Fluoride and Aluminium in Tea (Camellia sinensis L.)—Tea Quality Indicators and Risk Factors for Consumers
Abstract
:1. Introduction
2. Results and Discussion
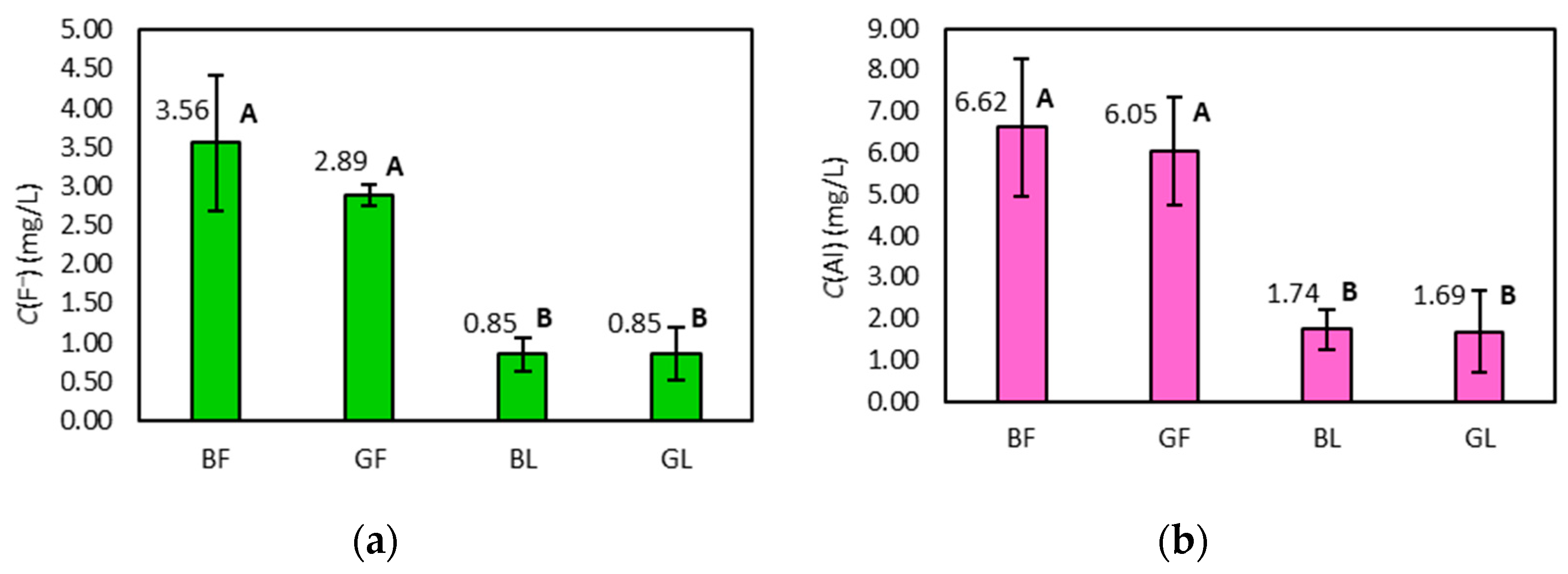
2.1. Concentration of Fluoride and Aluminium in Tea Infusions
2.2. Fluoride and Aluminium Concentrations in Infusions with Respect to the Form and Type of Tea
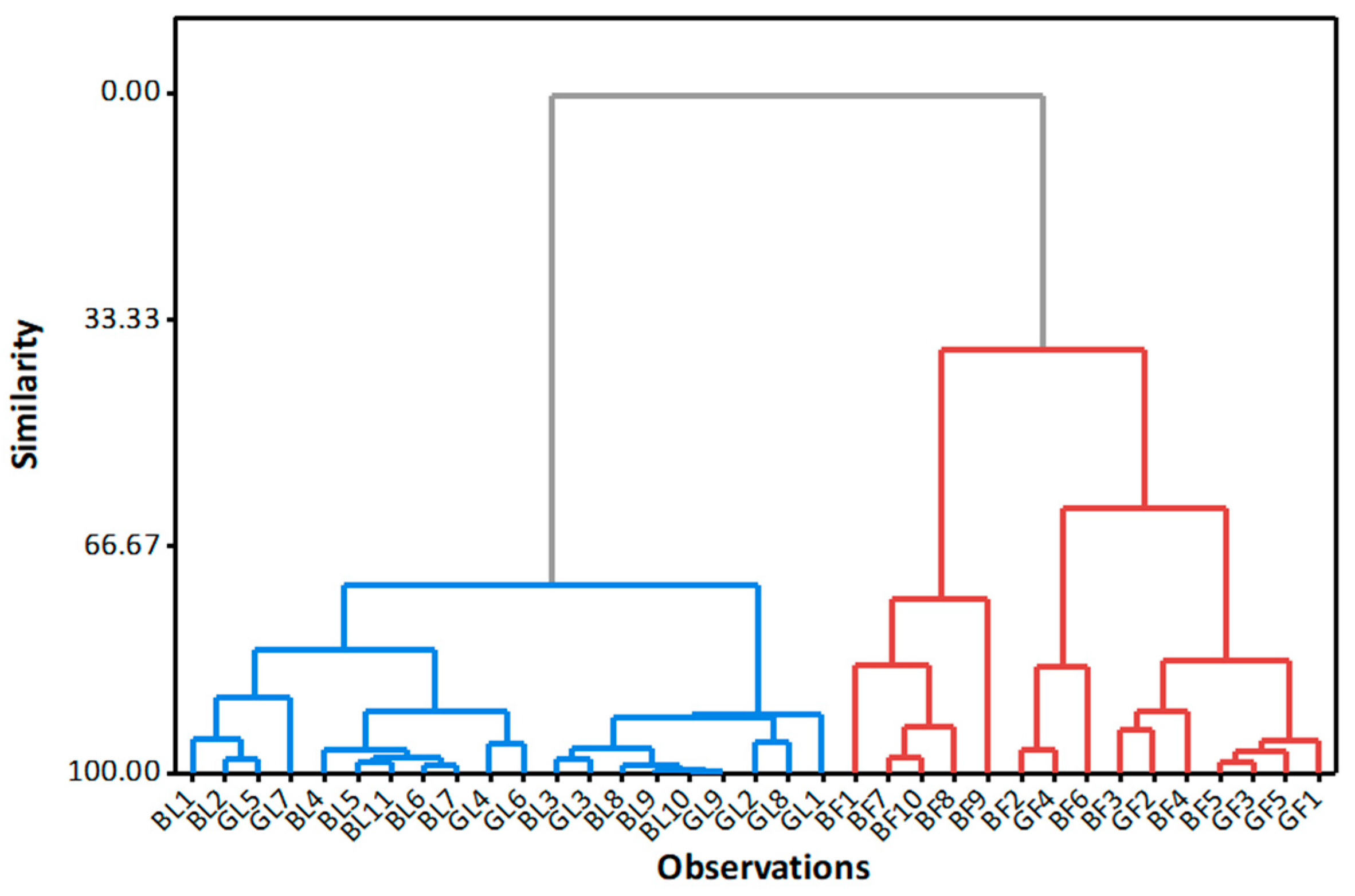
2.3. Relationship between Fluoride and Aluminium Concentrations in Tea Infusions
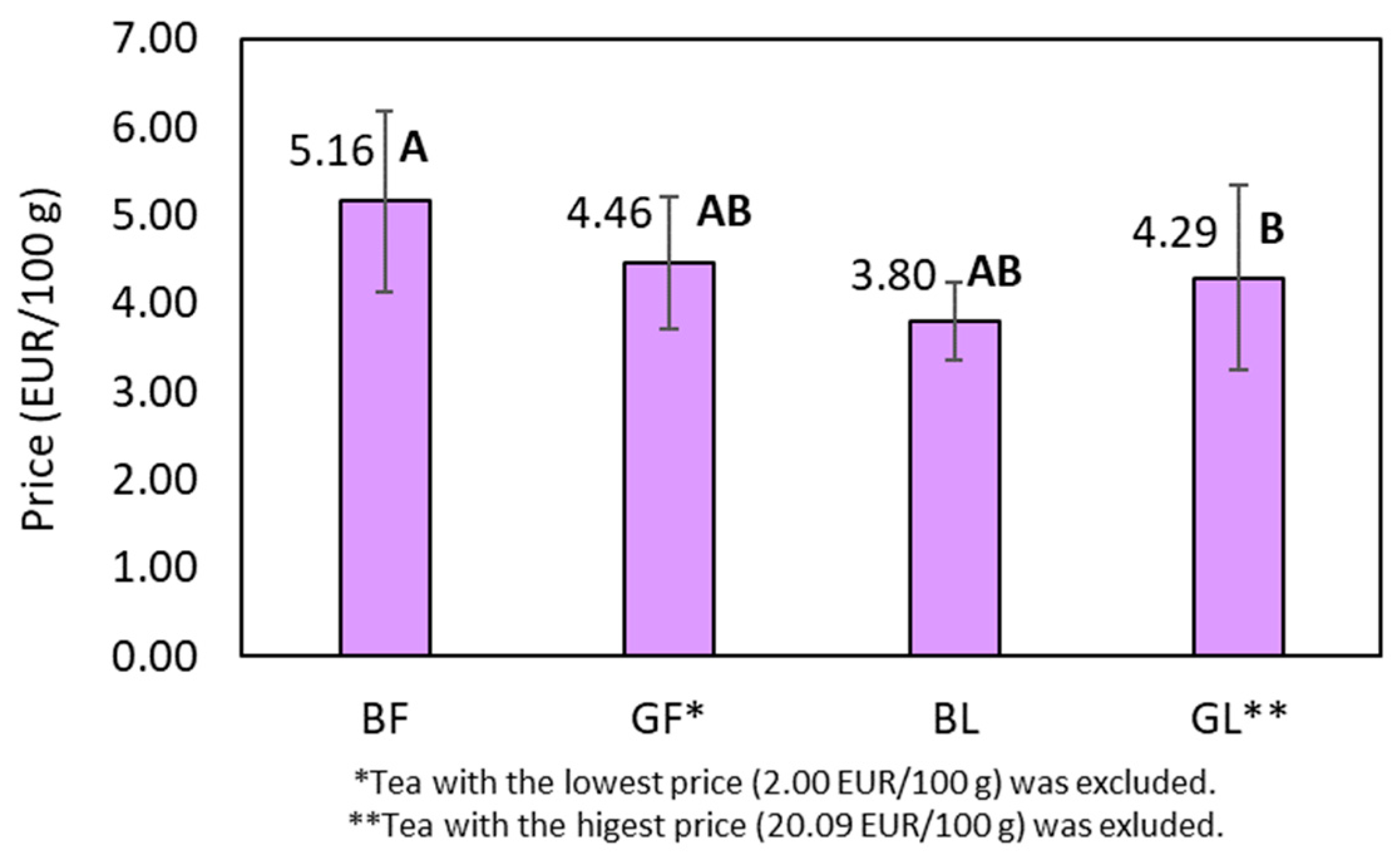
2.4. Price Performance
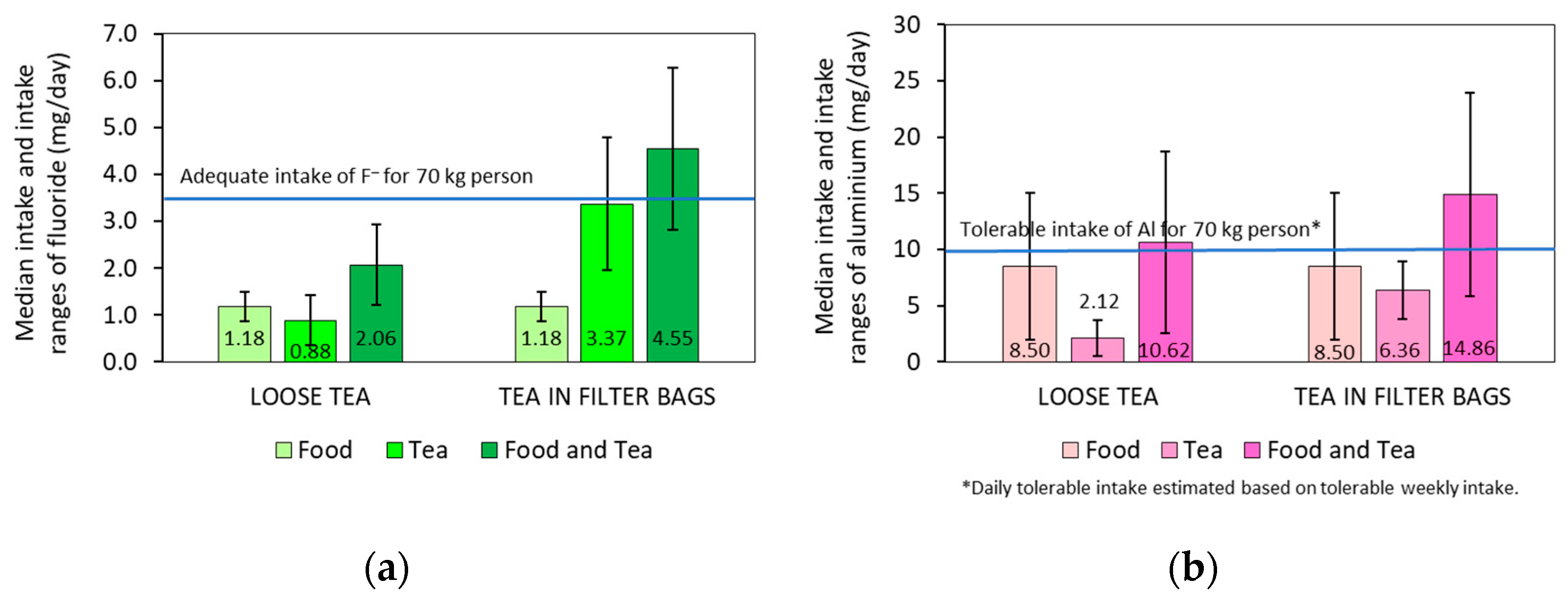
2.5. Exposure to Fluoride and Aluminium through Tea
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrumentation
3.3. Sample Collection and Preparation
3.4. Determination of Fluoride
3.4.1. Calibration Curve Technique
3.4.2. Standard Addition Technique
3.5. Determination of Aluminium+
3.5.1. Calibration Curve Technique
3.5.2. Standard Addition Technique
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A. Determination of Limit of Detection
Appendix B. List of Teas According to Brand
References
- Food and Agriculture Organization of the United Nations. Tea. Available online: https://www.fao.org/markets-and-trade/commodities/tea/en/ (accessed on 16 May 2023).
- Castellana, F.; De Nucci, S.; De Pergola, G.; Di Chito, M.; Lisco, G.; Triggiani, V.; Sardone, R.; Zupo, R. Trends in Coffee and Tea Consumption during the COVID-19 Pandemic. Foods 2021, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Sai, V.; Chaturvedula, P.; Prakash, I. The Aroma, Taste, Color and Bioactive Constituents of Tea. J. Med. Plants Res. 2011, 5, 2110–2124. [Google Scholar]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 May 2023).
- Trade Map. List of Imported Products for the Selected Product (Coffee, Tea, Maté and Spices). Available online: https://www.trademap.org/Product_SelProduct_TS.aspx?nvpm (accessed on 16 May 2023).
- Statista Market Forecast. Tea–Europe. Available online: https://www.statista.com/outlook/cmo/hot-drinks/tea/europe (accessed on 16 May 2023).
- Yang, Y.; Liu, Y.; Huang, C.F.; de Silva, J.; Zhao, F.J. Aluminium Alleviates Fluoride Toxicity in Tea (Camellia sinensis). Plant Soil 2016, 402, 179–190. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Takatsu, S.; Maeda, S.; Hiradate, S. Mechanism for the Detoxification of Aluminum in Roots of Tea Plant (Camellia sinensis (L.) Kuntze). Phytochemistry 2008, 69, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black Tea Source, Production, and Consumption: Assessment of Health Risks of Fluoride Intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Zannou, O.; Koca, I. Assessment of the Aluminium Contents of Black Tea and Black Tea Infusions. Discov. Food 2022, 2, 13. [Google Scholar] [CrossRef]
- Peng, A.; Yu, K.; Yu, S.; Li, Y.; Zuo, H.; Li, P.; Li, J.; Huang, J.; Liu, Z.; Zhao, J. Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety. Int. J. Mol. Sci. 2023, 24, 4640. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium in Tea—Concentrations, Speciation and Bioavailability. Coord. Chem. Rev. 2002, 228, 385–395. [Google Scholar] [CrossRef]
- Kralj, B.; Križaj, I.; Bukovec, P.; Slejko, S.; Milačič, R. Speciation of Aluminium in Tea Infusions by Use of SEC and FPLC with ICP-OES and ES-MS-MS Detection. Anal. Bioanal. Chem. 2005, 383, 467–475. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhao, Q.; Zhang, X.C.; Wan, X.C.; Mao, J.D. Localization of Fluoride and Aluminum in Subcellular Fractions of Tea Leaves and Roots. J. Agric. Food Chem. 2014, 62, 2313–2319. [Google Scholar] [CrossRef]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Koblar, A.; Tavčar, G.; Ponikvar-Svet, M. Fluoride in Teas of Different Types and Forms and the Exposure of Humans to Fluoride with Tea and Diet. Food Chem. 2012, 130, 286–290. [Google Scholar] [CrossRef]
- Chan, L.; Mehra, A.; Saikat, S.; Lynch, P. Human Exposure Assessment of Fluoride from Tea (Camellia Sinensis L.): A UK Based Issue? Food Res. Int. 2013, 51, 564–570. [Google Scholar] [CrossRef]
- Krishnankutty, N.; Storgaard Jensen, T.; Kjær, J.; Jørgensen, J.S.; Nielsen, F.; Grandjean, P. Public-Health Risks from Tea Drinking: Fluoride Exposure. Scand. J. Public Health 2022, 50, 355–361. [Google Scholar] [CrossRef]
- Szmagara, A.; Krzyszczak, A.; Stefaniak, E.A. Determination of Fluoride Content in Teas and Herbal Products Popular in Poland. J. Environ. Health Sci. Eng. 2022, 20, 717–727. [Google Scholar] [CrossRef]
- Erdemoglu, S.B.; Turkdemir, H.; Gucer, S. Determination of Total and Fluoride Bound Aluminium in Tea Infusions by Ion Selective Electrode and Flame Atomic Absorption Spectrometry. Anal. Lett. 2000, 33, 1513–1529. [Google Scholar] [CrossRef]
- Street, R.; Drábek, O.; Száková, J.; Mládková, L. Total Content and Speciation of Aluminium in Tea Leaves and Tea Infusions. Food Chem. 2007, 104, 1662–1669. [Google Scholar] [CrossRef]
- Polechońska, L.; Dambiec, M.; Klink, A.; Rudecki, A. Concentrations and Solubility of Selected Trace Metals in Leaf and Bagged Black Teas Commercialized in Poland. J. Food Drug Anal. 2015, 23, 486–492. [Google Scholar] [CrossRef]
- Troisi, J.; Richards, S.; Symes, S.; Ferretti, V.; Di Maio, A.; Amoresano, A.; Daniele, B.; Aliberti, F.; Guida, M.; Trifuoggi, M.; et al. A Comparative Assessment of Metals and Phthalates in Commercial Tea Infusions: A Starting Point to Evaluate Their Tolerance Limits. Food Chem. 2019, 288, 193–200. [Google Scholar] [CrossRef]
- Speirs, R.L. Correlations between the Concentrations of Fluoride and Some Other Constituents in Tea Infusions and Their Possible Dental Caries-Preventive Effect. Arch. Oral Biol. 1983, 28, 471–475. [Google Scholar] [CrossRef]
- Hayacibara, M.F.; Queiroz, C.S.; Tabchoury, C.P.M.; Cury, J.A. Fluoride and Aluminum in Teas and Tea-Based Beverages. Rev. Saude Publica 2004, 38, 100–105. [Google Scholar] [CrossRef]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Aluminium and Fluoride Concentrations of Three Tea Varieties Growing at Lantau Island, Hong Kong. Environ. Geochem. Health 2003, 25, 219–232. [Google Scholar] [CrossRef]
- Kronborg, A. Trace Elements in Norwegian and Polish Tea Infusions: Determined by High-Resolution Inductively Coupled Plasma Mass Spectrometry (HR ICP-MS) and Ion Selective Electrode (ISE). Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2013. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Fluoride. EFSA J. 2013, 11, 3332–3378. [Google Scholar] [CrossRef]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical Aspects of Human and Environmental Overload with Fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Safety of Aluminium from Dietary Intake-Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar] [CrossRef]
- Becaria, A.; Campbell, A.; Bondy, S. Aluminum as a Toxicant. Toxicol. Ind. Health 2002, 18, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding Aspects of Aluminum Exposure in Alzheimer’s Disease Development. Brain Pathol. 2016, 26, 139. [Google Scholar] [CrossRef]
- Waugh, D.T.; Potter, W.; Limeback, H.; Godfrey, M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and Its Implications for Public Health and Water Fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006-Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Cao, J.; Zhao, Y.; Li, Y.; Deng, H.J.; Yi, J.; Liu, J.W. Fluoride Levels in Various Black Tea Commodities: Measurement and Safety Evaluation. Food Chem. Toxicol. 2006, 44, 1131–1137. [Google Scholar] [CrossRef]
- Matsuura, H.; Hokura, A.; Katsuki, F.; Itoh, A.; Haraguchi, H. Multielement Determination and Speciation of Major-to-Trace Elements in Black Tea Leaves by ICP-AES and ICP-MS with the Aid of Size Exclusion Chromatography. Anal. Sci. 2001, 17, 391–398. [Google Scholar] [CrossRef]
- Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Truzzi, C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules 2023, 28, 3802. [Google Scholar] [CrossRef]
- de Silva, J.; Tuwei, G.; Zhao, F.J. Environmental Factors Influencing Aluminium Accumulation in Tea (Camellia sinensis L.). Plant Soil 2016, 400, 223–230. [Google Scholar] [CrossRef]
- Ponikvar, M.; Sedej, B.; Pihlar, B.; Žemva, B. Determination of Fluoride in M(SbF6)x Compounds. Anal. Chim. Acta 2000, 418, 113–118. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, W.F.; Yang, X.Q. Fluoride Content in Tea and Its Relationship with Tea Quality. J. Agric. Food Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef]
- Li, C.; Ni, D. Effect of Fluoride on Chemical Constituents of Tea Leaves. Fluoride 2009, 42, 237–243. [Google Scholar]
- Market Data Forecast. Europe Tea Market|2023–2028|UK, Germany, France, Italy, Spain. Available online: https://www.marketdataforecast.com/market-reports/europe-tea-market (accessed on 16 May 2023).
- Bu, X.; Nguyen, H.V.; Chou, T.P.; Chen, C.P. A Comprehensive Model of Consumers’ Perceptions, Attitudes and Behavioral Intention toward Organic Tea: Evidence from an Emerging Economy. Sustainability 2020, 12, 6619. [Google Scholar] [CrossRef]
- Allain, P.; Gauchard, F.; Krari, N. Enhancement of Aluminum Digestive Absorption by Fluoride in Rats. Res. Commun. Mol. Pathol. Pharmacol. 1996, 91, 225–231. [Google Scholar]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary Assessment Methods in Epidemiologic Studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Peters, M.A.; Ladd, D.M. Determination of Fluoride in Oxides with the Fluoride-Ion Activity Electrode. Talanta 1971, 18, 655–664. [Google Scholar] [CrossRef]
- Ponikvar, M.; Stibilj, V.; Žemva, B. Daily Dietary Intake of Fluoride by Slovenian Military Based on Analysis of Total Fluorine in Total Diet Samples Using Fluoride Ion Selective Electrode. Food Chem. 2007, 103, 369–374. [Google Scholar] [CrossRef]
- Štepec, D.; Tavčar, G.; Ponikvar-Svet, M. Measurement Uncertainty Evaluation and Traceability Assurance for Total Fluorine Determination in Vegetation by Fluoride Ion Selective Electrode. J. Fluor. Chem. 2019, 217, 22–28. [Google Scholar] [CrossRef]
- Senila, M.; Drolc, A.; Pintar, A.; Senila, L.; Levei, E. Validation and Measurement Uncertainty Evaluation of the ICP-OES Method for the Multi-Elemental Determination of Essential and Nonessential Elements from Medicinal Plants and Their Aqueous Extracts. J. Anal. Sci. Technol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Orion. User Guide Fluoride Ion Selective Electrode; Thermo Fisher Scientific: Beverly, MA, USA, 2011. [Google Scholar]

| Sample | n 1 | C(F–) (mg/L) 2 | C(Al) (mg/L) 2 | ||
|---|---|---|---|---|---|
| Calibration Curve | Standard Addition | Calibration Curve | Standard Addition | ||
| BF1 | 3 | 4.05 ± 0.03 | 4.04 ± 0.22 | 6.21 ± 0.20 | 6.12 ± 0.08 |
| BL5 | 3 | 0.96 ± 0.03 | 0.96 ± 0.08 | 1.79 ± 0.05 | 1.81 ± 0.06 |
| GF2 | 3 | 3.08 ± 0.02 | 3.16 ± 0.18 | 7.48 ± 0.12 | 7.52 ± 0.17 |
| GL3 | 3 | 0.71 ± 0.02 | 0.72 ± 0.03 | 1.48 ± 0.07 | 1.52 ± 0.07 |
| Symbol, Trade Name, Origin 1 | n 2 | C(F–) (mg/L) 3 | C(Al) (mg/L) 3 | Price (EUR/100 g) |
|---|---|---|---|---|
| Black filter (BF) tea | ||||
| BF1, English Breakfast, N/A 4 | 3 | 4.05 ± 0.03 | 6.21 ± 0.20 | 6.10 |
| BF2, English Breakfast, N/A | 2 | 2.71 ± 0.04 | 3.83 ± 0.02 | 5.00 |
| BF3, English Breakfast Supreme, N/A | 2 | 3.47 ± 0.15 | 7.51 ± 0.25 | 5.28 |
| BF4, Gold, N/A | 2 | 3.39 ± 0.05 | 6.56 ± 0.23 | 3.38 |
| BF5, Prince of Wales, N/A | 2 | 2.81 ± 0.19 | 6.25 ± 0.28 | 6.74 |
| BF6, English Tea No.1, N/A | 2 | 1.95 ± 0.03 | 3.93 ± 0.06 | 5.00 |
| BF7, Earl Grey, N/A | 2 | 4.15 ± 0.07 | 7.38 ± 0.18 | 6.38 |
| BF8, Earl Grey, N/A | 2 | 3.99 ± 0.17 | 8.11 ± 0.28 | 4.09 |
| BF9, Earl Grey Royal, N/A | 2 | 4.79 ± 0.07 | 8.90 ± 0.05 | 4.91 |
| BF10, Irish Cream, N/A | 2 | 4.26 ± 0.03 | 7.53 ± 0.30 | 4.76 |
| Black loose (BL) tea | ||||
| BL1, English Breakfast, India | 2 | 1.12 ± 0.07 | 2.65 ± 0.11 | 3.29 |
| BL2, Irish Morning, Sri Lanka | 2 | 0.88 ± 0.01 | 2.63 ± 0.16 | 3.65 |
| BL3, Earl Grey Decaffeine, Sri Lanka | 2 | 0.76 ± 0.03 | 1.25 ± 0.01 | 4.20 |
| BL4, Earl Grey Superior, India | 2 | 0.86 ± 0.04 | 1.80 ± 0.02 | 4.20 |
| BL5, Earl Grey, Sri Lanka | 3 | 0.96 ± 0.03 | 1.79 ± 0.05 | 3.65 |
| BL6, Keemun, China | 2 | 1.05 ± 0.07 | 1.59 ± 0.03 | 3.11 |
| BL7, Yunnan, China | 2 | 0.97 ± 0.08 | 1.63 ± 0.16 | 3.65 |
| BL8, Ceylon OP1 Kenilworth, Sri Lanka | 2 | 0.55 ± 0.01 | 1.41 ± 0.02 | 3.65 |
| BL9, Assam TGFOP1 Dirial, India | 2 | 0.54 ± 0.01 | 1.31 ± 0.01 | 3.65 |
| BL10, Darjeeling TGFOP1, India | 2 | 0.58 ± 0.01 | 1.29 ± 0.02 | 4.57 |
| BL11, Bio Black Tea Darjeeling Premium, India | 2 | 1.06 ± 0.02 | 1.80 ± 0.09 | 4.21 |
| Green filter (GF) tea | ||||
| GF1, Green Tea, N/A | 2 | 2.77 ± 0.07 | 6.58 ± 0.18 | 3.86 |
| GF2, Green Tea, N/A | 3 | 3.08 ± 0.02 | 7.48 ± 0.12 | 5.00 |
| GF3, Green Tea Superior, N/A | 2 | 2.76 ± 0.09 | 6.10 ± 0.06 | 5.20 |
| GF4, Green Tea, N/A | 2 | 2.90 ± 0.05 | 3.95 ± 0.05 | 2.00 |
| GF5, Green Tea, N/A | 2 | 2.95 ± 0.06 | 6.13 ± 0.09 | 3.78 |
| Green loose (GL) tea | ||||
| GL1, Kukicha Organic, Japan | 2 | 0.34 ± 0.01 | 0.78 ± 0.01 | 6.21 |
| GL2, Sencha Yamato, Japan | 2 | 0.86 ± 0.01 | 0.93 ± 0.02 | 5.48 |
| GL3, Yunnan Green, China | 3 | 0.71 ± 0.02 | 1.48 ± 0.07 | 4.38 |
| GL4, Earl Green, China | 2 | 1.43 ± 0.05 | 1.79 ± 0.01 | 3.65 |
| GL5, China Gunpowder Temple of Heaven, China | 2 | 0.82 ± 0.03 | 2.43 ± 0.01 | 3.11 |
| GL6, Jade Dragon Wings Organic, China | 2 | 1.29 ± 0.03 | 2.23 ± 0.10 | 3.84 |
| GL7, Korea Mystic Green Organic, South Korea | 2 | 0.96 ± 0.01 | 3.74 ± 0.19 | 4.02 |
| GL8, Premium Shincha Gyokuro Okumidori Kirishima 2021, Japan | 2 | 0.67 ± 0.03 | 0.51 ± 0.01 | 20.09 |
| GL9, Genmaicha, Japan | 2 | 0.57 ± 0.02 | 1.31 ± 0.08 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovič, A.; Tavčar, G.; Ponikvar-Svet, M. Fluoride and Aluminium in Tea (Camellia sinensis L.)—Tea Quality Indicators and Risk Factors for Consumers. Molecules 2023, 28, 6396. https://doi.org/10.3390/molecules28176396
Pavlovič A, Tavčar G, Ponikvar-Svet M. Fluoride and Aluminium in Tea (Camellia sinensis L.)—Tea Quality Indicators and Risk Factors for Consumers. Molecules. 2023; 28(17):6396. https://doi.org/10.3390/molecules28176396
Chicago/Turabian StylePavlovič, Anja, Gašper Tavčar, and Maja Ponikvar-Svet. 2023. "Fluoride and Aluminium in Tea (Camellia sinensis L.)—Tea Quality Indicators and Risk Factors for Consumers" Molecules 28, no. 17: 6396. https://doi.org/10.3390/molecules28176396
APA StylePavlovič, A., Tavčar, G., & Ponikvar-Svet, M. (2023). Fluoride and Aluminium in Tea (Camellia sinensis L.)—Tea Quality Indicators and Risk Factors for Consumers. Molecules, 28(17), 6396. https://doi.org/10.3390/molecules28176396







