Abstract
Identifying bioactive molecules from marine organisms is still vastly understudied. Fish remain an untapped source of bioactive molecules, even when considering species whose toxicity to other fish species has been noticed before. We assessed potential applications of crude body mucus of the Lusitanian toadfish (Halobratachus didactylus) and characterized its peptide fraction composition. Mucus samples from three individuals (two wild and one captive) revealed potential antioxidant, antihypertensive, and antimicrobial activities. For antioxidant activity, the best results of 2371 ± 97 µmol Trolox Equivalent/g protein for ORAC and 154 ± 6 µmol Trolox Equivalent/g protein for ABTS were obtained. For antihypertensive activity, the relevant inhibitory activity of ACE resulted in IC50 of 60 ± 7 µg protein/mL. Antimicrobial activity was also identified against the pathogenic bacteria Escherichia coli and Listeria monocytogenes. The peptide profile of the crude body mucus was obtained through size exclusion chromatography, with a conspicuous peak at ca. 800 Da. LC-MS/MS allowed the detection of the most probable peptide sequences of this dominant peptide. This is the first study where the bioactive potential of mucus from the Lusitanian toadfish is demonstrated. Peptides with such properties can be applied in the food and pharmaceutical industries.
1. Introduction
Multidrug-resistant bacteria pose a significant challenge for research on potential pharmacological agents. In recent years, little progress has been made in addressing this issue with fewer new effective molecules [1]. Additionally, chronic diseases such as diabetes, cardiovascular disease, cancer, and psychotic disorders have also demonstrated, in some patients, multidrug resistance, resulting in substantial societal costs and creating the need for new alternative molecules [2]. Several antimicrobial peptides derived from natural sources like plants, invertebrates, and mammals have demonstrated value, but only a few have been launched in the market [1,3]. The food industry also seeks high-quality and safer bioactive molecules to be used as preservatives or health enhancers, and nature is a rich source of new peptides with diverse bioactive potentials, including antimicrobial, antioxidant, and antihypertensive potential [4]. In contrast to their terrestrial counterparts, marine organisms represent a largely untapped potential for drug discovery. This represents a striking difference considering their substantial contribution to the Earth’s biodiversity, with oceans covering 70% of the planet [5,6].
Despite abundant marine biodiversity, researchers have not extensively explored marine organisms for drug discovery compared to plants, primarily due to historical challenges, such as natural constraints to access marine habitats and species [6]. Nevertheless, marine organisms thrive in harsh and challenging environments, making them valuable reservoirs of bioactive substances [7,8]. Fish are the most diverse group of vertebrates, occurring virtually across all aquatic habitats. They possess robust immune systems that have to tackle numerous pathogens [9]. The body mucus plays a crucial role in fish immunity as it acts as a protective barrier against the surrounding environment, serving as a first line of defense [9,10]. Fish secrete ichthyocrinotoxins composed of various immune-related molecules within the mucus, including C-reactive proteins, lysozymes, immunoglobulins, proteolytic enzymes, and antimicrobial peptides [10,11]. Although researchers have extensively studied fish mucus to evaluate immunity, most studies focus on fish in aquaculture systems, which face a higher risk of bacterial infections due to confinement. In turn, frequent treatments lead to the emergence of multidrug-resistant bacteria [12]. To address this concern, recent publications within the past six years have explored methods to enhance fish immunity by incorporating polyphenols, plant extracts, and probiotics into their diets [13,14,15]. When assessing immune parameters in fish, researchers prefer mucus collection over blood due to its less invasive nature [16]. Sparus aurata, Oncorhynchus mykiss, and Salmo salar have undergone extensive studies regarding mucus composition due to their prevalence in human diets and widespread farming in aquaculture systems. However, only a few studies have investigated the presence of novel bioactive molecules within fish mucus, resulting in a limited number of described antimicrobial peptides (AMPs), such as NK-lysin, CF-14, and hepcidin type 2-like AMPs from Salmo salar, catfish and Takifugu pardalis, respectively, which exhibited potent antibacterial effects against Gram-positive and Gram-negative fish bacteria strains [17,18,19].
To date, only the protein activity of a known western Atlantic venomous species from the family Batrachoididae (Thalassophryne nattereri) has been described [20,21]. Ichthyocrinotoxins have been reported in another western Atlantic species (Oyster toadfish—Opsanus tau) [22] but lack additional characterization.
The eastern Atlantic and Mediterranean Lusitanian toadfish, Halobatrachus didactylus, is found in estuaries along the west coast of Portugal [23]. Males of this species display parental care of eggs and larvae, suggesting a possible ecological role for mucus in protecting eggs [24]. Moreover, when held in captivity alongside other fish species, H. didactylus often causes the rapid death of other fish (personal observations). Our investigation aims to identify novel bioactive molecules within the mucus of H. didactylus, particularly peptides. While the existing literature has primarily focused on the ecology and ethology of this species, our study represents the first bioprospection of its body mucus pharmacological applications.
2. Results and Discussion
2.1. Peptide Profile
Body mucus was collected from three specimens of H. didactylus: one captive (HdTAG) and two wild specimens (HdSES1 and HdSES2). Mucus was collected with a synthetic sponge that was immediately frozen and later washed with a 100 mM KPB buffer solution and centrifuged. We initially employed high-performance size exclusion chromatography (HPSEC) to compare the peptide profiles between different specimens.
Figure 1 depicts the body mucus chromatograms of the three H. didactylus samples. Notably, all three chromatograms exhibited a comparable distribution of molecular sizes, ranging from ca. 20 kDa to 800 Da. A prominent peak at around 11 min retention time was consistently observed across all three chromatograms, indicating the presence of relevant and common molecules with a molecular size of approximately 800 Da. This analysis provides valuable insights into the molecular size distribution profile of H. didactylus, particularly highlighting the presence of putative bioactive peptides with smaller molecular sizes.
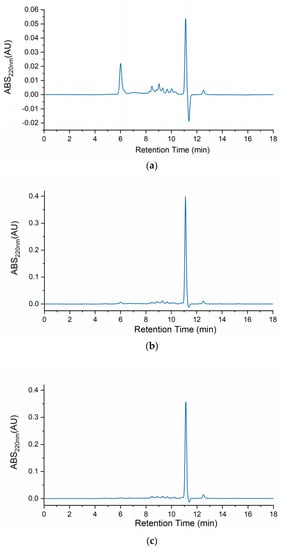
Figure 1.
HPSEC chromatograms of the body mucus from the three H. didactylus individuals (a)—HdTAG, (b)—HdSES1, and (c)—HdSES2.
In contrast with our results, the chromatographic profile of Opsanus tau (Batrachoididae) revealed three distinct peaks [22]. This species was also revealed to be toxic to other fish sharing the same tank while in captivity. These results, although highlighting possible differences between body mucus protein profiles from different species, were described more than 40 years ago. Therefore, before drawing any further conclusions, a comparative analysis using identical methodologies should be performed.
A previous study has also reported the identification of a single peak through HPLC analysis using a reverse-phase column for antibacterial fractions derived from the skin mucus of Takifugu pardalis (Tetraodontidae) [19]. Remarkably, this fraction exhibited antibacterial activity against Escherichia coli D31 [19]. The findings from the T. pardalis research indicated the presence of antimicrobial molecules with smaller molecular sizes within the mucus. These results are consistent with ours, suggesting the potential existence of bioactive antimicrobial peptides in H. didactylus mucus.
2.2. Total Protein and Antioxidant Activity
The body mucus samples from the three individuals were evaluated, HdTAG, HdSES1, and HdSES2, for soluble protein content (by BCA) and antioxidant activities (by ORAC and ABTS). Table 1 demonstrates that the HdTAG individual (captive) exhibited a higher protein concentration than the wild individuals HdSES1 and HdSES2. Additionally, the same individual demonstrated significantly higher antioxidant activity in the ORAC assay (p < 0.05). Notably, the ORAC antioxidant activity results indicated a potential relationship between antioxidant activity and protein concentration for the HdTAG individual. However, this correlation was absent for the ABTS assay. One possible explanation could be that these assays measure different reactions, with the ORAC assay focusing on hydrogen’s antioxidant donation capacity for radical neutralization and the ABTS assay operating through electron transfer [25]. The body mucus of H. didactylus exhibited antioxidant potential, which appears to be a crucial aspect of the fish’s immune defense system. That implies that reactive oxygen species may be overpopulated in stressful conditions, which can lead to cellular damage.

Table 1.
Protein concentration (BCA) and antioxidant (ORAC and ABTS) activity of mucus samples from H. didactylus individuals.
Antioxidant activity of Lusitanian toadfish mucus proved to be more effective than similar studies on other fish species (e.g., mackerel-Scomber sp. [26]). It is important to note that while we expressed our values based on protein concentration and used crude mucus, those authors did not determine the protein concentration of mackerel mucus and performed dialysis, resulting in two mucus fractions (H > 12,000–14,000 Da and L < 12,000–14,000 Da). Nonetheless, our captive and wild individuals can be compared to the farmed and wild mackerel. Regarding ORAC activity, the mucus from wild individuals in the current study (HDSES1 and HDSES2) exhibited higher values than the fraction L (less than 12,000–14,000 Da) of wild mackerel, surpassing 60 µmol TE/g. Additionally, the captive HdTAG individual showed higher ORAC activity than the fraction L (333 µmol TE/g) and the fraction H (178 µmol TE/g) of farmed mackerel. However, HdSES1 and HdSES2 did not exceed the value of 164 µmol TE/g for the fraction H of wild mackerel. Regarding ABTS, in this study results surpassed the values observed for wild and farmed mackerel mucus fractions, ranging from 2.9 to 7 µmol TE/g. The previous study on mackerel mucus suggested that farmed mackerel experiences higher stress levels, leading to increased antioxidant activity. This finding could be relevant to justify these results as the captive HdTAG individual also showed higher antioxidant activity results.
The HPSEC analysis of body mucus revealed the presence of low-molecular-weight compounds, suggesting the potential presence of antioxidant peptides responsible for the observed antioxidant activities. The significance of antioxidant peptides has been increasingly recognized in the food and pharmaceutical sectors [4,27]. Adding preservatives to avoid oxidation and extend product shelf life is crucial [27]. Therefore, there is a growing interest in identifying safe ingredients, and antioxidant peptides hold promise. These peptides have demonstrated their ability to scavenge free radicals, regulate the activities of endogenous antioxidant enzymes, and modulate antioxidant-related signaling pathways, thus playing a vital role in preventing oxidative damage that can be detrimental to human health [27].
2.3. Antihypertensive Activity
To our knowledge, the antihypertensive activity of fish body mucus remains largely unexplored. However, previous studies have extensively examined the angiotensin-converting enzyme (ACE) inhibitory potential of other fish materials such as salt-cured cod skin from Atlantic cod Gadus morhua, skin gelatin extracts from Alaska pollack Theragra chalcogramma, and muscle extracts from tuna Neothunnus macropterus and chum salmon Oncorhynchus keta [28,29]. Among those, hydrolysates and peptides derived from marine organisms ACE have proved to regulate blood pressure by converting angiotensin I into angiotensin II, a vasoconstrictor [29]. In this study, the inhibitory activity of ACE (iACE) using the HdTAG sample was evaluated because it exhibited higher protein concentration and antioxidant activity in the ORAC assay. This test resulted in an IC50 of 60 ± 7 µg mucus protein/mL, a promising outcome blocking ACE action even at low concentrations. In a comparative scale, the body mucus sample from H. didactylus exhibited higher ACE inhibition than the hydrolysates from other fish species such as the Skipjack tuna Katsuwonus pelamis (IC50 2500 µg/mL), Atlantic salmon Salmo Salar (IC50 110 µg/mL), and Chum salmon Oncorhynchus keta (IC50 1008 µg/mL) [30]. In general, peptides with inhibitory activity of ACE lower than 100 µg protein/mL are considered to have good potential antihypertensive activity [31,32].
The specific molecules responsible for this bioactivity remain unknown, but the predominance of the 800 Da peptide suggests that this is a promising candidate for future tests separating this fraction. Peptides can block ACE through competitive and non-competitive mechanisms. In the competitive mode, the peptide binds to the enzyme, causing inhibition, while in the non-competitive mode, the peptide–enzyme combination forms a dead-end complex [30,33]. Several ACE-blocking drugs available in the market (e.g., Captopril) have been associated with significant side effects, as reported in previous studies [30,31]. Antihypertensive peptides derived from fish could serve as an alternative, with some fish peptides already identified to have no side effects [29]. From a different perspective, these peptides can become useful in the food industry as nutraceuticals apart from their pharmaceutical applications.
2.4. Antimicrobial Activity
We utilized body mucus from the HdTAG individual to determine growth inhibition curves against pathogenic bacteria, including three Gram-negative bacteria, Escherichia coli ATCC 25922, Salmonella enterica serovar Enteritidis ATCC 13076, Pseudomonas aeruginosa ATCC 27853, and one Gram-positive bacteria, Listeria monocytogenes NCTC 10357. The HdTAG mucus sample exhibited inhibitory effects on all bacteria at concentrations ranging from 442 to 55 µg mucus protein/mL, as illustrated in Figure 2. Particularly notable were the significant reductions in E. coli growth by 76% at 442 µg mucus protein/mL and L. monocytogenes growth by 66% at 221 µg mucus protein/mL. Additionally, the HdTAG sample demonstrated reductions in S. enterica growth by 35% at a concentration of 442 µg mucus protein/mL and P. aeruginosa growth by 25% at 221 µg mucus protein/mL. While many studies on fish mucus evaluate antimicrobial activity against fish pathogenic bacteria for applications in the aquaculture industry, such as Aeromonas hydrophila, Yersinia ruckeri, Vibrio harveyi, and Vibrio parahaemolyticus [34,35,36], there are limited studies investigating the antimicrobial activity of fish mucus against human pathogenic bacteria. For instance, a glycoprotein extracted from the epidermal mucus of African sharptooth catfish Clarias gariepinus (CFG) displayed antimicrobial potential against E. coli, L. monocytogenes, and P. aeruginosa at an inhibitory concentration of 50 μg/mL of CFG [37].
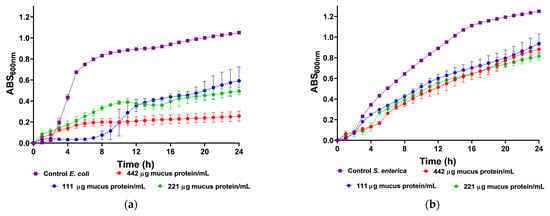
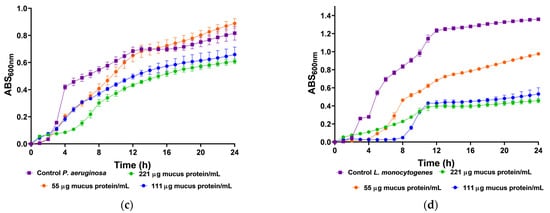
Figure 2.
Inhibition growth curves (measured by absorbance at 600 nm) of HdTAG body mucus samples tested at concentrations between 442 and 55 µg mucus protein/mL against pathogenic bacteria; Gram-negative: (a)—E. coli, (b)—S. enterica, and (c)—P. aeruginosa; and Gram-positive: (d)—L. monocytogenes.
Similarly, mucus derived from freshwater pool barb Puntius sophore demonstrated inhibitory effects upon E. coli and P. aeruginosa at concentrations ranging from 250 to 50 μg/mL of mucus [38]. These results are helpful to provide a framework for fish mucus antimicrobial activity. However, we cannot compare results directly because different authors use different methods. This study used crude venom extracts, which can be compared to the acid extraction technique used with the pool barb. This differed from the glycoprotein extraction on mucus from the African sharptooth catfish.
In comparison with another known venomous fish species, a study demonstrated a potent antimicrobial activity of the common stingray Dasyatis pastinaca skin mucus against the same strains of E. coli ATCC 25922 and P. aeruginosa ATCC 27853, with an inhibitory concentration of 16 µg/µL of skin mucus [39]. In this last example, the authors did not describe the mucus collection method. This finding on the ability of mucus from the body of H. didactylus to be antibacterial is a significant feature since there is an increase in antibiotic resistance within pathogens and a decline in the discovery rate of new drugs [40]. We tested it against the most common foodborne pathogens, such as E. coli, L. monocytogenes, and S. enterica. The discovery of antimicrobial peptides in mucus could be up-and-coming, as it could be applied as a preservative in the food industry. In addition, the antibacterial activity of mucus from the body of H. didactylus against P. aeruginosa is also relevant since this organism is difficult to control with antibiotics due to its high intrinsic resistance [41].
2.5. Fractionation by Size Exclusion Chromatography and Peptidomics of the Peptide Fraction
To isolate the most representative peak, which must contain the bioactive peptide fraction to identify the most representative peptides, particularly the fraction containing the most prominent body mucus samples underwent preparative size exclusion chromatography. Due to the limited volume of protein collected, we pooled the samples HdSES1 and HdSES2 (referred to as HdSESP), which previously showed identical molecular size distribution, protein content, and antioxidant activity.
The purification of the target fraction resulted in a fraction containing a most prominent peak below 1.2 kDa (ca. 800 Da), as illustrated in Figure 3.
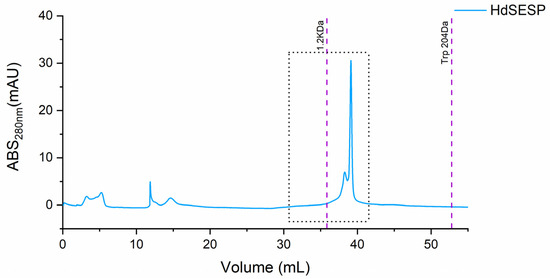
Figure 3.
SEC chromatogram of the mucus sample from the HdSESP pooled mucus sample of the HdSES1 individual with the HdSES2 individual.
Several studies have characterized and demonstrated promising bioactivities of fractions derived from fish mucus. Skin mucus fractions of climbing perch Anabas testudineus obtained a bioactive fraction through Sephadex G-25 column [42].
The obtained fraction of HdSESP, highlighted in Figure 3, was subjected to LC-MS/MS to characterize the sequence of the peptides. Due to the lack of information in proteomic databases for H. didactylus, we could not obtain reliable results for peptide identification using database search methods. Consequently, we employed de novo sequencing with the PEAKS Studio© software, considered one of the most reliable tools. The number of MS scans was 25686, with 3329 MS/MS scans. In the analysis process, the number of de novo peptides after the score filter (de novo score ≥ 80) was 37 (Figure 4). The analysis was satisfactory concerning the high degree of confidence in the sequencing.
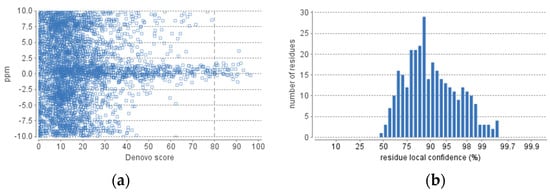
Figure 4.
De novo statistics representation. (a) Scatterplot of peptide de novo score versus precursor mass error. (b) Distribution of residue local confidence in the filtered result.
The analysis using the CAMPR3 database yielded the data presented in Table 2. We evaluated the listed peptides using the CAMPR3 classifiers (SVM, RF, ANN, and DA) to predict their potential antimicrobial activity. Our analysis categorized some peptides as potentially antimicrobial, while others were not found to exhibit antimicrobial activity (NAMP). These antimicrobial peptides are the most promising candidates for further exploration due to their predicted bioactivity against microorganisms. Among these, the ones that simultaneously offer reasonable estimates of antimicrobial activity for all classifiers are highlighted in Table 2.

Table 2.
Peptide classification based on antimicrobial potential predicted in silico.
We observed diversity of peptide sizes and abundance within the body mucus of H. didactylus, as indicated by the identification of peptides with distinct mass and area values. This diversity suggests that multiple antimicrobial compounds contribute to the fish’s defense against pathogens. The identified peptide sequences were subjected to a search of previous reports in the BIOPEP-UWM database, one of the largest databases of bioactive peptides, without finding any match. The results suggest that these peptides may be novel bioactive molecules.
Previous studies have emphasized the antimicrobial properties of specific peptides in fish mucus, such as NK-lysin, CF-14, and hepcidin type 2-like peptides. Li et al. (2019) reported the importance of proline in the middle of the peptide sequence CF-14 for its antimicrobial activity [18]. Similarly, Go et al. (2019) highlighted the antimicrobial properties of peptides with a positively charged structure, such as hepcidin type 2-like, which can disrupt cell membranes by interacting with negatively charged lipopolysaccharides or phospholipids [19]. The peptides we identified in this study share some of these features, particularly the presence of proline in their sequences.
Table 3 ranks the potential antimicrobial peptides based on their bioactivities, determined through PeptideRanker analysis and the BIOPEP-UWM database. PeptideRanker is a server that utilizes a novel N-to-1 neural network to predict bioactive peptides. PeptideRanker assigns a probability of bioactivity to each peptide, with a threshold of 0.5 indicating peptides predicted as bioactive [43]. Among the 15 sequences analyzed, only 7 exhibited potential bioactivity, with VYPFPGPLPN being the most promising candidate.

Table 3.
Peptide classification based on potential bioactivity predicted in silico.
Using the BIOPEP-UWM database, several peptides displayed a range of potential bioactivities. For instance, DPPNPKNL and DPPNPKLN (highlighted in Table 3) demonstrated significant bioactivity frequency in immunomodulating, neuropeptide, and renin inhibitor categories. Notably, these peptides exhibited inhibitory potential against dipeptidyl peptidase IV (DPP-IV), an enzyme involved in glucose metabolism [44]. The inhibitory properties of these peptides against DPP-IV are particularly interesting, considering the potential of DPP-IV inhibitors in treating type 2 diabetes [44,45].
PAPPPPPP showed a high frequency of bioactive fragments, suggesting its potential involvement in various bioactivities. Our analysis revealed its significant inhibitory activity against ACE and DPP-IV, providing valuable insights into the bioactive repertoire of H. didactylus mucus and suggesting potential therapeutic applications in cardiovascular and metabolic health.
In contrast, the peptide EDNSELGQETPTLR exhibited a lower frequency of bioactive fragments than the previously discussed peptides. However, it demonstrated partial inhibitory activity against dipeptidyl peptidase III (DPP-III) and displayed potential immunomodulatory properties. DPP-III inhibitors have been investigated for their potential role in various diseases, including cancer and inflammation [46,47]. The immunomodulatory potential of EDNSELGQETPTLR indicates its potential involvement in regulating the immune response, warranting further investigation for potential therapeutic applications [48].
VYPFPGPLPN, the highest-ranked peptide by PeptideRanker, displayed a high frequency of bioactive fragments, suggesting its potential involvement in multiple bioactivities. Our analysis revealed its potential inhibitory activity against various enzymes, including alpha-glucosidase, DPP-IV, and renin. Alpha-glucosidase inhibitors, similar to DPP-IV inhibitors, have garnered significant interest in managing type 2 diabetes [49], while renin inhibitors have implications in hypertension management [50]. The presence of multiple inhibitory activities in VYPFPGPLPN highlights its potential as a multi-target bioactive peptide.
Additionally, Table 3 highlights that ACE inhibition is the second most frequent bioactivity observed in the identified peptides. Structural characteristics, such as size, chain length, and amino acid sequence type and order, are crucial in determining bioactivity [33]. Previous studies have demonstrated the potent antihypertensive activity of peptides derived from fish hydrolysis, particularly those containing hydrophobic amino acids at the N-terminal and C-terminal ends. For instance, peptides with leucine at the N-terminal and proline at the C-terminal, derived from Alaska pollock Theragra chalcogramma skin, exhibited intense antihypertensive activity by inhibiting ACE [29]. Furthermore, peptides rich in proline have been shown to enhance the observed antihypertensive potential in snakehead hydrolysates [33]. These characteristics are also evident in the peptides listed in Table 3, with proline, leucine, and valine present at the terminals.
Overall, this study provides valuable insights into the potential antimicrobial properties of peptides found in the body mucus of H. didactylus, as predicted by the CAMPR3 database. The results obtained from PeptideRanker and the BIOPEP-UWM database offer valuable information regarding the potential bioactivities of the identified peptides. These findings suggest the presence of diverse and bioactive peptides within the body mucus of H. didactylus. Future experimental investigations are necessary to validate the predicted bioactivities [43] and explore these peptides’ specific mechanisms and applications in various therapeutic contexts.
3. Materials and Methods
3.1. Materials
Angiotensin-I converting enzyme (peptidyl-di-peptidase A, EC 3.4.15.1, 5.1 U/mg), Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), and AAPH [2,20-azobis (2-Int. J. Mol. Sci. 2022, 23, 2439 7 of 9 amidinopropane) dihydrochloride] were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Fluorescein [30,60-dihydroxyspiro (isobenzofuran-1 [3H], 90 [9H]-xanthen)-3-one] was purchased from Fisher Scientific (Hanover Park, IL, USA). The tripeptide Abz-Gly-Phe (NO2)-Pro was obtained from Bachem Feinchemikalien (Bubendorf, Switzerland). Tris [tris (hydroxymethyl) aminomethane] was obtained from Honeywell Fluka (Charlotte, NC, USA). Muller-Hinton Broth was purchased from Biokar Diagnostics (Beauvais, France). Pierce BCA Protein Assay Kit was purchased from Thermo Scientific (Vantaa, Finland).
3.2. Mucus Collection
The mucus was collected from Halobatrachus didactylus fish captured by fishermen in central west Portugal and later released by our team. Two wild individuals from Sesimbra (HdSES1 and HdSES2) and one captive in our fish facility for more than 3 months was previously captured from Tagus estuary (HdTAG). The dorso-lateral surface was washed with deionized water and the excess liquid was gently removed with a paper towel. A sponge was then used to collect the mucus from the fish skin. No anesthesia or chemical was used during the procedure. The sponges were washed with KPB buffer solution 100 mM and centrifuged to collect the mucus. The mucus samples were stored at −80 °C until further analysis.
3.3. Peptide Size Profile
The peptide profile was determined using high-performance size exclusion chromatography (HPSEC). The fish mucus samples were filtered with 0.22 µm filters before HPSEC analysis. An Agilent AdvanceBio SEC column (Agilent Technologies, London, UK), 2.7 µm particle size, 130 Å pore size, and 7.8 inner diameter × 300 mm length was used. The column was eluted isocratically with a phosphate buffer (0.15 M NaH2PO4 pH 7) at a flow rate of 1 mL/min. The sample injection volume was 10 µL. The instrument used was Waters 2690 with a photodiode array detector (PDA 190–600 nm). The software Empower 3 was used for the data collection. To determine molecular weights of the resulting chromatogram peaks, a calibration curve was made with the following protein standards: Ovalbumin (44,300 Da); Myoglobin (17,600 Da); Cytochrome C (12,327 Da); Aprotinin (6511 Da); Neurotensin (1672 Da); Angiotensin-II (1040 Da); Tyr-Phe dipeptide (328.4 Da); and L-tryptophan (204 Da).
3.4. Soluble Protein Content
Bicinchoninic acid (BCA) methodology (Pierce BCA Protein Assay Kit) determined the soluble protein concentration in mucus samples.
3.5. Antioxidant Activity
3.5.1. ABTS Assay
The ABTS (2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) radical activity is based on an antioxidant’s capacity to scavenge the ABTS’s oxidized state. For this, ABTS radical scavenging activity is performed in a 96-well microplate, following the method described by [51] with some modifications. For the assay, ABTS radical was prepared by the mixture solution of equal parts of ABTS (7 mM) with potassium persulfate (2.44 mM), and then the radical was generated after 16 h. Before performing the assay in the microplate, ABTS radical absorbance was adjusted to 0.70 (±0.02) at 734 nm. In each well, for 20 µL of mucus samples was added 180 µL of ABTS radical. Instead of the sample, ultrapure water was used as control, and seven calibration solutions using Trolox (25–175 µM). The mixture was incubated for 5 min at 30 °C, and the absorbance at 734 nm was measured with a Multidetection plate reader (Synergy H1, Winooski, VT, USA). All measurements were performed in duplicate. The final results were expressed in µmol of Trolox equivalents.
3.5.2. ORAC Assay
The oxygen radical absorbance capacity (ORAC) assay followed the protocol outlined by Coscueta et al. (2019) [52]. A black polystyrene 96-well microplate (Nunc, Roskilde, Denmark) was utilized, and measurements were taken using a Multidetection plate reader (Synergy H1, Vermont, USA) controlled by Gen5 Biotek software version 3.04. Fluorescence was monitored for 80 min at 1 min intervals. Each sample, standard, blank, or control analysis was duplicated. The final ORAC values were expressed as Trolox Equivalents (μmol TE).
3.6. Antihypertensive Activity
The angiotensin-converting enzyme-I inhibition (iACE) assay was conducted following the procedure outlined by Coscueta et al. (2019) [52]. The assay was performed using a black polystyrene 96-well microplate (Nunc, Denmark), and fluorescence was monitored at 1 min intervals for 80 min using a Multidetection plate reader (Synergy H1, Vermont, USA) controlled by Gen5 Biotek software version 3.04. Each sample, blank, and control analysis was carried out in triplicate. The iACE was quantified as the concentration required to inhibit 50% of the enzymatic activity (IC50). The IC50 values were determined using non-linear modelling of the data obtained.
3.7. Antimicrobial Activity
The antimicrobial activity of the mucus samples was evaluated following the method by [53] against the following pathogenic bacteria: Gram-negative Escherichia coli ATCC 25922, Salmonella enterica serovar Enteritidis ATCC 13076, and Pseudomonas aeruginosa ATCC 27853, and Gram-positive Listeria monocytogenes NCTC 10357. The mucus samples were combined with a 1/10 ratio of inoculum containing 1% (v/v) of bacteria cultured for 24 h in Mueller–Hinton Broth (MHB). For the positive control, deionized water was used instead of mucus samples with a 1% inoculum, while deionized water with MHB served as the negative control. The mixture was then transferred to a 96-well microplate (Sarstedt, Germany), and the optical density (OD) at 600 nm was measured every hour for a 24 h period at 37 °C using a microplate reader (Multiskan GO, Thermo Scientific, Finland). An increase in OD indicated bacterial growth.
3.8. Preparative Size Exclusion Chromatography
The mucus samples from HdTAG and HDSESP (pooled from HDSES1 with HDSES2) were loaded onto two size exclusion chromatography (SEC) columns, namely Superdex Peptide 10/300 GL and Superdex 200 Increase 10/300 GL, connected in series, using an ÄKTA pure chromatography system. Ultrapure water was used as the eluent for the columns, and the peaks were detected at an absorbance of 280 nm. The elution flow rate was set at 0.5 mL/min, and fractions of 5 mL were collected starting from the time of sample loading on the column. Two fractions containing the most intense peaks were pooled.
3.9. Peptidomic Analysis
Peptide identification and quantification were conducted using nanoLC-MS/MS, following the method described by Osório et al. (2021) [54]. Tandem mass spectra were processed with PEAKS Studio version 10.6 (Bioinformatics Solutions Inc., Waterloo, ON, Canada). The DENOVO analysis was configured assuming no digestion enzyme, with a fragment ion mass tolerance of 0.02 Da, a parent ion tolerance of 10.0 ppm, and a maximum of 3 variable PTMs per peptide.
The identified peptide sequences were categorized based on their potential antimicrobial activity, filtering out those not expected to possess such activity. To predict the bioactivity in silico, all identified peptides were analyzed using the CAMPR3 (Collection of Antimicrobial Peptides) [55] with Support Vector Machine (SVM) classifier, Random Forest (RF) classifier, Artificial Neural Network (ANN) classifier, and Discriminant Analysis classifier.
The potentially antimicrobial sequences were further analyzed to estimate their overall bioactive potential. For in silico bioactivity prediction of each peptide, a ranking was initially performed using PeptideRanker [56], a server that predicts bioactive peptides based on a novel N-to-1 neural network [57]. Subsequently, all these peptides were analyzed using the BIOPEP-UWM database [58] to estimate potential bioactivities [59,60].
3.10. Statistical Analysis
Statistical analysis was performed using RStudio v2023.06.0. The mean values obtained from two replicates were subjected to analysis of variance (ANOVA). Mean separation was determined using Tukey’s post hoc test at a 5% significance level. Homoscedasticity of variance was assessed using Cochran’s and Bartlett’s tests at a 5% significance level.
4. Conclusions
In conclusion, our study provides the first peptide profile for the Lusitanian toadfish Halobatrachus didactylus epidermal mucus. We also describe its antioxidant activity, antihypertensive activity, antimicrobial activity of H. didactylus’s body mucus, and the peptidomics obtained for the most representative chromatographic peak. The peptide profiles revealed the presence of bioactive peptides with smaller molecular sizes, indicating their potential role in various biological activities. The body mucus exhibited significant antioxidant activity, surpassing values reported for other fish species. The inhibitory activity against ACE highlighted the potential of H. didactylus mucus as an alternative source of antihypertensive peptides.
Moreover, the mucus displayed antimicrobial solid effects against pathogenic bacteria, expanding the understanding of its biological defense mechanisms. The fractionation and peptidomics analysis of the mucus led to identifying novel peptides with bioactive properties estimated in silico. Further in vitro assays and cytotoxicity tests are required to validate the potential applications of these peptides in the pharmaceutical and food industries. Overall, this research contributes to exploring and valorizing the properties of toxic H. didactylus epidermal mucus and highlighting the potential of fish ichthyocrinotoxins that remain a largely untapped source of bioactive molecules.
5. Patents
New peptides and uses thereof. Instituto Nacional da Propriedade Industrial. Patent Nº.: PT118365. 5 December 2022.
Author Contributions
Conceptualization, M.F.C. and E.R.C.; methodology, M.F.C., E.R.C., R.M. and J.N.; software, E.R.C.; validation, M.E.B., F.A., D.G. and M.P.; investigation, M.F.C., R.M. and J.N.; data curation, E.R.C.; writing—original draft preparation, M.F.C., M.E.B. and E.R.C.; writing—review and editing, M.F.C., E.R.C., M.E.B., R.M., J.N., F.A., D.G. and M.P.; supervision F.A., D.G. and M.P.; project administration, F.A., D.G. and M.P.; and funding acquisition, F.A., D.G. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from the FCT-FDCT “FISHMUC—Bioactive properties of external mucus isolated from coastal fish of Macao and Portugal”, project reference MACAU/0003/2019 and “Macao Science and Technology Development Fund (FDCT), project reference 0005/2019/APJ”. F.A. (Frederico Almada) had the support through researcher contract DL57/2016/CP1339/CT0003 and the FCT—Fundação para a Ciência e Tecnologia through the strategic project UIDB/04292/2020 awarded to MARE and through the project LA/P/0069/2020 granted to the Associate Laboratory ARNET. J.N. (José Neto) had the support through the PhD scholarship UI/BD/150955/2021 FCT—Fundação para a Ciência e Tecnologia.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as the patenting process is still ongoing at the time this article is submitted.
Acknowledgments
The authors thank the CBQF for its institutional support. The authors thank Hugo Osório from the i3S—Instituto de Investigação e Inovação em Saúde, Universidade do Porto—for his contribution to the analysis of MS/MS. The authors also thank Aquário Vasco da Gama and Fátima Gil for all their assistance with the collection of epidermal mucus from captive fish. Access to this fish facility was crucial for all preliminary tests that allowed us to fine tune the methodologies applied in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Grosberg, R.K.; Vermeij, G.J.; Wainwright, P.C. Biodiversity in Water and on Land. Curr. Biol. 2012, 22, R900–R903. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and Biological Activities of Marine-Derived Bioactive Peptides: A Review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Smith, W.L.; Wheeler, W.C. Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y. Applications of Antimicrobial Peptides from Fish and Perspectives for the Future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef]
- Sridhar, A.; Manikandan, D.B.; Marimuthu, S.K.; Murugesan, M.; Ramasamy, T. Methanol Skin Mucus Extract of Mrigal (Cirrhinus mrigala) Fish Peptide Targeting Viral Particles of Infectious Pancreatic Necrosis Virus (IPNV) and Infectious Salmon Anemia Virus (ISAV): An in Silico Approach. Int. J. Pept. Res. Ther. 2021, 27, 1429–1440. [Google Scholar] [CrossRef]
- Ziegman, R.; Alewood, P. Bioactive Components in Fish Venoms. Toxins 2015, 7, 1497–1531. [Google Scholar] [CrossRef] [PubMed]
- Qadiri, S.S.N.; Makesh, M.; Rajendran, K.V.; Rathore, G.; Purushothaman, C.S. Specific Immune Response in Mucosal and Systemic Compartments of Cirrhinus mrigala Vaccinated against Edwardsiella tarda: In Vivo Kinetics Using Different Antigen Delivery Routes. J. World Aquac. Soc. 2019, 50, 856–865. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Jahazi, M.A.; Nikdehghan, N.; Van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of Dietary Polyphenols from Agricultural By-Products on Mucosal and Humoral Immune and Antioxidant Responses of Convict Cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734790. [Google Scholar] [CrossRef]
- Heydari, M.; Firouzbakhsh, F.; Paknejad, H. Effects of Mentha longifolia Extract on Some Blood and Immune Parameters, and Disease Resistance against Yersiniosis in Rainbow Trout. Aquaculture 2020, 515, 734586. [Google Scholar] [CrossRef]
- Mohammadi, G.; Adorian, T.J.; Rafiee, G. Beneficial Effects of Bacillus subtilis on Water Quality, Growth, Immune Responses, Endotoxemia and Protection against Lipopolysaccharide-Induced Damages in Oreochromis niloticus under Biofloc Technology System. Aquac. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Oliveira, M.; Tvarijonaviciute, A.; Trindade, T.; Soares, A.; Tort, L.; Teles, M. Can Non-Invasive Methods Be Used to Assess Effects of Nanoparticles in Fish? Ecol. Indic. 2018, 95, 1118–1127. [Google Scholar] [CrossRef]
- Valero, Y.; Cortés, J.; Mercado, L. NK-Lysin from Skin-Secreted Mucus of Atlantic Salmon and Its Potential Role in Bacteriostatic Activity. Fish Shellfish Immunol. 2019, 87, 410–413. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Wang, D.; Li, J. Characterization and Antimicrobial Mechanism of CF-14, a New Antimicrobial Peptide from the Epidermal Mucus of Catfish. Fish Shellfish Immunol. 2019, 92, 881–888. [Google Scholar] [CrossRef]
- Go, H.-J.; Kim, C.-H.; Park, J.B.; Kim, T.Y.; Lee, T.K.; Oh, H.Y.; Park, N.G. Biochemical and Molecular Identification of a Novel Hepcidin Type 2-like Antimicrobial Peptide in the Skin Mucus of the Pufferfish Takifugu pardalis. Fish Shellfish Immunol. 2019, 93, 683–693. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Barbaro, K.C.; Cardoso, D.F.; Moura-Da-Silva, A.M.; Mota, I. Thalassophryne nattereri Fish Venom: Biological and Biochemical Characterixation and Serum Neutralization of Its Toxic Activities. Toxicon 1998, 36, 405–410. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Lopes-Ferreira, M.; Junqueira-De-Azevedo, I.L.M.; Spencer, P.J.; Araújo, M.S.; Portaro, F.C.V.; Ma, L.; Valente, R.H.; Juliano, L.; Fox, J.W.; et al. Natterins, a New Class of Proteins with Kininogenase Activity Characterized from Thalassophryne nattereri Fish Venom. Biochimie 2005, 87, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.R.; Leong, I.; Nayar, M.S.B. Ichthyotoxins from the Oyster Toadfish, Opsanus tau (Linnaeus). Toxicon 1982, 20, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.F.; Santos, M.J.; Costa, J.L.; Costa, M.J.; Cabral, H.N. Metazoan Parasites as Biological Indicators of Population Structure of Halobatrachus didactylus on the Portuguese Coast. J. Appl. Ichthyol. 2005, 21, 220–224. [Google Scholar] [CrossRef]
- Cotter, J.C.; Pereira, T.J.; Costa, M.J.; Costa, J.L. Distribution, Abundance, Population Structure and Activity of Halobatrachus didactylus in the Tagus Estuary (Portugal) and Adjacent Coastal Waters. J. Mar. Biol. Assoc. UK 2013, 93, 405–412. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tagami, M.; Kuwahara, J. Evaluation of Antioxidant Activity and Amino Acids in the Mucus of Mackerel for Cosmetic Applications. J. Oleo Sci. 2020, 69, 1133–1138. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Brassesco, M.E.; Pintado, M. Collagen-Based Bioactive Bromelain Hydrolysate from Salt-Cured Cod Skin. Appl. Sci. 2021, 11, 8538. [Google Scholar] [CrossRef]
- Kim, S.K.; Ngo, D.H.; Vo, T.S. Marine Fish-Derived Bioactive Peptides as Potential Antihypertensive Agents, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, ISBN 9780124160033. [Google Scholar]
- Abdelhedi, O.; Nasri, M. Basic and Recent Advances in Marine Antihypertensive Peptides: Production, Structure-Activity Relationship and Bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive Activity of Fish Protein Hydrolysates and Its Peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-Converting Enzyme Inhibitory Activity and Antioxidant Capacity of Bioactive Peptides Derived from Enzymatic Hydrolysis of Buffalo Milk Proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-i-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rajeshwaran, T.; Priya, P.; Kailasam, M.; Biswas, G.; Ghoshal, T.K.; Vijayan, K.K.; Arasu, A.R.T. Comparative Immunological and Biochemical Properties of the Epidermal Mucus from Three Brackishwater Fishes. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 95–103. [Google Scholar] [CrossRef]
- Guluarte, C.; Reyes-Becerril, M.; Gonzalez-Silvera, D.; Cuesta, A.; Angulo, C.; Esteban, M.Á. Probiotic Properties and Fatty Acid Composition of the Yeast Kluyveromyces lactis M3. In Vivo Immunomodulatory Activities in Gilthead Seabream (Sparus Aurata). Fish Shellfish Immunol. 2019, 94, 389–397. [Google Scholar] [CrossRef]
- Soltanian, S.; Gholamhosseini, A. The Effects of Starvation on Some Epidermal Mucus Immune Parameters in Rainbow Trout, Oncorhynchus Mykiss. Int. J. Aquat. Biol. 2019, 7, 291–300. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, Biological Characteristics and Antibacterial Activity of Glycoprotein Extracted from the Epidermal Mucus of African Catfish (Clarias gariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and Role of Bioactive Molecules from Puntius sophore (Freshwater/Brackish Fish) Skin Mucus with Its Potent Antibacterial, Antiadhesion, and Antibiofilm Activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef]
- Fuochi, V.; Li Volti, G.; Camiolo, G.; Tiralongo, F.; Giallongo, C.; Distefano, A.; Petronio, G.P.; Barbagallo, I.; Viola, M.; Furneri, P.M.; et al. Antimicrobial and Anti-Proliferative Effects of Skin Mucus Derived from Dasyatis pastinaca (Linnaeus, 1758). Mar. Drugs 2017, 15, 342. [Google Scholar] [CrossRef]
- Cantisani, M.; Finamore, E.; Mignogna, E.; Falanga, A.; Nicoletti, G.F.; Pedone, C.; Morelli, G.; Leone, M.; Galdiero, M.; Galdiero, S. Structural Insights into and Activity Analysis of the Antimicrobial Peptide Myxinidin. Antimicrob. Agents Chemother. 2014, 58, 5280–5290. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Gera, L.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides to Target Gram-Negative Pathogens, Acinetobacter Baumannii and Pseudomonas Aeruginosa: Utilization of Charge, “Specificity Determinants,” Total Hydrophobicity, Hydrophobe Type and Location as Design Parameters to Improve the Therapeutic Ratio. Chem. Biol. Drug Des. 2011, 77, 225–240. [Google Scholar] [CrossRef]
- Najm, A.A.K.; Azfaralariff, A.; Dyari, H.R.E.; Othman, B.A.; Shahid, M.; Khalili, N.; Law, D.; Syed Alwi, S.S.; Fazry, S. Anti-Breast Cancer Synthetic Peptides Derived from the Anabas Testudineus Skin Mucus Fractions. Sci. Rep. 2021, 11, 23182. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Batista, P.; Gomes, J.E.G.; da Silva, R.; Pintado, M.M. Screening of Novel Bioactive Peptides from Goat Casein: In Silico to In Vitro Validation. Int. J. Mol. Sci. 2022, 23, 2439. [Google Scholar] [CrossRef]
- Ma, C.; Liu, D.; Hao, H.; Wu, X. Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods 2022, 11, 2148. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. An in Silico Model to Predict the Potential of Dietary Proteins as Sources of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.C.; Chauhan, S.S. Dipeptidyl Peptidase III: A Multifaceted Oligopeptide N-End Cutter. FEBS J. 2011, 278, 3256–3276. [Google Scholar] [CrossRef]
- Abramić, M.; Agić, D. Survey of Dipeptidyl Peptidase III Inhibitors: From Small Molecules of Microbial or Synthetic Origin to Aprotinin. Molecules 2022, 27, 3006. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Massolini, B.D.; Contieri, S.S.G.; Lazarini, G.S.; Bellacosa, P.A.; Dobre, M.; Petroianu, G.; Brateanu, A.; Campos, L.A.; Baltatu, O.C. Therapeutic Renin Inhibition in Diabetic Nephropathy—A Review of the Physiological Evidence. Front. Physiol. 2020, 11, 190. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of Elevated CO2 on Grapevine (Vitis vinifera L.): Volatile Composition, Phenolic Content, and in Vitro Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic Soy Protein Hydrolysis: A Tool for Biofunctional Food Ingredient Production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Osório, H.; Silva, C.; Ferreira, M.; Gullo, I.; Máximo, V.; Barros, R.; Mendonça, F.; Oliveira, C.; Carneiro, F. Proteomics Analysis of Gastric Cancer Patients with Diabetes Mellitus. J. Clin. Med. 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- CAMP: Collection of Sequences and Structures of Antimicrobial Peptides. Available online: http://www.camp3.bicnirrh.res.in/ (accessed on 25 July 2022).
- PeptideRanker. Available online: http://distilldeep.ucd.ie/PeptideRanker/ (accessed on 25 July 2022).
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- No BIOPEP-UWM—Katedra Biochemii Zywno’sci. Available online: https://biochemia.uwm.edu.pl/biopep-uwm/ (accessed on 25 July 2022).
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP Database and Other Programs for Processing Bioactive Peptide Sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).