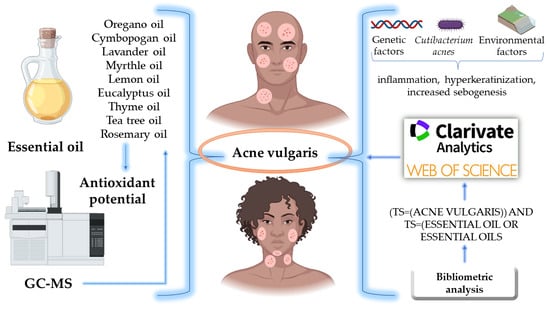
Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris
Abstract
:1. Introduction
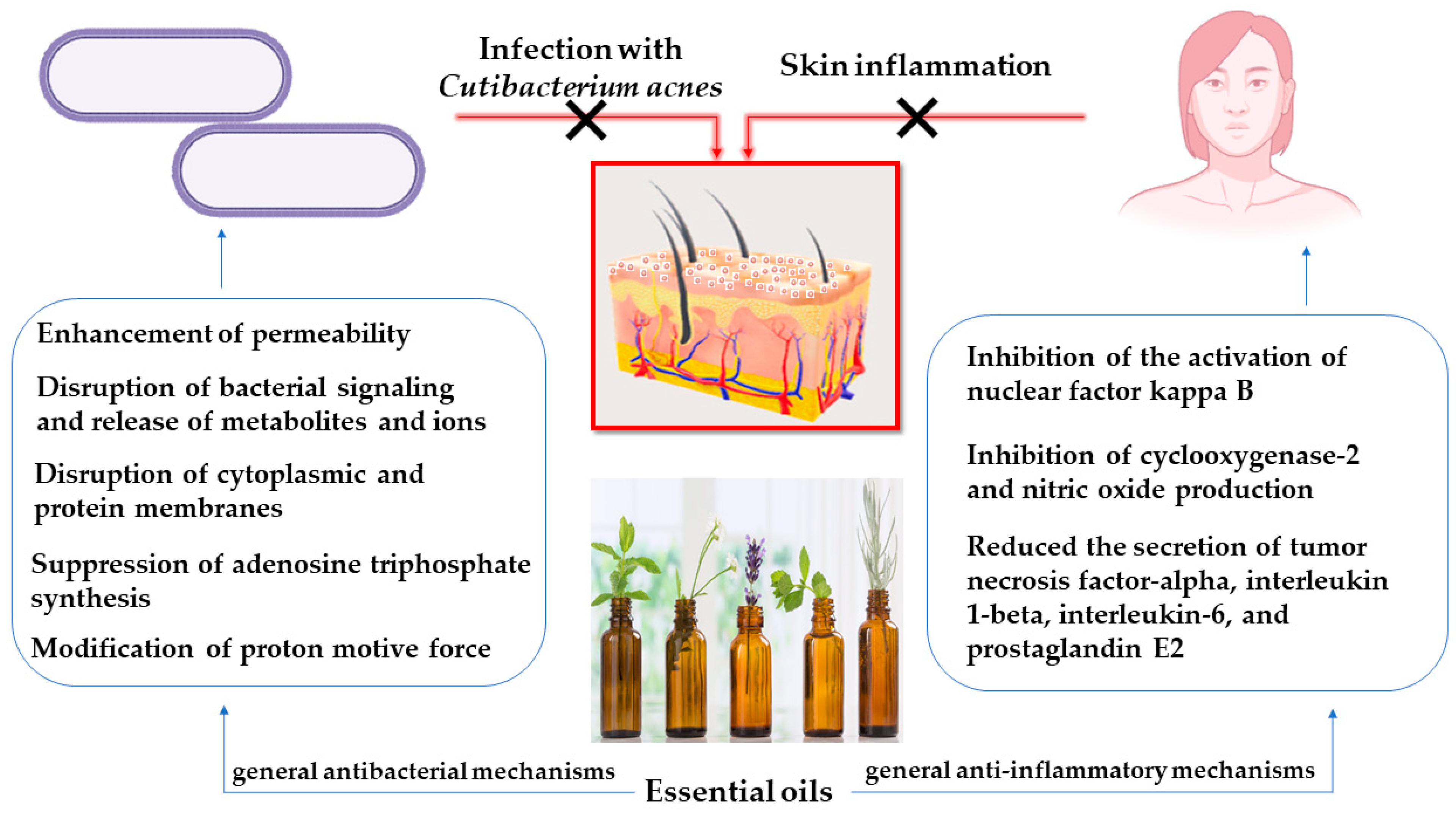
2. Pathophysiology of Acne Vulgaris
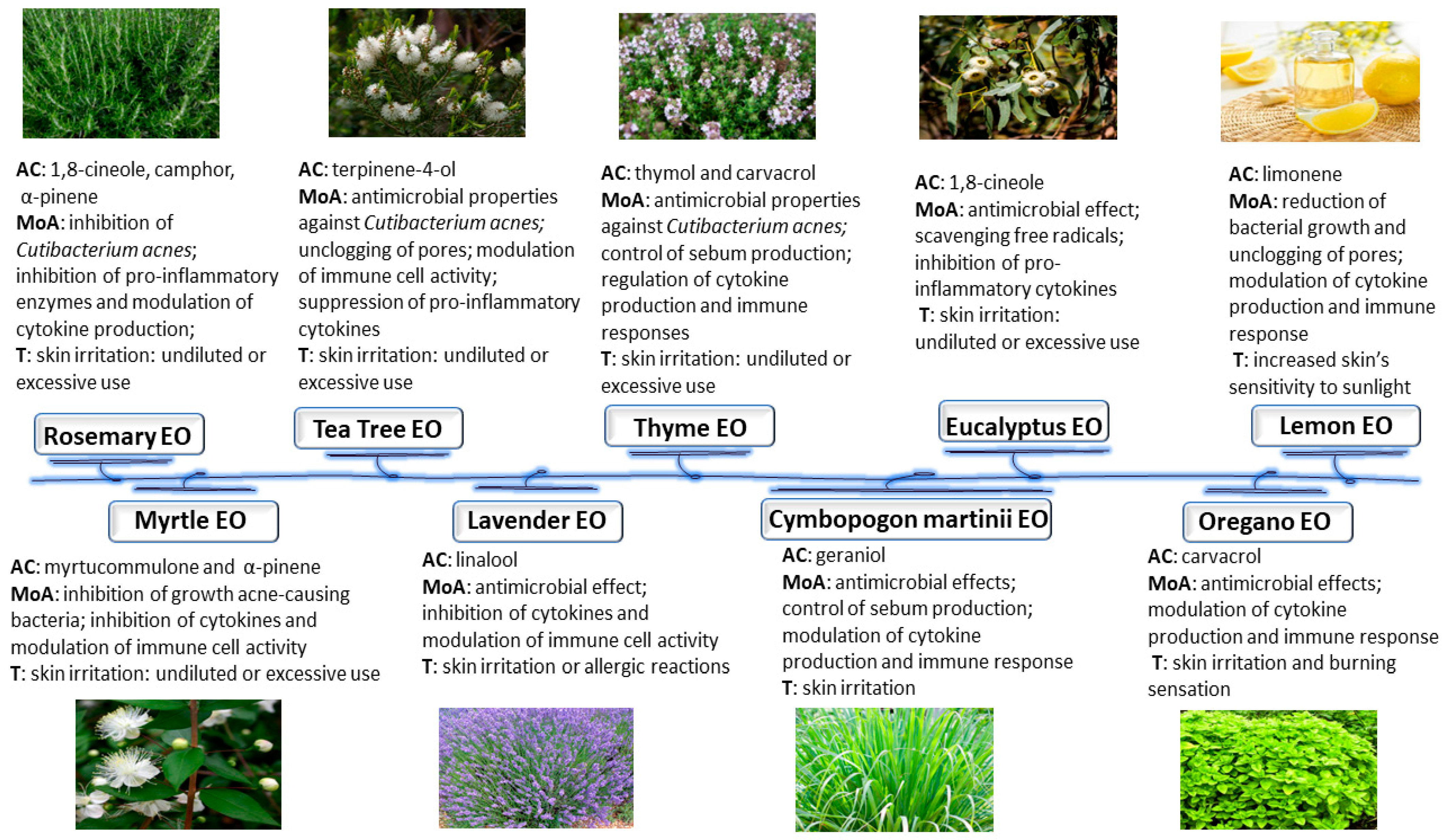
3. Anti-Inflammatory and Anti-Microbial Activity of Certain Essential Oils
3.1. Oregano Essential Oil
3.2. Cymbopogon Martinii Essential Oil
3.3. Lavender Essential Oil
3.4. Myrtle Essential Oil
3.5. Lemon and Other Citrus Essential Oil
3.6. Eucalyptus Essential Oil
3.7. Thyme Essential Oil
3.8. Tea Tree Essential Oil
3.9. Rosemary Essential Oil
4. Antioxidant Activity of Certain Essential Oils
4.1. Oregano Essential Oil
4.2. Cymbopogon Martinii Essential Oil
4.3. Lavender Essential Oil
4.4. Myrtle Essential Oil
4.5. Lemon and Other Citrus Essential Oil
4.6. Eucalyptus Essential Oil
4.7. Thyme Essential Oil
4.8. Tea Tree Essential Oil
4.9. Rosemary Essential Oil
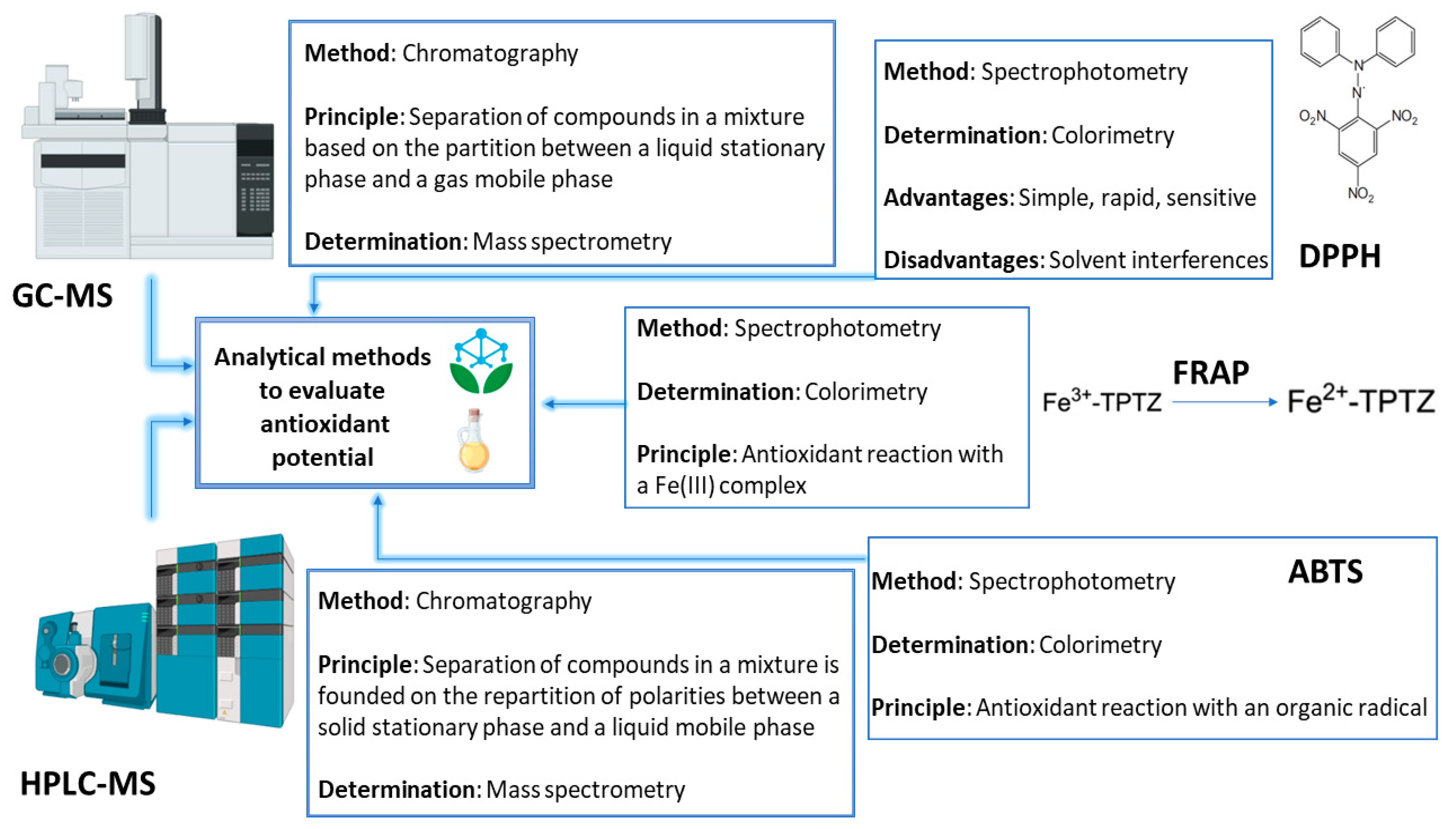
5. Analysis of Essential Oils
5.1. Techniques Employed for the Extraction, Isolation, and Purification of Essential Oils
5.2. Common Analytical Methods for Determining the Antioxidant Activity of Essential Oils
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.C.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to Manage Acne Vulgaris. Drugs Context 2021, 10, 2021-8-6. [Google Scholar] [CrossRef]
- Bungau, S.G.; Tit, D.M.; Vesa, C.M.; Abid, A.; Szilagyi, D.V.; Radu, A.F.; Bungau, A.F.; Tarce, A.G.; Behl, T.; Stoicescu, M.; et al. Non-Conventional Therapeutical Approaches to Acne Vulgaris Related to Its Association with Metabolic Disorders. Eur. J. Pharmacol. 2022, 923, 174936. [Google Scholar] [CrossRef]
- Kraft, J.; Freiman, A. Management of Acne. CMAJ 2011, 183, 430–435. [Google Scholar] [CrossRef]
- Kurokawa, I.; Nakase, K. Recent Advances in Understanding and Managing Acne. F1000Research 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Firlej, E.; Kowalska, W.; Szymaszek, K.; Roliński, J.; Bartosińska, J. The Role of Skin Immune System in Acne. J. Clin. Med. 2022, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Kircik, L.H. The Sequence of Inflammation, Relevant Biomarkers, and the Pathogenesis of Acne Vulgaris: What Does Recent Research Show and What Does It Mean to the Clinician? J. Drugs Dermatol. 2013, 12, 109–115. [Google Scholar]
- Elsaie, M.L. Hormonal Treatment of Acne Vulgaris: An Update. Clin. Cosmet. Investig. Dermatol. 2016, 9, 241–248. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An Update on the Role of the Sebaceous Gland in the Pathogenesis of Acne. Dermato-Endocrinology 2011, 3, 41–49. [Google Scholar] [CrossRef]
- Ju, Q.; Tao, T.; Hu, T.; Karadağ, A.S.; Al-Khuzaei, S.; Chen, W. Sex Hormones and Acne. Clin. Dermatol. 2017, 35, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Stoll, S.; Shalita, A.R.; Webster, G.F.; Kaplan, R.; Danesh, S.; Penstein, A. The Effect of the Menstrual Cycle on Acne. J. Am. Acad. Dermatol. 2001, 45, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Zauli, S.; Virgili, A. Is Hormonal Treatment Still an Option in Acne Today? Br. J. Dermatol. 2015, 172, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Benner, N.; Sammons, D. Overview of the Treatment of Acne Vulgaris. Osteopath. Fam. Physician 2013, 5, 185–190. [Google Scholar] [CrossRef]
- Layton, A.M.; Ravenscroft, J. Adolescent Acne Vulgaris: Current and Emerging Treatments. Lancet Child Adolesc. Health 2023, 7, 136–144. [Google Scholar] [CrossRef]
- Bungau, S.G.; Popa, V.C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–19. [Google Scholar]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.-F.; Behl, T.; Sharifi-Rad, J. Theoretical Evaluation of Cleome Species’ Bioactive Compounds and Therapeutic Potential: A Literature Review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef]
- Sharma, V.; Nath, D.; Gautam, S.; Radu, A.-F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia Ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef]
- Proença, A.C.; Luís, Â.; Duarte, A.P. The Role of Herbal Medicine in the Treatment of Acne Vulgaris: A Systematic Review of Clinical Trials. Evid. Based Complement. Altern. Med. 2022, 2022, 2011945. [Google Scholar] [CrossRef]
- Decker, A.; Graber, E.M. Over-the-Counter Acne Treatments. J. Clin. Aesthet. Dermatol. 2012, 5, 32–40. [Google Scholar]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Winkelman, W.J. Aromatherapy, Botanicals, and Essential Oils in Acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Stevensen, C.J. Aromatherapy in Dermatology. Clin. Dermatol. 1998, 16, 689–694. [Google Scholar] [CrossRef]
- Asnaashari, S.; Kazemnezhad, M.; Masoud, F.; Javadzadeh, Y. An Overview on the Anti-Acne Properties of Herbal Essential Oils. J. Herb. Med. 2023, 38, 100642. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris. Microorganisms 2022, 10, 1874. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R.; Pietrasik, D.; Walasek-Janusz, M. Essential Oils in the Treatment of Various Types of Acne-A Review. Plants 2022, 12, 90. [Google Scholar] [CrossRef]
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of Essential Oil from Citrus Reticulate Blanco Peel and Its Antibacterial Activity against Cutibacterium Acnes (Formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Zhu, L.; Qian, C.; Tian, H.; Zhao, Z.; Jin, L.; Yang, D. Antibacterial Activity of the Essential Oil from Litsea cubeba against Cutibacterium acnes and the Investigations of Its Potential Mechanism by Gas Chromatography-Mass Spectrometry Metabolomics. Front. Microbiol. 2022, 13, 823845. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.; Puziol, P.d.F.; Leitao, R.N.; Gomes, T.R.; Scherer, R.; Martins, M.L.L.; Cavalcanti, A.S.S.; Cavalcanti, L.C. Application of the Essential Oil from Copaiba (Copaifera langsdori Desf.) for Acne Vulgaris: A Double-Blind, Placebo-Controlled Clinical Trial. Altern. Med. Rev. 2012, 17, 69–75. [Google Scholar]
- Orafidiya, L.; Agbani, E.; Oyedele, A.; Babalola, O.; Onayemi, O. Preliminary Clinical Tests on Topical Preparations of Ocimum Gratissimum Linn Leaf Essential Oil for the Treatment of Acne Vulgaris. Clin. Drug Investig. 2002, 22, 313–319. [Google Scholar] [CrossRef]
- Orafidiya, L.O.; Agbani, E.O.; Oyedele, A.O.; Babalola, O.O.; Onayemi, O.; Aiyedun, F.F. The Effect of Aloe Vera Gel on the Anti-Acne Properties of the Essential Oil of Ocimum Gratissimum Linn Leaf—A Preliminary Clinical Investigation. Int. J. Aromather. 2004, 14, 15–21. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-EL-Sooud, K.; Aleya, L.; Bungau, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β Drives Inflammatory Responses to Propionibacterium acnes In Vitro and In Vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kardeh, S.; Moein, S.A.; Namazi, M.R.; Kardeh, B. Evidence for the Important Role of Oxidative Stress in the Pathogenesis of Acne. Galen Med. J. 2019, 8, 1291. [Google Scholar] [CrossRef]
- Ghumra, W.; Lee, N.; Whitehouse, H.; Bhutani, R.; Lagos, D.; Layton, A.M. MicroRNAs as Biomarkers of Atrophic Scarring in Acne: A Cross-sectional Analysis of 41 Patients. Clin. Exp. Dermatol. 2021, 46, 1495–1503. [Google Scholar] [CrossRef]
- Caliş, B.; Yerlikaya, F.H.; Ataseven, A.; Temiz, S.A.; Onmaz, D.E. Oxidative Stress-Related MiRNAs in Patients with Severe Acne Vulgaris. Indian J. Dermatol. 2022, 67, 657–661. [Google Scholar] [PubMed]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altinyazar, C.; Koca, R.; Tekin, N.S. Oxidative Stress in Acne Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 763–767. [Google Scholar] [CrossRef]
- Abdel Fattah, N.S.A.; Shaheen, M.A.; Ebrahim, A.A.; El Okda, E.S. Tissue and Blood Superoxide Dismutase Activities and Malondialdehyde Levels in Different Clinical Severities of Acne Vulgaris. Br. J. Dermatol. 2008, 159, 1086–1091. [Google Scholar] [CrossRef]
- Abulnaja, K.O. Oxidant/Antioxidant Status in Obese Adolescent Females with Acne Vulgaris. Indian J. Dermatol. 2009, 54, 36–40. [Google Scholar] [CrossRef]
- Antiga, E.; Verdelli, A.; Bonciani, D.; Bonciolini, V.; Caproni, M.; Fabbri, P. Acne: A New Model of Immune-Mediated Chronic Inflammatory Skin Disease. G. Ital. Di Dermatol. E Venereol. Organo Uff. Soc. Ital. Di Dermatol. E Sifilogr. 2015, 150, 247–254. [Google Scholar]
- Ozlu, E.; Karadag, A.S.; Ozkanli, S.; Oguztuzun, S.; Kilic, M.; Zemheri, E.; Akbulak, O.; Akdeniz, N. Comparison of TLR-2, TLR-4, and Antimicrobial Peptide Levels in Different Lesions of Acne Vulgaris. Cutan. Ocul. Toxicol. 2016, 35, 300–309. [Google Scholar] [CrossRef]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of Toll-like Receptors by Propionibacterium acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shou, Y.-H.; Yang, Y.-S.; Xu, J.-H. Elucidating the Immune Infiltration in Acne and Its Comparison with Rosacea by Integrated Bioinformatics Analysis. PLoS ONE 2021, 16, e0248650. [Google Scholar] [CrossRef] [PubMed]
- Khondker, L.; Khan, S.I. Acne Vulgaris Related to Androgens—A Review. Mymensingh Med. J. 2014, 23, 181–185. [Google Scholar]
- Nickles, M.A.; Sharma, D.; Tsoukas, M.M.; Ashack, K.A. Acne and Insulin Resistance: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2022, 87, 687–688. [Google Scholar] [CrossRef]
- Briganti, S.; Flori, E.; Mastrofrancesco, A.; Ottaviani, M. Acne as an Altered Dermato-Endocrine Response Problem. Exp. Dermatol. 2020, 29, 833–839. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion-In Vitro and In Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef]
- Oniga, I.; Pus, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Genus Origanum. Phyther. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Chuang, L.T.; Tsai, T.H.; Lien, T.J.; Huang, W.C.; Liu, J.J.; Chang, H.; Chang, M.L.; Tsai, P.J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Conti, B.J.; Santiago, K.B.; Fernandes, A.J.; Sforcin, J.M. Cymbopogon martinii Essential Oil and Geraniol at Noncytotoxic Concentrations Exerted Immunomodulatory/Anti-Inflammatory Effects in Human Monocytes. J. Pharm. Pharmacol. 2014, 66, 1491–1496. [Google Scholar] [CrossRef]
- Mahant, S.; Sahajpal, N.S.; Nanda, S. Insights into the Mechanism of Cymbopogan martinii Essential Oil in Topical Therapy of Acne Vulgaris. Future Microbiol. 2021, 16, 1181–1193. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based. Complement. Alternat. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential Oil Exerts Antibacterial and Anti-Inflammatory Effect in Macrophage Mediated Immune Response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Feuillolay, C.; Pecastaings, S.; Gac, C.L.; Fiorini-Puybaret, C.; Luc, J.; Joulia, P.; Roques, C. A Myrtus communis Extract Enriched in Myrtucummulones and Ursolic Acid Reduces Resistance of Propionibacterium acnes Biofilms to Antibiotics Used in Acne Vulgaris. Phytomedicine 2016, 23, 307–315. [Google Scholar] [CrossRef]
- Feißt, C.; Franke, L.; Appendino, G.; Werz, O. Identification of Molecular Targets of the Oligomeric Non-Prenylated Acylphloroglucinols from Myrtus communis and Their Implication as Anti-Inflammatory Compounds. J. Pharmacol. Exp. Ther. 2005, 315, 389–396. [Google Scholar] [CrossRef]
- Gorjian, H.; Khaligh, N.G. Myrtle: A Versatile Medicinal Plant. Nutrire 2023, 48, 10. [Google Scholar] [CrossRef]
- Hennia, A.; Miguel, M.; Nemmiche, S. Antioxidant Activity of Myrtus communis L. and Myrtus nivellei Batt. & Trab. Extracts: A Brief Review. Medicines 2018, 5, 89. [Google Scholar]
- Rafiee, F.; Mazhari, M.; Ghoreishi, M.; Esmaeilipour, O. Effect of Lemon Verbena Powder and Vitamin C on Performance and Immunity of Heat-Stressed Broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Fongnzossie, E.F.; Tize, Z.; Fogang Nde, P.J.; Nyangono Biyegue, C.F.; Bouelet Ntsama, I.S.; Dibong, S.D.; Nkongmeneck, B.A. Ethnobotany and Pharmacognostic Perspective of Plant Species Used as Traditional Cosmetics and Cosmeceuticals among the Gbaya Ethnic Group in Eastern Cameroon. S. Afr. J. Bot. 2017, 112, 29–39. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Zonfrillo, M.; Andreola, F.; Krasnowska, E.K.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages. Molecules 2022, 27, 3488. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, A.E.; Lamson, D.W. Immune-Modifying and Antimicrobial Effects of Eucalyptus Oil and Simple Inhalation Devices. Altern. Med. Rev. 2010, 15, 33–47. [Google Scholar]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and Anti-Inflammatory Effects of Essential Oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based. Complement. Alternat. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-Biofilm Activities of Essential Oils Rich in Carvacrol and Thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Bassett, I.B.; Pannowitz, D.L.; Barnetson, R.S. A Comparative Study of Tea-Tree Oil versus Benzoylperoxide in the Treatment of Acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The Efficacy of 5% Topical Tea Tree Oil Gel in Mild to Moderate Acne Vulgaris: A Randomized, Double-Blind Placebo-Controlled Study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar]
- Malhi, H.K.; Tu, J.; Riley, T.V.; Kumarasinghe, S.P.; Hammer, K.A. Tea Tree Oil Gel for Mild to Moderate Acne; a 12 Week Uncontrolled, Open-Label Phase II Pilot Study. Australas. J. Dermatol. 2017, 58, 205–210. [Google Scholar] [CrossRef]
- Mazzarello, V.; Donadu, M.G.; Ferrari, M.; Piga, G.; Usai, D.; Zanetti, S.; Sotgiu, M.A. Treatment of Acne with a Combination of Propolis, Tea Tree Oil, and Aloe Vera Compared to Erythromycin Cream: Two Double-Blind Investigations. Clin. Pharmacol. 2018, 10, 175–181. [Google Scholar] [CrossRef]
- Baumann, L.S. Less-Known Botanical Cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef]
- Peng, C.H.; Su, J.D.; Chyau, C.C.; Sung, T.Y.; Ho, S.S.; Peng, C.C.; Peng, R.Y. Supercritical Fluid Extracts of Rosemary Leaves Exhibit Potent Anti-Inflammation and Anti-Tumor Effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Efferth, T.; Liang, H.; Liu, Z.; Liu, W. Investigation of Antibacterial Activity of Rosemary Essential Oil against Propionibacterium acnes with Atomic Force Microscopy. Planta Med. 2007, 73, 1275–1280. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y.R.; Chen, W.Y.; Tsai, P.J. Rosmarinus Officinalis Extract Suppresses Propionibacterium acnes-Induced Inflammatory Responses. J. Med. Food 2013, 16, 324–333. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Clinical Studies on the Use of Essential Oils in the Management of Acne Vulgaris. Available online: https://clinicaltrials.gov/ct2/results?cond=Acne+Vulgaris&term=Essential+oil&cntry=&state=&city=&dist= (accessed on 13 May 2023).
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative Stress and Metabolic Syndrome in Acne Vulgaris: Pathogenetic Connections and Potential Role of Dietary Supplements and Phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and Antioxidant Analysis of Medicinal and Food Plants towards Bioactive Food and Pharmaceutical Resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; Vostinaru, O.; Rigano, D.; de Aguiar Andrade, E.H. Bioactive Compounds Present in Essential Oils: Advances and Pharmacological Applications. Front. Pharmacol. 2023, 14, 1130097. [Google Scholar] [CrossRef]
- Wei, H.; Chen, G.; Wang, R.-J.; Peng, J. Oregano Essential Oil Decreased Susceptibility to Oxidative Stress-Induced Dysfunction of Intestinal Epithelial Barrier in Rats. J. Funct. Foods 2015, 18, 1191–1199. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. vulgare and Subsp. hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and Antioxidant Activities of Mexican Oregano Essential Oils (Lippia graveolens H. B. K.) with Different Composition When Microencapsulated in Beta-Cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural Diversity in Phenolic Components and Antioxidant Properties of Oregano (Origanum vulgare L.) Accessions, Grown under the Same Conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical Analysis and Evaluation of Its Antimalarial, Antioxidant, and Cytotoxic Activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In Vitro Pro-Oxidant/Antioxidant Role of Carvacrol, Thymol and Their Mixture in the Intestinal Caco-2 Cell Line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ma, G.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.; Zhao, Z.; Xu, H. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Alinkina, E.S.; Fatkulina, L.D.; Vorob’yova, A.K. Oregano Essential Oil as an Inhibitor of Higher Fatty Acid Oxidation. Appl. Biochem. Microbiol. 2011, 47, 445–449. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef]
- Lawrence, K.; Lawrence, R.; Parihar, D.; Srivastava, R.; Charan, A. Antioxidant Activity of Palmarosa Essential Oil (Cymbopogon martini) Grown in North Indian Plains. Asian Pac. J. Trop. Biomed. 2012, 2, S888–S891. [Google Scholar] [CrossRef]
- Smitha, G.R.; Rana, V.S. Variations in Essential Oil Yield, Geraniol and Geranyl Acetate Contents in Palmarosa (Cymbopogon martinii, Roxb. Wats. Var. Motia) Influenced by Inflorescence Development. Ind. Crops Prod. 2015, 66, 150–160. [Google Scholar] [CrossRef]
- Danh, L.T.; Triet, N.D.A.; Han, L.T.N.; Zhao, J.; Mammucari, R.; Foster, N. Antioxidant Activity, Yield and Chemical Composition of Lavender Essential Oil Extracted by Supercritical CO2. J. Supercrit. Fluids 2012, 70, 27–34. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x Intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Kozics, K.; Srancikova, A.; Sedlackova, E.; Horvathova, E.; Melusova, M.; Melus, V.; Krajcovicova, Z.; Sramkova, M. Antioxidant Potential of Essential Oil from Lavandula angustifolia in In Vitro and Ex Vivo Cultured Liver Cells. Neoplasma 2017, 64, 485–493. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, F.; Huang, H.; Ji, T.; Li, C.; Tan, W.; Chen, Y.; Ma, L. Evaluation on Bioactivities of Total Flavonoids from Lavandula angustifolia. Pak. J. Pharm. Sci. 2015, 28, 1245–1251. [Google Scholar]
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of Chemical Composition, Antioxidant and Antimicrobial Activity of Lavender (Lavandula angustifolia L.) Essential Oils Extracted by Supercritical CO2, Hexane and Hydrodistillation. Food Bioprocess Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Mohammed Salamatullah, A.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Deng, X.; Lu, Z.; Chen, J.; Chen, W. Essential Oil Compositions, Antioxidant Activities, and Procollagen Synthesis Abilities of Four Lavandula angustifolia Varieties. Curr. Pharm. Biotechnol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis Essential Oils: Insecticidal, Antioxidant and Antimicrobial Activities: A Review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Petretto, G.L.; Maldini, M.; Addis, R.; Chessa, M.; Foddai, M.; Rourke, J.P.; Pintore, G. Variability of Chemical Composition and Antioxidant Activity of Essential Oils between Myrtus communis Var. Leucocarpa DC and Var. Melanocarpa DC. Food Chem. 2016, 197, 124–131. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Fallahi, S.; Mahmoudvand, H.; Shakibaie, M.; Harandi, M.F.; Dezaki, E.S. Efficacy of Myrtus communis L. to Inactivate the Hydatid Cyst Protoscoleces. J. Investig. Surg. 2016, 29, 137–143. [Google Scholar] [CrossRef]
- Neves, A.; Marto, J.; Duarte, A.; Gonçalves, L.; Pinto, P.; Figueiredo, A.; Ribeiro, H. Characterization of Portuguese Thymbra Capitata, Thymus Caespititius and Myrtus communis Essential Oils in Topical Formulations. Flavour Fragr. J. 2017, 32, 392–402. [Google Scholar] [CrossRef]
- Jabri, M.A.; Hajaji, S.; Marzouki, L.; El-Benna, J.; Sakly, M.; Sebai, H. Human Neutrophils ROS Inhibition and Protective Effects of Myrtus communis Leaves Essential Oils against Intestinal Ischemia/Reperfusion Injury. RSC Adv. 2016, 6, 16645–16655. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria Monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Misharina, T.A.; Samusenko, A.L. Antioxidant Properties of Essential Oils from Lemon, Grapefruit, Coriander, Clove, and Their Mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Li, G.; Xiang, S.; Pan, Y.; Long, X.; Cheng, Y.; Han, L.; Zhao, X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-Volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021, 8, 689094. [Google Scholar] [CrossRef] [PubMed]
- Surbhi; Kumar, A.; Singh, S.; Kumari, P.; Rasane, P. Eucalyptus: Phytochemical Composition, Extraction Methods and Food and Medicinal Applications. Adv. Tradit. Med. 2021, 23, 369–380. [Google Scholar]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant Activity, Neuroprotective Properties and Bioactive Constituents Analysis of Varying Polarity Extracts from Eucalyptus globulus Leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas Aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Wang, X.; Xu, X.; Yan, X.; Li, X.; Sun, S.; Dong, Y.; Ren, X.; Liu, X.; et al. Chemical Composition and Anti-Oxidant Potential on Essential Oils of Thymus Quinquecostatus Celak. from Loess Plateau in China, Regulating Nrf2/Keap1 Signaling Pathway in Zebrafish. Sci. Rep. 2020, 10, 11280. [Google Scholar] [CrossRef] [PubMed]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.-T. α-Terpinene, an Antioxidant in Tea Tree Oil, Autoxidizes Rapidly to Skin Allergens on Air Exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.; Gomes, D.; Simões, R.; da Graça Miguel, M. Tea Tree Oil: Properties and the Therapeutic Approach to Acne—A Review. Antioxidants 2023, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Mezza, G.N.; Borgarello, A.V.; Grosso, N.R.; Fernandez, H.; Pramparo, M.C.; Gayol, M.F. Antioxidant Activity of Rosemary Essential Oil Fractions Obtained by Molecular Distillation and Their Effect on Oxidative Stability of Sunflower Oil. Food Chem. 2018, 242, 9–15. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Zahra, S.M.; Irfan, S.; Shafique, B.; Noreen, R.; Alahmad, U.F.; Liaqat, S.; Umar, S. Extraction and Analysis of Essential Oils: Extraction Methods Used at Laboratory and Industrial Level and Chemical Analysis. In Essential Oils Extraction, Characterization and Applications; Nayik, G.A., Ansari, M.J.B.T.-E.O., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 37–52. ISBN 978-0-323-91740-7. [Google Scholar]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction Methods, Identification and Detection of Bioactive Compounds from Plant Extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36. [Google Scholar]
- Rasul, M. Extraction, Isolation and Characterization of Natural Products from Medicinal Plants. Int. J. Basic Sci. Appl. Comput. 2018, 2, F0076122618. [Google Scholar]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas Chromatographic Technologies for the Analysis of Essential Oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Simon, J.E.; Lou, S.-N.; Ho, C.-T. Authenticity Analysis of Citrus Essential Oils by HPLC-UV-MS on Oxygenated Heterocyclic Components. J. Food Drug Anal. 2015, 23, 30–39. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A Validated High Performance Liquid Chromatography Method for the Analysis of Thymol and Carvacrol in Thymus vulgaris L. Volatile Oil. Pharmacogn. Mag. 2010, 6, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Cerrón-Mercado, F.; Perez-Alvarez, J.A.; Nolazco-Cama, D.; Salva-Ruíz, B.; Tellez-Monzon, L.; Fernández-López, J.; Viuda-Martos, M. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oil Obtained from Chincho (Tagetes elliptica Sm) Leaves Grown in the Peruvian Andes. Foods 2023, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oil. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Umaru, I.J.; Badruddin, F.A.; Umaru, H.A. Phytochemical Screening of Essential Oils and Antibacterial Activity and Antioxidant Properties of Barringtonia asiatica (L.) Leaf Extract. Biochem. Res. Int. 2019, 2019, 7143989. [Google Scholar] [CrossRef]
- Copolovici, D.; Bungau, S.; Boscencu, R.; Tit, D.M.; Copolovici, L. The Fatty Acids Composition and Antioxidant Activity of Walnut Cold Press Oil. Rev. Chim. 2017, 68, 507–509. [Google Scholar] [CrossRef]
- Rajabian, A.; Hassanzadeh Khayyat, M.; Emami, S.A.; Tayarani-Najaran, Z.; Oskooie, R.; Asili, J. Phytochemical Evaluation and Antioxidant Activity of Essential Oil, and Aqueous and Organic Extracts of Artemisia Dracunculus. Jundishapur J. Nat. Pharm. Prod. 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–115. [Google Scholar]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An Appraisal of Eighteen Commonly Consumed Edible Plants as Functional Food Based on Their Antioxidant and Starch Hydrolase Inhibitory Activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef]
- Goodarzi, S.; Hadjiakhoondi, A.; Yassa, N.; Khanavi, M.; Tofighi, Z. Essential Oils Chemical Composition, Antioxidant Activities and Total Phenols of Astrodaucus Persicus. Iran. J. Basic Med. Sci. 2016, 19, 159–165. [Google Scholar] [PubMed]
- Xie, Q.; Liu, Z.; Li, Z. Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China. Molecules 2015, 20, 9380–9392. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH Free Radical Method. LWT -Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kokina, M.; Salević, A.; Kalušević, A.; Lević, S.; Pantić, M.; Pljevljakušić, D.; Šavikin, K.; Shamtsyan, M.; Nikšić, M.; Nedović, V. Characterization, Antioxidant and Antibacterial Activity of Essential Oils and Their Encapsulation into Biodegradable Material Followed by Freeze Drying. Food Technol. Biotechnol. 2019, 57, 282–289. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.K.; Koul, A.; Gupta, S.; Singh, G.; Razdan, V.K. Antioxidant and Antimicrobial Properties of the Essential Oil and Extracts of Zanthoxylum alatum Grown in North-Western Himalaya. Sci. World J. 2013, 2013, 790580. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Sudha, G.; Priya, M.S.; Shree, R.B.I.; Vadivukkarasi, S. Antioxidant Activity of Ripe and Unripe Pepino Fruit (Solanum muricatum Aiton). J. Food Sci. 2012, 77, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.C.; Parra, C.; Said, B.; Werner, E.; Flores, S.; Villena, J.; Russo, A.; Caro, N.; Montenegro, I.; Madrid, A. Antioxidant and Anti-Proliferative Activity of Essential Oil and Main Components from Leaves of Aloysia Polystachya Harvested in Central Chile. Molecules 2020, 26, 131. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of Antioxidant Activity with the Thiobarbituric Acid Reactive Substances Assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Arambewela, L.S.R.; Arawwawala, L.D.A.M.; Athauda, N. Antioxidant and Antifungal Activities of Essential Oil of Alpinia calcarata Roscoe Rhizomes. J. Ayurveda Integr. Med. 2010, 1, 199–202. [Google Scholar]
- Rahman, M.A.; Islam, M.S. Alpinia Calcarata Roscoe: A Potential Phytopharmacological Source of Natural Medicine. Pharmacogn. Rev. 2015, 9, 55–62. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application to Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Luna, H.; Garrido, S. Antioxidant Activity of Tagetes Erecta Essential Oil. J. Chil. Chem. Soc. 2006, 51, 883–886. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative Properties and Stability of Ethanolic Extracts of Holy Basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Fd. Sci. Technol. 2014, 11, 31–42. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, A.F.; Radu, A.-F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Purza, A.L.; Endres, L.M. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules 2023, 28, 6395. https://doi.org/10.3390/molecules28176395
Bungau AF, Radu A-F, Bungau SG, Vesa CM, Tit DM, Purza AL, Endres LM. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules. 2023; 28(17):6395. https://doi.org/10.3390/molecules28176395
Chicago/Turabian StyleBungau, Alexa Florina, Andrei-Flavius Radu, Simona Gabriela Bungau, Cosmin Mihai Vesa, Delia Mirela Tit, Anamaria Lavinia Purza, and Laura Maria Endres. 2023. "Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris" Molecules 28, no. 17: 6395. https://doi.org/10.3390/molecules28176395
APA StyleBungau, A. F., Radu, A.-F., Bungau, S. G., Vesa, C. M., Tit, D. M., Purza, A. L., & Endres, L. M. (2023). Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules, 28(17), 6395. https://doi.org/10.3390/molecules28176395