A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Almond Skin (AS) and Decoction Preparation: Parameters Selection
2.2. DSA Preliminary Chemical Characterization and Markers Choosing
2.3. Preliminary DSA In Vitro Biological Activities and Safety
2.3.1. Antioxidant Assays
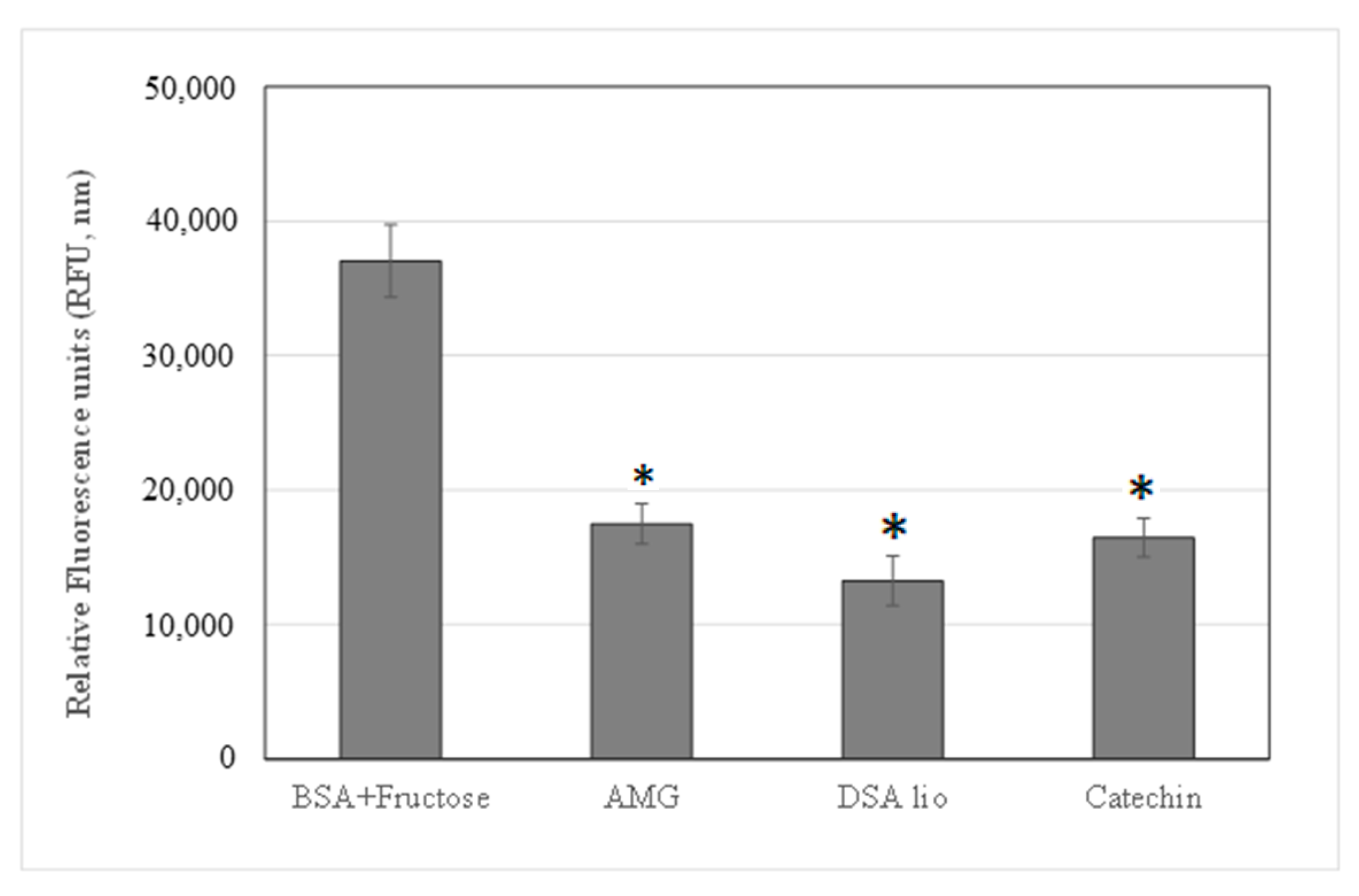
2.3.2. Advanced Glycation End Products (AGEs) and Metalloproteinases’ (MMPs’) Inhibition
2.3.3. DSA Safety Evaluation
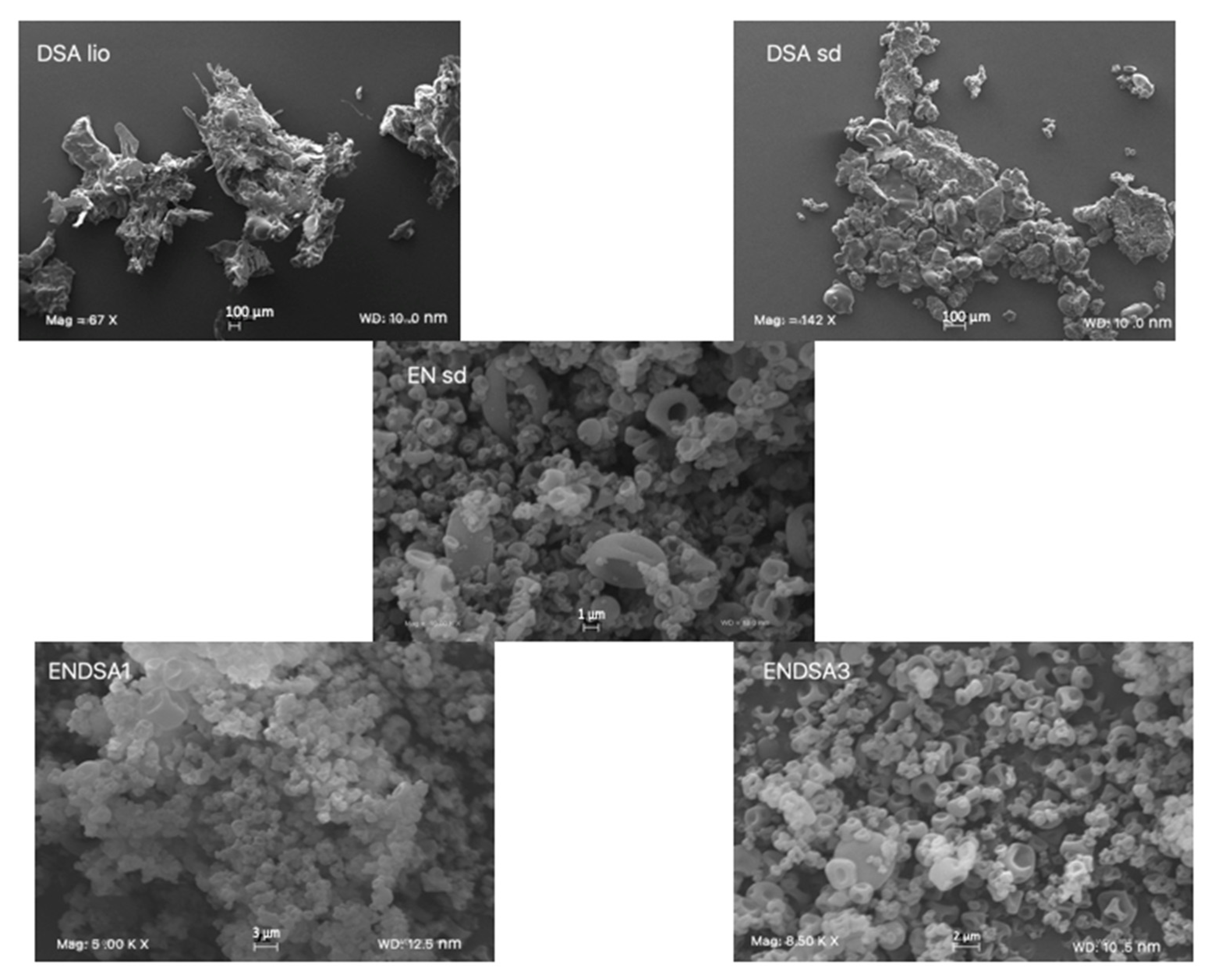
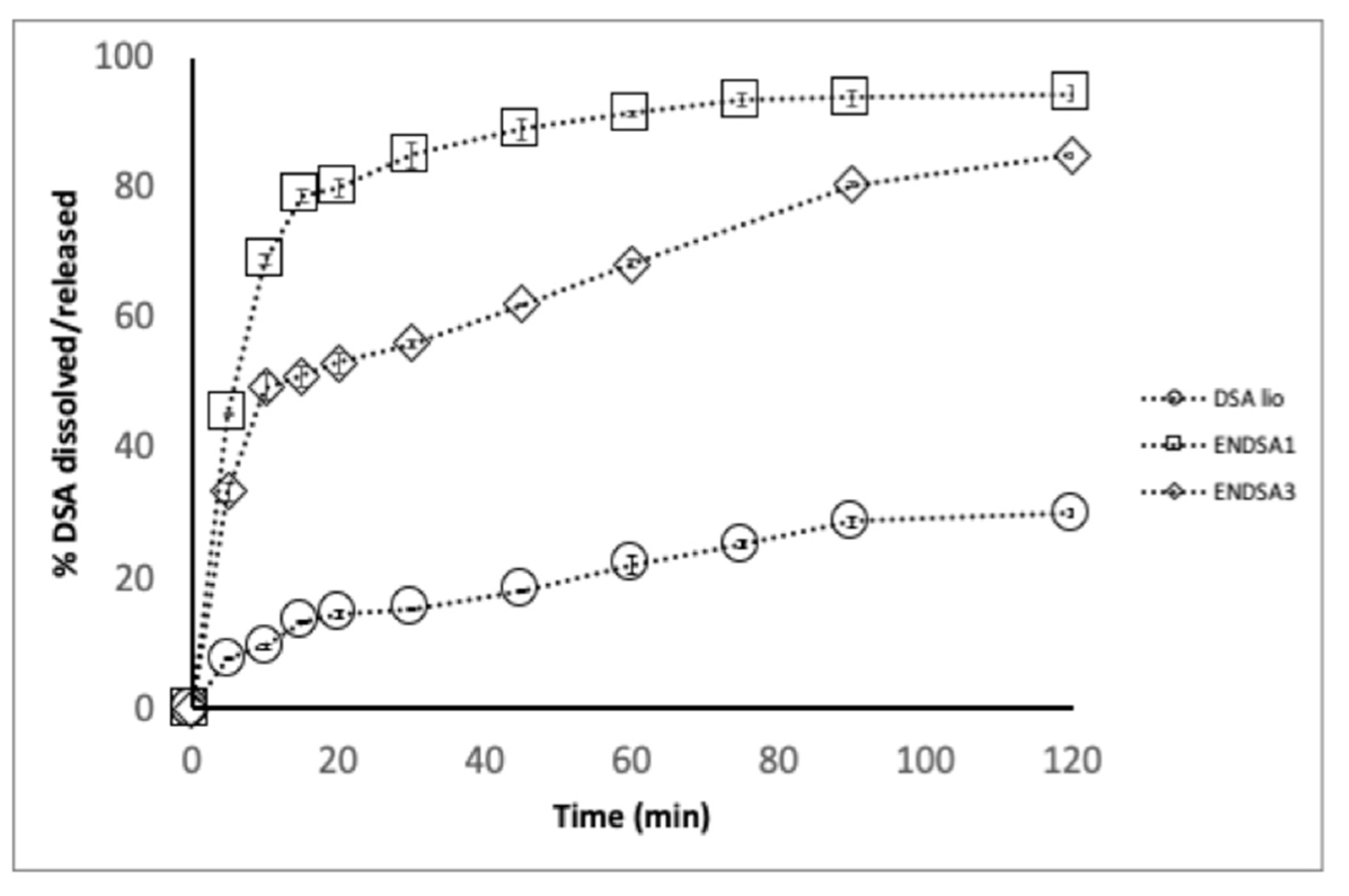
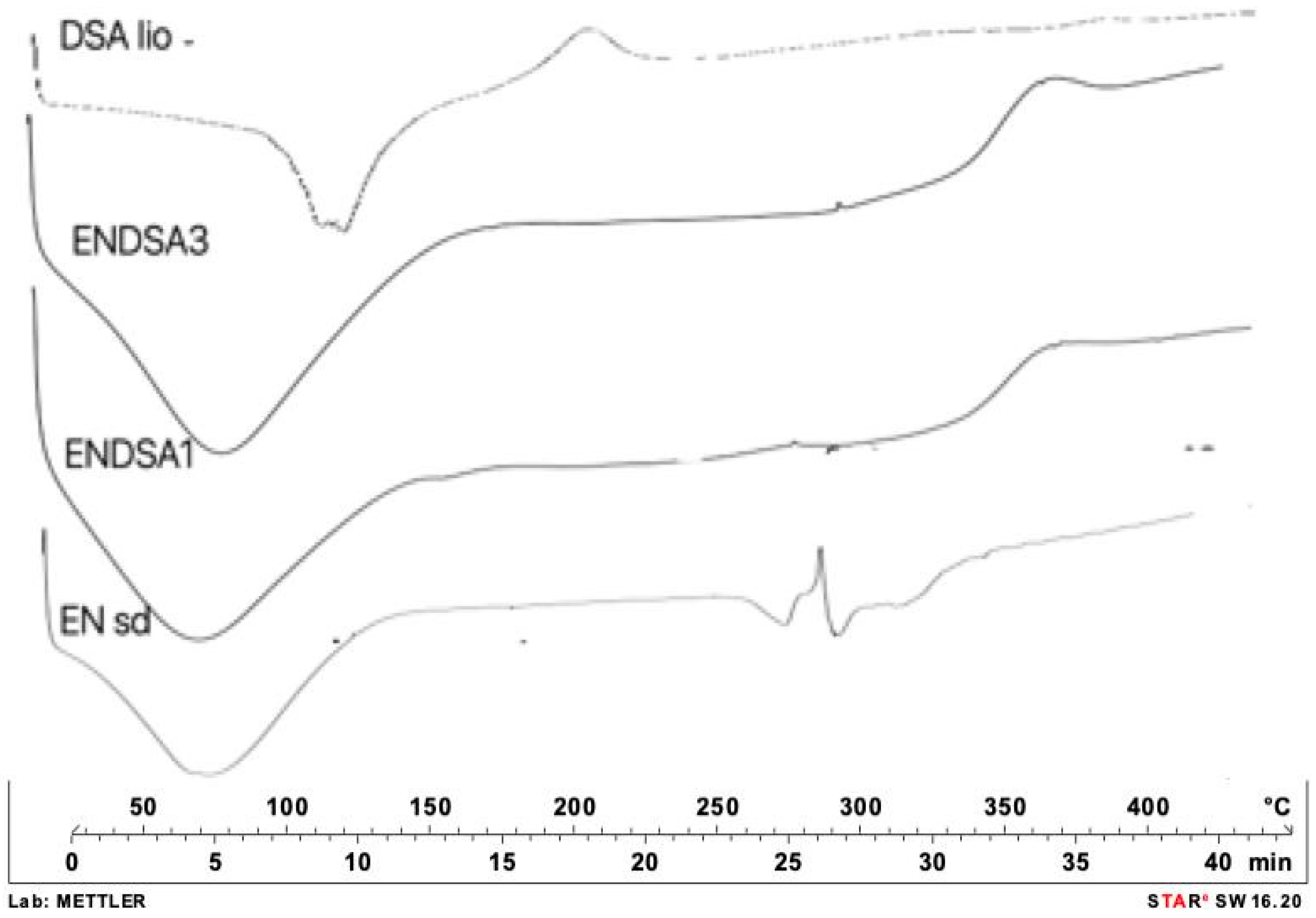
2.4. DSA and Microparticles Technological Characterization
2.4.1. DSA
2.4.2. Microparticle Characterization
2.5. Accelerate (ICH Guidelines) and Functional Stability
- -
- after the microencapsulation process (T0) to verify if the spray-dried process was able to keep DSA functional activity;
- -
- under harsh storage conditions (t7), as previously reported.
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extract Preparation
Dried Blanched Almond Skin Preparation and Relative Humidity Control
Decoction Procedure and Production Yields
3.2.2. Chemical Characterization
Chemical Profile and Quantitative Analysis of DSA
ESI-FT-ICR-MS Analysis
Quantitative Analysis Using HPLC Method and Total Phenols Content (TPC)
UV-Vis Method
3.2.3. In Vitro Biological Activity
Bleaching of the Free Radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH Test)
TEAC (Trolox Equivalent Antioxidant Capacity) Assay
ORAC Assay
Nitric Oxide (NO) Radical Scavenger Assay
Antiglycation Effect
MMP-2 and MMP-9 Inhibitory Assay
3.2.4. Cell Viability Assay
Cell Culture
Viability Assay
3.2.5. Statistical Analysis
3.2.6. Formulation Studies and Technological Characterization
Feed Preparation and Microparticle Preparation
Solubility Studies
In Vitro Dissolution Test
Morphology
Spray-Drying Process Yield (SPY), Actual Active Content (AAC), and Inclusion Efficiency (IE)
Fourier-Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Gu, L.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Dong, Q.; Banaich, M.S.; O’brien, P.J. Cytoprotection by almond skin extracts or catechins of hepatocyte cytotoxicity induced by hydroperoxide (oxidative stress model) versus glyoxal or methylglyoxal (carbonylation model). Chem. Interact. 2010, 185, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics 2020, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Ragno, A.; Cordaro, M.; D’amico, R.; Siracusa, R.; Fusco, R.; Merlo, F.M.; Smeriglio, A.; Impellizzeri, D.; Cuzzocrea, S.; et al. Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12115. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A.M. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Ahston Acton, Q. Fluoresceins. Patent Issued for Antioxidant Composition and methods Thereto in: Spiro Compounds. Adv. Res Appl 2013, 145, General Editor, Ahston Acton Q., Scholarly Editions TM, Atlanta, Georgia. Available online: https://www.google.it/books/edition/Spiro_Compounds_Advances_in_Research_and/3HUkiDFai7kC?hl=it&gbpv=1&dq=Spiro+Compounds:+Advances+in+Research+and+Application:+2013+Edition&printsec=frontcover (accessed on 25 November 2023).
- Lauro, M.R.; Crascì, L.; Sansone, F.; Cardile, V.; Panico, A.M.; Puglisi, G. Development and In Vitro Evaluation of an Innovative “Dietary Flavonoid Supplement” on Osteoarthritis Process. Oxidative Med. Cell Longev. 2017, 2017, 7503240. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2022, 63, 9816–9842. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Qiao, Z.; Sang, S.; Zhang, J.; Zhan, S.; Wu, Z.; Liu, L. Research advances of advanced glycation end products in milk and dairy products: Formation, determination, control strategy and immunometabolism via gut microbiota. Food Chem. 2023, 417, 135861. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Thebo, N.; Simair, A.; Sheikh, W.; Abbasi, A.R.; Nizamani, H. Clinical Study of the Prunus dulcis (Almond) Shell Extract on Tinea capitis Infection. Nat. Prod. Chem. Res. 2014, 2, 2–5. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Gravina, A.; Mereu, R.; Palumbo, I.; Randazzo, S.; et al. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in combined dyslipidemia. Clin. Investig. Med. 2009, 32, E124–E132. [Google Scholar] [CrossRef]
- EUDRAGUARD®Portfolio. Available online: https://healthcare.evonik.com/en/nutraceuticals/supplement-coatings/eudraguard-portfolio (accessed on 3 March 2023).
- Lauro, M.R.; Picerno, P.; Franceschelli, S.; Pecoraro, M.; Aquino, R.P.; Pignatello, R. Eudraguard® Natural and Protect: New “Food Grade” Matrices for the Delivery of an Extract from Sorbus domestica L. Leaves Active on the α-Glucosidase Enzyme. Pharmaceutics 2023, 15, 295. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Lapsley, K.; Ellis, P.R. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef]
- Available online: http://www.cargohandbook.com/index.php/Almonds (accessed on 20 April 2023).
- Kalogiouri, N.P.; Mitsikaris, P.D.; Klaoudatos, D.; Papadopoulos, A.N.; Samanidou, V.F. A Rapid HPLC-UV Protocol Coupled to Chemometric Analysis for the Determination of the Major Phenolic Constituents and Tocopherol Content in Almonds and the Discrimination of the Geographical Origin. Molecules 2021, 26, 5433. [Google Scholar] [CrossRef]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Timón, M.; Andrés, A.I.; Sorrentino, L.; Cardenia, V.; Petrón, M.J. Effect of Phenolic Compounds from Almond Skins Obtained by Water Extraction on Pork Patty Shelf Life. Antioxidants 2022, 11, 2175. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.; Hibbert, D.; Gülçin, İ.; Demirci Çekiç, S.; Güçlü, K.; Özyürek, M.; Çelik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Sarwar, R.; Farooq, U.; Khan, A.; Naz, S.; Khan, S.; Khan, A.; Rauf, A.; Bahadar, H.; Uddin, R. Evaluation of Antioxidant, Free Radical Scavenging, and Antimicrobial Activity of Quercus incana Roxb. Front. Pharmacol. 2015, 6, 277. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Hanssen, K.F.; Laakso, M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arter. Thromb. Vasc. Biol. 2005, 25, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Caimi, G. Matrix metalloproteinases in metabolic syndrome. Eur. J. Intern. Med. 2012, 23, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Natural Polymeric Compound Based on High Thermal Stability Catechin from Green Tea. Biomolecules 2020, 10, 1191. [Google Scholar] [CrossRef]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M.; et al. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2019, 35, 3095–3099. [Google Scholar] [CrossRef]
- Crascì, L.; Vicini, P.; Incerti, M.; Cardile, V.; Avondo, S.; Panico, A. 2-Benzisothiazolylimino-5-benzylidene-4-thiazolidinones as protective agents against cartilage destruction. Bioorg. Med. Chem. 2015, 23, 1551–1556. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio, G.P.; Straquadanio, C.; Crascì, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2017, 33, 843–850. [Google Scholar] [CrossRef]
- Kerbab, K.; Sansone, F.; Zaiter, L.; Esposito, T.; Celano, R.; Franceschelli, S.; Pecoraro, M.; Benayache, F.; Rastrelli, L.; Picerno, P.; et al. Halimium halimifolium: From the Chemical and Functional Characterization to a Nutraceutical Ingredient Design. Planta Med. 2019, 85, 1024–1033. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 5th ed.; Pharmaceutical Press: London, UK, 2011. [Google Scholar]
| Compounds | [M − H]− (m/z) (FT- ICR) | Mass Error (ppm) | Diagnostic MS/MS Ions (m/z) | Formula |
|---|---|---|---|---|
| gallic acid (1) | 169.01436 | −0.52 | 125.17 | C7H6O5 |
| procyanidin B3 (2) | 577.1359 | −1.30 | 425.10; 289.07 | C30H26O12 |
| procyanidin B1 (3) | 577.1357 | −1.0 | 425.10; 289.07 | C30H26O12 |
| catechin (4) | 289.0722 | −1.51 | 245.11; 205.08 | C15H14O6 |
| epicatechin (5) | 289.0721 | −1.38 | 245.11; 205.08 | C15H14O6 |
| procyanidin C1 (6) | 865.1987 | −0.19 | 577.13; 289.07 | C45H38O18 |
| kaempferol 3-O-rutinoside (7) | 593.1519 | −1.34 | 285.14 | C27H30O15 |
| isorhamnetin-3-O-rutinoside (8) | 623.1618 | −1.7 | 315.11; 300.01 | C28H32O16 |
| quercetin (9) | 301.0353 | −0.02 | 179.18; 151.18 | C15H10O7 |
| AAC a,b | ||||
|---|---|---|---|---|
| T0 | T7days | |||
| samples | procyanidin B3 | catechin | procyanidin B3 | catechin |
| DSA lio | A,c 1.3 ± 0.1 | A,c 0.9 ± 0.1 | A,d 1.8 ± 0.2 | A,d 0.1 ± 0.01 |
| ENDSA1 | A,c 1.3 ± 0.2 | A,c 0.8 ± 0.1 | B,c 1.4 ± 0.2 | B,c 0.7 ± 0.1 |
| ENDSA3 | A,c 1.4 ± 0.3 | A,c 1.0 ± 0.2 | B,c 1.4 ± 0.5 | B,c 0.8 ± 0.2 |
| Samples | DPPH Assay (SC50 = μg/mL) a | TEAC (mM TE/mg Extract or mM Compound) b | ORAC (ORAC Units = μmol TE/μg Sample) c | NO Scavenger (% Inhibition of NO) | ||||
|---|---|---|---|---|---|---|---|---|
| T0 | T7 | T0 | T7 | T0 | T7 | T0 | T7 | |
| DSA lio | A,e 211,6 ± 1,9 | A,f 232.8 ± 1.3 | A,e 0.27 ± 0.01 | A,f 0.24 ± 0.02 | A,e 4.23 ± 0.20 | A,f 3.50 ± 0.50 | A,e 63.12 ± 0.19 | A,f 56.51 ± 1.10 |
| ENDSA1 | B,e 203.2 ± 1.7 | B,e 207.3 ± 2.3 | A,e 0.30 ± 0.04 | B,e 0.29 ± 0.05 | A,e 4.26 ± 0.41 | B,e 4.20 ± 0.35 | A,e 65.10 ± 0.20 | A,e 63.81 ± 0.60 |
| ENDSA3 | B,e 202.0 ± 2.1 | B,e 206.8 ± 2.1 | A,e 0.28 ± 0.02 | B,e 0.27 ± 0.03 | A,e 4.25 ± 0.33 | B,e 4.19 ± 0.25 | A,e 64.01 ± 0.31 | A,e 62.8 ± 0.24 |
| CA | 5.9 ± 0.9 | 2.8 ± 0.50 | 2.00 ± 0.50 | 59.00 ± 0.42 | ||||
| α-tocopherol d | 10.1 ± 1.2 | |||||||
| BHT d | 0.36 ± 0.03 | |||||||
| Trolox d | 1.00 ± 0.07 | |||||||
| Curcumina d | 42 ± 0.28 | |||||||
| Samples | AGEs (% Inhibition) | MMP-2 (IC50 µg/mL) | MMP-9 (IC50 µg/mL) |
|---|---|---|---|
| DSA lio | 64.23 ± 0.23 | 8.10 ± 0.17 | 45.23 ± 0.35 |
| CA | 55.59 ± 0.45 | 22.23 ± 0.26 | n.a. |
| AMG | 56.83 ± 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picerno, P.; Crascì, L.; Iannece, P.; Esposito, T.; Franceschelli, S.; Pecoraro, M.; Giannone, V.; Panico, A.M.; Aquino, R.P.; Lauro, M.R. A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity. Molecules 2023, 28, 7913. https://doi.org/10.3390/molecules28237913
Picerno P, Crascì L, Iannece P, Esposito T, Franceschelli S, Pecoraro M, Giannone V, Panico AM, Aquino RP, Lauro MR. A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity. Molecules. 2023; 28(23):7913. https://doi.org/10.3390/molecules28237913
Chicago/Turabian StylePicerno, Patrizia, Lucia Crascì, Patrizia Iannece, Tiziana Esposito, Silvia Franceschelli, Michela Pecoraro, Virgilio Giannone, Anna Maria Panico, Rita Patrizia Aquino, and Maria Rosaria Lauro. 2023. "A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity" Molecules 28, no. 23: 7913. https://doi.org/10.3390/molecules28237913
APA StylePicerno, P., Crascì, L., Iannece, P., Esposito, T., Franceschelli, S., Pecoraro, M., Giannone, V., Panico, A. M., Aquino, R. P., & Lauro, M. R. (2023). A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity. Molecules, 28(23), 7913. https://doi.org/10.3390/molecules28237913









