Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies
Abstract
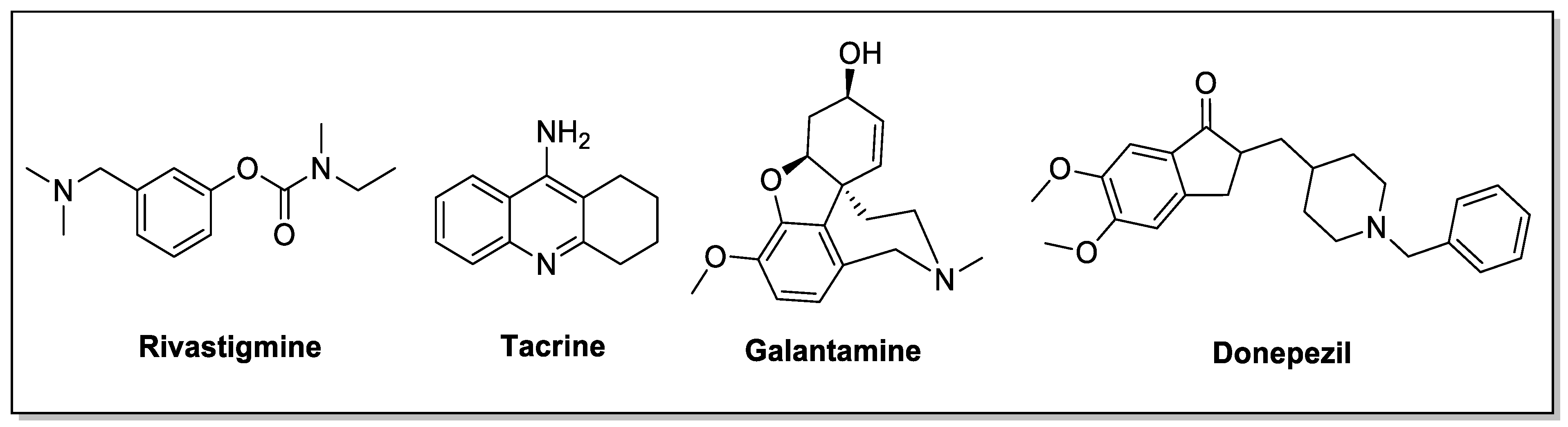
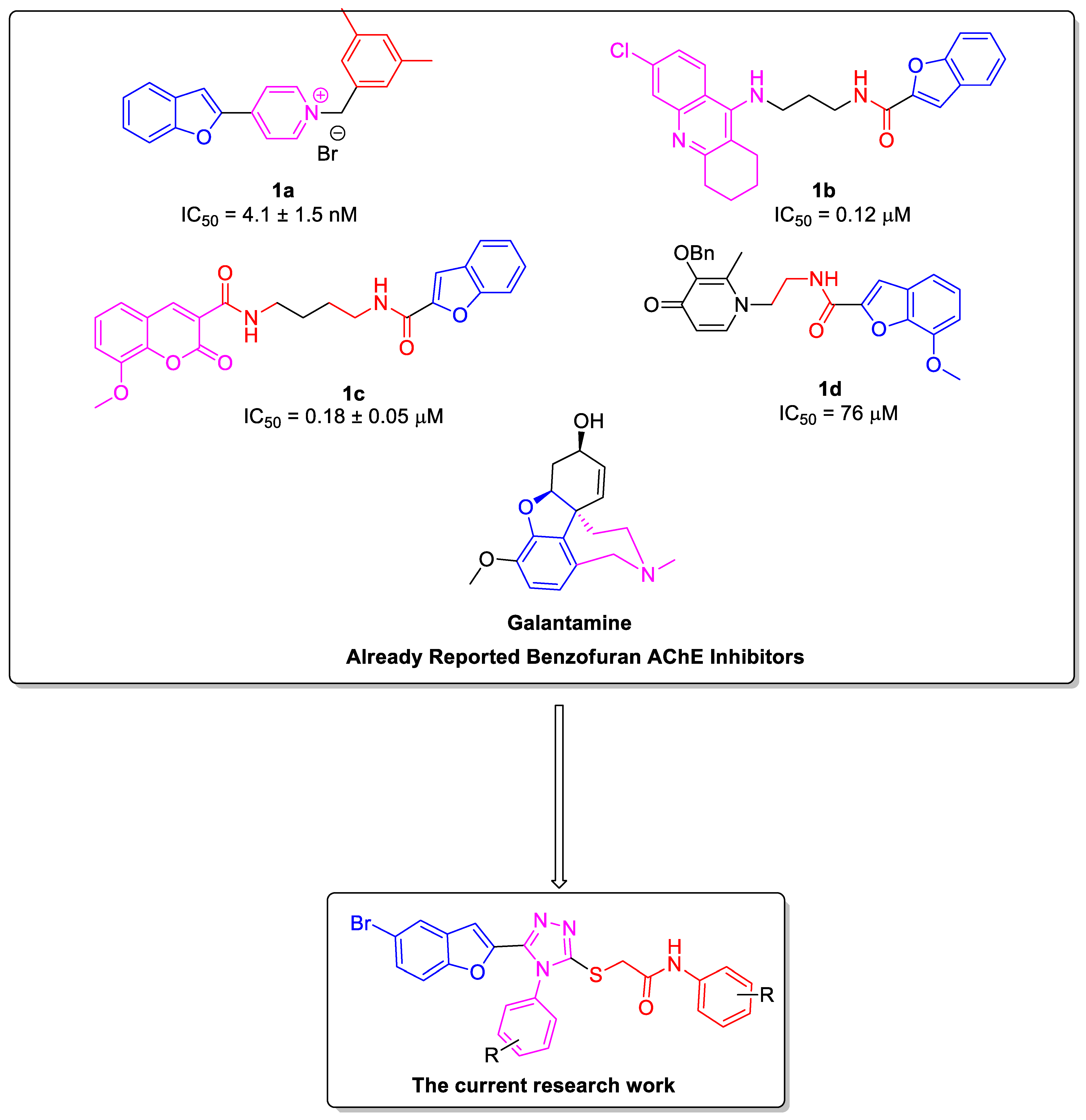
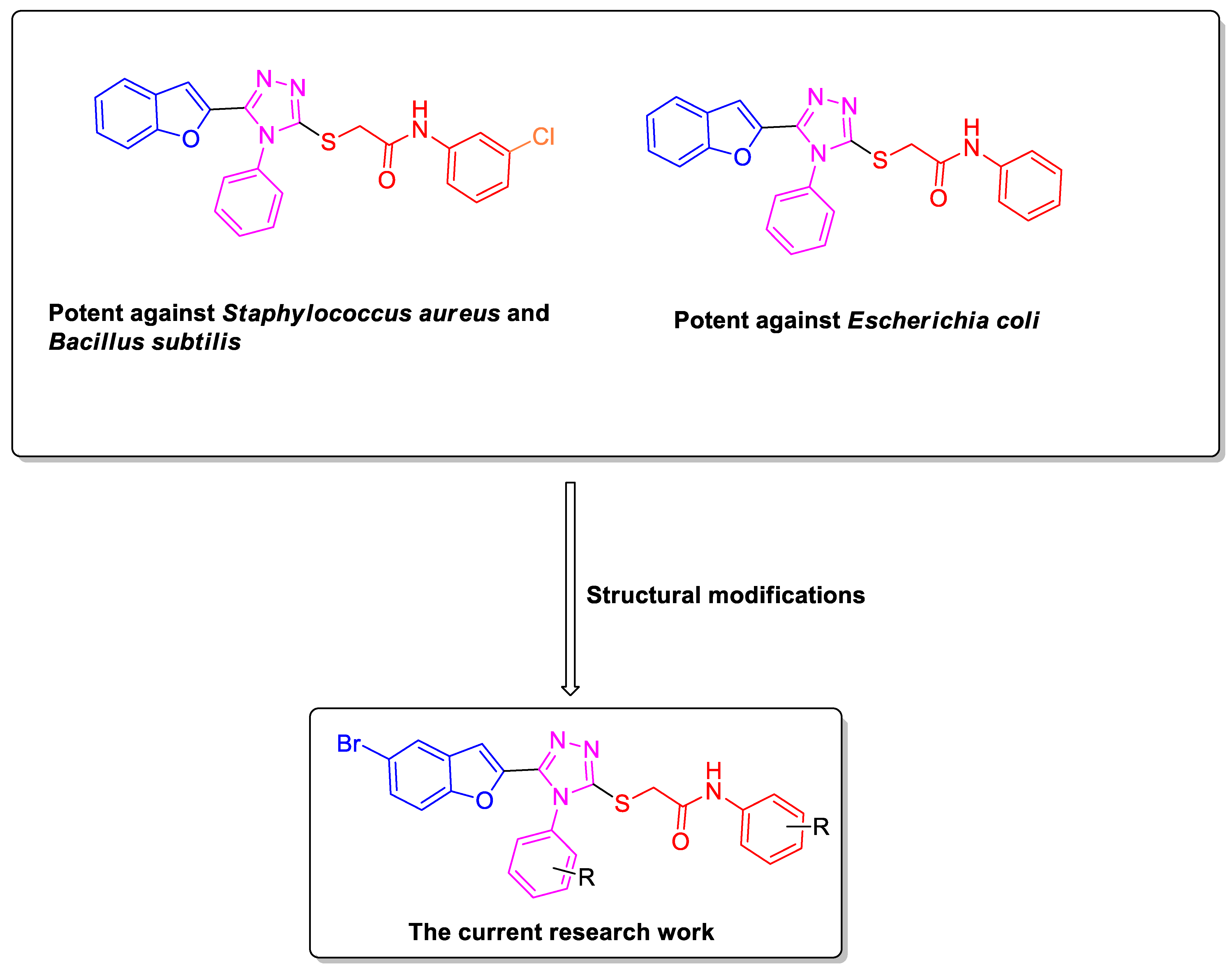
1. Introduction
2. Results and Discussion
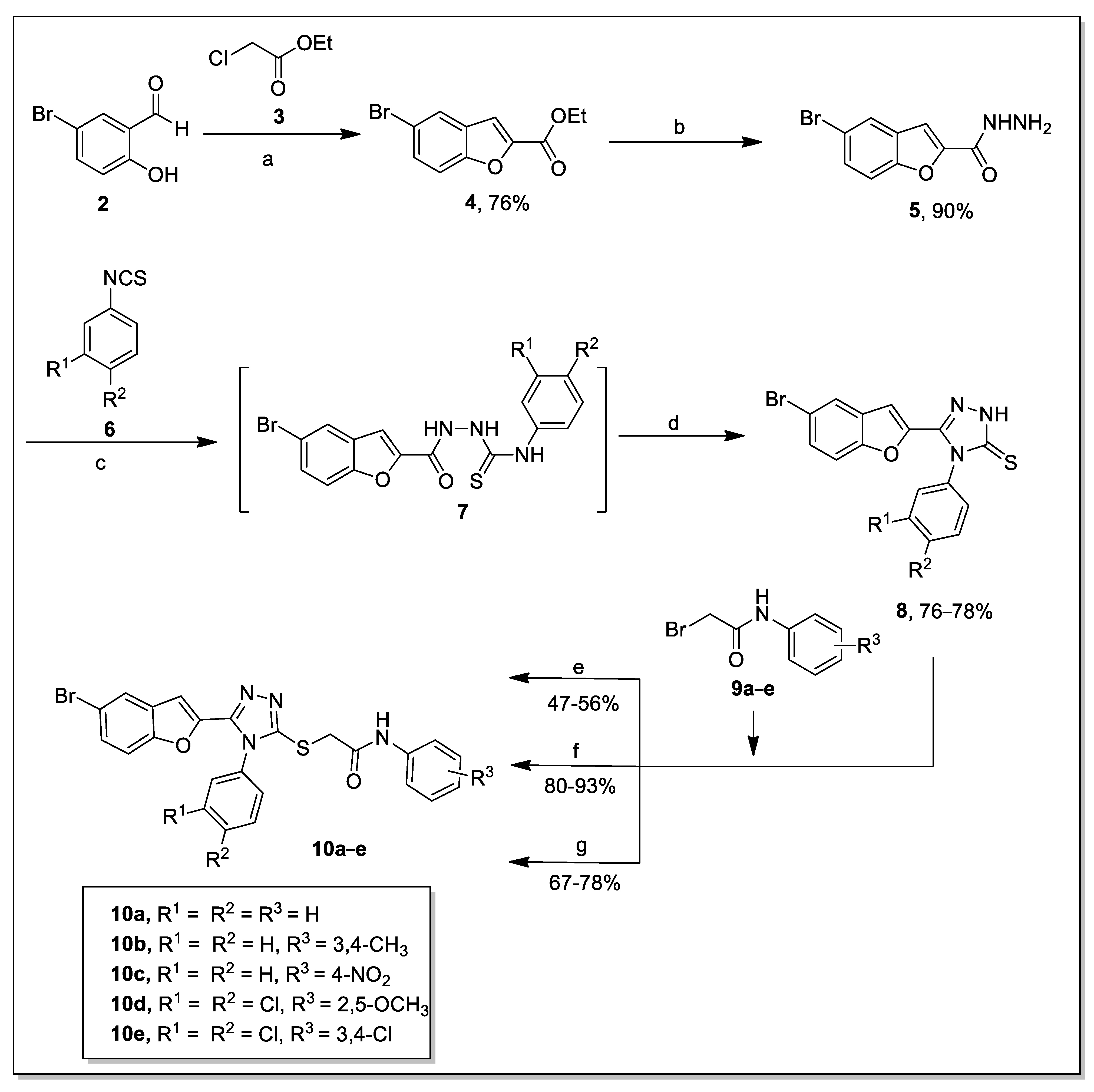
2.1. Chemistry
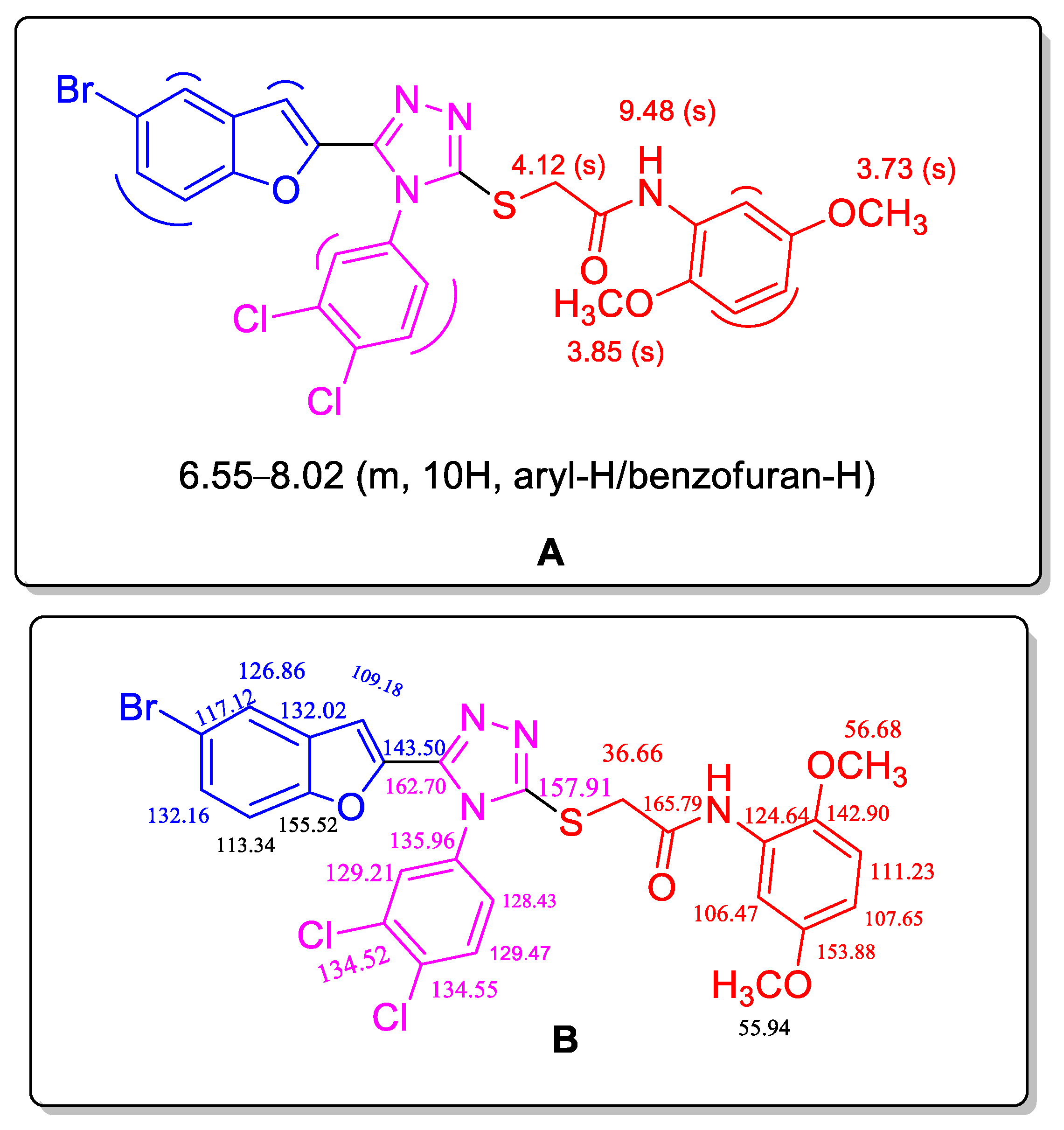
2.2. Spectral Interpretation of Compound (10d)
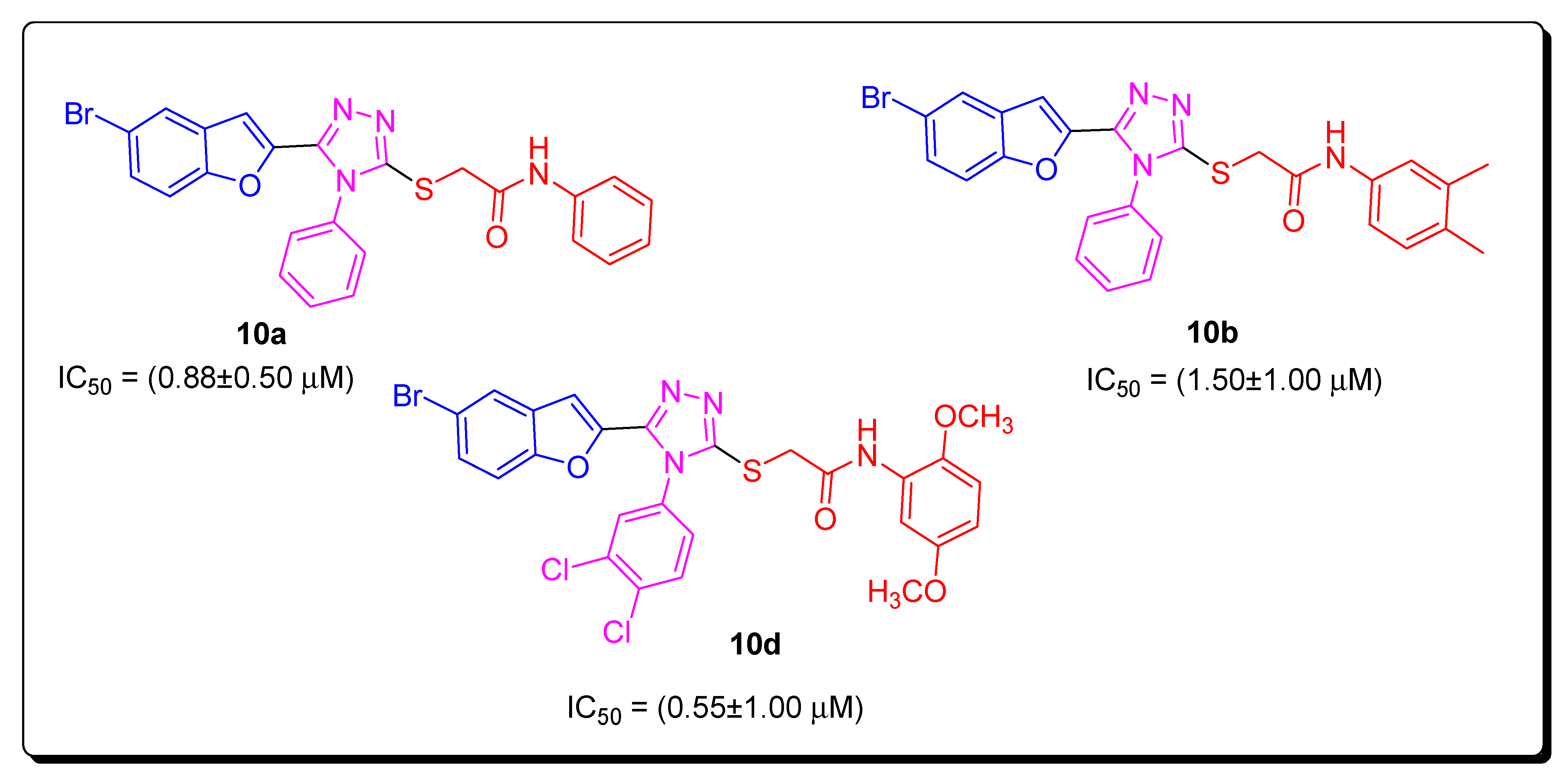
2.3. AChE Inhibitory Activity
2.4. Structure–Activity Relationship of the Potent AChE Inhibitors
2.5. Antibacterial Activity
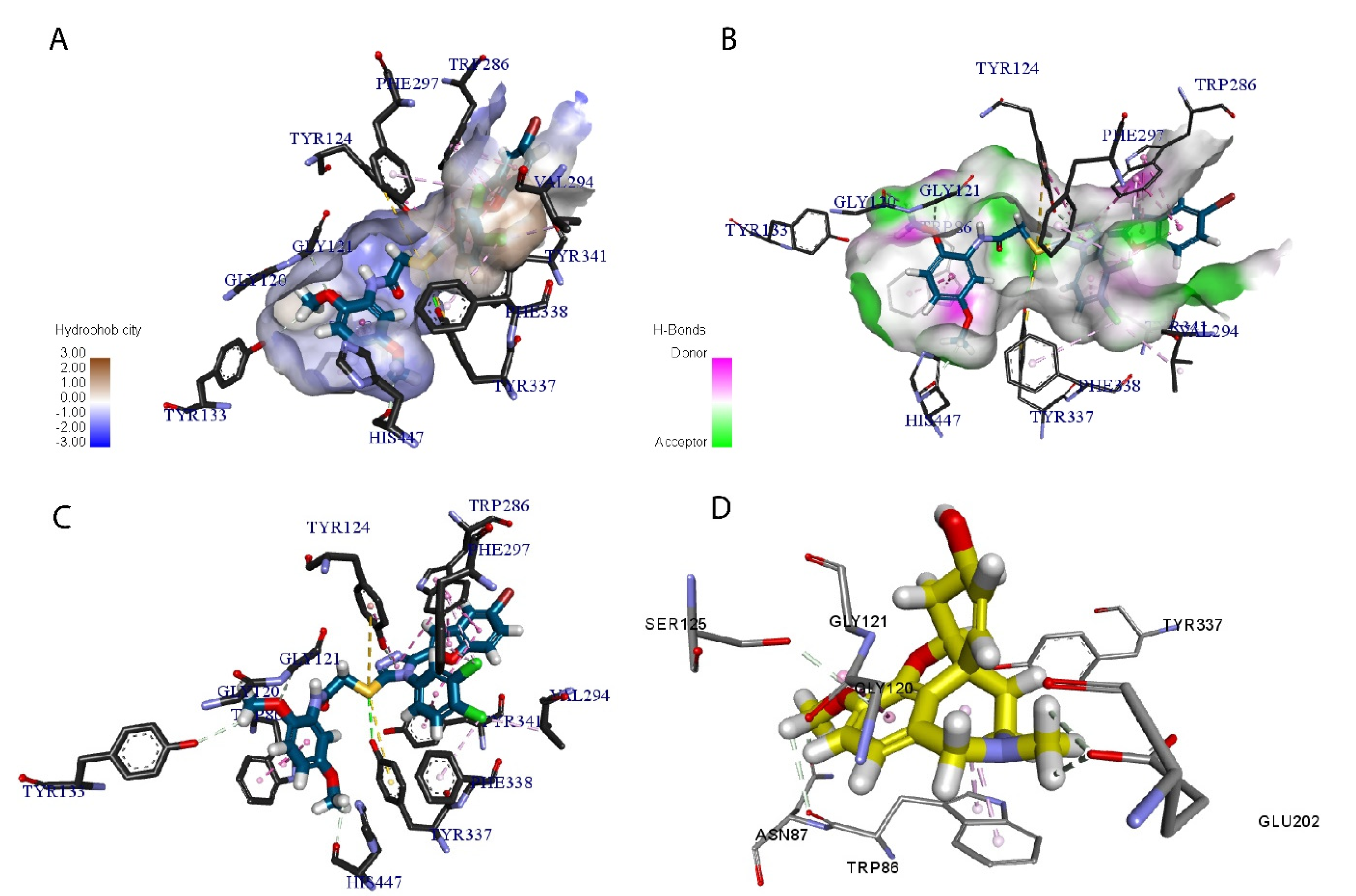
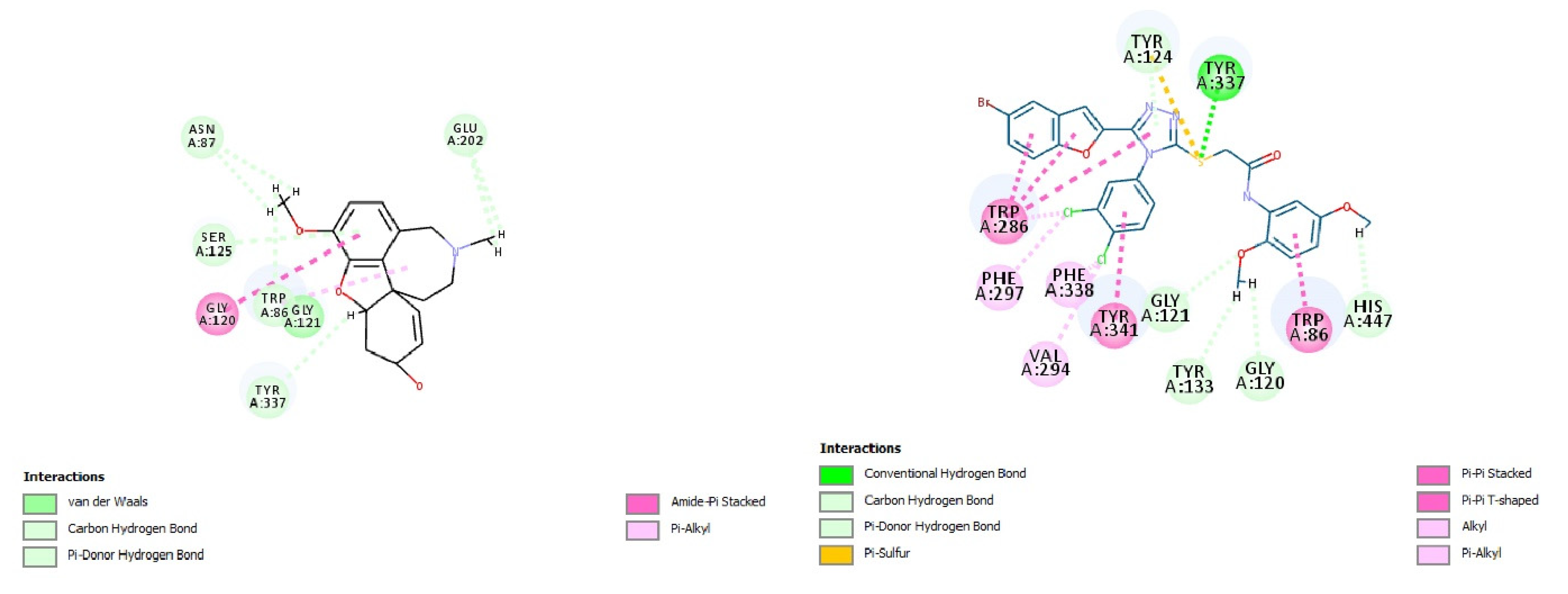
2.6. In Silico Modeling of the Most Potent Compound (10d)
3. Experimental
3.1. General Protocol for the Synthesis of Derivatives
3.1.1. Synthesis of Ethyl 5-Bromobenzofuran-2-Carboxylate (4)
3.1.2. Synthesis of 5-Bromobenzofuran-2-Carbohydrazide (5)
3.1.3. General Procedure for the Synthesis of 5-(5-Bromobenzofuran-2-yl)-4-phenyl-4H-1,2,4-triazole-3-thione (8)
3.1.4. General Synthetic Procedures for the Synthesis of 2-((5-(5-Bromobenzofuran-2-yl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)-N-phenylacetamide (10a–e)
3.2. Characterization Data
3.3. AChE Inhibition Assay
3.4. Antibacterial Assay
3.5. In Silico Modeling Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Synthesis, biological evaluation and molecular modeling study of novel tacrine–carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 21–30. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, L.; Peng, S.; Liao, H.; Lehmann, J.; Zhang, Y. Discovery of a novel acetylcholinesterase inhibitor by structure-based virtual screening techniques. Bioorg. Med. Chem. Lett. 2012, 22, 3181–3187. [Google Scholar] [CrossRef]
- Hussein, W.; Sağlık, B.N.; Levent, S.; Korkut, B.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new cholinesterase inhibitors for Alzheimer’s disease. Molecules 2018, 23, 2033. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Sang, Z.; Yuan, W.; Li, Y.; Liu, Q.; Bai, P.; Shi, Y.; Ang, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 76, 314–331. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Yin, W.; Li, J.; Wang, W.; Bai, F.; Xu, S.; Gong, Q.; Peng, T.; Hong, Y.; et al. Kinetics-driven drug design strategy for next-generation acetylcholinesterase inhibitors to clinical candidate. J. Med. Chem. 2021, 64, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Chioua, M.; Bolea, I.; De Los Ríos, C.; Iriepa, I.; Moraleda, I.; Bastida, A.; Esteban, G.; Unzeta, M.; Gálvez, E.; et al. Synthesis, biological assessment and molecular modeling of new multipotent MAO and cholinesterase inhibitors as potential drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2011, 46, 4665–4668. [Google Scholar] [CrossRef] [PubMed]
- Khoobi, M.; Alipour, M.; Sakhteman, A.; Nadri, H.; Moradi, A.; Ghandi, M.; Emami, S.; Foroumadi, A.; Shafiee, A. Design, synthesis, biological evaluation and docking study of 5-oxo-4, 5-dihydropyrano [3, 2-c] chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 68, 260–269. [Google Scholar] [CrossRef]
- Dighe, S.N.; Tippana, M.; Van Akker, S.; Collet, T.A. Structure-based scaffold repurposing toward the discovery of novel cholinesterase inhibitors. ACS Omega 2020, 5, 30971–30979. [Google Scholar] [CrossRef]
- Hemaida, A.Y.; Hassan, G.S.; Maarouf, A.R.; Joubert, J.; El-Emam, A.A. Synthesis and biological evaluation of thiazole-based derivatives as potential acetylcholinesterase inhibitors. ACS Omega 2021, 6, 19202–19211. [Google Scholar] [CrossRef]
- Samadi, A.; Estrada, M.; Pérez, C.; Rodríguez-Franco, M.I.; Iriepa, I.; Moraleda, I.; Chioua, M.; Marco-Contelles, J. Pyridonepezils, new dual AChE inhibitors as potential drugs for the treatment of Alzheimer’s disease: Synthesis, biological assessment, and molecular modeling. Eur. J. Med. Chem. 2012, 57, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Vignaux, P.A.; Minerali, E.; Lane, T.R.; Foil, D.H.; Madrid, P.B.; Puhl, A.C.; Ekins, S. The antiviral drug tilorone is a potent and selective inhibitor of acetylcholinesterase. Chem. Res. Toxicol. 2021, 34, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Anukanon, S.; Pongpamorn, P.; Tiyabhorn, W.; Chatwichien, J.; Niwetmarin, W.; Sessions, R.B.; Ruchirawat, S.; Thasana, N. In silico-guided rational drug design and semi-synthesis of C(2)-functionalized huperzine A derivatives as acetylcholinesterase inhibitors. ACS Omega 2021, 6, 19924–19939. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Shinde, A.; Kambhampati, R.S.; Mohammed, A.R.; Saraf, S.K.; Badange, R.K.; Bandyala, T.R.; Bhatta, V.; Bojja, K.; Reballi, V.; et al. Discovery and development of 1-[(2-Bromophenyl) sulfonyl]-5-methoxy-3-[(4-methyl-1-piperazinyl) methyl]-1H-indole dimesylate monohydrate (SUVN-502): A novel, potent, selective and orally active serotonin 6 (5-HT6) receptor antagonist for potential treatment of Alzheimer’s disease. J. Med. Chem. 2017, 60, 1843–1859. [Google Scholar]
- Cheng, Z.Q.; Zhu, K.K.; Zhang, J.; Song, J.L.; Muehlmann, L.A.; Jiang, C.S.; Liu, C.L.; Zhang, H. Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BChE inhibitors. Bioorg. Chem. 2019, 83, 277–288. [Google Scholar] [CrossRef]
- Stavrakov, G.; Philipova, I.; Lukarski, A.; Atanasova, M.; Zheleva, D.; Zhivkova, Z.D.; Ivanov, S.; Atanasova, T.; Konstantinov, S.; Doytchinova, I. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules 2020, 25, 3341. [Google Scholar] [CrossRef]
- Kumawat, A.; Raheem, S.; Ali, F.; Dar, T.A.; Chakrabarty, S.; Rizvi, M.A. Organoselenium compounds as acetylcholinesterase inhibitors: Evidence and mechanism of mixed inhibition. J. Phys. Chem. B 2021, 125, 1531–1541. [Google Scholar] [CrossRef]
- Irfan, A.; Faisal, S.; Zahoor, A.F.; Noreen, R.; Al-Hussain, S.A.; Tuzun, B.; Javaid, R.; Elhenawy, A.A.; Zaki, M.E.A.; Ahmad, S.; et al. In silico development of novel benzofuran-1,3,4-oxadiazoles as lead inhibitors of M. tuberculosis polyketide synthase 13. Pharmaceuticals 2023, 16, 829. [Google Scholar] [CrossRef]
- Aziz, H.; Zahoor, A.F.; Shahzadi, I.; Irfan, A. Recent synthetic methodologies towards the synthesis of pyrazoles. Polycycl. Aromat. Compd. 2019, 41, 698–720. [Google Scholar] [CrossRef]
- Akhtar, R.; Yousaf, M.; Zahoor, A.F.; Naqvi, S.A.R.; Abbas, N. Synthesis of lamivudine (3TC) and its derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 989–1001. [Google Scholar] [CrossRef]
- Mandala, D.; Chada, S.; Watts, P. Semi-continuous multi-step synthesis of lamivudine. Org. Biomol. Chem. 2017, 15, 3444–3454. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Rasul, A.; Khan, S.G.; Ali, K.G. In-vitro cytotoxic evaluation of newly designed ciprofloxacin-oxadiazole hybrids against human liver tumor cell line (Huh7). Pak. J. Pharm. Sci. 2021, 34, 1143–1148. [Google Scholar] [PubMed]
- Irfan, A.; Faisal, S.; Ahmad, S.; Al-Hussain, S.A.; Javed, S.; Zahoor, A.F.; Parveen, B.; Zaki, M.E.A. Structure-based virtual screening of furan-1,3,4-oxadiazole tethered N-phenylacetamide derivatives as novel class of hTYR and hTYRP1 inhibitors. Pharmaceuticals 2023, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Zahoor, A.F.; Rasul, A.; Al-Hussain, S.A.; Faisal, S.; Ahmad, S.; Noor, R.; Muhammed, M.T.; Zaki, M.E.A. BTEAC catalyzed ultrasonic-assisted synthesis of bromobenzofuran-oxadiazoles: Unravelling anti-HepG-2 cancer therapeutic potential through in vitro and in silico studies. Int. J. Mol. Sci. 2023, 24, 3008. [Google Scholar] [CrossRef]
- Irfan, A.; Zahoor, A.F.; Kamal, S.; Hassan, M.; Kloczkowski, A. Ultrasonic-assisted synthesis of benzofuran appended oxadiazole molecules as tyrosinase inhibitors: Mechanistic approach through enzyme inhibition, molecular docking, chemoinformatics, ADMET and drug-likeness studies. Int. J. Mol. Sci. 2022, 23, 10979. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Rasul, A.; Mansha, A.; Ahmad, S.; Raza, Z. Synthesis, hemolytic studies, and in silico modeling of novel acefylline–1, 2, 4-triazole hybrids as potential anti-cancer agents against MCF-7 and A549. ACS Omega 2021, 6, 11943–11953. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Tüzün, B.; Mansha, A.; Anjum, M.N.; Rasul, A.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Repositioning of acefylline as anti-cancer drug: Synthesis, anticancer and computational studies of azomethines derived from acefylline tethered 4-amino-3-mercapto-1,2,4-triazole. PLoS ONE 2022, 17, e0278027. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, F.; Zahoor, A.F.; Rasul, A.; Ahmad, S.; Mansha, A. Synthesis and anticancer evaluation of 2-oxo-2-(arylamino) ethyl 4-phenylpiperazine-1-carbodithioates. Pak. J. Pharm. Sci. 2021, 34, 353–357. [Google Scholar]
- Hafeez, F.; Zahoor, A.F.; Rasul, A.; Mansha, A.; Noreen, R.; Raza, Z.; Ali, K.G.; Irfan, A.; El-Hiti, G.A. Ultrasound-assisted synthesis and in silico modeling of methanesulfonyl-piperazine-based dithiocarbamates as potential anticancer, thrombolytic, and hemolytic structural motifs. Molecules 2022, 27, 4776. [Google Scholar] [CrossRef]
- Hafeez, F.; Zahoor, A.F.; Irfan, M.; Kamal, S.; Ahmad, S. Facile one-pot approach to the synthesis of alkyl piperazine-1-carbodithioates as hemolytic and thrombolytic agents. Russ. J. Org. Chem. 2022, 58, 884–890. [Google Scholar] [CrossRef]
- Irfan, A.; Ullah, S.; Anum, A.; Jabeen, N.; Zahoor, A.F.; Kanwal, H.; Kotwica-Mojzych, K.; Mojzych, M. Synthetic transformations and medicinal significance of 1,2,3-thiadiazoles derivatives: An update. Appl. Sci. 2021, 11, 5742. [Google Scholar] [CrossRef]
- Mondal, S.; Mohanty, B.; Nurhuda, M.; Dalapati, S.; Jana, R.; Addicoat, M.; Datta, A.; Jena, B.K.; Bhaumik, A. A thiadiazole-based covalent organic framework: A metal-free electrocatalyst toward oxygen evolution reaction. ACS Catal. 2020, 10, 5623–5630. [Google Scholar] [CrossRef]
- Hatami, M.; Basri, Z.; Sakhvidi, B.K.; Mortazavi, M. Thiadiazole–A promising structure in design and development of anti-Alzheimer agents. Int. Immunopharmacol. 2023, 118, 110027. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Heterocycles in medicinal chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef]
- Dawood, K.M. An update on benzofuran inhibitors: A patent review. Expert. Opin. Ther. Pat. 2019, 29, 841–870. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Baharloo, F.; Moslemin, M.H.; Nadri, H.; Asadipour, A.; Mahdavi, M.; Emami, S.; Firoozpour, L.; Mohebat, R.; Shafiee, A.; Foroumadi, A. Benzofuran-derived benzylpyridinium bromides as potent acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2015, 93, 196–201. [Google Scholar] [CrossRef]
- Fancellu, G.; Chand, K.; Tomás, D.; Orlandini, E.; Piemontese, L.; Silva, D.F.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel tacrine–benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease. J. Enzym. Inhib. Med. Chem. 2020, 35, 211–226. [Google Scholar] [CrossRef]
- Hiremathad, A.; Chand, K.; Keri, R.S. Development of coumarin–benzofuran hybrids as versatile multitargeted compounds for the treatment of Alzheimer’s disease. Chem. Biol. Drug. Des. 2018, 92, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Chand, K.; Tolayan, L.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Hydroxypyridinone-benzofuran hybrids with potential protective roles for Alzheimer s disease therapy. J. Inorg. Biochem. 2018, 179, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, R.; Türkan, F.; Yildiko, Ü.; Aras, A.; Evren, E.; Onkol, T. Synthesis and in silico studies of novel Ru (II) complexes of schiff base derivatives of 3-[(4-amino-5-thioxo-1, 2, 4-triazole-3-yl) methyl]-2 (3H)-benzoxazolone compounds as potent glutathione S-transferase and cholinesterases inhibitor. J. Mol. Struct. 2021, 1231, 129943. [Google Scholar] [CrossRef]
- Özil, M.; Balaydın, H.T.; Şentürk, M. Synthesis of 5-methyl-2, 4-dihydro-3H-1, 2, 4-triazole-3-one’s aryl Schiff base derivatives and investigation of carbonic anhydrase and cholinesterase (AChE, BuChE) inhibitory properties. Bioorg. Chem. 2019, 86, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Poojary, B.; Prasad, D.J.; Naik, P.; Holla, B.S. Synthesis and antitumor activity studies of some new fused 1, 2, 4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066–5070. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Khurana, J.M. Synthesis, antimicrobial evaluation and QSAR analysis of novel nalidixic acid based 1, 2, 4-triazole derivatives. Eur. J. Med. Chem. 2021, 46, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Mehr-un-Nisa; Munawar, M.A.; Chattha, F.A.; Kousar, S.; Munir, J.; Ismail, T.; Ashraf, M.; Khan, M.A. Synthesis of novel triazoles and a tetrazole of escitalopram as cholinesterase inhibitors. Bioorgan. Med. Chem. 2015, 23, 6014–6024. [Google Scholar] [CrossRef]
- Mermer, A.; Demirbaş, N.; Şirin, Y.; Uslu, H.; Özdemir, Z.; Demirbaş, A. Conventional and microwave prompted synthesis, antioxidant, anticholinesterase activity screening and molecular docking studies of new quinolone-triazole hybrids. Bioorg. Chem. 2018, 78, 236–248. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1, 2, 4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Faiz, S.; Zahoor, A.F.; Ajmal, M.; Kamal, S.; Ahmad, S.; Abdelgawad, A.M.; Elnaggar, M.E. Design, synthesis, antimicrobial evaluation, and laccase catalysis effect of novel benzofuran–oxadiazole and benzofuran–triazole hybrids. J. Heterocycl. Chem. 2019, 56, 2839–2852. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1, 2, 4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar] [CrossRef]
- Irfan, A.; Faiz, S.; Rasul, A.; Zafar, R.; Zahoor, A.F.; Kotwica-Mojzych, K.; Mojzych, M. Exploring the synergistic anticancer potential of benzofuran–oxadiazoles and triazoles: Improved ultrasound-and microwave-assisted synthesis, molecular docking, hemolytic, thrombolytic and anticancer evaluation of furan-based molecules. Molecules 2022, 27, 1023. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Rasul, A.; Rasool, N.; Raza, Z.; Faisal, S.; Parveen, B.; Kamal, S.; Zia-ur-Rehman, M.; Zahid, F.M. Synthesis, anticancer, and computational studies of 1, 3, 4-oxadiazole-purine derivatives. J. Heterocycl. Chem. 2020, 57, 2782–2794. [Google Scholar] [CrossRef]
- Javid, J.; Abbasi, M.A.; Siddiqui, S.Z.; Iqbal, J.; Virk, N.A.; Rasool, S.; Ali Shah, S.A. Comparative conventional and microwave assisted synthesis of heterocyclic oxadiazole analogues having enzymatic inhibition potential. J. Heterocycl. Chem. 2021, 58, 93–110. [Google Scholar] [CrossRef]
- Rezki, Z.; Aouad, M.R. Green ultrasound-assisted three-component click synthesis of novel 1H-1, 2, 3-triazole carrying benzothiazoles and fluorinated-1, 2, 4-triazole conjugates and their antimicrobial evaluation. Acta Pharm. 2017, 67, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Y.; Göçer, H.; Göksu, S.; Gülçin, İ. Synthesis and carbonic anhydrase isoenzymes I and II inhibitory effects of novel benzylamine derivatives. J Enzym. Inhib. Med. Chem. 2014, 29, 168–174. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Naqvi, S.A.R.; Mukhtar, A.; Hussain, Z.; Shahzad, S.A.; Mansha, A.; Ahmad, M.; Zahoor, A.F.; Yar, M. Antioxidant and antibacterial activities of Hibiscus Rosa-sinensis Linn flower extracts. Pak. J. Pharm. Sci. 2014, 27, 469–475. [Google Scholar] [PubMed]
| Compounds | Reaction Yield | Reaction Time | ||||
|---|---|---|---|---|---|---|
| Conventional (%) | Microwave (%) | Ultrasound (%) | Conventional (h) | Microwave (s) | Ultrasound (min) | |
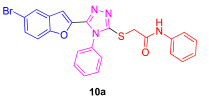 | 47 | 85 | 69 | 18 | 69 | 25 |
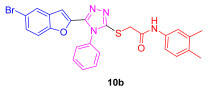 | 56 | 90 | 77 | 22 | 75 | 30 |
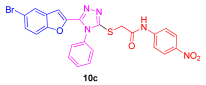 | 49 | 88 | 67 | 15 | 63 | 20 |
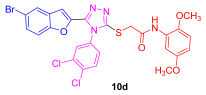 | 52 | 93 | 78 | 24 | 72 | 15 |
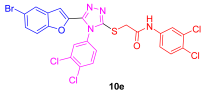 | 55 | 80 | 73 | 20 | 60 | 22 |
| Compounds | Inhibition % (0.5 mM) | IC50 (µM) |
|---|---|---|
| 10a | 65.84 ± 1.50 | 0.88 ± 0.50 |
| 10b | 61.7 ± 1.50 | 1.50 ± 1.00 |
| 10c | 55.90 ± 1.25 | 2.28 ± 1.75 |
| 10d | 81.45 ± 0.50 | 0.55 ± 1.00 |
| 10e | 51.23 ± 1.24 | 1.98 ± 0.25 |
| Eserine | 93.20 ± 1.15 | 0.06 ± 0.01 |
| Compounds | B. subtilis | E. coli | ||
|---|---|---|---|---|
| Inhibition Zone (mm) | MIC (µg/mL) | Inhibition Zone (mm) | MIC (µg/mL) | |
| 10a | Nd | Nd | 38 ± 1.50 | 1.80 ± 0.25 |
| 10b | 42 ± 1.00 | 1.25 ± 0.60 | 11.5 ± 1.40 | 3.8 ± 2.1 |
| 10c | nd * | nd * | 28 ± 0.00 | 2.5 ± 0.70 |
| 10d | 11 ± 0.50 | 9.25 ± 1.25 | 12 ± 0.65 | 16 ± 1.00 |
| 10e | 8 ± 0.75 | 14 ± 1.80 | 24 ± 0.50 | 2.5 ± 1.40 |
| Penicillin | 18 ± 0.50 | 1 ± 1.50 | 24 ± 0.50 | 2.4 ± 1.00 |
| Ligand | Binding Score (S) Kcal/mol | Interacting Residues | Interaction Type |
|---|---|---|---|
| Galantamine | −7.07 | TRP86, GLY121, TYR337, GLU202, SER125, GLY120, ASN87 | H-bonding, Amide-π stacked, π-Alkyl |
| 10d | −9.34 | HIS447, TRP86, GLY121, GLY120, TYR133, TYR341, PHE338, VAL294, PHE297, TRP286, TYR124, TYR337 | H-bonding, π-sulfur, π-π stacked, π-π T-shaped, alkyl, π-alkyl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Zahoor, A.F.; Kamal, S.; Raza, Z.; Bhat, M.A. Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies. Molecules 2023, 28, 6007. https://doi.org/10.3390/molecules28166007
Saeed S, Zahoor AF, Kamal S, Raza Z, Bhat MA. Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies. Molecules. 2023; 28(16):6007. https://doi.org/10.3390/molecules28166007
Chicago/Turabian StyleSaeed, Sadaf, Ameer Fawad Zahoor, Shagufta Kamal, Zohaib Raza, and Mashooq Ahmad Bhat. 2023. "Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies" Molecules 28, no. 16: 6007. https://doi.org/10.3390/molecules28166007
APA StyleSaeed, S., Zahoor, A. F., Kamal, S., Raza, Z., & Bhat, M. A. (2023). Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies. Molecules, 28(16), 6007. https://doi.org/10.3390/molecules28166007







