Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams
Abstract
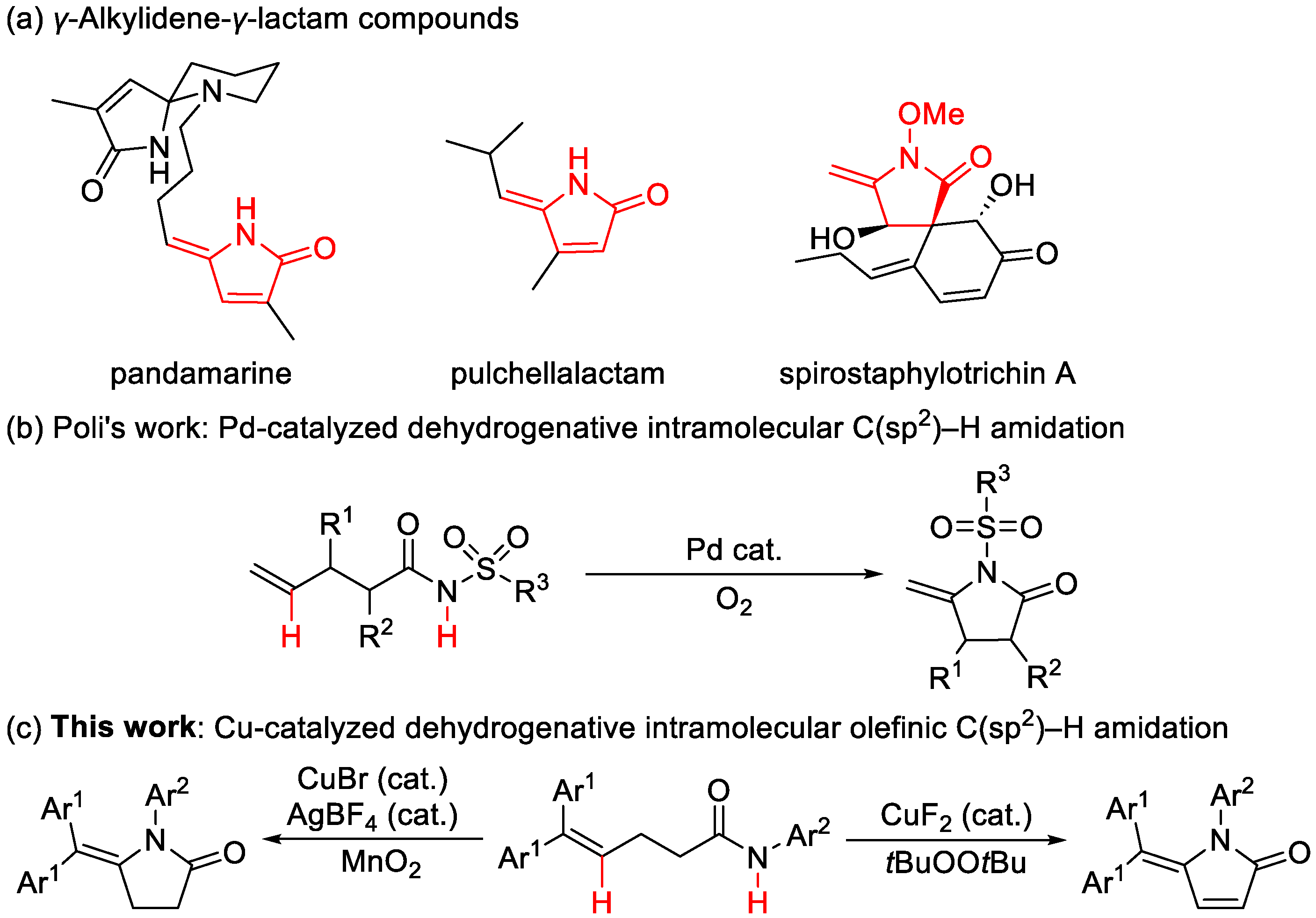
1. Introduction
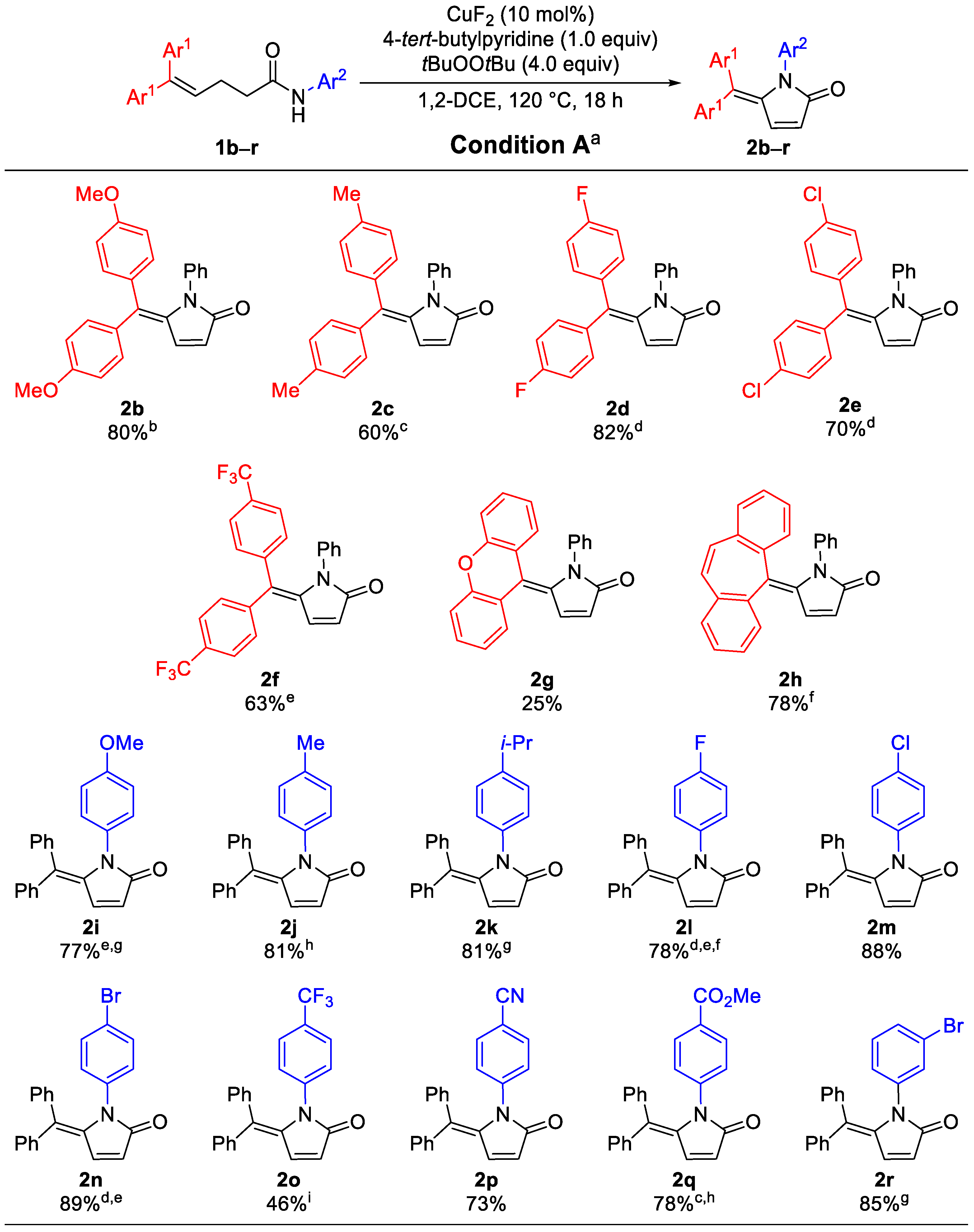
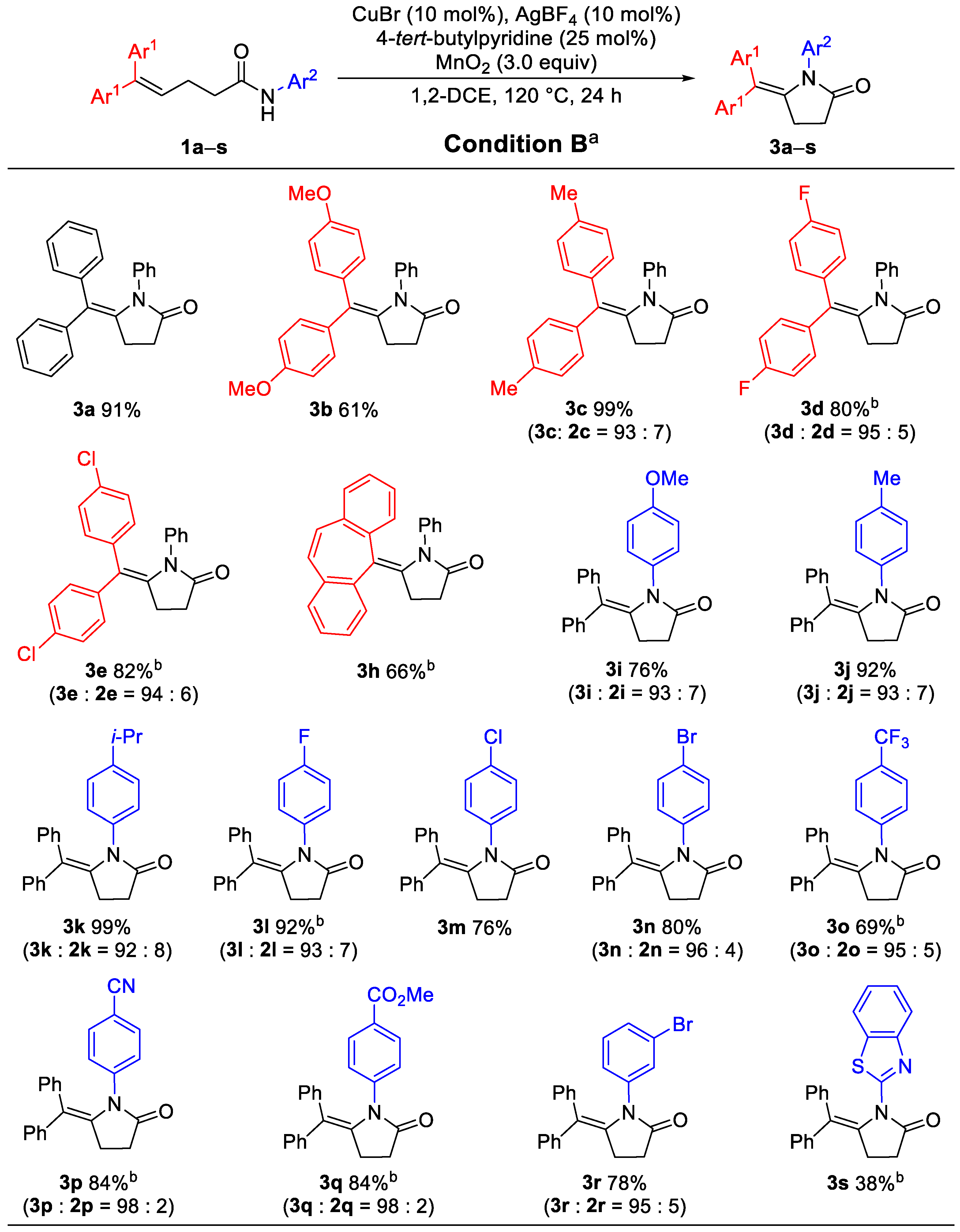
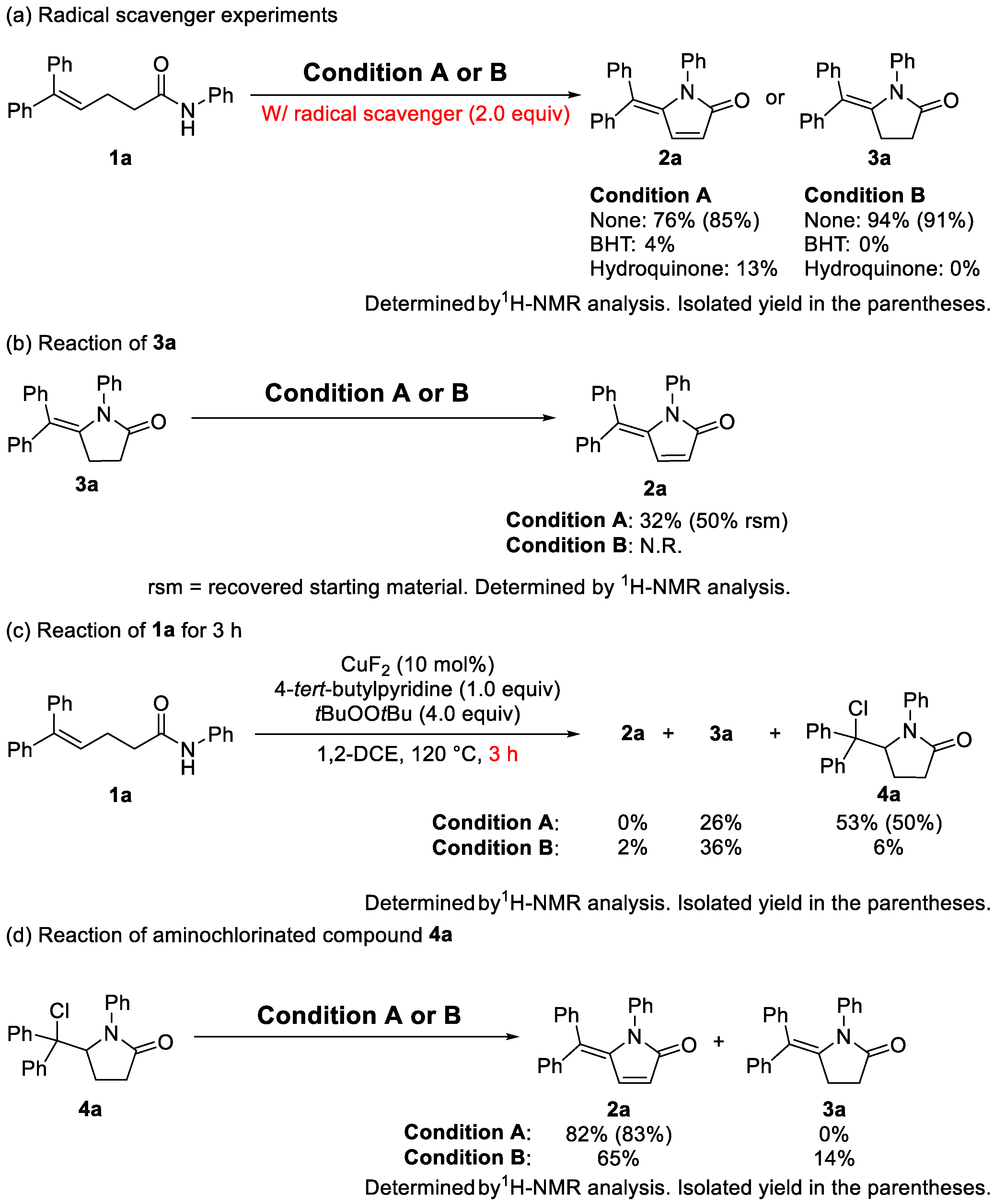
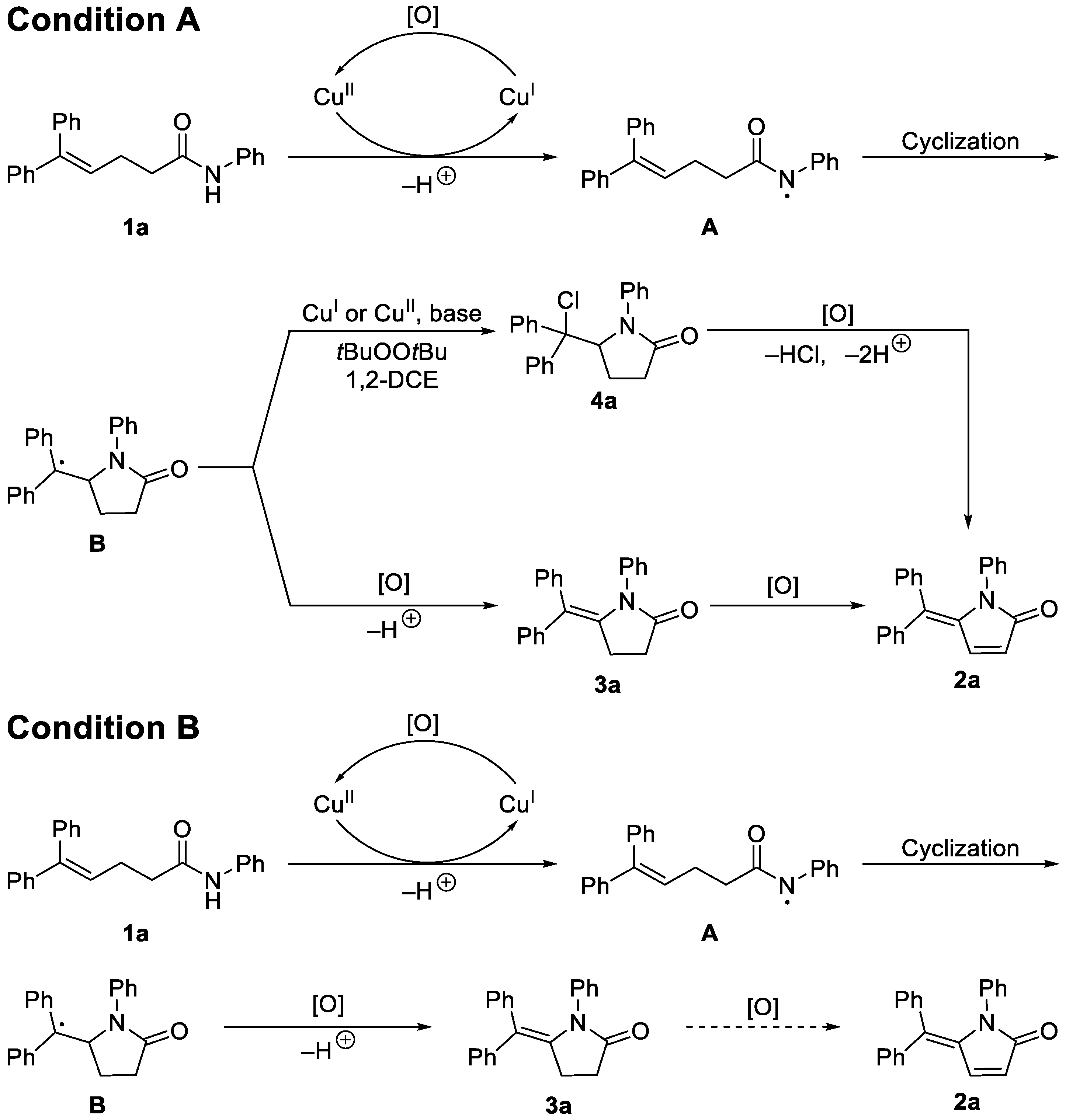
2. Results
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Synthesis of 5-Alkylidene-pyrrolin-2-ones
3.3. General Procedure for the Synthesis of 5-Alkylidene-pyrrolidin-2-ones
3.4. Spectroscopic Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, X.; Li, L.; Wan, C.; Liu, J.-B. FeCl3-catalyzed sequential cyclization for the construction of 12-aryl 5,7-dihydropyrido[2,3-b:6,5-b’]diindoles. Tetrahedron Lett. 2020, 61, 152334. [Google Scholar] [CrossRef]
- Liu, J.-B.; Ren, M.; Lai, X.; Qiu, G. Iron-catalyzed stereoselective haloamidation of amide-tethered alkynes. Chem. Commun. 2021, 57, 4259–4262. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Huang, K.; Liu, J.-B.; Zhang, L.; Hou, M.; Qiu, G. Palladium-catalyzed cyclization of 1-alkynyl-8-iodonaphthalene and double isocyanides for the synthesis of acenaphtho[1,2-b]pyrroles. Chin. Chem. Lett. 2022, 33, 4870–4873. [Google Scholar] [CrossRef]
- Ren, M.; Yan, X.; Lai, X.; Liu, J.-B.; Zhou, H.; Qiu, G. Nitrenium ion-based ipso-addition and ortho-cyclization of arenes under photo and iron dual-catalysis. Mol. Catal. 2022, 528, 112413. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, C.; Jiao, N. Recent advances in copper–catalyzed dehydrogenative functionalization via a single electron transfer (SET) process. Chem. Soc. Rev. 2012, 41, 3464–3484. [Google Scholar] [CrossRef]
- Louillat, M.-L.; Patureau, F.W. Oxidative C–H amination reactions. Chem. Soc. Rev. 2014, 43, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, S. Transition-Metal-Mediated Direct C–H Amination of Hydrocarbons with Amine Reactants: The Most Desirable but Challenging C–N Bond-Formation Approach. ACS Catal. 2016, 6, 2341–2351. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef]
- Bozell, J.J.; Hegedus, L.S. Palladium-Assisted Functionalization of Olefins: A New Amination of Electron-Deficient Olefins. J. Org. Chem. 1981, 46, 2561–2563. [Google Scholar] [CrossRef]
- Hosokawa, T.; Takano, M.; Kuroki, Y.; Murahashi, S. Palladium(II)-Catalyzed Amidation of Alkenes. Tetrahedron Lett. 1992, 33, 6643–6646. [Google Scholar] [CrossRef]
- Beller, M.; Eichberger, M.; Trauthwein, H. Anti-Markovnikov Functionalization of Olefins: Rhodium-Catalyzed Oxidative Aminations of Styrenes. Angew. Chem. Int. Ed. 1997, 36, 2225–2227. [Google Scholar] [CrossRef]
- Timokhin, V.I.; Anastasi, N.R.; Stahl, S.S. Dioxygen-Coupled Oxidative Amination of Styrene. J. Am. Chem. Soc. 2003, 125, 12996–12997. [Google Scholar] [CrossRef] [PubMed]
- Brice, J.L.; Harang, J.E.; Timokhin, V.I.; Anastasi, N.R.; Stahl, S.S. Aerobic Oxidative Amination of Unactivated Alkenes Catalyzed by Palladium. J. Am. Chem. Soc. 2005, 127, 2868–2869. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.V.; Pérez-Torrente, J.J.; Bartolomé, M.I.; Lahoz, F.J.; Oro, L.A. Rational design of efficient rhodium catalysts for the anti-markovnikov oxidative amination of styrene. Chem. Commun. 2010, 46, 5322–5324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Xue, Y.; Sun, K.; Wu, L.; Zhang, B. An organoselenium-catalyzed N1- and N2-selective aza-Wacker reaction of alkenes with benzotriazoles. Chem. Commun. 2020, 56, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, N.; Dai, C.; Han, Y.; Yang, S.; Huang, Z.; Zhao, Y. Rh(III)-Catalyzed Direct Amination of Aromatic Ketoximes Enabled by Potassium Acetate. Eur. J. Org. Chem. 2019, 2019, 7857–7863. [Google Scholar] [CrossRef]
- Yu, L.; Yang, C.; Yu, Y.; Liu, D.; Hu, L.; Xiao, Y.; Song, Z.-N.; Tan, Z. Ammonia as Ultimate Amino Source in Synthesis of Primary Amines via Nickel-Promoted C–H Bond Amination. Org. Lett. 2019, 21, 5634–5638. [Google Scholar] [CrossRef]
- Grandhi, G.S.; Dana, S.; Mandal, A.; Baidya, M. Copper-Catalyzed 8-Aminoquinoline-Directed Oxidative C–H/N–H Coupling for N-Arylation of Sulfoximines. Org. Lett. 2020, 22, 2606–2610. [Google Scholar] [CrossRef]
- Vu, H.-M.; Yong, J.-Y.; Chen, F.-W.; Li, X.-Q.; Shi, G.-Q. Rhodium-Catalyzed C(sp2)–H Amidation of Azine with Sulfonamides. J. Org. Chem. 2020, 85, 4963–4972. [Google Scholar] [CrossRef]
- Kärkäs, M.D. Photochemical Generation of Nitrogen-Centered Amidyl, Hydrazonyl, and Imidyl Radicals: Methodology Developments and Catalytic Applications. ACS Catal. 2017, 7, 4999–5022. [Google Scholar] [CrossRef]
- Chen, N.; Xu, H.-C. Electrochemical generation of nitrogen-centered radicals for organic synthesis. Green Synth. Catal. 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Kwon, K.; Simons, R.T.; Nandakumar, M.; Roizen, J.L. Strategies to Generate Nitrogen-centered Radicals That May Rely on Photoredox Catalysis: Development in Reaction Methodology and Applications in Organic Synthesis. Chem. Rev. 2022, 122, 2353–2428. [Google Scholar] [CrossRef] [PubMed]
- Pratley, C.; Fenner, S.; Murphy, J.A. Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev. 2022, 122, 8181–8260. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, Q.; Li, L.; Ma, Y.-N. Synthesis of Six-Membered N-Heterocycle Frameworks Based on Intramolecular Metal-Free N-Centered Radical Chemistry. Chem. Rec. 2022, 22, e202100218. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016, 45, 3069–3087. [Google Scholar] [CrossRef]
- Alvi, K.A.; Casey, A.; Nair, B.G. Pulchellalactam: A CD45 Protein Tyrosine Phosphatase Inhibitor from the Marine Fungus Corollospora pulchella. J. Antibiot. 1998, 51, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Lin, S.T.; Hsu, N.-M.; Chern, M.-S. Efficient Total Synthesis of Pulchellalactam, a CD45 Protein Tyrosine Phosphatase Inhibitor. J. Org. Chem. 2002, 67, 4702–4706. [Google Scholar] [CrossRef]
- Nay, B.; Riache, N.; Evanno, L. Chemistry and biology of non-tetramic γ-hydroxy-γ-lactams and γ-alkylidene-γ-lactams from natural sources. Nat. Prod. Rep. 2009, 26, 1044–1062. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Noutsias, D.; Vassilikogiannakis, G. First Total Synthesis of Pandamarine. Org. Lett. 2015, 17, 3596–3599. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Liu, Y.; Liu, Y.; Li, K.; Yang, X.; Liu, S.; Zhou, X.; Yang, J. Spirostaphylotrichin X from a Marine-Derived Fungus as an Anti-influenza Agent Targeting RNA Polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730. [Google Scholar] [CrossRef]
- Abell, A.D.; Oldham, M.D.; Taylor, J.M. Synthesis of Cyclic Acylated Enamino Esters from Enol Lactones, 4-Keto Amides, and 5-Hydroxy Lactams. J. Org. Chem. 1995, 60, 1214–1220. [Google Scholar] [CrossRef]
- Park, B.R.; Lim, C.H.; Lim, J.W.; Kim, J.N. Facile Synthesis of 5-Alkylidene-1,5-dihydropyrrol-2-ones from Morita-Baylis-Hillman Adducts. Bull. Korean Chem. Soc. 2012, 33, 1337–1340. [Google Scholar] [CrossRef][Green Version]
- Mardjan, M.I.D.; Mayooufi, A.; Parrain, J.-L.; Thibonnet, J.; Commeiras, L. Straightforward Access to a Great Diversity of Complex Biorelevant γ-Lactams Thanks to a Tunable Cascade Multicomponent Process. Org. Process Res. Dev. 2020, 24, 606–614. [Google Scholar] [CrossRef]
- Saito, T.; Sugizaki, K.; Osada, H.; Kutsumura, N.; Otani, T. A Hetero Pauson-Khand Reaction of Ketenimines: A New Synthetic Method for γ-Exomethylene-α,β-unsaturated γ-Lactams. Heterocycles 2010, 80, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-C.; Parthasarathy, K.; Cheng, C.-H. Cobalt-Catalyzed Regioselective Synthesis of Pyrrolidinone Derivatives by Reductive Coupling of Nitriles and Acrylamides. J. Am. Chem. Soc. 2009, 131, 18252–18253. [Google Scholar] [CrossRef] [PubMed]
- Kise, N.; Kinameri, S.; Sakurai, T. Reductive coupling of aliphatic cyclic imides and ω-amidoesters with benzophenones by low-valent titanium: Synthesis of 5-diarylmethylene-1,5-dihydropyrrol-2-ones, 6-diarylmethyl-2-pyridones, and ω-(diarylmethylene)lactams. Tetrahedron 2019, 75, 3553–3569. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Kampouropoulos, I.; Sofiadis, M.; Montagnon, T.; Vassilikogiannakis, G. Access to high value sp3-rich frameworks using photocatalyzed [2 + 2]-cycloadditions of γ-alkylidene-γ-lactams. Chem. Commun. 2022, 58, 8085–8088. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Bosveli, A.; Montagnon, T.; Vassilikogiannakis, G. Sequential Visible Light-Induced Reactions Using Different Photocatalysts: Transformation of Furans into 2-Pyridones via γ-Lactams Using a New Ring Expansion Reaction. Chem. Eur. J. 2022, 28, e202200322. [Google Scholar] [CrossRef]
- Lorion, M.M.; Duarte, F.J.S.; Calhorda, M.J.; Oble, J.; Poli, G. Opening the Way to Catalytic Aminopalladation/Proxicyclic Dehydropalladation: Access to Methylidene γ-Lactams. Org. Lett. 2016, 18, 1020–1023. [Google Scholar] [CrossRef]
- Ishida, Y.; Nakamura, I.; Terada, M. Copper-Catalyzed Domino [1,3]/[1,2] Rearrangement for the Efficient Synthesis of Multisubstituted ortho-Anisidines. J. Am. Chem. Soc. 2018, 140, 8629–8633. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Dang, L. Mechanistic Study of Domino Rearrangement-Promoted Meta C–H Activation in 2-Methyl-N-methoxyaniline via Cu(NHC)+: Motivation and Selectivity. Org. Lett. 2020, 22, 9178–9183. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Masukawa, K.; Ishida, Y.; Terada, M. Cu-Catalyzed [1,3]-Alkoxy Rearrangement/Diels–Alder Cascade Reactions via in Situ Generation of Functionalized ortho-Quinol Imines. Org. Lett. 2021, 23, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Chemler, S.R. Evolution of copper(II) as a new alkene amination promoter and catalyst. J. Organomet. Chem. 2011, 696, 150–158. [Google Scholar] [CrossRef][Green Version]
- Liwosz, T.W.; Chemler, S.R. Copper-Catalyzed Oxidative Amination and Allylic Amination of Alkenes. Chem. Eur. J. 2013, 19, 12771–12777. [Google Scholar] [CrossRef]
- Nozawa-Kumada, K.; Ono, K.; Kurosu, S.; Shigeno, M.; Kondo, Y. Copper-catalyzed aerobic benzylic C(sp3)–H lactonization of 2-alkylbenzamides via N-centered radicals. Org. Biomol. Chem. 2022, 20, 5948–5952. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ishida, S.; Nishikata, T. Dichloromethane as a Chlorination Reagent for α-Bromocarbonyl Compounds in the Presence of a Copper Catalyst. Chem. Lett. 2017, 46, 644–646. [Google Scholar] [CrossRef]
- Bhakat, M.; Khatua, B.; Guin, J. Photocatalytic Aerobic Coupling of Azaarenes and Alkanes via Nontraditional Cl• Generation. Org. Lett. 2022, 24, 5276–5280. [Google Scholar] [CrossRef]
- Wang, H.-W.; Wu, J.-X.; Huang, X.-Q.; Li, D.-C.; Wang, S.-N.; Lu, Y.; Dou, J.-M. RhIII-Catalyzed C–H N-Heteroarylation and Esterification Cascade of Carboxylic Acid with Organoboron Reagents and 1,2-Dichloroethane in One-Pot Synthesis. Org. Lett. 2022, 24, 5704–5709. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jiang, L.; Wang, Z.; Bin, Z.; You, J.; Yang, Y. Copper-Catalyzed Oxidative C–H Annulation of Quinolines with Dichloroethane toward Benzoquinoliziniums Using an In Situ Activation Strategy. Org. Lett. 2022, 24, 6256–6260. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Huang, T.; Chen, J.; Chen, T. Direct Dehydrogenation for the Synthesis of α,β-Unsaturated Carbonyl Compounds. Adv. Synth. Catal. 2020, 362, 3332–3346. [Google Scholar] [CrossRef]
- Gnaim, S.; Vantourout, J.C.; Serpier, F.; Echeverria, P.-G.; Baran, P.S. Carbonyl Desaturation: Where Does Catalysis Stand? ACS Catal. 2021, 11, 883–892. [Google Scholar] [CrossRef]
- Gnaim, S.; Takahira, Y.; Wilke, H.R.; Yao, Z.; Li, J.; Delbrayelle, D.; Echeverria, P.-G.; Vantourout, J.C.; Baran, P.S. Electrochemically driven desaturation of carbonyl compounds. Nat. Chem. 2021, 13, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard JA, K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard JA, K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. A Found. Adv. 2015, A71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
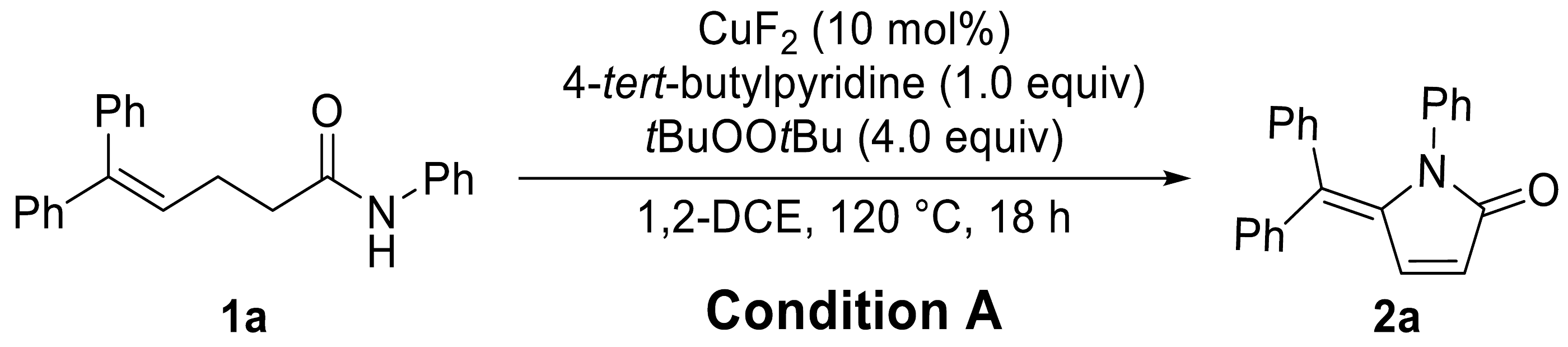 | ||
|---|---|---|
| Entry | Variation from Standard Conditions | Yield (%) b |
| 1 | None | 76 (85) |
| 2 | CuCl, CuCl2, or Cu(OAc)2 instead of CuF2 | 39–75 |
| 3 | Pyridine, DMAP, or 1,10-phen instead of 4-tert-butylpyridine | 21–66 |
| 4 | W/O 4-tert-butylpyridine | 29 |
| 5 | tBuOOH, tBuOOAc, or PIDA instead of tBuOOtBu | 0–35 |
| 6 | MnO2 instead of tBuOOtBu | 5 c |
| 7 | DCM, toluene, or PhCF3 instead of 1,2-DCE | 0–44 |
| 8 | At 100 °C | 73 |
| 9 | 1.0 mmol Scale | 83 (87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozawa-Kumada, K.; Hayashi, M.; Kwon, E.; Shigeno, M.; Yada, A.; Kondo, Y. Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams. Molecules 2023, 28, 6682. https://doi.org/10.3390/molecules28186682
Nozawa-Kumada K, Hayashi M, Kwon E, Shigeno M, Yada A, Kondo Y. Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams. Molecules. 2023; 28(18):6682. https://doi.org/10.3390/molecules28186682
Chicago/Turabian StyleNozawa-Kumada, Kanako, Masahito Hayashi, Eunsang Kwon, Masanori Shigeno, Akira Yada, and Yoshinori Kondo. 2023. "Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams" Molecules 28, no. 18: 6682. https://doi.org/10.3390/molecules28186682
APA StyleNozawa-Kumada, K., Hayashi, M., Kwon, E., Shigeno, M., Yada, A., & Kondo, Y. (2023). Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams. Molecules, 28(18), 6682. https://doi.org/10.3390/molecules28186682






