Abstract
Diazo compounds are organic substances that are often used as precursors in organic synthesis like cyclization reactions, olefinations, cyclopropanations, cyclopropenations, rearrangements, and carbene or metallocarbene insertions into C−H, N−H, O−H, S−H, and Si−H bonds. Typically, reactions from diazo compounds are catalyzed by transition metals with various ligands that modulate the capacity and selectivity of the catalyst. These ligands can modify and enhance chemoselectivity in the substrate, regioselectivity and enantioselectivity by reflecting these preferences in the products. Porphyrins have been used as catalysts in several important reactions for organic synthesis and also in several medicinal applications. In the chemistry of diazo compounds, porphyrins are very efficient as catalysts when complexed with low-cost metals (e.g., Fe and Co) and, therefore, in recent years, this has been the subject of significant research. This review will summarize the advances in the studies involving the field of diazo compounds catalyzed by metalloporphyrins (M−Porph, M = Fe, Ru, Os, Co, Rh, Ir) in the last five years to provide a clear overview and possible opportunities for future applications. Also, at the end of this review, the properties of artificial metalloenzymes and hemoproteins as biocatalysts for a broad range of applications, namely those concerning carbene-transfer reactions, will be considered.
1. Introduction
Diazo compounds have been known since the synthesis of ethyl diazoacetate (EDA), which was the first aliphatic organic substance to have a diazo group, in 1883 by Curtius [1,2,3]. Since then, they have fascinated organic chemists due to their synthetic versatility through many bond-forming reactions (C−C, C=C, C−O, C−N, C−S, etc.), including the formation of carbocyclic and heterocyclic compounds. Reactions with diazo compounds involve the formation of intermediate carbenes (R1R2C:) that can be produced under different reaction conditions such as heating, light irradiation, or Lewis and Brönsted acids decomposition. Metal–carbene complexes or metallocarbenes (R1R2C=M) are carbenes bonded to a metal. The first of these metallocarbenes was discovered by Schrock [Ta(CH2tBu)3(CHtBu)] [4], and since then, it has expanded to many other metals, mainly to complexes of the early transition metals from groups 4–6, with the most diverse types of ligands and became important reactive synthons that were used in the most diverse syntheses. For a long time, and up to the present day, these compounds continue to be of interest for organic synthesis and have been reviewed by many researchers in scientific journals and books, mainly in using more efficient and selective catalysts [5,6,7,8]. The scope of reactions involving carbenes and metallocarbenes represents a significantly broader and more versatile approach in organic synthesis compared to reactions with diazo compounds [9,10,11,12]. Carbenes are species containing only two groups covalently bonded to a carbon and two free non-bonding electrons that can be spin-paired in the same orbital (singlet state) or unpaired in two separate orbitals (triplet state) [13]. Therefore, they are electrically neutral species that can simultaneously form two bonds. Metal–carbene complexes are species made up of carbenes that are covalently bonded to a metal, usually with expensive transition metals (M = Ru, Os, Co, Rh, Ir) [14,15]; however, there are several works with iron, an abundant metal, which is cheap, environmentally benign, and with low toxicity [16,17,18,19,20,21,22]. The chemical behavior of carbenes and metal–carbene complexes differ in reactivity and selectivity. Scheme 1 shows the reaction of carbenes and metal–carbene complexes with olefins, forming two C−C bonds in one step leading to cyclopropanes. Depending on the specific combination of initial diazo compounds and olefins, this reaction can yield a vast array of organic compounds. Moreover, beyond intermolecular reactions involving diazo compounds, they also demonstrate the potential to engage in diverse intramolecular reactions, including cyclopropanations, C−H, O−H, S−H, N−H bond insertions, and aromatic cycloaddition reactions.
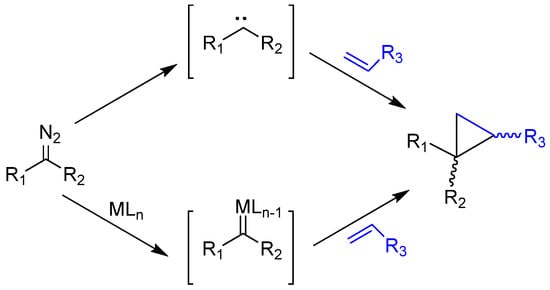
Scheme 1.
Synthesis of cyclopropanes involving carbenes or metal–carbene complexes.
Diazo compounds are used in syntheses that produce drugs, agrochemicals, pesticides, and derivatives that can be used to prepare other substances. The chemistry of these compounds is widely recognized precisely for having significant importance in total or partial syntheses. They are the best synthetic building blocks for required target molecules due to their high reactivity, versatility, multiplicity of reactions and preparation methods. As amphiphilic reagents, they can undergo geminal difunctionalization by replacing the corresponding diazo function with two new substituents, which are favored by the release of nitrogen [23]. In Scheme 2, some reactions are highlighted, among many others, that can be carried out with diazo compounds, under heating, catalysis with metals, Lewis and Bronsted acid catalysis, and photochemistry to form carbocycles and heterocycles. In Scheme 2, R1 can represent alkyl, aryl, H, CHO, CO2R, and R2 can also be alkyl, aryl, H, CHO, CO2R. Additionally, R1 can be different or equal to R2 in Scheme 2.
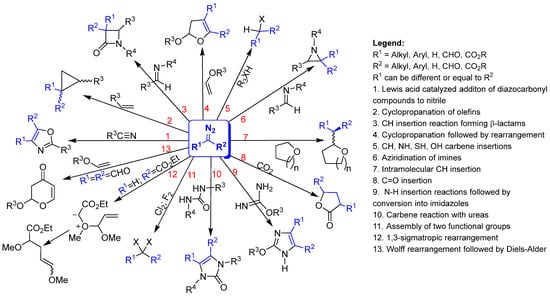
Scheme 2.
Some highlighted reactions that can be carried out with diazo compounds.
Discovered in the 1970s, transition metal carbene radicals (R1R2•C=M) turned out to be fundamental in contemporary catalysis, usually referred to as metalloradical catalysis (MRC). MRC flourishing applications led to the synthesis of countless organic compounds, from simple cyclopropanes to trickier eight-membered rings. So far, the emphasis has mostly been put on Co(II) based systems (R1R2•C=Co), which are highly appropriate for catalytic carbene transfer reactions [24,25,26,27].
2. Porphyrin-Based Catalysts
Porphyrins are macrocyclic organic compounds with a general structure with a tetrapyrrole macrocycle as the central part, the four pyrrolic subunits connected by methine bridges. The general skeleton of porphyrins has been extensively modified in several positions to adjust their reactivity, envisaging multiple applications, including catalysis (Figure 1).
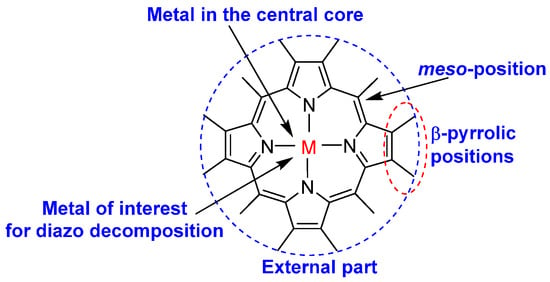
Figure 1.
General structure of the metalloporphyrins’ core.
The outer part of these heterocyclic macrocycles can present several topologies and numerous substitutions, whereas the central core has rigid planarity in several macrocycles (Figure 1). These molecules present structural arrangements that form cavities, which have enough space to accommodate several metal ions bound to the nitrogens in the central cavity. They perform important biochemical functions (e.g., myoglobin, hemoglobin, cytochromes P450) and have been used in medicinal chemistry, in catalysis for various reactions, for the inactivation of microorganisms, as detection probes and materials science. It has been known for many years that metalloporphyrins are important metal catalysts for various transformations. These metal-containing macrocycles react with diazo compounds and induce the formation of carbenes or metallocarbenes, which are helpful in various synthetic transformations. In this sense, diazo compounds are excellent reactants for interacting with porphyrins in inter- or intramolecular reactions and structural modifications in the outer part of the macrocyclic ring. As metalloporphyrins have a metal ion in the center of each macrocycle, they can form metallocarbene intermediates (mainly from diazo compounds) that can catalyze various reactions, such as inter- or intramolecular cyclopropanations, cyclopropenation reactions, carbene carbonylation, olefination of carbonyl compounds, formation of C−C, C−N, C−O, C−Si, C−S bonds, intramolecular Buchner reaction, among others [10,11,17,28,29,30,31,32].
3. Reactions through Unusual Strategies
Nitrogen heterocyclic chemical entities are present in many drugs used in clinical medicine. Any efficient method focusing on their efficient preparation is very important in Medicinal Chemistry. One of these prominent N-heterocycles is piperidine, which is present as a pharmacophore group in several drugs in the therapeutic arsenal. There are many attractive synthetic routes for the synthesis of piperidines and their analogues.
Based on recent syntheses of 5-membered N−heterocycles by metalloradical cyclization catalyzed by Co(II) porphyrins [33], de Bruin and collaborators reported a robust method with high global yields for the synthesis of piperidines directly starting from γ-amino substituted linear aldehydes. The reaction occurs by ring-closing C−C bond formation after the in-situ formation of N−tosylhydrazones from aldehydes. This is followed by in situ deprotonation of the hydrazones to afford the corresponding diazo compounds (Scheme 3). In this reaction, there is also the formation of linear alkenes as secondary products in small amounts [34]. It is crucial to emphasize that in the absence of the Co complex of meso-tetraphenyl porphyrin (CoTPP), only the diazo compound would be formed, and the cyclization product would not be obtained.
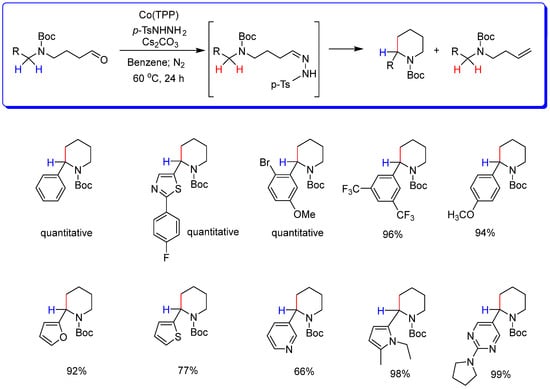
Scheme 3.
Piperidines synthesized from linear aldehydes and p-TsNHNH2 in one-pot synthesis catalyzed by Co(TPP) [33].
A unique route to prepare eight-membered rings was also developed by de Bruin and collaborators, based on metalloradical catalyzed reactions, to construct a series of novel dibenzocyclooctenes, monobenzocyclooctadienes and 8-membered heterocyclic enol ethers, which have been synthesized in good to excellent yields and with excellent substituent tolerance using Co(TPP) as a catalyst, thus producing cobalt(III)-carbene radicals as intermediates [35,36,37]. The metalloradical activation of N-tosyl hydrazones with Co(TPP) offered a novel route to build a series of dibenzocyclooctenes, in good to excellent yields through selective Ccarbene−Caryl cyclization (Scheme 4A).
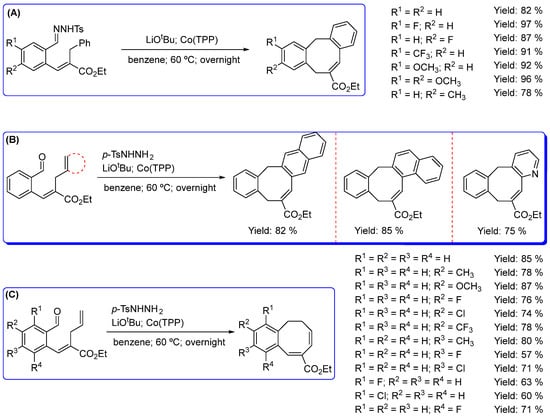
Scheme 4.
Metalloradical catalyzed reactions, using Co(TPP) as catalyst, for the synthesis of (A) several dibenzocyclooctenes from N-tosyl hydrazones as carbene precursor; (B) different dibenzocyclooctenes with in situ generation of the carbene precursor from o-aryl aldehydes and tosylhydrazide; (C) various monobenzocyclooctadienes with in situ generation of the carbene precursor from o-aryl aldehydes and tosylhydrazide [35,36].
Moreover, various aromatic substituents were tolerated on both benzene rings. The proposed mechanism consists of intramolecular hydrogen atom transfer (HAT) to Co(III)–carbene radical intermediates, followed by the dissociation of an ortho-quinodimethane that undergoes 8π cyclization. The presence of radical-type intermediates was confirmed by trapping experiments [35]. The protocol was extended to the synthesis of the novel monobenzocyclooctadienes that can be synthesized by ring-closure of o-aryl aldehydes containing bis-allylic C−H bonds. A different mechanism is present here, suggesting that ring-closure to the monobenzocyclooctadienes involves a direct radical-rebound step within the coordination sphere of cobalt, thus tolerating enantioselective formation of chiral monobenzocyclooctadienes (Scheme 4B,C) [36].
4. X−H Functionalization Catalyzed by Metalloporphyrins
The functionalization of C−H bonds with diazo compounds catalyzed by high-cost transition metals has been explored for many years, mainly with Rh, and many examples can be found in the literature [8,10,38,39]. However, developing efficient and sustainable reactions with non-precious metals is still of little use in processes involving diazo compounds; in this sense, iron, being an abundant and low-cost metal, is an excellent candidate for catalysis. Hock et al. [40] developed highly efficient functionalization reactions on C−H bonds by diazoacetonitrile with N-heterocycles catalyzed by the iron tetraphenylporphyrin Fe(TPP)Cl (Scheme 5). This protocol makes it possible to prepare important precursors of indole and indazole heterocycles. The authors investigated the possibility of this reaction going through a mechanism via free radicals, conducting the reaction in the presence of TEMPO. This classic radical scavenger completely inhibited the C−H functionalization reactions.
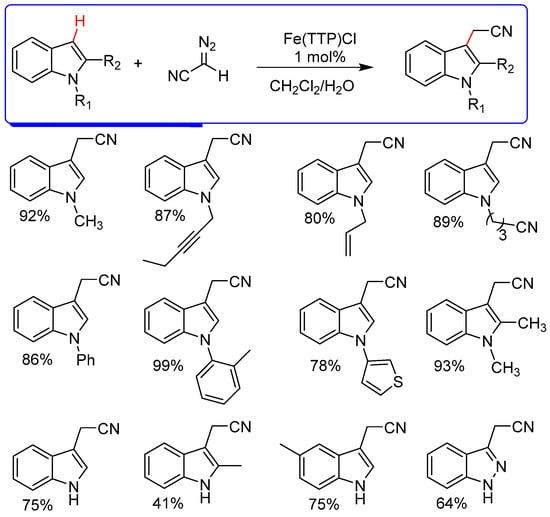
Scheme 5.
C−H functionalization of indole and indazole heterocycles with diazoacetonitrile catalyzed by Fe(TPP)Cl [40].
Fe(TPP)Cl also catalyzes the insertion of benzylic carbenes, generated in situ from N-tosylhydrazones derived from different benzaldehydes, into X−H (X = Si, Sn, Ge). Toluene and NaH were shown to be the best solvent and base, respectively. Silanes with tertiary, secondary, and primary Si−H bonds afforded the corresponding insertion products in moderate to high yields, and a stepwise double insertion strategy was developed to synthesize unsymmetrical tetrasubstituted silanes. Moreover, this reaction could be extended to Sn−H and Ge−H bonds, affording the insertion products in good to high yields (Scheme 6) [41].
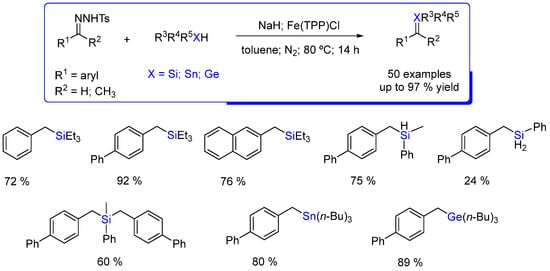
Scheme 6.
Fe(TPP)Cl catalyzed insertion reactions of in situ generated benzylic carbenes from N-tosylhydrazones into X−H (X = Si, Sn, Ge) [41].
Recently, the synthesis and characterization of several donor-acceptor iron porphyrin carbene complexes obtained from an iron porphyrin and the corresponding donor-acceptor diazo compounds were reported. Furthermore, the crystal structure of a donor-acceptor iron porphyrin carbene complex derived from a morpholine-substituted diazo amide was obtained. The carbene transfer reactivity of the iron porphyrin carbenes was studied for the N−H insertion reaction with aniline or morpholine (Scheme 7). However, the morpholine-derived amide complex could only react with aniline to deliver the corresponding N−H insertion product with very poor efficiency. Hence, iron porphyrin carbenes were identified as the real intermediates for iron porphyrin-catalyzed carbene transfer reactions from donor-acceptor diazo compounds [42].
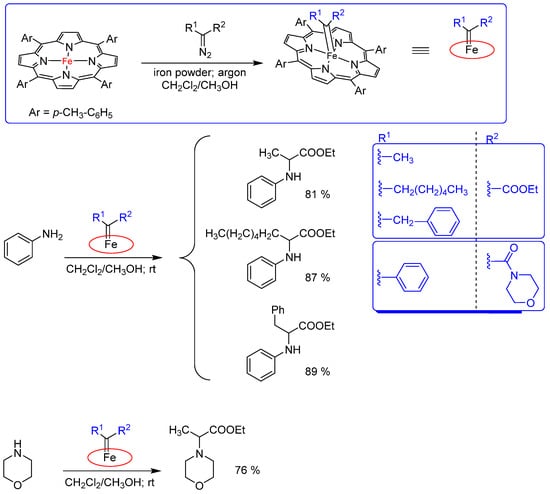
Scheme 7.
Synthesis of iron porphyrin carbene complexes and the corresponding catalyzed N−H insertion reactions with aniline or morpholine [42].
Phenols and naphthols are present in many natural products, dyes, pharmaceuticals, functional polymers, etc. These versatile building blocks readily engage in diverse transformations through conventional reactions and exhibit the unique ability to undergo insertion into vicinal C−H bonds adjacent to OH groups. The importance of these substances stimulates research into new direct functionalization strategies on the C−H bond in a chemoselective manner in the presence of other functionalities. For example, the chemo- and site-selective C−H functionalization of unprotected phenols and naphthols. However, this type of functionalization presents considerable challenges. Yu et al. developed a novel iron-catalyzed chemo and regioselective ortho C−H bond functionalization of phenols and naphthols with diazoesters. In this transformation, several iron porphyrins were used as catalysts, the best results being obtained for tetrakis-(2,4,6-trifluorophenyl)porphyrin chloride Fe(2,4,6-TFPP)Cl (Scheme 8) [43].
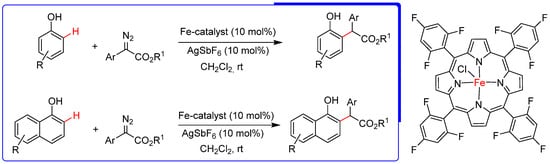
Scheme 8.
Iron catalyzed ortho C–H bond functionalization of phenols and 1-naphthols with α-aryl α-diazoesters [43].
Metalloporphyrin dialkylcarbene or bis(dialkylcarbene) complexes prepared from diazo compounds as carbene sources are rare in the literature and with little structural information by NMR spectroscopy and X-ray crystallography. They are generally difficult to use in synthetic methodologies due to their low stability. Furthermore, dialkylcarbene metalloporphyrin species may be prone to undergo a 1,2-hydride migration side reaction. However, Che and collaborators [44] were able to solve these problems by synthesizing stable porphyrin complexes of mono and bis(dialkylcarbene) group 8 metals (Fe, Ru, Os) with the linker adamantane (generated from photolysis of aziadamantane, a diazirine compound, as the carbene source). The steric effect of the bulky ligand on the porphyrin macrocycle makes the Fe−porphyrin complex an effective, active, and robust catalyst for the intermolecular transfer of diarylcarbene in reactions including cyclopropanation and S−H, N−H, O−H, and C(sp3)-H insertion. The authors synthesized several dialkylcarbene carbene complexes, but for this article, FeII(TPFPP) = adamantane (bright red solid) is highlighted in Scheme 9. Its preparation involves treating FeII(TPFPP) with aziadamantane at room temperature under UV irradiation (365 nm) for 15 min (Scheme 9).
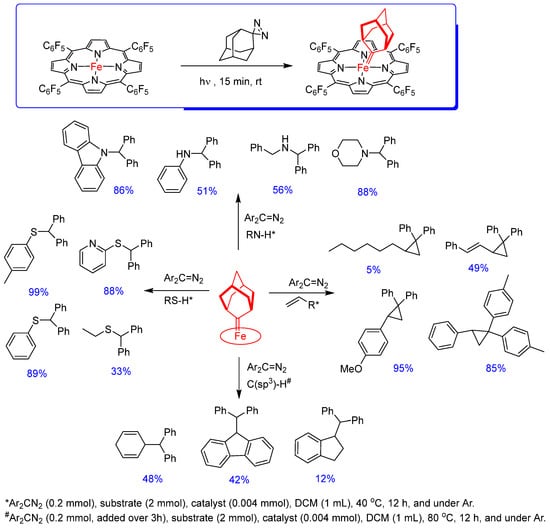
Scheme 9.
Examples of intermolecular diarylcarbene transfer reactions catalyzed by the iron(II) complex FeII(TPFPP) = adamantane [44].
The same research group described the Ir(III) porphyrin-catalyzed intermolecular C(sp3)−H insertion reaction of a quinoid carbene (QC), which showed to be efficient for the arylation of activated hydrocarbons such as 1,4-cyclohexadienes, thus giving functionalized phenol moieties anchored onto 1,4-cyclohexadienes (Scheme 10). Moreover, mechanistic studies point out a radical mechanism for these insertion reactions, this methodology being enabled by the hydrogen-atom transfer (HAT) reactivity of the Ir(III)-QC intermediate. The system exhibits distinctive regioselectivity, mainly ruled by steric effects since the insertion into primary C−H bonds is favored over secondary and/or tertiary C−H bonds in substituted cyclohexene substrates [45].
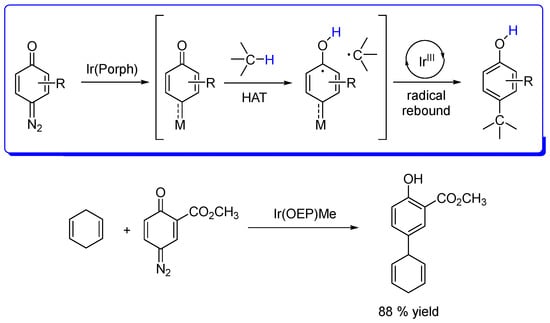
Scheme 10.
Direct C(sp3)−H arylation based on an Ir(III) porphyrin-catalyzed quinoid carbene (QC) intermolecular insertion reaction, illustrated for 1,4-cyclohexadiene as substrate [45].
Combining metallo- and photocatalysis, Ir(III) porphyrin-based porous MOFs catalyzed intermolecular C(sp3)−H insertion reaction under visible light was also reported, allowing the isolation and structural characterization of an Ir(III) porphyrin-carbene species. Different inert substrates, including cyclic (cyclopentane or cyclohexane) and acyclic (pentane, 2,3-dimethylbutane, 3-methylpentane or hexane) gave satisfactory yields both with ethyl diazoacetate (EDA) or with quinoid carbene (QC) (Figure 2) [46].
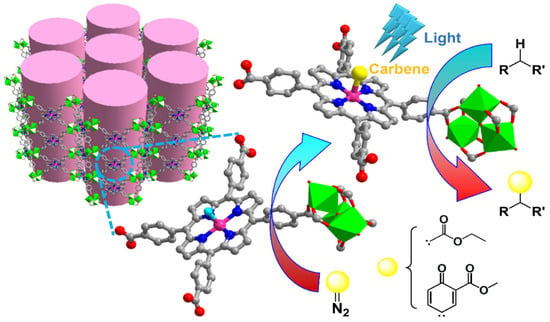
Figure 2.
Schematic of the Ir(III) porphyrin-based MOF for the photocatalytic activation of inert C(sp3)−H bonds via Ir(III) porphyrin carbene intermediate [46]. Reproduced with permission from the American Chemical Society.
Based on Ir(TCPP)Cl (TCPP = tetrakis(4-carboxyphenyl)porphyrin), the metal-organic framework (MOF), Ir-PMOF-1(Hf), which can resist acid conditions, has been examined in the O−H insertion reaction of carboxylic acids with diazo compounds, results showing that Ir-PMOF-1(Hf) is an efficient heterogeneous catalyst for these reactions (Scheme 11). Moreover, Ir-PMOF-1(Hf) maintained its framework after the catalytic reactions and could be recycled and reused for at least ten runs. For other carboxylic acids, the yields range from 57% after 10 min (for 2-hydroxybenzoic acid as substrate) to 91% after 6 min of reaction (for trifluoroacetic acid) [47].
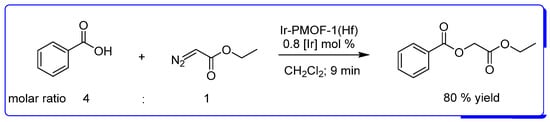
Scheme 11.
O−H insertion reaction of benzoic acid with EDA catalyzed by a heterogeneous MOF catalyst based on Ir(TCPP)Cl [47].
As reported by the same research group, the heterogeneous catalyst Ru-PMOF-1(Hf), based on Ru(TCPP)(CO) (TCPP = tetrakis(4-carboxyphenyl)porphyrin), evidenced catalytic efficiency for the N−H insertion reactions of EDA into a range of secondary amines with up to 92% yield (Scheme 12). Due to its 3D structure with orthogonal 1D open channels, Ru-PMOF-1(Hf) induces size selectivity, displaying an apparent yield-decreasing tendency along the chain lengthening (yield for diethylamine > dipropylamine > dibutylamine > dipentylamine). Moreover, Ru-PMOF-1(Hf) could be recovered and reused for at least ten runs with negligible loss of catalytic activity [48].
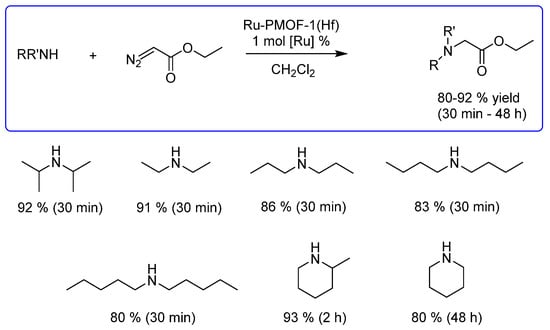
Scheme 12.
N−H insertion reaction of secondary amines with EDA catalyzed by a heterogeneous MOF catalyst based on Ru(TCPP)(CO) [48].
The development of metalloporphyrin-based capsules showed to be a promising strategy to create nanoreactors, real nanoscale chemical environments where chemical transformations can occur, thus changing the reactivity of molecules upon binding inside the cavity, for example, in carbene transfer/insertion reactions. A recent example deals with a Ru(II) porphyrin-based molecular nanoreactor, bearing a stable and inert diphenylcarbene axial ligand as a catalyst in selective N−H carbene insertion reactions, where no signs of dimerization side processes nor double insertions were observed in the rotaxane assembly reaction, hence leading to the quantitative formation of rotaxanes by active-metal-template synthetic methodology. Only the internal axial position of the Ru(II) catalyst is available for activation of the substrates, explaining the registered high selectivity for rotaxanes (Scheme 13) [49].
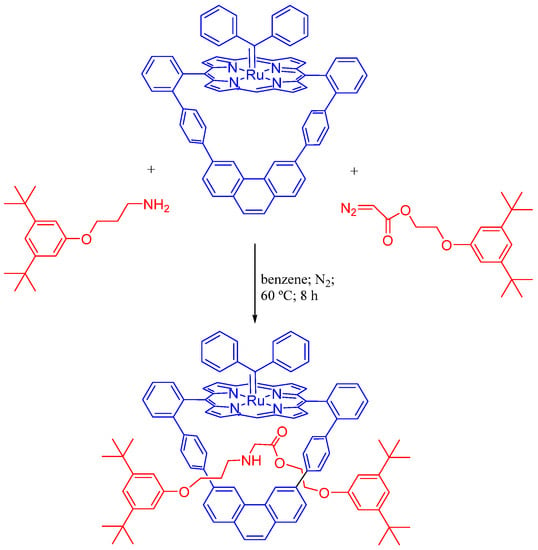
Scheme 13.
Synthesis of asymmetrical rotaxanes by the active-metal template methodology based on N−H carbene insertion reactions catalyzed a Ru(II) porphyrin [49].
5. Cyclopropanation Reactions Catalyzed by Metalloporphyrins
Of all the reactions using diazo compounds, cyclopropanations (Scheme 1) have been the most studied as they simultaneously form two C−C bonds. Cyclopropanes are very useful building blocks in organic synthesis and the best method for their synthesis is, via one-pot reaction, the addition of carbene (generated by several methods) to an olefin. Versatility regarding olefins and diazo compounds is quite diverse, generating many different compounds [38,50,51,52,53].
There are reports in the literature that hydrophobic pockets can accelerate cyclopropanation reactions. For example, a hybrid system of cationic iron porphyrin and DNA accelerates the cyclopropanation reactions due to the concentration of reagents occupying hydrophobic spaces close to DNA [54]. Considering the premise that DNA acts similarly to a micelle, Roelfes and collaborators [55] studied the combination of cationic Fe-porphyrins with anionic surfactants, such as sodium dodecyl sulphate (SDS), in concentrations above their critical micelle concentrations in water (Scheme 14). It was concluded that micellar catalysis with surfactants eliminates the need for organic solvents and accelerates the cyclopropanation reaction of p-methoxystyrene with ethyl diazoacetate (the carbene precursor) with iron porphyrins. Moreover, the catalytic cyclopropanation of other styrene derivatives with different diazoacetates was considerably accelerated in the presence of the cationic iron porphyrin bearing four para-N-methylpyridinium groups at the meso-positions. In most of the cases, without the addition of surfactant, the reaction yield was below 5%. Highlighted in Scheme 14 are some examples and the best catalysts that can be used for these reactions. The acceleration of these micellar reactions, similar to the cyclopropanation reactions catalyzed by heme and DNA enzymes, suggests that the main effect is the increase in molarity within the hydrophobic cavities.
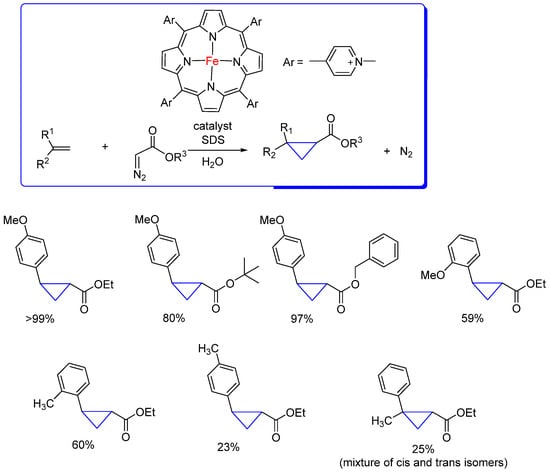
Scheme 14.
Cyclopropanation reaction of styrene derivatives with diazo compounds in the presence of a cation iron-porphyrin under micellar catalysis conditions [55].
Asymmetric cyclopropanation reactions using chiral catalysts and catalyzed N−H insertion reactions in the presence of diazo compounds are well-established methodologies. However, reactions using organometallic diazo derivatives are still a challenge, as the influence of the metal complex on the course of the reaction with these diazo compounds is not well understood. Specifically, the influence of the ferrocene group is not known in these reactions, nor the best reaction conditions. To investigate the influence of the ferrocenyl metal complex, Simonneaux and collaborators [56] investigated (1) the asymmetric cyclopropanation of styrene derivatives with diazoacetylferrocene in the presence of the Halterman ruthenium chiral porphyrin (Scheme 15); (2) N−H insertion of aminoesters with diazoacetylferrocene catalyzed by Fe(TPP)Cl (Scheme 16).
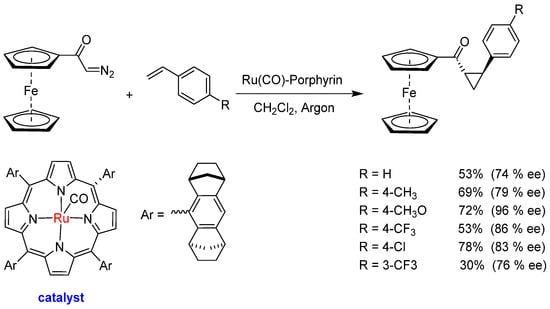
Scheme 15.
Asymmetric cyclopropanation of olefins by diazoacetylferrocene catalyzed by a Ru(CO)−porphyrin.
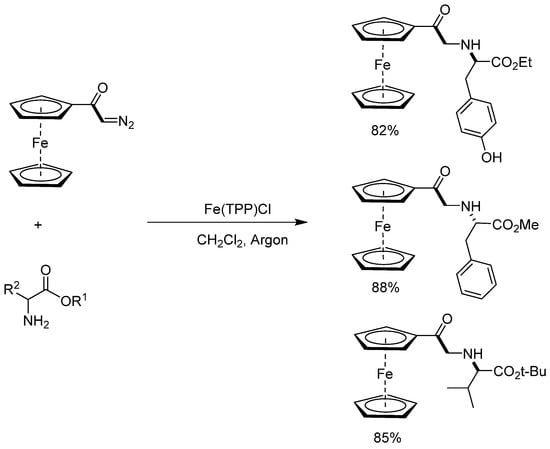
Scheme 16.
N–H insertion of aminoesters by diazoacetylferrocene catalyzed by Fe(TPP)Cl.
In asymmetric cyclopropanation, the reaction between diazoacetylferrocene and different styrene was used to form the corresponding optically active ferrocenyl 2-arylcyclopropyl ketones (30–78% yield). The ferrocene group remained intact in the final cyclopropyl ketones, and the enantiomeric excesses found for the trans-isomer stayed between 74% and 96%. The N−H insertion reaction between diazoacetylferrocene and aminoesters was catalyzed by the tetraphenylporphyrin iron chloride Fe(TPP)Cl. The insertion is regioselective onto the NH2 group and occurs in high yields (82–87%), being chemoselective even in the presence of the O−H group of tyrosine.
Asymmetric cyclopropanation is one of the areas of the chemistry of diazo compounds that has evolved the most over the last few years through Rh and Ru-based catalysts [10,38,39]. However, there is always room to create new chiral catalysts and new reaction pathways to improve chiral induction under more favourable conditions [57]. Gallo and collaborators reported the synthesis of iron and ruthenium glycoporphyrins and their catalytic activity in cyclopropanation reactions by using diazo compounds as carbene precursors, thus concluding that the number and location of carbohydrate units (a cellobioside) on the porphyrin skeleton modulate the diastereoselectivity of the reactions. However, none of the complexes studied induced enantiocontrol, probably due to the long distance between the chiral carbohydrates and the active metal centre [58].
Zhang and collaborators [59] developed the asymmetric radical cyclopropanation of alkenes using N-arylsulfonyl hydrazones (as diazo precursors) to generate metalloradicals from chiral Co−porphyrins, followed by their insertion into the double bond of the alkene. This reaction produced cyclopropanes in high yields with effective control of diastereo- and enantioselectivity. Following this line of research, Zhang, and collaborators [57,60] also developed a highly asymmetric system for radical cyclopropanation with asymmetric diazomalonates. The asymmetric reaction of 1,1-cyclopropanediesters was quite effective with several types of alkenes. The study of the mechanism of this reaction indicated that it happens via metalloradical catalysis (MRC). These optically active 1,1-cyclopropanediesters are important chiral building blocks in organic syntheses. The catalysts for the reactions are chiral Co−porphyrins containing chiral groups in an arrangement with D2 symmetry. The products obtained from 1,1-cyclopropanation can react with alkenes with various functional groups (Scheme 17).
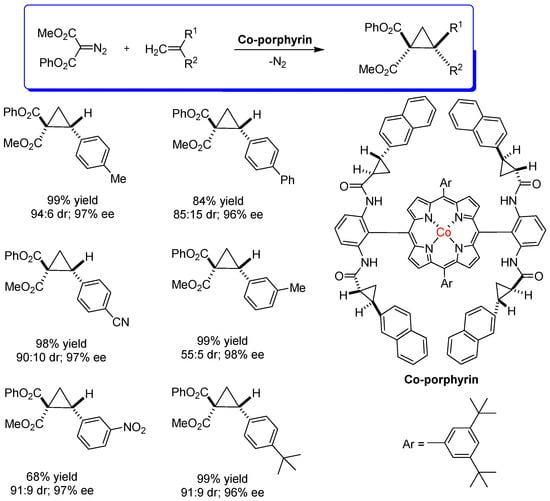
Scheme 17.
Asymmetric radical cyclopropanation of styrenes with diazomalonates catalyzed by chiral Co−porphyrins via metalloradical catalysis (MRC) [60].
The origin of enantioselectivity is due to the non-covalent interactions of the catalytic system, which is very efficient in various styrene derivatives, regardless of the electronic nature of the aromatic alkene used. The reaction yields were high (up to 99%), as diastereoselectivity (ranged up to 94:6 dr) and enantioselectivity (up to 97% ee). Increasing steric hindrance did not affect the enantioselectivity of the reaction, but it did affect the reaction yield and diastereoselectivity. Unsaturated substrates reacted well but under different catalytic conditions.
Entities that have cavities or cages can function as catalysts and carry out selective reactions inside them through non-covalent host-guest interactions, or the cavities can allow the encapsulation of catalysts, in some cases enhancing their reactivity. This is how most enzymes catalyze reactions in their cavities, and because of the supramolecular interactions of substrates within these cavities, they exhibit high specificity and efficiency [61]. Many natural products, such as chiral cyclodextrins, have different cavity sizes and catalyze reactions (e.g., β-cyclodextrin). Many of these supramolecular entities were designed and synthesized to mimic the structure and functionality of discrete coordination enzymes to accelerate substrate-specific reactions and manipulate regio- and enantioselectivity [62]. In this line of research, the calixarene macrocycle can be highlighted, which has phenolic units connected by methylene bridges in the ortho-positions relative to the hydroxyl group. These caged entities combine a hydrophobic cavity and a hydrophilic external surface that includes various substrates, accommodating metallic compounds and catalyzing reactions with results that surpass reactions with different types of catalysis [63].
Mouarrawis et al. were able to synthesize three cubic cages with different exopolarities attributed to the different groups on the periphery (Figure 3). These cages were used as hosts to encapsulate the catalytic active cobalt(II) meso-tetra(4-pyridyl)porphyrin, the guest. The resulting caged catalysts were studied in the cobalt-catalyzed cyclopropanation reaction of styrene with EDA (Figure 3) involving cobalt-carbene radical intermediates. The exofunctionalized cage catalyst with apolar icosyl groups evidenced higher activity than the non-functionalized or the polar (PEG-4) exofunctionalized counterparts. However, the polar PEG exofunctionalized catalyst evidenced higher selectivity for the cyclopropane product than the non-functionalized or the apolar icosyl exofunctionalized catalyst. On the other hand, encapsulation of the cobalt(II) meso-tetra(4-pyridyl)porphyrin guest into the cage with apolar icosyl groups led to a three times more active catalyst than Co(TPP) and a significantly increased TON if compared to both Co(TPP) and non-encapsulated cobalt(II) meso-tetra(4-pyridyl)porphyrin. According to the authors, the increased local concentration of the substrates in the hydrophobic cage compared to the bulk explains the higher catalytic activities registered [64,65].
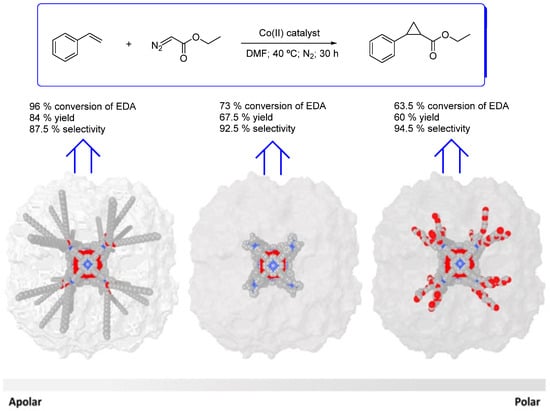
Figure 3.
Modeled structures of the apolar cage (functionalized with 24 icosyl groups in the periphery; left), the non-functionalized cage (middle), and the polar cage (functionalized with 24 PEG-4 groups in the periphery; right), showing their inner cavity (red) and the different peripheral substituents (grey) [64,65]. Reproduced with permission from John Wiley and Sons.
Fischer and collaborators reported a catalytic process where the diastereoselectivity remarkably rests on specific local confinement effects, which can be modified by the careful choice of the MOF catalyst. The heterogeneous porphyrin-based MOF catalysts, PCN-222(Rh) and PCN-224(Rh) (Figure 4), that contain no stereocenters, were studied in the diastereoselective cyclopropanation of different styrenes with EDA, demonstrating good catalytic activity. When styrene and other non-coordinating olefins were the substrates, no diastereoselectivity was registered. Remarkably, styrenes carrying coordinating amino and hydroxy groups exhibited a high diastereomeric ratio (dr) of up to 23:1 (trans:cis) over PCN-224(Rh), which was attributed to substrate coordination to neighboring Rh centers caused by local cavity confinement effects. For 4-aminostyrene, the diastereoselectivity was increased to a dr of 42:1 (trans:cis) over PCN-222(Rh), a structural analogue of PCN-224(Rh), although featuring shorter Rh–Rh distances, that is 9.7 Å for PCN-222(Rh) and 13.6 Å for PCN-224(Rh) [66].
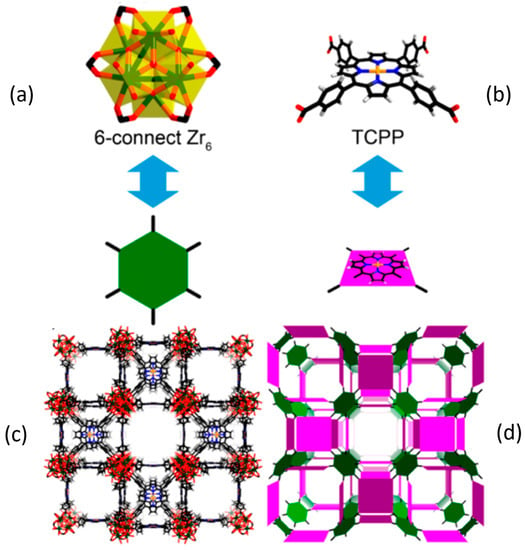
Figure 4.
Crystal structure, structural components, and underlying network topology of PCN-224(Rh): (a) the 6-connected D3d symmetric Zr6 in PCN-224. (b) TCPP ligands (violet square) with twisted dihedral angles generate a framework with 3-D nanochannels (c,d). Color scheme: Zr, green spheres; C, gray; O, red; N, blue; Rh, orange; and H, white [67]. Adapted with permission from J. Am. Chem. Soc. 2013, 135, 17105−17110. Copyright 2013 American Chemical Society.
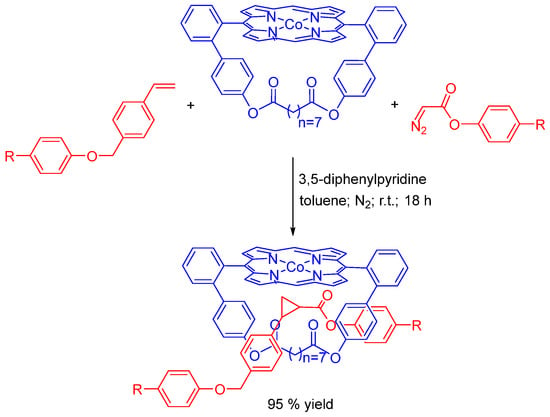
Olefin cyclopropanation was studied in the synthesis of rotaxanes by radical carbene transfer reactions promoted by Co(II) porphyrin-based semi-rigid macrocycle, and the highest yield (95%) was obtained in the presence of 3,5-diphenylpyridine as axial ligand. The active-metal-template strategy, which includes the radical-type activation of ligands by the cobalt ion of the porphyrin, is the basis for the reported methodology (Scheme 18) [68,69].
Scheme 18.
Synthesis of rotaxanes promoted by Co(II)−porphyrins using the radical-carbene-transfer cyclopropanation reaction as a metal-active-template methodology [68,69].
6. Catalytic Properties of Metalloenzymes and Hemoproteins
Metalloporphyrins are recognized as useful supports for oxene, carbene, and nitrene transfer reactions. The development of artificial hemoproteins, which can be seen as non-natural oxene, carbene, and nitrene transferases, was inspired by natural heme monooxygenases. Hence, these activities were originally revealed by testing hemoproteins for their ability to mimic the identified activities of metalloporphyrin catalysts [70,71]. Being aware that the first evidence of hemoproteins acting as biocatalysts for carbene transfer reactions was published online on 12 December 2012 [72], we may realize why engineered metalloenzymes and hemoproteins (e.g., myoglobin, cytochrome P450s) only recently arose as a highly promising tool to create biocatalysts for a broad range of applications involving non-native substrates, namely those concerning carbene-transfer reactions [20,70,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
One example is the cyclopropanation of 5-chloropent-1-ene with diazoacetone to produce the corresponding cyclopropyl ketone in the presence of hemoprotein biocatalysts derived from thermophilic bacterial globins (Scheme 19a). These biocatalysts were developed as variants of the mutant heme protein derived from the thermophile Methylacidophilium infernorum (named Hell’s Gate globin I–HGG), reaching high diastereoselectivity (cis/trans ratio up to 1:99) and good enantioselectivity for (1R,2R) enantiomer (75% ee) [77].
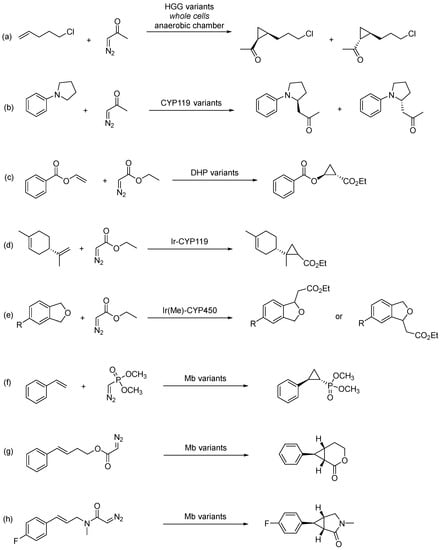
Scheme 19.
Transformations involving carbene-transfer reactions catalyzed by artificial biocatalysts.
In recent years, Fasan and co-workers have demonstrated the ability of engineered metalloenzymes for several efficient and selective biocatalytic transformations. The development of an iron-based biocatalyst for enantioselective α-C−H functionalization of pyrrolidines, via carbene transfer reaction with diazoacetone, is a possible strategy (Scheme 19b). This transformation could be achieved in high yields, high catalytic activity, and high stereoselectivity (up to 99% ee and over 20,000 TON) using engineered variants of CYP119 from Sulfolobus solfataricus [87]. Furthermore, an engineered dehaloperoxidase (DHP) enzyme (from Amphitrite ornata) was used as carbene transferase for the stereoselective synthesis of cyclopropanol esters (with up to 99% de and ee) through the biocatalytic asymmetric cyclopropanation of vinyl esters with EDA (Scheme 19c) [88].
More recently, an engineered artificial metalloenzyme containing an Ir–porphyrin complex was reported. In this work, E. coli cells expressing Ir–CYP119 catalyzed the cyclopropanation of (−)-limonene with high diastereoselectivity. So, by using a heterologous heme transport system, the authors constructed an artificial biosynthetic pathway incorporating an Ir-porphyrin-based metalloenzyme in E. coli (Scheme 19d) [79]. The same research group described artificial metalloenzymes generated from the combination of a CYP450 scaffold and an Ir-porphyrin cofactor that catalyze the intermolecular insertion of carbenes into the C−H bonds of a range of phthalan derivatives containing substituents that render the two methylene positions in each phthalan nonequivalent (Scheme 19e). These reactions occur with site selectivity ratios of up to 17.8:1 and, in most cases, with pairs of enzyme mutants that preferentially form each of the two constitutional isomers [89]. Finally, the authors have shown that the non-pathogenic E. coli strain Nissle 1917 (EcN), possessing a genetically encoded transport system, is a suitable host for the efficient uptake of an Ir-porphyrin complex and the in vivo assembly of Ir-CYP119. This strain enabled stereoselective and site-selective functionalization by carbene insertion into benzylic C−H bonds of phthalan derivatives catalyzed by an artificial metalloenzyme in whole cells. It was shown to accelerate the directed evolution of Ir-CYP119 by enabling high-throughput screening of reactions with new substrates in whole cells [90].
The intermolecular cyclopropanation reaction using a phosphorus-containing diazo compound [dimethyl(diazomethyl)phosphonate)] as carbene precursor, developed by Fasan and co-workers, was considered to be the first example of an efficient and enantioselective synthesis of enantioenriched cyclopropylphosphonate esters (up to 99% de and ee for the (1S,2R) stereoisomer), catalyzed by myoglobin-based biocatalysts (Mb variants; Scheme 19f) [91]. Using also engineered myoglobin catalysts, the same research group reported the cyclopropanation of α-difluoromethyl alkenes in the presence of EDA, affording CHF2-containing cyclopropanes in high yield (up to >99%) and with high stereoselectivity (up to >99% de and ee) [92], along with the construction of 2,3-dihydrobenzofurans in high enantiopurity (>99.9% de and ee) and high yields via benzofuran cyclopropanation [93]. Additionally, a myoglobin-based carbene transferase incorporating a non-native iron-porphyrin cofactor and axial ligand showed to be highly efficient as a catalyst for the asymmetric cyclopropanation of electron-rich and electron-poor alkenes, reaching high diastereo- and enantioselectivity (up to >99% de and ee). Mechanistic studies showed that the reaction depends on radical-type carbene-transfer reactivity due to the reconfigured primary coordination sphere around the iron center [94].
Moreover, biocatalysts derived from sperm whale myoglobin proved to be active for carbene transfer in the asymmetric synthesis of fused cyclopropane-δ-lactones via intramolecular cyclopropanation of homoallylic diazoacetates in high yields and with up to 99% ee (Scheme 19g) [95], and in the asymmetric synthesis of fused cyclopropane-γ-lactams via cyclization of allyl diazoacetamides into the corresponding bicyclic lactams, as can be seen in Scheme 19h for (E)-2-diazo-N-(3-(4-fluorophenyl)allyl)-N-methylacetamide, in high yields and up to 99% ee [96].
Most recently, Arnold and co-workers reported that engineered hemoproteins derived from a bacterial cytochrome P450 can catalyze the synthesis of chiral 1,2,3-polysubstituted cyclopropanes, regardless of the stereo purity of the olefin substrates used. Cytochrome P450BM3 variant P411-INC-5185 exclusively converts (Z)-enol acetates into enantio- and diastereo-enriched cyclopropanes if starting from mixtures of (Z/E)-olefins. So, in the end, the reaction delivers a leftover (E)-enol acetate with 98% stereo purity (Scheme 20a) [97].
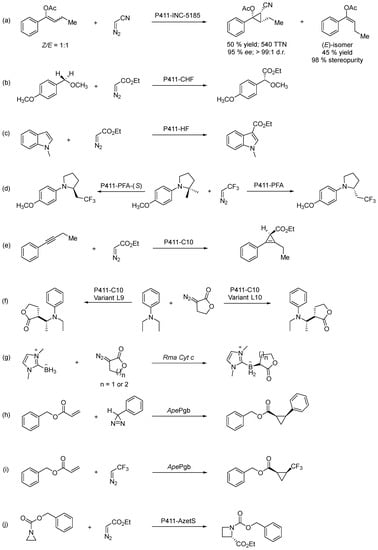
Scheme 20.
Transformations involving carbene-transfer reactions catalyzed by engineered enzymes.
This very recent work is just the extension of a path that began a decade ago in Professor F. Arnold’s research lab, which gave rise to a remarkable journey based on directed evolution, offering new meanings to the world of enzymatic catalysis, of which we highlight below some of the most recent discoveries. One example is the results obtained for the construction of C−C bonds through sp3 C–H functionalization, achieved by using E. coli expressing cytochrome P411-CHF iron-based catalyst, derived from a cytochrome P450 enzyme in which the native cysteine axial ligand was substituted by serine (cytochrome P411). This engineered iron-based catalyst evidenced enantio-, regio- and chemoselectivity for the intermolecular alkylation of sp3 C–H bonds through carbene C–H insertion (Scheme 20b) [98]. On the other hand, the P411-HF variant stood out as a highly active alkylation enzyme catalyst for the alkylation of indoles, as exemplified for 1-methylindole (Scheme 20c). Moreover, no N−H insertion products were observed, and alkylated products were isolated in good yields across a range of substituted, unprotected indoles, knowing how transferring carbene moieties to heterocycles to obtain C(sp2)−H alkylation products are valuable transformations in organic synthesis [99]. Likewise, by engineering cytochrome P450 enzymes, Arnold and co-workers were able to develop several P411 variants able to catalyze the insertion of fluoroalkyl carbenes into α-amino C(sp3)−H bonds, as shown for N-phenylpyrrolidine and 2,2,2-trifluoro-1-diazoethane. The enantiodivergent synthesis of fluoroalkyl-containing molecules turned out to be possible with P411-PFA and P411-PFA-(S) variants as biocatalysts, originating selectively the (R)- and the (S)-enantiomer, respectively (Scheme 20d). Finally, these variants could install a trifluoroethyl group onto various N-aryl pyrrolidine substrates by directly activating the α-amino C−H bonds, thus achieving excellent turnovers and enantiomeric excess (ee) up to 99% [100]. Following the stereoselective carbene addition to terminal alkynes to produce cyclopropenes (P411-C6 variant as biocatalyst) and bicyclo [1.1.0]butanes (P411-E10 variant as biocatalyst) [101], the more challenging carbene transfer to internal alkynes for cyclopropene synthesis was achieved with P411-C10 variants (which belong to the family of P411-CHF) with impressive efficiency and stereoselectivity (all with >99.9% ee), as illustrated for 1-phenylbutyne and EDA (Scheme 20e) [102]. This P411-C10 engineered enzyme was also shown to be efficient for lactone carbene insertion into primary and secondary α-amino C−H bonds, thus allowing chiral lactone derivatives synthesis with high catalytic efficiency. Moreover, for carbene insertion into secondary C−H bonds, a single mutation was uncovered to invert the two contiguous chiral centers, hence leading to the opposite enantiomers of the same major diastereomers, thus in a stereo divergent manner (Scheme 20f) [103]. Based on their previous work, which demonstrated that variants of a heme protein, Rhodothermus marinus cytochrome c (Rma cyt c), catalyze abiological carbene boron–hydrogen (B–H) bond insertion with high efficiency and selectivity [104], the authors explored a similar approach with lactone-based carbenes. One of the Rma cyt c variants showed high selectivity and efficiency for B–H insertion of 5- and 6-membered lactone carbenes (Scheme 20g) [104].
Engineered variants of Aeropyrum pernix protoglobin (ApePgb) represent the first example of a biocatalyst for carbene transfer from diazirines (cyclic isomers of diazo compounds) at ambient temperature, not requiring exogenous heat or light. Moderate yields and modest diastereo- or enatioselectivity values were reached, as exemplified for benzyl acrylate, using 3-phenyl-3H-diazirine as a carbene precursor (Scheme 20h) [105]. The same ApePgb variant was shown to catalyze the cyclopropanation of unactivated alkenes using EDA, yielding the corresponding cis-cyclopropanes [106], which was the basis for the development of a method for the challenging synthesis of cis-trifluoromethyl-substituted cyclopropanes (Scheme 20i) using ApePgb that can catalyze the reaction with low-to-excellent yield (6–55%) and enantioselectivity (17–99% ee), depending on the substrate [107]. The same research group reported the enantioselective one-carbon ring enlargement of aziridines into azetidines, where two new bonds are formed (one C−C and one C−N bond) through a [1,2]-Stevens rearrangement strategy, catalyzed by P411-AzetS, an engineered variant of cytochrome P450BM3, which exhibited carbene transferase activity with utmost stereo control, favoring the (S)-enantiomer (99:1 er) (Scheme 20j) [108]. Very recently, a high-affinity heme-binding protein with an open coordination site adjacent to a large reconfigurable substrate binding cavity was designed from scratch, and its catalytic activity tested for the enantioselective cyclopropanation of styrene with EDA (up to 93% isolated yield, 5000 TON, 97:3 e.r.) [85].
7. Final Remarks
The chemistry of diazo compounds has always aroused the interest of synthetic organic chemists due to the many reactions that can be carried out through the decomposition of these compounds. Some of these reactions are very difficult to perform by other methods. These reactions proceed through the formation of carbenes or metallocarbenes, depending on the reaction conditions. Reactions catalyzed by low-cost metal-complexed porphyrins offer advantages over rarer transition metal-complexed porphyrins. Porphyrins complexed with these metals are powerful tools for creating new C−C, C−H, C=C, O−H, N−H, S−H, Se−H bonds, etc. Depending on the porphyrin system involved, the carbene insertion can be efficiently targeted to a specific functional group for the synthesis of a broad portfolio of fine chemicals. For this reason, in recent years, porphyrins have been highly efficient and low-cost catalysts. Particularly, those Fe and Co complexes have promoted the alkene cyclopropanations, C−H and X−H functionalizations (X = N, O, S, Se, Si, Sn, Ge).
Metalloenzyme-catalyzed carbene transformations are potent routes for creating tricky molecules. These lab-designed enzymes harness proteins’ ability to control reactive carbene species, ensuring precise outcomes. Novel artificial carbene transferases enable diverse methodologies, even unprecedented ones, which cover various reactions such as cyclopropanation, cyclopropenation, and carbene X–H insertion. In these conversions, biocatalysts surpass small-molecule catalysts in selectivity and turnover. The integration of these enzymatic reactions into synthesis, biological pathways, and chemo-enzymatic cascades is promising despite current limitations.
The new achievements involving diazo compounds discussed in this updated review and resulting from the studies carried out in the last five to six years provide an overview of the significance of such compounds in novel organic synthesis procedures.
8. Future Research Directions and Perspectives
The use of porphyrin-based catalysts in the decomposition of diazo compounds is an interesting research area with potential future directions and perspectives. Diazo compounds are versatile synthetic intermediates that can undergo various transformations, and their controlled decomposition is a crucial step in many organic synthesis processes. Several potential future research directions and perspectives can be highlighted in utilizing porphyrin-based catalysts for diazo compound decomposition, such as mechanistic insights and the pathways involved in the decomposition of diazo compounds catalyzed by porphyrin-based catalysts. Investigating reaction intermediates and transition states can provide insights into the factors influencing catalytic efficiency and selectivity, ligand design that coordinate to the metal core of porphyrins and could enhance the catalytic activity and selectivity in diazo compound decomposition. Ligand modifications can influence the electronic and steric properties of the catalyst, affecting its reactivity; substrate scope of porphyrin-based catalysts in diazo compound decomposition is essential and can provide valuable information about the catalyst’s versatility; enantioselective catalysis using chiral porphyrin-based catalysts is an emerging research direction and an opportunities for the synthesis of chiral building blocks and molecules; catalytic site engineering to explore site-specific modifications of the porphyrin catalyst’s active site can lead to enhanced catalytic properties; porphyrin-based catalysts can also be utilized in photocatalytic diazo compound decomposition reactions and sustainable catalysis that focus on the development of sustainable and environmentally friendly processes using porphyrin-based catalysts coupled with green solvents, mild reaction conditions, and renewable resources could be integrated into the catalytic systems.
Author Contributions
M.M.Q.S., J.A.S.C. and V.F.F. have been engaged in writing the initial and final manuscript versions and have been involved in developing the idea of the review and contributing suggestions to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
V.F.F. thanks FAPERJ and CNPQ for their research grants. The work was supported through the project UIDB/50006/2020|UIDP/50006/2020, funded by FCT/MCTES through national funds.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Curtius, T. Ueber die Einwirkung von salpetriger Säure auf salzsauren Glycocolläther. Berichte Dtsch. Chem. Ges. 1883, 16, 2230–2231. [Google Scholar] [CrossRef]
- Burtoloso, A.C.B.; Momo, P.B.; Novais, G.L. Traditional and New methods for the Preparation of Diazocarbonyl Compounds. Ann. Braz. Acad. Sci. 2018, 90, 859–893. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K.; Koenigs, R.M. Diazoacetonitrile (N 2 CHCN): A Long Forgotten but Valuable Reagent for Organic Synthesis. Chem.-Eur. J. 2020, 26, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Alkylcarbene complex of tantalum by intramolecular alpha-hydrogen abstraction. J. Am. Chem. Soc. 1974, 96, 6796–6797. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, C.; Ding, Q.; Peng, Y. Diazo Compounds: Versatile Synthons for the Synthesis of Nitrogen Heterocycles via Transition Metal-Catalyzed Cascade C–H Activation/Carbene Insertion/Annulation Reactions. Adv. Synth. Catal. 2019, 361, 919–944. [Google Scholar] [CrossRef]
- Ciszewski, Ł.W.; Rybicka-Jasińska, K.; Gryko, D. Recent developments in photochemical reactions of diazo compounds. Org. Biomol. Chem. 2019, 17, 432–448. [Google Scholar] [CrossRef]
- Candeias, N.R.; Paterna, R.; Gois, P.M.P. Homologation Reaction of Ketones with Diazo Compounds. Chem. Rev. 2016, 116, 2937–2981. [Google Scholar] [CrossRef]
- DeAngelis, A.; Panish, R.; Fox, J.M. Rh-Catalyzed Intermolecular Reactions of α-Alkyl-α-Diazo Carbonyl Compounds with Selectivity over β-Hydride Migration. Acc. Chem. Res. 2016, 49, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Longevial, J.F.; Rose, C.; Poyac, L.; Clément, S.; Richeter, S. Molecular Systems Combining Porphyrinoids and N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2021, 2021, 776–791. [Google Scholar] [CrossRef]
- Simões, M.M.Q.; Gonzaga, D.T.G.; Cardoso, M.F.C.; Forezi, L.D.S.M.; Gomes, A.T.P.C.; Da Silva, C.F.D.C.; Ferreira, V.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Carbene transfer reactions catalysed by dyes of the metalloporphyrin group. Molecules 2018, 23, 792. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.P.C.; Forezi, L.D.S.M.; Simões, M.M.Q.; Gonzaga, D.T.; Cardoso, M.F.C.; Da Silva, F.D.C.; Neves, M.G.P.M.S.; Ferreira, V.F.; Cavaleiro, J.A.S. Carbene-Type Species in the Functionalization of Porphyrin Derivatives. Synthesis 2018, 50, 2678–2692. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef]
- Wilkinson, A.; McNaught, A.D. The IUPAC Compendium of Chemical Terminology 2nd ed. (the “Gold Book”); Blackwell Scientific Publications: Research Triangle Park, NC, USA, 2019; ISBN 0-9678550-9-8. [Google Scholar]
- Ferreira, V.F. Synthesis of Heterocyclic Compounds by Carbenoid Transfer Reactions. Curr. Org. Chem. 2007, 11, 177–193. [Google Scholar] [CrossRef]
- Da Silva, F.D.C.; Jordao, A.K.; da Rocha, D.R.; Ferreira, S.B.; Cunha, A.C.; Ferreira, V.F. Recent Advances on the Synthesis of Heterocycles from Diazo Compounds. Curr. Org. Chem. 2012, 16, 224–251. [Google Scholar] [CrossRef]
- Carreras, V.; Tanbouza, N.; Ollevier, T. The Power of Iron Catalysis in Diazo Chemistry. Synthesis 2021, 53, 79–94. [Google Scholar]
- Damiano, C.; Sonzini, P.; Gallo, E. Iron catalysts with N-ligands for carbene transfer of diazo reagents. Chem. Soc. Rev. 2020, 49, 4867–4905. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.F.; G A Pinto, D.C.; Silva, A.M.S. Iron: A Worthy Contender in Metal Carbene Chemistry. ACS Catal. 2020, 10, 10096–10116. [Google Scholar] [CrossRef]
- Weissenborn, M.J.; Koenigs, R.M. Iron-porphyrin Catalyzed Carbene Transfer Reactions—An Evolution from Biomimetic Catalysis towards Chemistry-inspired Non-natural Reactivities of Enzymes. ChemCatChem 2020, 12, 2171–2179. [Google Scholar] [CrossRef]
- Empel, C.; Jana, S.; Koenigs, R.M. C-H functionalization via iron-catalyzed carbene-transfer reactions. Molecules 2020, 25, 880. [Google Scholar] [CrossRef]
- Wenger, O.S. Is Iron the New Ruthenium? Chem.-Eur. J. 2019, 25, 6043–6052. [Google Scholar] [CrossRef] [PubMed]
- Empel, C.; Koenigs, R.M. Sustainable Carbene Transfer Reactions with Iron and Light. Synlett 2019, 30, 1929–1934. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, L. Reactions between Diazo Compounds and Hypervalent Iodine(III) Reagents. Angew. Chem. Int. Ed. 2020, 59, 12282–12292. [Google Scholar] [CrossRef]
- Epping, R.F.J.; Vesseur, D.; Zhou, M.; de Bruin, B. Carbene Radicals in Transition-Metal-Catalyzed Reactions. ACS Catal. 2023, 13, 5428–5448. [Google Scholar] [CrossRef] [PubMed]
- Snabilié, D.D.; Meeus, E.J.; Epping, R.F.J.; He, Z.; Zhou, M.; de Bruin, B. Understanding Off-Cycle and Deactivation Pathways in Radical-Type Carbene Transfer Catalysis. Chem.-Eur. J. 2023, 29, e202300336. [Google Scholar] [CrossRef]
- Grotenhuis, C.T.; de Bruin, B. Radical-type Reactions Controlled by Cobalt: From Carbene Radical Reactivity to the Catalytic Intermediacy of Reactive o-Quinodimethanes. Synlett 2018, 29, 2238–2250. [Google Scholar]
- Dzik, W.I.; Zhang, X.P.; de Bruin, B. Redox Noninnocence of Carbene Ligands: Carbene Radicals in (Catalytic) C−C Bond Formation. Inorg. Chem. 2011, 50, 9896–9903. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, C.Y.; Che, C.M. Cobalt-Porphyrin-Catalyzed Intramolecular Buchner Reaction and Arene Cyclopropanation of In Situ Generated Alkyl Diazomethanes. Adv. Synth. Catal. 2017, 359, 2253–2258. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Leão, R.A.C.; Alonso, C.M.A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Silva, A.M.S.; Pinheiro, S.; De Souza, M.C.B.V.; Ferreira, V.F.; et al. A new insight into the catalytic decomposition of ethyl diazoacetate in the presence of meso-tetraarylporphyrin (=5,10,15,20-tetraaryl-21H,23H-porphine) complexes. Helv. Chim. Acta 2008, 91, 2270–2283. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Diazo compounds in the functionalization of porphyrin macrocycles. J. Porphyrins Phthalocyanines 2011, 15, 835–847. [Google Scholar] [CrossRef]
- Epping, R.F.J.; Hoeksma, M.M.; Bobylev, E.O.; Mathew, S.; de Bruin, B. Cobalt(II)–tetraphenylporphyrin-catalysed carbene transfer from acceptor–acceptor iodonium ylides via N-enolate–carbene radicals. Nat. Chem. 2022, 14, 550–557. [Google Scholar] [CrossRef]
- Che, C.-M.; Lo, V.K.-Y.; Zhou, C.-Y.; Huang, J.-S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 2011, 40, 1950–1975. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Cui, X.; Zhang, X.P. Enantioselective Radical Cyclization for Construction of 5-Membered Ring Structures by Metalloradical C-H Alkylation. J. Am. Chem. Soc. 2018, 140, 4792–4796. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, M.; Olivares, A.M.; de Bruin, B. [Co(TPP)]-Catalyzed Formation of Substituted Piperidines. Chem.-Eur. J. 2019, 25, 5658–5663. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, C.T.; van den Heuvel, N.; van der Vlugt, J.I.; de Bruin, B. Catalytic Dibenzocyclooctene Synthesis via Cobalt(III)-Carbene Radical and ortho -Quinodimethane Intermediates. Angew. Chem. Int. Ed. 2018, 57, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lankelma, M.; Vlugt, J.I.; Bruin, B. Catalytic Synthesis of 8-Membered Ring Compounds via Cobalt(III)-Carbene Radicals. Angew. Chem. Int. Ed. 2020, 59, 11073–11079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wolzak, L.A.; Li, Z.; De Zwart, F.J.; Mathew, S.; De Bruin, B. Catalytic Synthesis of 1 H-2-Benzoxocins: Cobalt(III)-Carbene Radical Approach to 8-Membered Heterocyclic Enol Ethers. J. Am. Chem. Soc. 2021, 143, 20501–20512. [Google Scholar] [CrossRef] [PubMed]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef]
- Caballero, A.; Prieto, A.; Díaz-Requejo, M.M.; Pérez, P.J. Metal-catalyzed olefin cyclopropanation with ethyl diazoacetate: Control of the diastereoselectivity. Eur. J. Inorg. Chem. 2009, 2009, 1137–1144. [Google Scholar] [CrossRef]
- Hock, K.J.; Knorrscheidt, A.; Hommelsheim, R.; Ho, J.; Weissenborn, M.J.; Koenigs, R.M. Tryptamine Synthesis by Iron Porphyrin Catalyzed C−H Functionalization of Indoles with Diazoacetonitrile. Angew. Chem. Int. Ed. 2019, 58, 3630–3634. [Google Scholar] [CrossRef]
- Wang, E.-H.; Ping, Y.-J.; Li, Z.-R.; Qin, H.; Xu, Z.-J.; Che, C.-M. Iron Porphyrin Catalyzed Insertion Reaction of N -Tosylhydrazone-Derived Carbenes into X–H (X = Si, Sn, Ge) Bonds. Org. Lett. 2018, 20, 4641–4644. [Google Scholar] [CrossRef]
- Ma, C.; Wang, S.; Sheng, Y.; Zhao, X.-L.; Xing, D.; Hu, W. Synthesis and Characterization of Donor–Acceptor Iron Porphyrin Carbenes and Their Reactivities in N–H Insertion and Related Three-Component Reaction. J. Am. Chem. Soc. 2023, 145, 4934–4939. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, G.; Zhang, J.; Liu, L. Iron-catalysed chemo- and ortho -selective C–H bond functionalization of phenols with α-aryl-α-diazoacetates. Org. Chem. Front. 2021, 8, 3770–3775. [Google Scholar] [CrossRef]
- Wang, H.X.; Wan, Q.; Low, K.H.; Zhou, C.Y.; Huang, J.S.; Zhang, J.L.; Che, C.M. Stable group 8 metal porphyrin mono- and bis(dialkylcarbene) complexes: Synthesis, characterization, and catalytic activity. Chem. Sci. 2020, 11, 2243–2259. [Google Scholar] [CrossRef]
- Wang, H.; Richard, Y.; Wan, Q.; Zhou, C.; Che, C. Iridium(III)-Catalyzed Intermolecular C(sp 3)−H Insertion Reaction of Quinoid Carbene: A Radical Mechanism. Angew. Chem. Int. Ed. 2020, 59, 1845–1850. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Zhao, L.; He, C.; Sun, W.; Duan, C. Ir-Porphyrin-Based Metal–Organic Framework as a Dual Metallo- and Photocatalyst for Inert Alkyl C(sp 3)−H Bond Activation and Direct Functionalization. ACS Appl. Mater. Interfaces 2021, 13, 10925–10932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Zhang, L.; Su, C.Y. An Acid Stable Metal-Organic Framework as an Efficient and Recyclable Catalyst for the O−H Insertion Reaction of Carboxylic Acids. ChemCatChem 2018, 10, 3901–3906. [Google Scholar] [CrossRef]
- Chen, L.; Cui, H.; Wang, Y.; Liang, X.; Zhang, L.; Su, C.-Y. Carbene insertion into N–H bonds with size-selectivity induced by a microporous ruthenium–porphyrin metal–organic framework. Dalt. Trans. 2018, 47, 3940–3946. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.A.; Almeida, M.P.; Alcântara, A.F.P.; Rigolin, V.H.; Ribeiro, M.A.; Barros, W.P.; Megiatto, J.D. Ru(II)Porphyrinate-based molecular nanoreactor for carbene insertion reactions and quantitative formation of rotaxanes by active-metal-template syntheses. Nat. Commun. 2020, 11, 6370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Xuan, J. Visible Light-Mediated Cyclopropanation: Recent Progress. Eur. J. Org. Chem. 2022, 2022, e202201066. [Google Scholar] [CrossRef]
- Allouche, E.M.D.; Charette, A.B. Cyclopropanation Reactions of Semi-stabilized and Non-stabilized Diazo Compounds. Synthesis 2019, 51, 3947–3963. [Google Scholar] [CrossRef]
- Goswami, M.; de Bruin, B.; Dzik, W.I. Difluorocarbene transfer from a cobalt complex to an electron-deficient alkene. Chem. Commun. 2017, 53, 4382–4385. [Google Scholar] [CrossRef]
- Chanthamath, S.; Iwasa, S. Enantioselective Cyclopropanation of a Wide Variety of Olefins Catalyzed by Ru(II)-Pheox Complexes. Acc. Chem. Res. 2016, 49, 2080–2090. [Google Scholar] [CrossRef]
- Rioz-Martínez, A.; Oelerich, J.; Ségaud, N.; Roelfes, G. DNA-Accelerated Catalysis of Carbene-Transfer Reactions by a DNA/Cationic Iron Porphyrin Hybrid. Angew. Chem. Int. Ed. 2016, 55, 14136–14140. [Google Scholar] [CrossRef]
- Maaskant, R.V.; Polanco, E.A.; Van Lier, R.C.W.; Roelfes, G. Cationic iron porphyrins with sodium dodecyl sulphate for micellar catalysis of cyclopropanation reactions. Org. Biomol. Chem. 2020, 18, 638–641. [Google Scholar] [CrossRef]
- Carrié, D.; Roisnel, T.; Simonneaux, G. Asymmetric intermolecular cyclopropanation of alkenes and N–H insertion of aminoesters by diazoacetylferrocene catalyzed by ruthenium and iron porphyrins. Polyhedron 2021, 205, 115294. [Google Scholar] [CrossRef]
- Damiano, C.; Gallo, E. Challenging asymmetric alkene cyclopropanation by unsymmetrical diazomalonates. Chem Catal. 2022, 2, 229–231. [Google Scholar] [CrossRef]
- Damiano, C.; Gadolini, S.; Intrieri, D.; Lay, L.; Colombo, C.; Gallo, E. Iron and Ruthenium Glycoporphyrins: Active Catalysts for the Synthesis of Cyclopropanes and Aziridines. Eur. J. Inorg. Chem. 2019, 2019, 4412–4420. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Cui, X.; Wojtas, L.; Zhang, X.P. Asymmetric Radical Cyclopropanation of Alkenes with In Situ-Generated Donor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc. 2017, 139, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, J.; Cindy Lee, W.-C.; Wang, D.-S.; Zhang, X.P. Radical differentiation of two ester groups in unsymmetrical diazomalonates for highly asymmetric olefin cyclopropanation. Chem Catal. 2022, 2, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, B.; Zhang, D.; Yu, M.; Chang, Z.; Bu, X. Recent progress in host–guest metal–organic frameworks: Construction and emergent properties. Coord. Chem. Rev. 2023, 476, 214921. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Sun, Y.; Liu, Y. Organic supramolecular aggregates based on water-soluble cyclodextrins and calixarenes. Aggregate 2020, 1, 31–44. [Google Scholar] [CrossRef]
- Mouarrawis, V.; Bobylev, E.O.; de Bruin, B.; Reek, J.N.H. Controlling the Activity of a Caged Cobalt-Porphyrin-Catalyst in Cyclopropanation Reactions with Peripheral Cage Substituents. Eur. J. Inorg. Chem. 2021, 2021, 2890–2898. [Google Scholar] [CrossRef]
- Mouarrawis, V.; Bobylev, E.O.; Bruin, B.; Reek, J.N.H. A Novel M 8 L 6 Cubic Cage That Binds Tetrapyridyl Porphyrins: Cage and Solvent Effects in Cobalt-Porphyrin-Catalyzed Cyclopropanation Reactions. Chem.-Eur. J. 2021, 27, 8390–8397. [Google Scholar] [CrossRef]
- Epp, K.; Bueken, B.; Hofmann, B.J.; Cokoja, M.; Hemmer, K.; De Vos, D.; Fischer, R.A. Network topology and cavity confinement-controlled diastereoselectivity in cyclopropanation reactions catalyzed by porphyrin-based MOFs. Catal. Sci. Technol. 2019, 9, 6452–6459. [Google Scholar] [CrossRef]
- Feng, D.; Chung, W.-C.; Wei, Z.; Gu, Z.-Y.; Jiang, H.-L.; Chen, Y.-P.; Darensbourg, D.J.; Zhou, H.-C. Construction of Ultrastable Porphyrin Zr Metal–Organic Frameworks through Linker Elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, A.F.P.; Fontana, L.A.; Rigolin, V.H.; Andrade, Y.F.S.; Ribeiro, M.A.; Barros, W.P.; Ornelas, C.; Megiatto, J.D. Olefin Cyclopropanation by Radical Carbene Transfer Reactions Promoted by Cobalt(II)/Porphyrinates: Active-Metal-Template Synthesis of [2]Rotaxanes. Angew. Chem. Int. Ed. 2018, 57, 8979–8983. [Google Scholar] [CrossRef]
- Alcântara, A.F.P.; Fontana, L.A.; Almeida, M.P.; Rigolin, V.H.; Ribeiro, M.A.; Barros, W.P.; Megiatto, J.D. Control over the Redox Cooperative Mechanism of Radical Carbene Transfer Reactions for the Efficient Active-Metal-Template Synthesis of [2]Rotaxanes. Chem.-Eur. J. 2020, 26, 7808–7822. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, K.; Ricoux, R.; Mahy, J.-P. Recent advances in the field of artificial hemoproteins: New efficient eco-compatible biocatalysts for nitrene-, oxene- and carbene-transfer reactions. J. Porphyrins Phthalocyanines 2019, 23, 1273–1285. [Google Scholar] [CrossRef]
- Liu, Z.; Arnold, F.H. New-to-nature chemistry from old protein machinery: Carbene and nitrene transferases. Curr. Opin. Biotechnol. 2021, 69, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.S.; Brustad, E.M.; Kannan, A.; Arnold, F.H. Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes. Science 2013, 339, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Schwizer, F.; Okamoto, Y.; Heinisch, T.; Gu, Y.; Pellizzoni, M.M.; Lebrun, V.; Reuter, R.; Köhler, V.; Lewis, J.C.; Ward, T.R. Artificial Metalloenzymes: Reaction Scope and Optimization Strategies. Chem. Rev. 2018, 118, 142–231. [Google Scholar] [CrossRef] [PubMed]
- Jeschek, M.; Panke, S.; Ward, T.R. Artificial Metalloenzymes on the Verge of New-to-Nature Metabolism. Trends Biotechnol. 2018, 36, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Natoli, S.N.; Hartwig, J.F. Noble-Metal Substitution in Hemoproteins: An Emerging Strategy for Abiological Catalysis. Acc. Chem. Res. 2019, 52, 326–335. [Google Scholar] [CrossRef]
- Davis, H.J.; Ward, T.R. Artificial Metalloenzymes: Challenges and Opportunities. ACS Cent. Sci. 2019, 5, 1120–1136. [Google Scholar] [CrossRef]
- Kim, T.; Kassim, A.M.; Botejue, A.; Zhang, C.; Forte, J.; Rozzell, D.; Huffman, M.A.; Devine, P.N.; McIntosh, J.A. Hemoprotein-Catalyzed Cyclopropanation En Route to the Chiral Cyclopropanol Fragment of Grazoprevir. Chembiochem 2019, 20, 1129–1132. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Bloomer, B.J.; Clark, D.S.; Mukhopadhyay, A.; Keasling, J.D.; Hartwig, J.F. Unnatural biosynthesis by an engineered microorganism with heterologously expressed natural enzymes and an artificial metalloenzyme. Nat. Chem. 2021, 13, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Fasan, R. Engineered and artificial metalloenzymes for selective C–H functionalization. Curr. Opin. Green Sustain. Chem. 2021, 31, 100494. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Arnold, F.H. Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer. Acc. Chem. Res. 2021, 54, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Roelfes, G. Repurposed and artificial heme enzymes for cyclopropanation reactions. J. Inorg. Biochem. 2021, 222, 111523. [Google Scholar] [CrossRef]
- Lovelock, S.L.; Crawshaw, R.; Basler, S.; Levy, C.; Baker, D.; Hilvert, D.; Green, A.P. The road to fully programmable protein catalysis. Nature 2022, 606, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Tomás-Gamasa, M.; Mascareñas, J.L. Organometallic catalysis in aqueous and biological environments: Harnessing the power of metal carbenes. Chem. Sci. 2022, 13, 6478–6495. [Google Scholar] [CrossRef] [PubMed]
- Kalvet, I.; Ortmayer, M.; Zhao, J.; Crawshaw, R.; Ennist, N.M.; Levy, C.; Roy, A.; Green, A.P.; Baker, D. Design of Heme Enzymes with a Tunable Substrate Binding Pocket Adjacent to an Open Metal Coordination Site. J. Am. Chem. Soc. 2023, 145, 14307–14315. [Google Scholar] [CrossRef]
- Huang, J.; Quest, A.; Cruz-Morales, P.; Deng, K.; Pereira, J.H.; Van Cura, D.; Kakumanu, R.; Baidoo, E.E.K.; Dan, Q.; Chen, Y.; et al. Complete integration of carbene-transfer chemistry into biosynthesis. Nature 2023, 617, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Couture, B.M.; Liu, N.; Lall, M.S.; Kohrt, J.T.; Fasan, R. Enantioselective Single and Dual α-C–H Bond Functionalization of Cyclic Amines via Enzymatic Carbene Transfer. J. Am. Chem. Soc. 2023, 145, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Siriboe, M.G.; Vargas, D.A.; Fasan, R. Dehaloperoxidase Catalyzed Stereoselective Synthesis of Cyclopropanol Esters. J. Org. Chem. 2023, 88, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Natoli, S.N.; Liu, Z.; Clark, D.S.; Hartwig, J.F. Site-Selective Functionalization of (sp3)C-H Bonds Catalyzed by Artificial Metalloenzymes Containing an Iridium-Porphyrin Cofactor. Angew. Chem. Int. Ed. Engl. 2019, 58, 13954–13960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Gu, Y.; Clark, D.S.; Mukhopadhyay, A.; Keasling, J.D.; Hartwig, J.F. Assembly and Evolution of Artificial Metalloenzymes within E. coli Nissle 1917 for Enantioselective and Site-Selective Functionalization of C─H and C═C Bonds. J. Am. Chem. Soc. 2022, 144, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chandgude, A.L.; Carminati, D.M.; Shen, Z.; Khare, S.D.; Fasan, R. Highly stereoselective and enantiodivergent synthesis of cyclopropylphosphonates with engineered carbene transferases. Chem. Sci. 2022, 13, 8550–8556. [Google Scholar] [CrossRef] [PubMed]
- Carminati, D.M.; Decaens, J.; Couve-Bonnaire, S.; Jubault, P.; Fasan, R. Biocatalytic Strategy for the Highly Stereoselective Synthesis of CHF 2 -Containing Trisubstituted Cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 7072–7076. [Google Scholar] [CrossRef]
- Vargas, D.A.; Khade, R.L.; Zhang, Y.; Fasan, R. Biocatalytic Strategy for Highly Diastereo- and Enantioselective Synthesis of 2,3-Dihydrobenzofuran-Based Tricyclic Scaffolds. Angew. Chem. Int. Ed. Engl. 2019, 58, 10148–10152. [Google Scholar] [CrossRef] [PubMed]
- Carminati, D.M.; Fasan, R. Stereoselective Cyclopropanation of Electron-Deficient Olefins with a Cofactor Redesigned Carbene Transferase Featuring Radical Reactivity. ACS Catal. 2019, 9, 9683–9697. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, N.; Chandgude, A.L.; Fasan, R. An Enzymatic Platform for the Highly Enantioselective and Stereodivergent Construction of Cyclopropyl-δ-lactones. Angew. Chem. Int. Ed. 2020, 59, 21634–21639. [Google Scholar] [CrossRef]
- Ren, X.; Chandgude, A.L.; Fasan, R. Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation. ACS Catal. 2020, 10, 2308–2313. [Google Scholar] [CrossRef]
- Mao, R.; Wackelin, D.J.; Jamieson, C.S.; Rogge, T.; Gao, S.; Das, A.; Taylor, D.M.; Houk, K.N.; Arnold, F.H. Enantio- and Diastereoenriched Enzymatic Synthesis of 1,2,3- Polysubstituted Cyclopropanes from (Z/E)-Trisubstituted Enol Acetates. J. Am. Chem. Soc. 2023, 145, 16176–16185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.K.; Chen, K.; Huang, X.; Wohlschlager, L.; Renata, H.; Arnold, F.H. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization. Nature 2019, 565, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, O.F.; Chen, K.; Arnold, F.H. Directed Evolution of a Cytochrome P450 Carbene Transferase for Selective Functionalization of Cyclic Compounds. J. Am. Chem. Soc. 2019, 141, 8989–8995. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, R.K.; Arnold, F.H. Enantiodivergent α-Amino C-H Fluoroalkylation Catalyzed by Engineered Cytochrome P450s. J. Am. Chem. Soc. 2019, 141, 9798–9802. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, X.; Kan, S.B.J.; Zhang, R.K.; Arnold, F.H. Enzymatic construction of highly strained carbocycles. Science 2018, 360, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arnold, F.H. Engineering Cytochrome P450s for Enantioselective Cyclopropenation of Internal Alkynes. J. Am. Chem. Soc. 2020, 142, 6891–6895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Z.; Chen, K.; Arnold, F.H. Enzymatic Lactone-Carbene C–H Insertion to Build Contiguous Chiral Centers. ACS Catal. 2020, 10, 5393–5398. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Huang, X.; Gumulya, Y.; Chen, K.; Arnold, F.H. Genetically programmed chiral organoborane synthesis. Nature 2017, 552, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.J.; Danelius, E.; Gonen, T.; Arnold, F.H. Biocatalytic Carbene Transfer Using Diazirines. J. Am. Chem. Soc. 2022, 144, 8892–8896. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.M.; Kan, S.B.J.; Lewis, R.D.; Brandenberg, O.F.; Chen, K.; Arnold, F.H. Diverse Engineered Heme Proteins Enable Stereodivergent Cyclopropanation of Unactivated Alkenes. ACS Cent. Sci. 2018, 4, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Schaus, L.; Das, A.; Knight, A.M.; Jimenez-Osés, G.; Houk, K.N.; Garcia-Borràs, M.; Arnold, F.H.; Huang, X. Protoglobin-Catalyzed Formation of cis-Trifluoromethyl-Substituted Cyclopropanes by Carbene Transfer. Angew. Chem. Int. Ed. 2023, 62, e202208936. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Lal, R.G.; Marchetti, L.A.; Arnold, F.H. Biocatalytic One-Carbon Ring Expansion of Aziridines to Azetidines via a Highly Enantioselective [1,2]-Stevens Rearrangement. J. Am. Chem. Soc. 2022, 144, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).