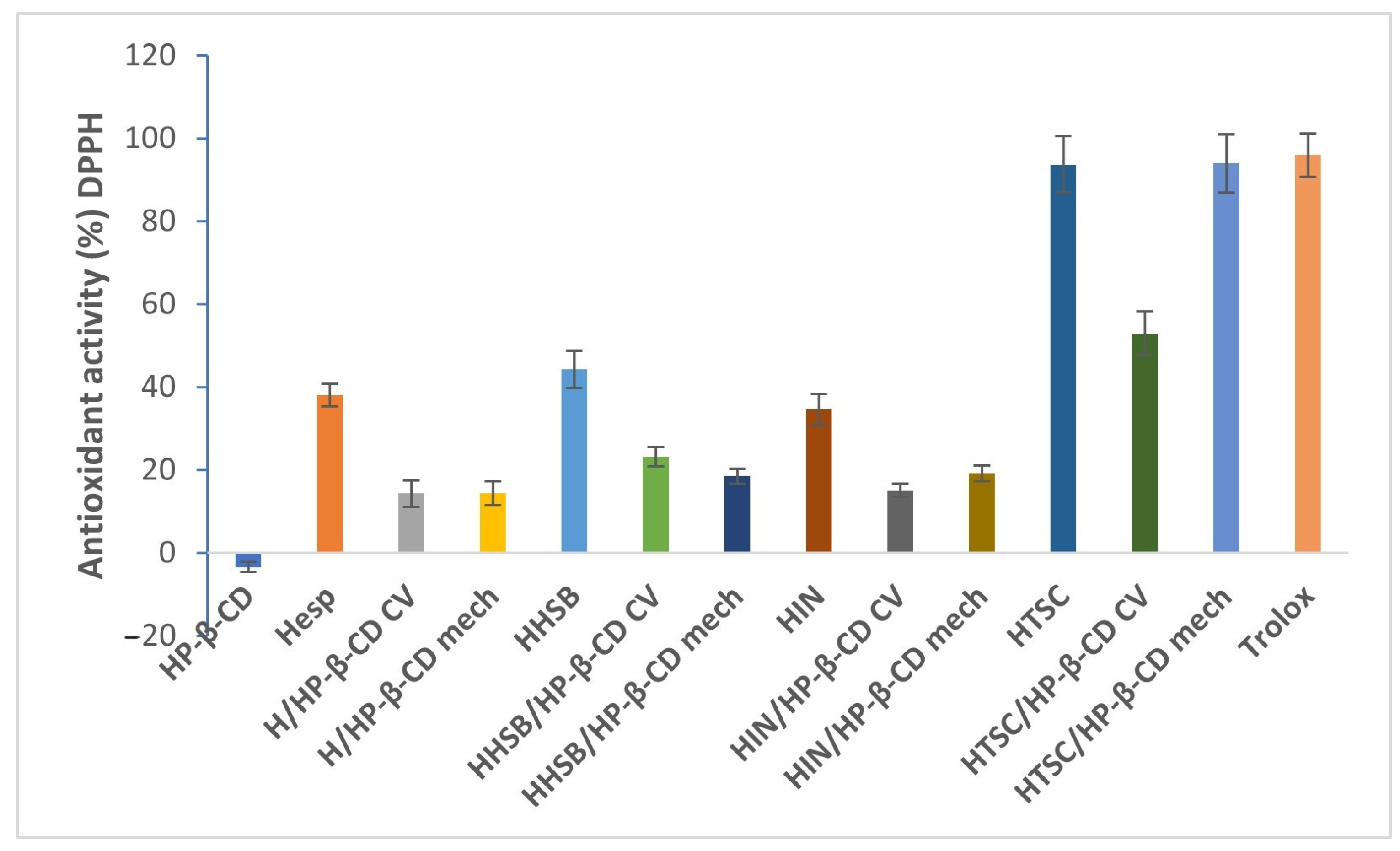
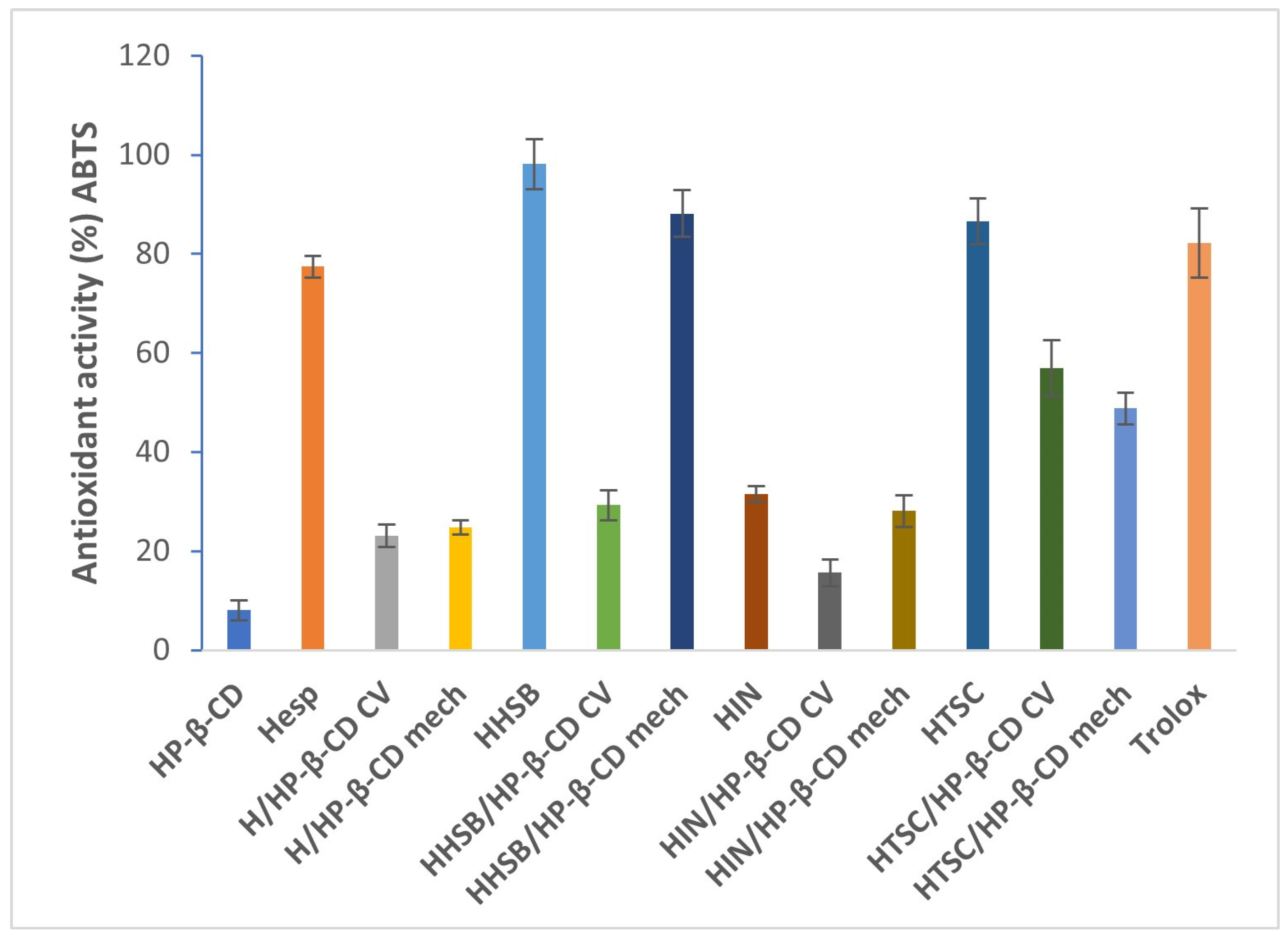
2.4. Antioxidant Activity Analysis
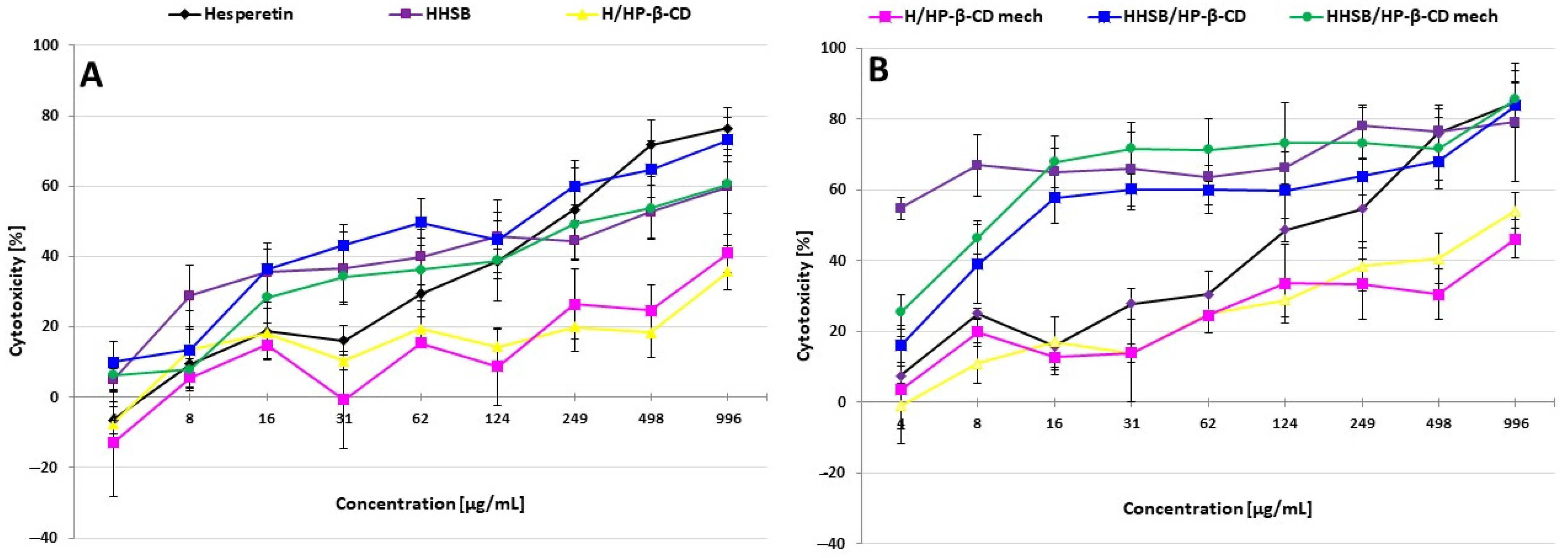
2.5. Cytotoxic Activity of Tested Chemicals
2.6. Antibacterial Activity of Tested Chemicals
3. Materials and Methods
3.1. Materials
3.2. Encapsulation in the Solution
3.3. Preparation of Physical Mixture
3.4. Phase Solubility Studies
3.5. Physico-Chemical Inclusion Compounds’ Characterization
3.6. Antioxidant Activities Research in Vitro
3.6.1. DPPH Assay
3.6.2. ABTS•+ Radical Cation Decolorization Assay
3.6.3. Ferric-Ion Reducing Antioxidant Power
3.7. Cytotoxic Activity
3.7.1. Preparation of Test Chemicals
3.7.2. Cell Culture
3.7.3. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Assay
3.8. Antibacterial Activity
Minimal Inhibition Concentration (MIC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.T.; de Franco, É.P.D.; de Araújo, M.E.M.B.; Messias, M.C.F.; Priviero, F.B.M.; Frankland Sawaya, A.C.H.; de Oliveira Carvalho, P. Characterization of the Antioxidant Activity of Aglycone and Glycosylated Derivatives of Hesperetin: An in Vitro and in Vivo Study. J. Mol. Recognit. 2016, 29, 80–87. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Jayaraman, R.; Subramani, S.; Sheik Abdullah, S.H.; Udaiyar, M. Antihyperglycemic Effect of Hesperetin, a Citrus Flavonoid, Extenuates Hyperglycemia and Exploring the Potential Role in Antioxidant and Antihyperlipidemic in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef]
- Sak, K.; Lust, H.; Kase, M.; Saar, M.; Jaal, J. Suppression of Taxanes Cytotoxicity by Citrus Flavonoid Hesperetin in PPC-1 Human Prostate Cancer Cells. Anticancer. Res. 2018, 38, 6209–6215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, S.; Zhang, L.; Tian, L.; Li, L.; Liu, Z.; Dong, Q.; Lv, X.; Mu, H.; Zhang, Q.; et al. Hesperetin as an Adjuvant Augments Protective Anti-tumour Immunity Responses in B16F10 Melanoma by Stimulating Cytotoxic CD8+ T Cells. Scand. J. Immunol. 2020, 91, 12867. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.-B.; Kim, S.-H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Denny, S.; West, P.W.J.; Mathew, T.C. Antagonistic Interactions between the Flavonoids Hesperetin and Naringenin and β-Lactam Antibiotics against Staphylococcus Aureus. Br. J. Biomed. Sci. 2008, 65, 145–147. [Google Scholar] [CrossRef]
- Napier, I.; Ponka, P.; Richardson, D.R. Iron Trafficking in the Mitochondrion: Novel Pathways Revealed by Disease. Blood 2005, 105, 1867–1874. [Google Scholar] [CrossRef]
- Richardson, D.; Tran, E.; Ponka, P. The Potential of Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class as Effective Antiproliferative Agents. Blood 1995, 86, 4295–4306. [Google Scholar] [CrossRef]
- Richardson, D.R.; Ponka, P. Orally Effective Iron Chelators for the Treatment of Iron Overload Disease: The Case for a Further Look at Pyridoxal Isonicotinoyl Hydrazone and Its Analogs. J. Lab. Clin. Med. 1998, 132, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.T.; Bhavsar, Z.A.; Jethava, D.J.; Patel, D.B.; Patel, H.D. A Review on Development of Bio-Active Thiosemicarbazide Derivatives: Recent Advances. J. Mol. Struct. 2021, 1226, 129268. [Google Scholar] [CrossRef]
- Brodowska, K.; Correia, I.; Garribba, E.; Marques, F.; Klewicka, E.; Łodyga-Chruscińska, E.; Pessoa, J.C.; Dzeikala, A.; Chrusciński, L. Coordination Ability and Biological Activity of a Naringenin Thiosemicarbazone. J. Inorg. Biochem. 2016, 165, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lodyga-Chruscinska, E.; Symonowicz, M.; Sykula, A.; Bujacz, A.; Garribba, E.; Rowinska-Zyrek, M.; Oldziej, S.; Klewicka, E.; Janicka, M.; Krolewska, K.; et al. Chelating Ability and Biological Activity of Hesperetin Schiff Base. J. Inorg. Biochem. 2015, 143, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, K.; Sykuła, A.; Garribba, E.; Lodyga-Chruścińska, E.; Sójka, M. Naringenin Schiff Base: Antioxidant Activity, Acid-Base Profile, and Interactions with DNA. Transit. Met. Chem. 2016, 41, 179–189. [Google Scholar] [CrossRef]
- Casas, J.S.; Garcıa-Tasende, M.S.; Sordo, J. Main Group Metal Complexes of Semicarbazones and Thiosemicarbazones. A Structural Review. Coord. Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- Doğan, M.; Koçyiğit, Ü.M.; Gürdere, M.B.; Ceylan, M.; Budak, Y. Synthesis and Biological Evaluation of Thiosemicarbazone Derivatives. Med. Oncol. 2022, 39, 157. [Google Scholar] [CrossRef]
- Sykula, A.; Kowalska-Baron, A.; Dzeikala, A.; Bodzioch, A.; Lodyga-Chruscinska, E. An Experimental and DFT Study on Free Radical Scavenging Activity of Hesperetin Schiff Bases. Chem. Phys. 2019, 517, 91–103. [Google Scholar] [CrossRef]
- Sykuła, A.; Nowak, A.; Garribba, E.; Dzeikala, A.; Rowińska-Żyrek, M.; Czerwińska, J.; Maniukiewicz, W.; Łodyga-Chruścińska, E. Spectroscopic Characterization and Biological Activity of Hesperetin Schiff Bases and Their Cu(II) Complexes. Int. J. Mol. Sci. 2023, 24, 761. [Google Scholar] [CrossRef]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Mircea, C.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Marangoci, N. Antibacterial and Antioxidant Properties of Hesperidin: Βcyclodextrin Complexes Obtained by Different Techniques. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 71–84. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dhamija, I.; Meehenian, H.; Kumar, N.; Simran, S.; Muskan, M.; Verma, M.L.; Kumar, S. Chronicle Updates in Cyclodextrin-Based Carriers for Drug Delivery. Bull. Natl. Res. Cent. 2022, 46, 202. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.E.; Pyo, Y.C.; Tran, P.; Park, J.S. Solubility Enhancement and Application of Cyclodextrins in Local Drug Delivery. J. Pharm. Investig. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review; Springer International Publishing: Cham, Switzerland, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Tang, W.; Ng, S.; Sun, D. Modifi Ed Cyclodextrins for Chiral Separation; Springer International Publishing: Cham, Switzerland, 2013; ISBN 9783642376474. [Google Scholar]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Wada-Hirai, A.; Shimizu, S.; Ichii, R.; Tsunoda, C.; Hiroshige, R.; Fujita, M.; Li, Y.-P.; Shimada, Y.; Otsuka, Y. Stabilization of the Metastable α–Form of Indomethacin Induced by the Addition of 2-Hydroxypropyl-β-Cyclodextrin, Causing Supersaturation (Spring) and Its Sustaining Deployment (Parachute). J. Pharm. Sci. 2021, 110, 3623–3630. [Google Scholar] [CrossRef]
- Shimizu, S.; Wada-Hirai, A.; Li, Y.; Shimada, Y.; Otsuka, Y.; Goto, S. Relationship Between Phase Solubility Diagrams and Crystalline Structures During Dissolution of Cimetidine/Cyclodextrin Complex Crystals. J. Pharm. Sci. 2020, 109, 2206–2212. [Google Scholar] [CrossRef]
- Pitha, J.; Milecki, J.; Fales, H.; Pannell, L.; Uekama, K. Hydroxypropyl-β-Cyclodextrin: Preparation and Characterization; Effects on Solubility of Drugs. Int. J. Pharm. 1986, 29, 73–82. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Silva, I.R.; Kronenberger, T.; Gomes, E.C.L.; César, I.C.; Oliveira, R.B.; Maltarollo, V.G. Improving the Solubility of an Antifungal Thiazolyl Hydrazone Derivative by Cyclodextrin Complexation. Eur. J. Pharm. Sci. 2021, 156, 105575. [Google Scholar] [CrossRef]
- Beraldo, H.; Sinisterra, R.D.; Teixeira, L.R.; Vieira, R.P.; Carolina Doretto, M. An Effective Anticonvulsant Prepared Following a Host–Guest Strategy That Uses Hydroxypropyl-β-Cyclodextrin and Benzaldehyde Semicarbazone. Biochem. Biophys. Res. Commun. 2002, 296, 241–246. [Google Scholar] [CrossRef]
- Ogawa, N.; Kaga, M.; Endo, T.; Nagase, H.; Furuishi, T.; Yamamoto, H.; Kawashima, Y.; Ueda, H. Quetiapine Free Base Complexed with Cyclodextrins to Improve Solubility for Parenteral Use. Chem. Pharm. Bull. 2013, 61, 809–815. [Google Scholar] [CrossRef]
- Zoghbi, A.; Geng, T.; Wang, B. Dual Activity of Hydroxypropyl-β-Cyclodextrin and Water-Soluble Carriers on the Solubility of Carvedilol. AAPS PharmSciTech 2017, 18, 2927–2935. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, H. Characterization of Hydroxypropyl-β-Cyclodextrins with Different Substitution Patterns via FTIR, GC–MS, and TG–DTA. Carbohydr Polym 2015, 118, 36–40. [Google Scholar] [CrossRef]
- Savic-Gajic, I.; Savic, I.M.; Nikolic, V.D.; Nikolic, L.B.; Popsavin, M.M.; Kapor, A.J. Study of the Solubility, Photostability and Structure of Inclusion Complexes of Carvedilol with β-Cyclodextrin and (2-Hydroxypropyl)-β-Cyclodextrin. J Incl Phenom Macrocycl Chem 2016, 86, 7–17. [Google Scholar] [CrossRef]
- Colthup, B.N.; Daly, H.L.; Wiberley, E.S. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Shi, Y.; Zhang, T.; Ren, H.; Kruse, A.; Cui, R. Polyethylene Imine Modified Hydrochar Adsorption for Chromium (VI) and Nickel (II) Removal from Aqueous Solution. Bioresour Technol 2018, 247, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and Ammonia as Probes for FTIR Analysis of Solid Acid Catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Gao, S.; Bie, C.; Ji, Q.; Ling, H.; Li, C.; Fu, Y.; Zhao, L.; Ye, F. Preparation and Characterization of Cyanazine-Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex. RSC Adv. 2019, 9, 26109–26115. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Tavakoli, O.; Khoobi, M.; Wu, Y.S.; Faramarzi, M.A.; Gholibegloo, E.; Farkhondeh, S. Beta-Carotene/Cyclodextrin-Based Inclusion Complex: Improved Loading, Solubility, Stability, and Cytotoxicity. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 55–64. [Google Scholar] [CrossRef]
- Catauro, M.; Ciprioti, S.V. Thermodynamics and Biophysics of Biomedical Nanosystems; Springer: Singapore, 2019; ISBN 978-981-13-0988-5. [Google Scholar]
- Kim, J.-S. Study of Flavonoid/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes by UV-Vis, FT-IR, DSC, and X-Ray Diffraction Analysis. Prev. Nutr. Food Sci. 2020, 25, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bao, Y.; Wang, D.; Wang, X.; Liu, H.; Li, Z.; Chen, M.; Wang, C.; Guo, Q. Water-Soluble Complexes of Orange Pigments from Monascus Sp. with HP-β-CD: Preparation, Inclusion Mechanism, and Improved Stability. J. Mol. Liq. 2020, 300, 1–9. [Google Scholar] [CrossRef]
- Pu, H.; Sun, Q.; Tang, P.; Zhao, L.; Li, Q.; Liu, Y.; Li, H. Characterization and Antioxidant Activity of the Complexes of Tertiary Butylhydroquinone with β-Cyclodextrin and Its Derivatives. Food Chem. 2018, 260, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Xu, X.; Jin, Z. Co-Encapsulation of Curcumin and Quercetin with Zein/HP-β-CD Conjugates to Enhance Environmental Resistance and Antioxidant Activity. NPJ Sci. Food 2023, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical Characterisation of the Supramolecular Structure of Luteolin/Cyclodextrin Inclusion Complex. Food Chem. 2013, 141, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Xia, S.; Ma, S.X.; Zhou, S.Y.; Zhao, X.Q.; Wang, S.H.; Li, M.Y.; Yang, X.D. Host-Guest System of Hesperetin and β-Cyclodextrin or Its Derivatives: Preparation, Characterization, Inclusion Mode, Solubilization and Stability. Mater. Sci. Eng. C 2016, 59, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Martini, M.F.; Glisoni, R.J.; Sosnik, A.; Moglioni, A.; Pickholz, M. Insights on Self-Aggregation Phenomena of 1-Indanone Thiosemicarbazones and the Formation of Inclusion Complexes with Hydroxypropyl-β-Cyclodextrin by Molecular Dynamics Simulations. J. Mol. Liq. 2016, 222, 963–971. [Google Scholar] [CrossRef]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabrò, M.L.; Di Bella, M.R.; Rustichelli, C.; Gamberini, M.C.; Ficarra, P. Study of Flavonoids/β-Cyclodextrins Inclusion Complexes by NMR, FT-IR, DSC, X-ray Investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Tommasini, S.; Calabrò, M.L.; Stancanelli, R.; Donato, P.; Costa, C.; Catania, S.; Villari, V.; Ficarra, P.; Ficarra, R. The Inclusion Complexes of Hesperetin and Its 7-Rhamnoglucoside with (2-Hydroxypropyl)-β-Cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 572–580. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Pérez-Abril, M.; Castillo, J.; Serrano, A.; Mercader, M.T.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of Temperature, PH, β- and HP-β-Cds on the Solubility and Stability of Flavanones: Naringenin and Hesperetin. LWT 2019, 108, 233–239. [Google Scholar] [CrossRef]
- Pérez-Abril, M.; Lucas-Abellán, C.; Castillo-Sánchez, J.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; Fortea, I.; Gabaldón, J.A.; Núñez-Delicado, E. Systematic Investigation and Molecular Modelling of Complexation between Several Groups of Flavonoids and HP-β-Cyclodextrins. J. Funct. Foods 2017, 36, 122–131. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Na, X.; Wang, H.; Xie, Y.; Cong, S.; Song, Y.; Xu, X.; Zhu, B.W.; Tan, M. Fluorescent Carbon Dots Derived from Maillard Reaction Products: Their Properties, Biodistribution, Cytotoxicity, and Antioxidant Activity. J. Agric. Food Chem. 2018, 66, 1569–1575. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, D.; Ni, Z.; Cao, M.; Cai, L. Preparation, Characterization of Naringenin, β-Cyclodextrin and Carbon Quantum Dot Antioxidant Nanocomposites. Food Chem. 2022, 375, 131646. [Google Scholar] [CrossRef]
- Jo, Y.J.; Cho, H.S.; Chun, J.Y. Antioxidant Activity of β-Cyclodextrin Inclusion Complexes Containing Trans-Cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci. Biotechnol. 2021, 30, 807–814. [Google Scholar] [CrossRef]
- Nowak, A.; Matusiak, K.; Borowski, S.; Bakuła, T.; Opaliński, S.; Kołacz, R.; Gutarowska, B. Cytotoxicity of Odorous Compounds from Poultry Manure. Int. J. Environ. Res. Public Health 2016, 13, 1046. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G.B. Hesperetin Protects Testicular Toxicity of Doxorubicin in Rat: Role of NFκB, P38 and Caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective Effects of Flavonoids against Microbes and Toxins: The Cases of Hesperidin and Hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic Flavonoids with Antimicrobial Activity: A Review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Ioannou, I.; Barboza, E.; Willig, G.; Marié, T.; Texeira, A.; Darme, P.; Renault, J.-H.; Allais, F. Implementation of an Enzyme Membrane Reactor to Intensify the α-O-Glycosylation of Resveratrol Using Cyclodextrins. Pharmaceuticals 2021, 14, 319. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- López-Nicolás, J.M.; Núñez-Delicado, E.; Pérez-López, A.J.; Barrachina, Á.C.; Cuadra-Crespo, P. Determination of Stoichiometric Coefficients and Apparent Formation Constants for β-Cyclodextrin Complexes of Trans-Resveratrol Using Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2006, 1135, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Boukamp, P. Normal Keratinization in a Spontaneously Immortalized. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]




| δ [ppm] | |||||||
|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | H-Me | |
| HP-β-CD | 5.0582 | 3.6362 | 3.9353 | 3.5774 | 3.8558 | 3.8558 | 1.1316 |
| H/HP-β-CD-(mech) | 5.0617 | 3.6367 | 3.9341 | 3.5803 | 3.8187 | 3.8535 | 1.1367 |
| H/HP-β-CD | 5.0603 | 3.6366 | 3.9304 | 3.5757 | 3.8230 | 3.8505 | 1.1362 |
| HHSB/HP-β-CD-(mech) | 5.0550 | 3.6363 | 3.9276 | 3.5748 | 3.8483 | 3.8483 | 1.1282 |
| HHSB/HP-β-CD | 5.0555 | 3.6374 | 3.9294 | 3.5494 | 3.8489 | 3.8489 | 1.1297 |
| HTSC/HP-β-CD-(mech) | 5.0545 | 3.6327 | 3.9316 | 3.5732 | 3.8521 | 3.8521 | 1.1271 |
| HTSC/HP-β-CD | 5.0684 | 3.6418 | 3.9463 | 3.5629 | 3.8661 | 3.8661 | 1.1419 |
| HIN/HP-β-CD-(mech) | 5.0517 | 3.6301 | 3.9293 | 3.5720 | 3.8490 | 3.8490 | 1.1243 |
| HIN/HP-β-CD | 5.0685 | 3.6468 | 3.9432 | 3.5858 | 3.8637 | 3.8637 | 1.1418 |
| pH 3.6 | pH 6.5 | pH 8.5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. | Compound/HP-β-CD | S0 [mM] | Kc [M−1] | CE [%] | Molar Ratio | S0 [mM] | Kc [M−1] | CE [%] | Molar Ratio | S0 [mM] | Kc [M−1] | CE [%] | Molar Ratio |
| 25 °C | Hesp | 0.108 ± 0.004 (0.05 ± 0.002) | 3221 ± 358 | 34.7 | 1:30 | 0.208 ± 0.11 (0.049 ± 0.002) | 11933 ± 1949 | 248 | 1:1 | 0.32 ± 0.032 (0.28 ± 0.002) | 2513 ± 143 | 80.4 | 1:3 |
| HTSC | 0.054 ± 0.03 (0.017 ± 0.006) | 13 ± 2.9 | 0.07 | 1:1430 | 0.061 ± 0.020 (0.011 ± 0.004) | 11 ± 1.6 | 0.07 | 1:1431 | 0.07 ± 0.03 (0.02 ± 0.001) | 12.5 ± 0.3 | 0.1 | 1:1001 | |
| HHSB | 0.041 ± 0.021 (0.018 ± 0.008) | 341 ± 79 | 1.4 | 1:72 | 0.045 ± 0.005 (0.025 ± 0.004) | 150 ± 21 | 0.7 | 1:144 | 0.045 ± 0.005 (0.025 ± 0.004) | 150 ± 19 | 0.7 | 1:144 | |
| HIN | 0.068 ± 0.04 (0.029 ± 0.009) | 102 ± 43 | 0.7 | 1:144 | 0.052 ± 0.024 (0.017 ± 0.003) | 39 ± 3.6 | 0.2 | 1:501 | 0.09 ± 0.05 (0.02 ± 0.007) | 143 ± 1.6 | 1.3 | 1:78 | |
| 37 °C | Hesp | 0.14 ± 0.04 (0.08 ± 0.008) | 3171 ± 887 | 44.4 | 1:3 | 0.110 ± 0.03 (0.085 ± 0.001) | 2280 ± 152 | 25 | 1:5 | 0.11 ± 0.01 (0.098 ± 0.005) | 1703 ± 163 | 19 | 1:6 |
| HTSC | 0.076 ± 0.035 (0.028 ± 0.02) | 16 ± 1.7 | 0.1 | 1:1001 | 0.068 ± 0.033 (0.027 ± 0.025) | 16 ± 1.7 | 0.1 | 1:1001 | 0.16 ± 0.054 (0.22 ± 0.003) | 18 ± 4.2 | 0.3 | 1:334 | |
| HHSB | 0.05 ± 0.01 (0.02 ± 0.01) | 226 ± 92 | 1.1 | 1:88 | 0.041 ± 0.023 (0.005 ± 0.004) | 618 ± 36 | 2.5 | 1:40 | 0.015 ± 0.0061 (0.005 ± 0.005) | 100 ± 34 | 0.15 | 1:668 | |
| HIN | 0.09 ± 0.05 (0.02 ± 0.006) | 145 ± 13 | 1.3 | 1:78 | 0.015 ± 0.009 (0.001 ± 0.0025) | 25 ± 3.9 | 0.04 | 1:2501 | 0.014 ± 0.01(0.001 ± 0.003) | 28 ± 3.9 | 0.04 | 1:2501 | |
| 60 °C | Hesp | 0.13 ± 0.02 (0.095 ± 0.002) | 2566 ± 144 | 34 | 1:4 | 0.096 ± 0.009 (0.082 ± 0.001) | 1179 ± 888 | 11 | 1:1 | 0.15 ± 0.027 (0.12 ± 0.002) | 2365 ± 221 | 37 | 1:4 |
| HTSC | 0.17 ± 0.065 (0.037 ± 0.002) | 28 ± 0.3 | 0.5 | 1:201 | 0.18 ± 0.06 (0.16 ± 0.05) | 2.2 ± 0.6 | 0.04 | 1:251 | 0.27 ± 0.089 (0.25 ± 0.006) | 3.2 ± 0.6 | 0.1 | 1:1001 | |
| HHSB | 0.036 ± 0.014 (0.012 ± 0.002) | 250 ± 20 | 0.9 | 1:112 | 0.115 ± 0.053 (0.027 ± 0.002) | 155 ± 4 | 1.8 | 1:57 | 0.096 ± 0.042 (0.018 ± 0.0009) | 728 ± 82 | 7 | 1:15 | |
| HIN | 0.041 ± 0.02 (0.008 ± 0.002) | 62 ± 1.7 | 0.3 | 1:334 | 0.041 ± 0.02 (0.008 ± 0.002) | 61 ± 2.6 | 0.3 | 1:334 | 0.283 ± 0.027 (0.143 ± 0.1) | 132 ± 23 | 3.7 | 1:28 | |
| Chemical Compound | IC50 [µg/mL] | |
|---|---|---|
| After 24 h | After 48 h | |
| Hesperetin | 220.9 | 150.0 |
| HTSC | Nd a | 11.9 |
| HHSB | 417.5 | 163.5 b |
| HIN | 350.4 | 59.7 |
| HP-β-CD | nd | nd |
| H/HP-β-CD | nd | 842.9 |
| H/HP-β-CDmech | nd | nd |
| HTSC/HP-β-CD | 710.8 | 160.4 |
| HTSC/HP-β-CDmech | 351.7 | 182.7 |
| HHSB/HP-β-CD | 166.7 | 12.3 |
| HHSB/HP-β-CDmech | 347.3 | 9.0 |
| HIN/HP-β-CD | 862.9 | 530.3 |
| HIN/HP-β-CDmech | 364.8 | 239.6 |
| Compounds | Zone of Inhibition [mm] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Hesperetin | HTSC | HHSB | HIN | HP-β-CD | H/HP-β-CD | H/HP-β-CD-m | HTSC/HP-β-CD | HTSC/HP-β-CD-m | HHSB/HP-β-CD | HHSB/HP-β-CD-m | HIN/HP-β-CD | HIN/HP-β-CD-m | |
| Escherichia coli ATCC 11303 | 9.5 ac ± 0.71 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 9.0 a ± 0.00 | 10.0 ac ± 0.00 | 10.0 ac ± 0.00 | 10.0 ac ± 0.00 | 10.0 ac ± 1.41 | 10.0 ac ± 0.00 | 9.0 ac ± 0.00 | 11.0 c ± 0.00 | 9.0 a ± 0.00 | 9.0 a ± 0.00 | |
| Escherichia coli ATCC 35218 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 9.0 a ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 9.0 a ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | 0.0 b ± 0.00 | |
| Listeria monocytogenes ATCC 19111 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Listeria monocytogenes ATCC 19112 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Listeria monocytogenes ATCC 19115 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Staphylococcus aureus ATCC 29737 | 0.0 c ± 0.00 | 10.0 ab ± 0.00 | 0.0 c ± 0.00 | 0.0c ± 0.00 | 12.0 a ± 0.00 | 9.0 ab ± 0.00 | 0.0 c ± 0.00 | 10.0 ab ± 1.41 | 9.5 b ± 0.71 | 10.0 ab ± 0.00 | 0.0 c ± 0.00 | 0.0 c ± 0.00 | 12.0 a ± 0.00 | |
| Staphylococcus aureus ATCC 25923 | 0.0 a ± 0.00 | 9.0 bd ± 0.00 | 12.0 c ± 0.00 | 11.0 bcd ± 0.00 | 8.5 d ± 0.71 | 9.0 bd ± 0.00 | 9.0 bd ± 0.00 | 8.5 d ± 0.71 | 9.5 bd ± 0.71 | 10.0 bcd ± 1.41 | 9.0 bd ± 0.00 | 9.5 bd ± 0.71 | 9.0 bd ± 0.00 | |
| Staphylococcus aureus ATCC 27734 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Salmonella Typhimurium ATCC 14028 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Salmonella Enteritidis ATCC 13076 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Salmonella Choleraesuis ATCC 7001 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| Compounds | MIC [µM] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | H | HTSC | HHSB | HIN | HP-β-CD | H/HP-β-CD | H/HP-β-CD mech | HTSC/HP-β-CD | HTSC/HP-β-CD-mech | HHSB/HP-β-CD | HHSB/HP-β-CD mech | HIN/HP-β-CD | HIN/HP-β-CD-mech | |
| Escherichia coli ATCC 11303 | 2.5 | nd | nd | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | |
| Escherichia coli ATCC 35218 | nd | nd | 2.5 | nd | nd | nd | nd | nd | 2.5 | nd | nd | nd | nd | |
| Staphylococcus aureus ATCC 29737 | nd | 2.5 | nd | nd | 2.5 | 2.5 | nd | 2.5 | 2.5 | 2.5 | nd | nd | 2.5 | |
| Staphylococcus aureus ATCC 25923 | nd | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sykuła, A.; Bodzioch, A.; Nowak, A.; Maniukiewicz, W.; Ścieszka, S.; Piekarska-Radzik, L.; Klewicka, E.; Batory, D.; Łodyga-Chruścińska, E. Encapsulation and Biological Activity of Hesperetin Derivatives with HP-β-CD. Molecules 2023, 28, 6893. https://doi.org/10.3390/molecules28196893
Sykuła A, Bodzioch A, Nowak A, Maniukiewicz W, Ścieszka S, Piekarska-Radzik L, Klewicka E, Batory D, Łodyga-Chruścińska E. Encapsulation and Biological Activity of Hesperetin Derivatives with HP-β-CD. Molecules. 2023; 28(19):6893. https://doi.org/10.3390/molecules28196893
Chicago/Turabian StyleSykuła, Anna, Agnieszka Bodzioch, Adriana Nowak, Waldemar Maniukiewicz, Sylwia Ścieszka, Lidia Piekarska-Radzik, Elżbieta Klewicka, Damian Batory, and Elżbieta Łodyga-Chruścińska. 2023. "Encapsulation and Biological Activity of Hesperetin Derivatives with HP-β-CD" Molecules 28, no. 19: 6893. https://doi.org/10.3390/molecules28196893
APA StyleSykuła, A., Bodzioch, A., Nowak, A., Maniukiewicz, W., Ścieszka, S., Piekarska-Radzik, L., Klewicka, E., Batory, D., & Łodyga-Chruścińska, E. (2023). Encapsulation and Biological Activity of Hesperetin Derivatives with HP-β-CD. Molecules, 28(19), 6893. https://doi.org/10.3390/molecules28196893










