Synthesis and Characterization of Bulky Substituted Hemihexaphyrazines Bearing 2,6-Diisopropylphenoxy Groups
Abstract
1. Introduction
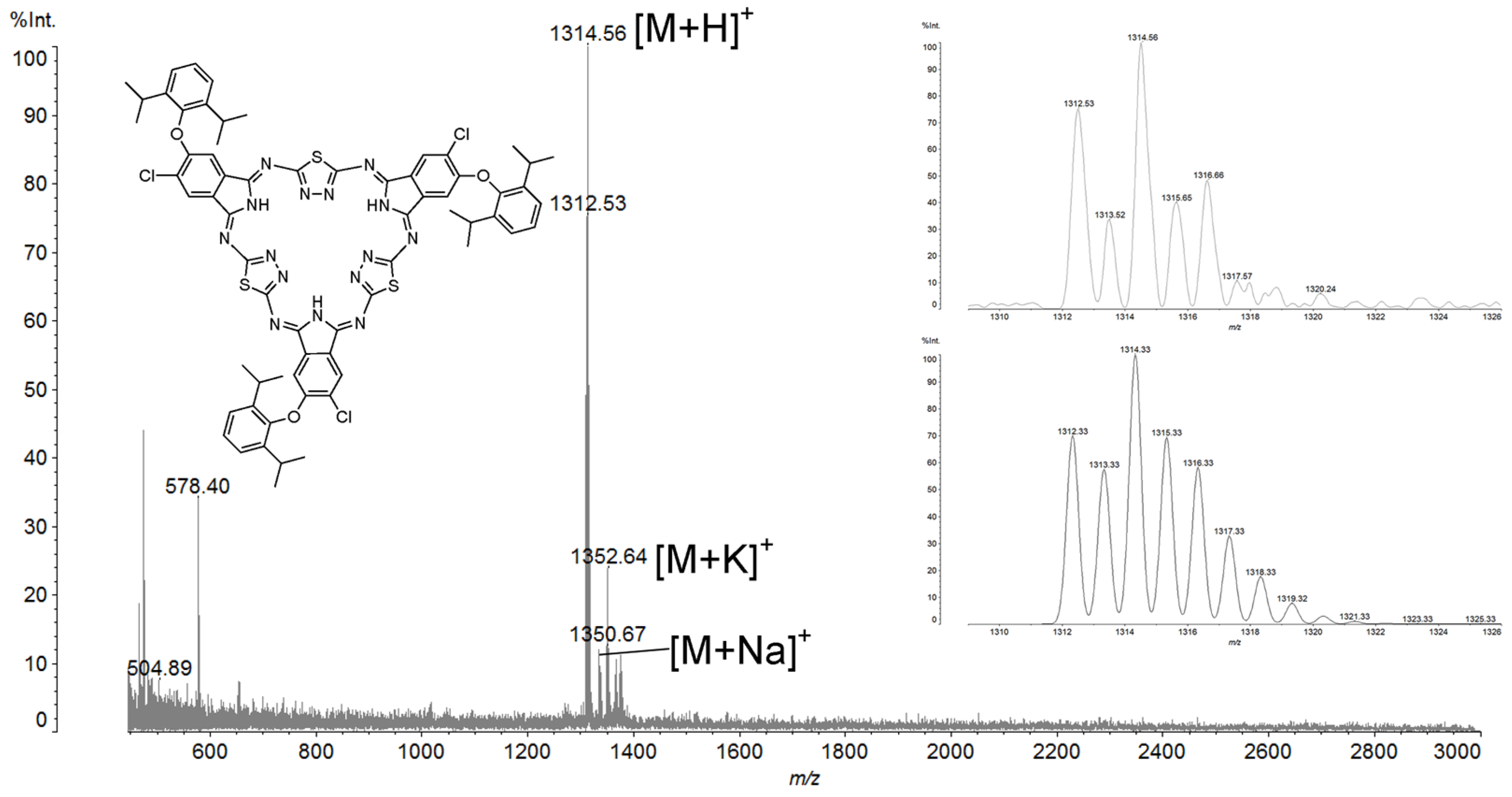
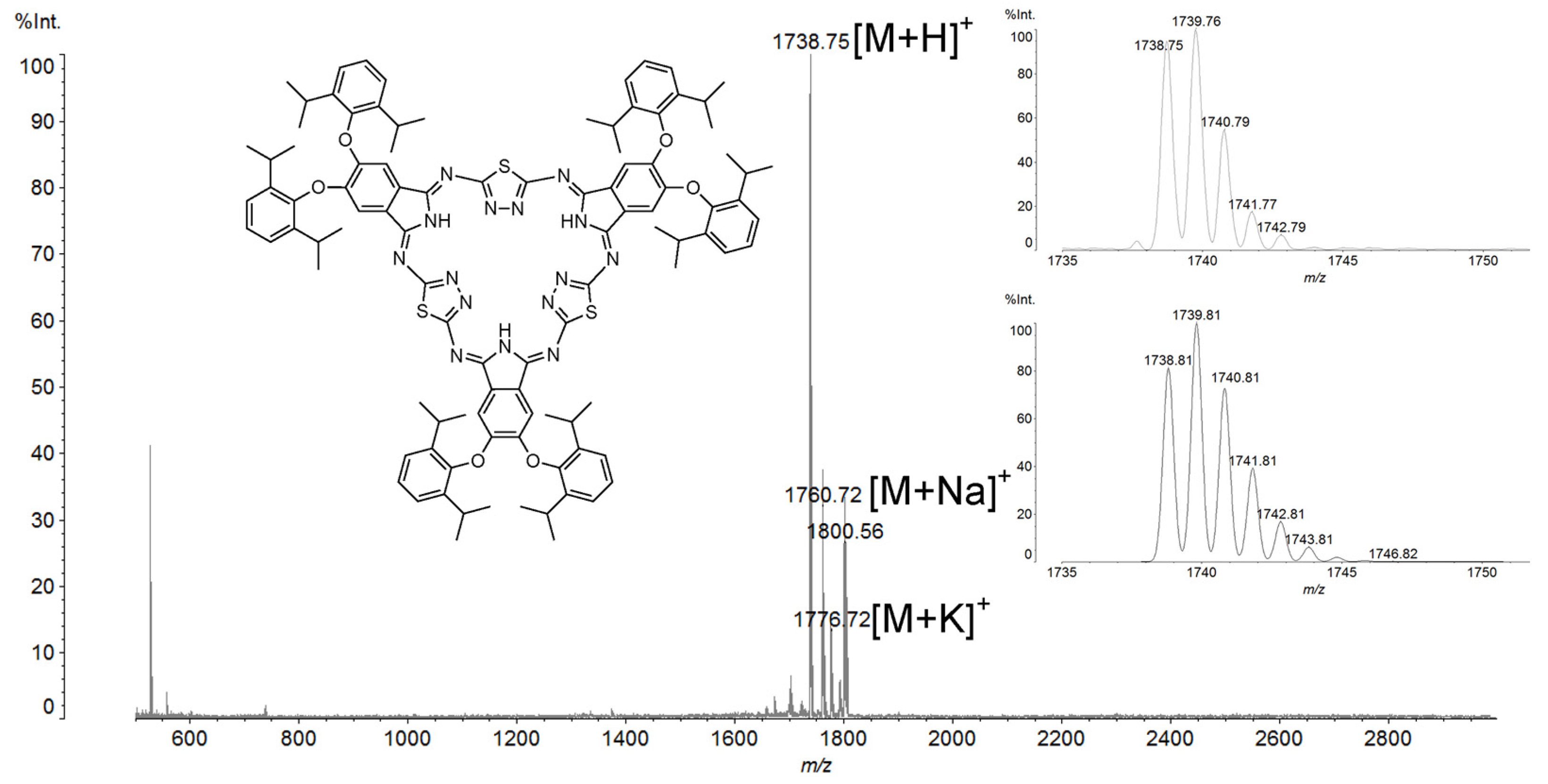
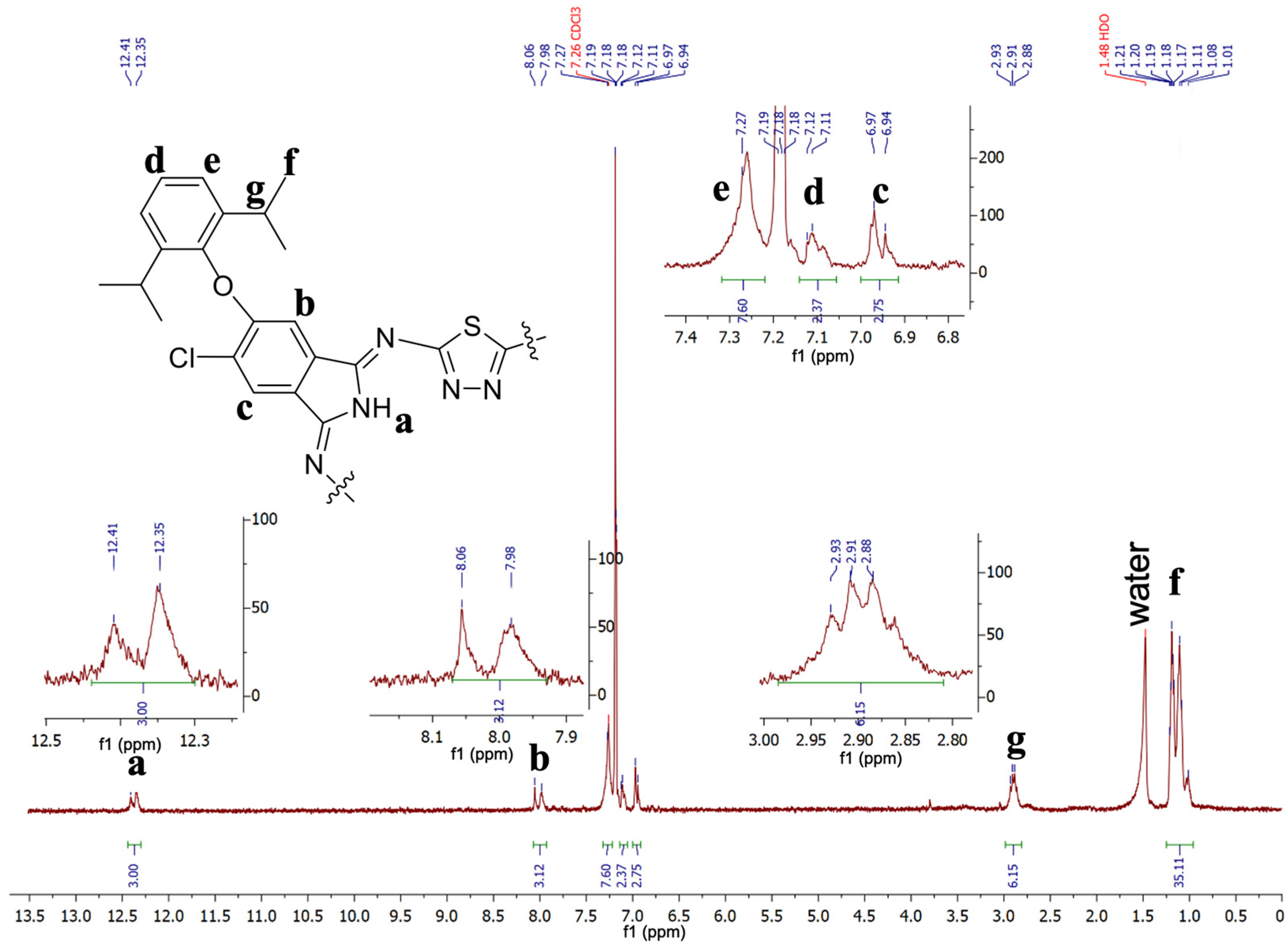
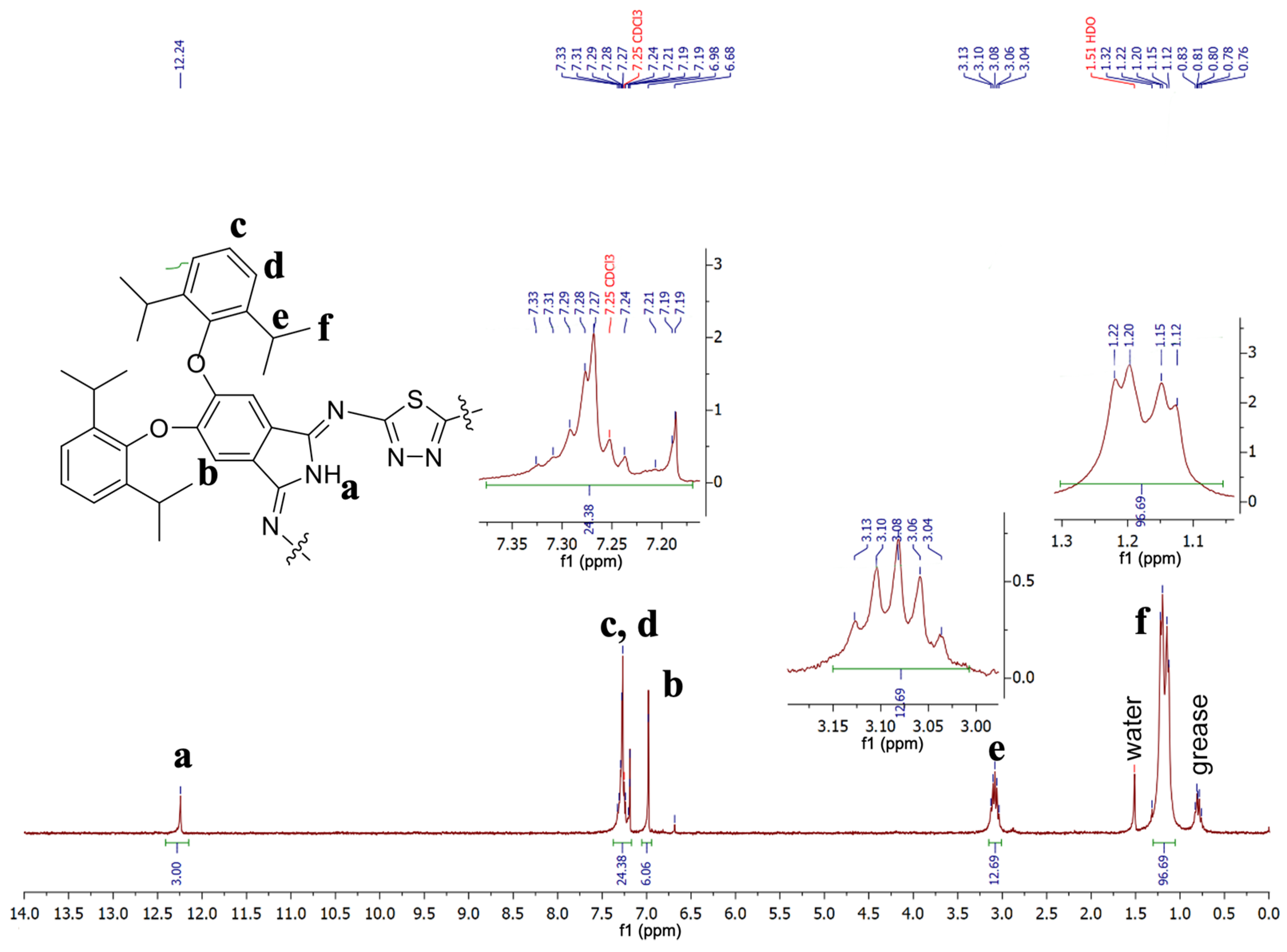
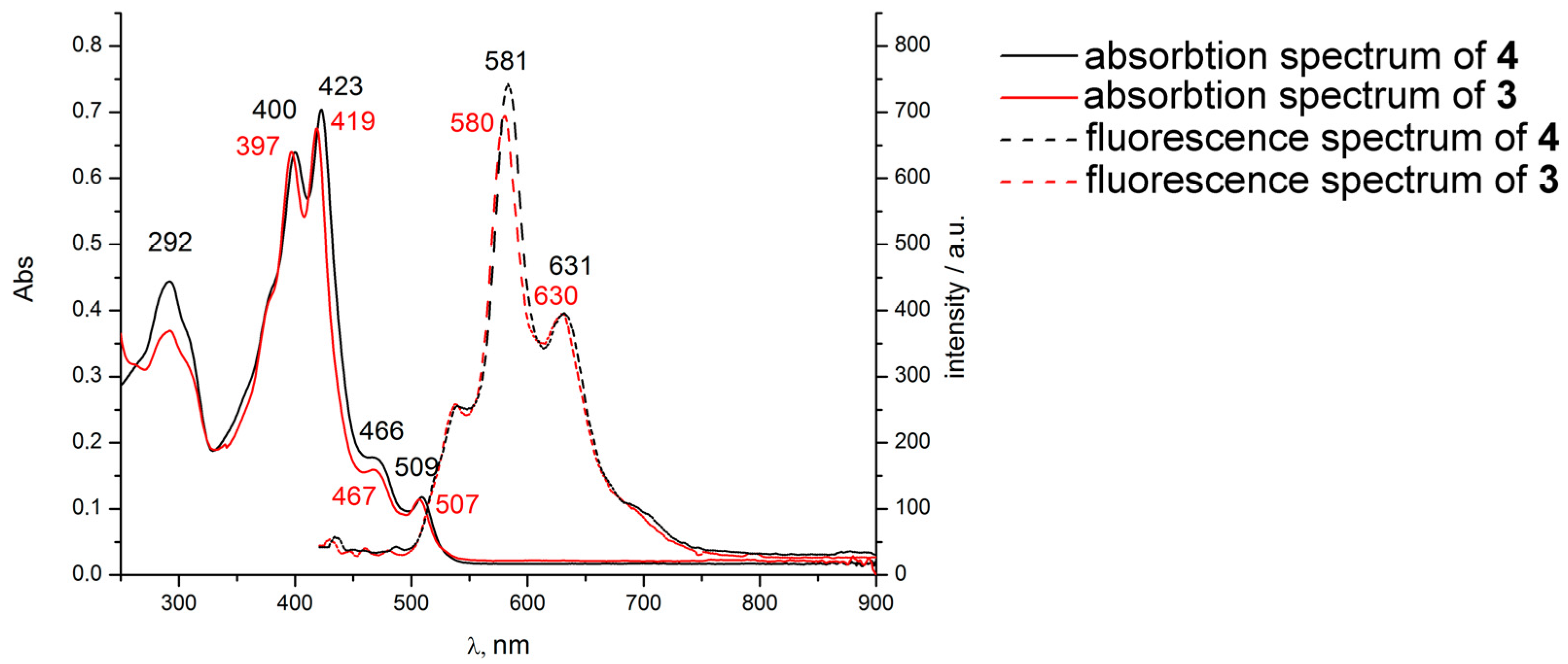
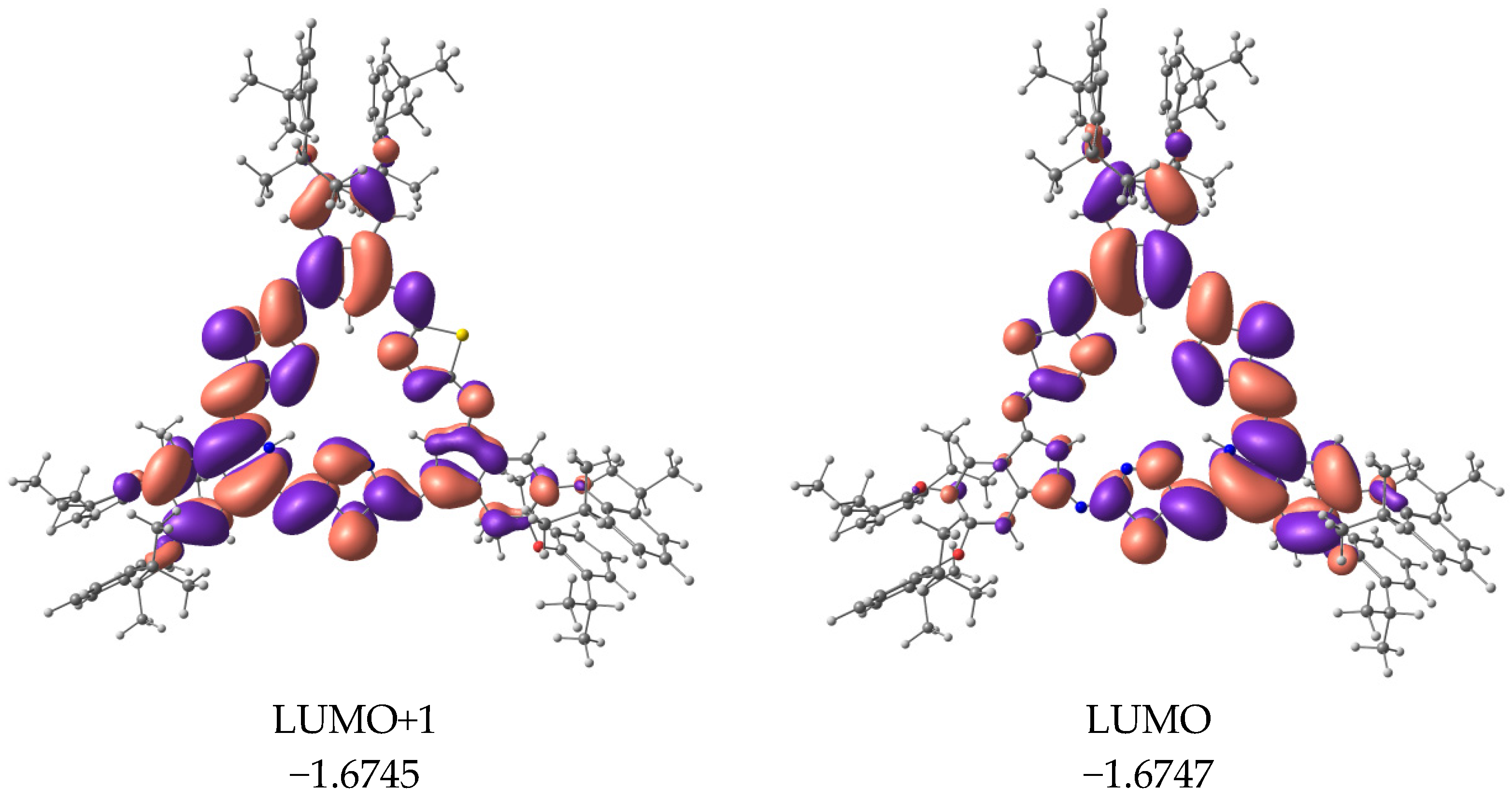
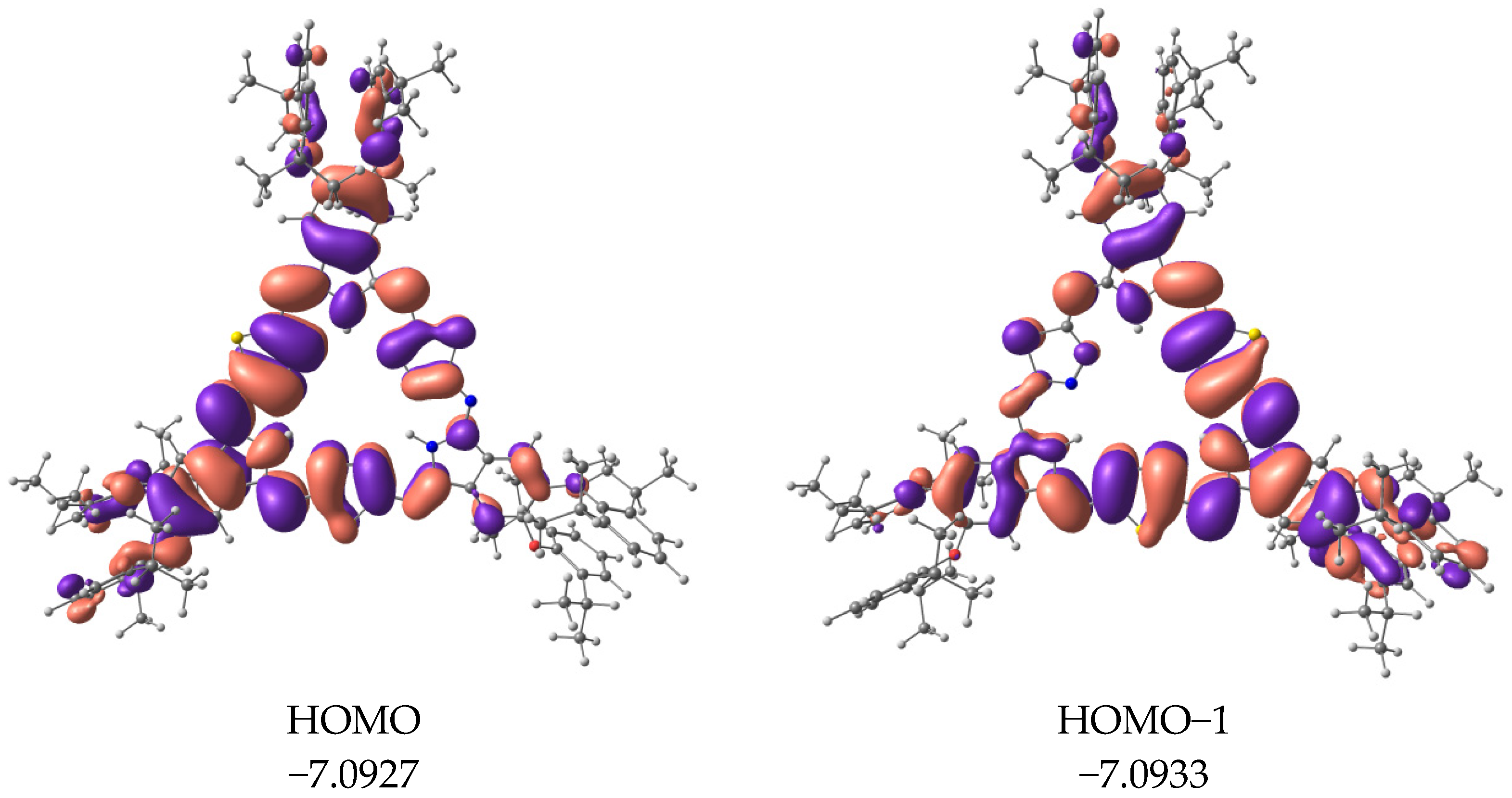
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis
- 4-Chloro-5-(2,6-diisopropylphenoxy) phthalonitrile (1) [37]. Anhydrous potassium carbonate (2.4 g, 15.5 mmol) was added to a solution of 2,6-diisopropylphenol (0.9 g, 5 mmol) and 4,5-dichlorophthalonitrile (0.98 g, 5 mmol) in dry DMF (75 mL). The reaction mixture was heated in an argon atmosphere at 45 °C for 24 h. It was then cooled down and poured into water and the precipitate was filtered off and washed with water. After drying, the crude product was purified by column chromatography using a mixture of heptane/ethyl acetate (5:1) as an eluent. Yield: 64% (1.09 g); mp 181 °C. 1H-NMR (300 MHz, CDCl3): δ (ppm) = 7.83 (s, H), 7.32–7.21 (m, 3 H), 6.68 (s, H), 2.68 (sept, J = 6.9 Hz, 2H), 1.12 (d, J = 6.9 Hz, 12H).
- 4,5-Bis(2,6-diisopropylphenoxy) phthalonitrile (2) [38]. Anhydrous potassium carbonate (2.4 g, 15.5 mmol) was added to a solution of 2,6-diisopropylphenol (1.8 g, 10 mmol) and 4,5-dichlorophthalonitrile (0.5 g, 2.5 mmol) in dry DMF (40 mL). The reaction mixture was heated at 80 °C in an argon atmosphere for 48 h. It was then cooled down and poured into water and the precipitate was filtered off and washed with water. After drying, it was purified by column chromatography using a mixture of heptane/ethyl acetate (5:1) as an eluent. Yield: 34% (0.41 g); mp 179-180 °C. 1H-NMR (300 MHz, CDCl3): δ (ppm) = 7.31 − 7.19 (m, 6H), 6.68 (s, 2H), 2.88 (sept, J = 6.9 Hz, 4H), 1.15 (d, J = 6.9 Hz, 24H).
- 2,14,26-Trichloro-3,15,27-tri[2′,6′-diisopropylphenoxy]-5,36:12,17: 24,29-triimino-7,10: 19,22: 31,34–tritio-[f,p,z]–tribenzo-1,2,4,9,11,12,14,19,21,22,24,29-dodecazacyclotriaconta-2,4,6,8,10,12,14,16,18,20,22,24,26,28,30-pentadecaene (3)
- 2,3,14,15,26,27-Hexa[2′,6′-diisopropylphenoxy]-5,36:12,17:24,29-triimino-7,10:19,22:31,34-trithio-[f,p,z]-tribenzo-1,2,4,9,11,12,14,19,21,22,24,29-dodecazacyclotriaconta-2,4,6,8,10,12,14,16,18,20,22,24,26,28,30-pentadecaene (4)
3.3. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hardin, B.E.; Yum, J.-H.; Hoke, E.T.; Jun, Y.C.; Péchy, P.; Torres, T.; Brongersma, M.L.; Nazeeruddin, M.K.; Grätzel, M.; McGehee, M.D. High excitation transfer efficiency from energy relay dyes in dye-sensitized solar cells. Nano Lett. 2010, 10, 3077–3083. [Google Scholar] [CrossRef]
- Morandeira, A.; López-Duarte, I.; O’Regan, B.; Martínez-Díaz, M.V.; Forneli, A.; Palomares, E.; Torres, T.; Durrant, J.R. Ru(II)-phthalocyanine sensitized solar cells: The influence of co-adsorbents upon interfacial electron transfer kinetics. J. Mater. Chem. 2009, 19, 5016. [Google Scholar] [CrossRef]
- Cho, K.T.; Trukhina, O.; Roldán-Carmona, C.; Ince, M.; Gratia, P.; Grancini, G.; Gao, P.; Marszalek, T.; Pisula, W.; Reddy, P.Y.; et al. Molecularly Engineered Phthalocyanines as Hole-Transporting Materials in Perovskite Solar Cells Reaching Power Conversion Efficiency of 17.5%. Adv. Energy Mater. 2017, 7, 1601733. [Google Scholar] [CrossRef]
- Bottari, G.; de La Torre, G.; Guldi, D.M.; Torres, T. An exciting twenty-year journey exploring porphyrinoid-based photo- and electro-active systems. Coord. Chem. Rev. 2021, 428, 213605. [Google Scholar] [CrossRef]
- Koifman, O.I.; Ageeva, T.A.; Beletskaya, I.P.; Averin, A.D.; Yakushev, A.A.; Tomilova, L.G.; Dubinina, T.V.; Tsivadze, A.Y.; Gorbunova, Y.G.; Martynov, A.G.; et al. Macroheterocyclic Compounds—A Key Building Block in New Functional Materials and Molecular Devices. Macroheterocycles 2020, 13, 311–467. [Google Scholar] [CrossRef]
- Pinzón, J.R.; Gasca, D.C.; Sankaranarayanan, S.G.; Bottari, G.; Torres, T.; Guldi, D.M.; Echegoyen, L. Photoinduced charge transfer and electrochemical properties of triphenylamine I(h)-Sc3N@C80 donor-acceptor conjugates. J. Am. Chem. Soc. 2009, 131, 7727–7734. [Google Scholar] [CrossRef]
- Rodríguez-Morgade, M.S.; Plonska-Brzezinska, M.E.; Athans, A.J.; Carbonell, E.; de Miguel, G.; Guldi, D.M.; Echegoyen, L.; Torres, T. Synthesis, characterization, and photoinduced electron transfer processes of orthogonal ruthenium phthalocyanine-fullerene assemblies. J. Am. Chem. Soc. 2009, 131, 10484–10496. [Google Scholar] [CrossRef] [PubMed]
- Lavarda, G.; Zirzlmeier, J.; Gruber, M.; Rami, P.R.; Tykwinski, R.R.; Torres, T.; Guldi, D.M. Tuning Intramolecular Förster Resonance Energy Transfer and Activating Intramolecular Singlet Fission. Angew. Chem. Int. Ed. 2018, 57, 16291–16295. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; González-Rodríguez, D.; Torres, T.; Martín, G.; Agulló-López, F.; Ledoux, I.; Zyss, J.; Ferro, V.R.; La García de Vega, J.M. Structural modulation of the dipolar-octupolar contributions to the NLO response in subphthalocyanines. J. Phys. Chem. B 2005, 109, 3800–3806. [Google Scholar] [CrossRef]
- García-Iglesias, M.; Cid, J.-J.; Yum, J.-H.; Forneli, A.; Vázquez, P.; Nazeeruddin, M.K.; Palomares, E.; Grätzel, M.; Torres, T. Increasing the efficiency of zinc-phthalocyanine based solar cells through modification of the anchoring ligand. Energy Environ. Sci. 2011, 4, 189–194. [Google Scholar] [CrossRef]
- Duan, C.; Zango, G.; García Iglesias, M.; Colberts, F.J.M.; Wienk, M.M.; Martínez-Díaz, M.V.; Janssen, R.A.J.; Torres, T. The Role of the Axial Substituent in Subphthalocyanine Acceptors for Bulk-Heterojunction Solar Cells. Angew. Chem. Int. Ed. 2017, 56, 148–152. [Google Scholar] [CrossRef]
- Bottari, G.; de La Torre, G.; Torres, T. Phthalocyanine-nanocarbon ensembles: From discrete molecular and supramolecular systems to hybrid nanomaterials. Acc. Chem. Res. 2015, 48, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Verreet, B.; Rand, B.P.; Cheyns, D.; Hadipour, A.; Aernouts, T.; Heremans, P.; Medina, A.; Claessens, C.G.; Torres, T. A 4% Efficient Organic Solar Cell Using a Fluorinated Fused Subphthalocyanine Dimer as an Electron Acceptor. Adv. Energy Mater. 2011, 1, 565–568. [Google Scholar] [CrossRef]
- Almeida-Marrero, V.; González-Delgado, J.A.; Torres, T. Emerging Perspectives on Applications of Porphyrinoids for Photodynamic Therapy and Photoinactivation of Microorganisms. Macroheterocycles 2019, 12, 8–16. [Google Scholar] [CrossRef]
- Sessler, J.L.; Jayawickramarajah, J.; Gouloumis, A.; Dan Pantos, G.; Torres, T.; Guldi, D.M. Guanosine and fullerene derived de-aggregation of a new phthalocyanine-linked cytidine derivative. Tetrahedron 2006, 62, 2123–2131. [Google Scholar] [CrossRef]
- Almeida-Marrero, V.; van de Winckel, E.; Anaya-Plaza, E.; Torres, T.; de La Escosura, A. Porphyrinoid biohybrid materials as an emerging toolbox for biomedical light management. Chem. Soc. Rev. 2018, 47, 7369–7400. [Google Scholar] [CrossRef]
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Sessler, J.L.; Seidel, D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Ed. 2003, 42, 5134–5175. [Google Scholar] [CrossRef]
- Zakharov, A.V.; Shlykov, S.A.; Bumbina, N.V.; Danilova, E.A.; Krasnov, A.V.; Islyaikin, M.K.; Girichev, G.V. The structure of a thiadiazole-containing expanded heteroazaporphyrinoid determined by gas electron diffraction and density functional theory calculations. Chem. Commun. 2008, 30, 3573–3575. [Google Scholar] [CrossRef]
- Zakharov, A.V.; Shlykov, S.A.; Danilova, E.A.; Krasnov, A.V.; Islyaikin, M.K.; Girichev, G.V. Thiadiazole-containing expanded heteroazaporphyrinoids: A gas-phase electron diffraction and computational structural study. Phys. Chem. Chem. Phys. 2009, 11, 8570–8579. [Google Scholar] [CrossRef]
- Zhabanov, Y.A.; Zakharov, A.V.; Shlykov, S.A.; Trukhina, O.N.; Danilova, E.A.; Koifman, O.I.; Islyaikin, M.K. Molecular structure and tautomers of [30]trithia-2,3,5,10,12,13,15,20,22,23,25,30-dodecaazahexaphyrin. J. Porphyr. Phthalocyanines 2013, 17, 220–228. [Google Scholar] [CrossRef]
- Trukhina, O.N.; Rodríguez-Morgade, M.S.; Wolfrum, S.; Caballero, E.; Snejko, N.; Danilova, E.A.; Gutiérrez-Puebla, E.; Islyaikin, M.K.; Guldi, D.M.; Torres, T. Scrutinizing the Chemical Nature and Photophysics of an Expanded Hemiporphyrazine: The Special Case of [30]Trithia-2,3,5,10,12,13,15,20,22,23,25,30-dodecaazahexaphyrin. J. Am. Chem. Soc. 2010, 132, 12991–12999. [Google Scholar] [CrossRef] [PubMed]
- Trukhina, O.N.; Zhabanov, Y.A.; Krasnov, A.V.; Danilova, E.A.; Islyaikin, M.K. Synthesis and thermal behavior of unsubstituted [30]trithia-2,3,5,10,12,13,15,20,22,23,25,30- dodecaazahexaphyrin. J. Porphyr. Phthalocyanines 2011, 15, 1287–1291. [Google Scholar] [CrossRef]
- Cirera, B.; Trukhina, O.; Björk, J.; Bottari, G.; Rodríguez-Fernández, J.; Martin-Jimenez, A.; Islyaikin, M.K.; Otero, R.; Gallego, J.M.; Miranda, R.; et al. Long-Range Orientational Self-Assembly, Spatially Controlled Deprotonation, and Off-Centered Metalation of an Expanded Porphyrin. J. Am. Chem. Soc. 2017, 139, 14129–14136. [Google Scholar] [CrossRef]
- Ivanov, E.N.; Trukhina, O.N.; Koifman, O.I.; Islyaikin, M.K. Homotrinuclear Complexes of [30]Trithiadodecaazahexaphyrine with Transition Metals: Ni(II), Cu(II) and Mn(II). Macroheterocycles 2016, 9, 225–229. [Google Scholar] [CrossRef]
- Kobayashi, N.; Inagaki, S.; Nemykin, V.N.; Nonomura, T. A Novel Hemiporphyrazine Comprising Three Isoindolediimine and Three Thiadiazole Units. Angew. Chem. Int. Ed. 2001, 40, 2710–2712. [Google Scholar] [CrossRef]
- Islyaikin, M.K.; Danilova, E.A.; Yagodarova, L.D.; Rodríguez-Morgade, M.S.; Torres, T. Thiadiazole-derived expanded heteroazaporphyrinoids. Org. Lett. 2001, 3, 2153–2156. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Islyaikin, M.K.; Otsuka, A.; Yamochi, H.; Kitagawa, H.; Lyubovskaya, R.N.; Ivanov, E.N.; Koifman, O.I.; Zhabanov, Y.A. Double-Decker Paramagnetic {(K)(H3Hhp)2}2− Radical Dianions Comprising Two 30Trithia-2,3,5,10,12,13,15,20,22,23,25,30-Dodecaazahexaphyrins and a Potassium Ion. Chem. Asian J. 2020, 15, 61–65. [Google Scholar] [CrossRef]
- Nazarov, D.I.; Khasanov, S.S.; Rompanen, I.A.; Ivanov, E.N.; Koifman, O.I.; Islyaikin, M.K.; Konarev, D.V. Preparation of double decker {Li3(Cl−)(Hhp)}22− dianions and H2TPCor− monoanions by deprotonation of free-base trithiododecaazahexaphyrin (H3Hhp) and triphenylcorrole (H3TPCor) macrocycles. Polyhedron 2021, 202, 115198. [Google Scholar] [CrossRef]
- Islyaikin, M.K.; Ivanov, E.N.; Koifman, O.I.; Konarev, D.V. New expanded porphyrinoids: Synthesis, structure and properties of hemihexaphyrazines and their reduced metal containing derivatives. J. Porphyr. Phthalocyanines 2023, 27, 55–67. [Google Scholar] [CrossRef]
- Nazarov, D.I.; Islyaikin, M.K.; Ivanov, E.N.; Koifman, O.I.; Batov, M.S.; Zorina, L.V.; Khasanov, S.S.; Shestakov, A.F.; Yudanova, E.I.; Zhabanov, Y.A.; et al. Dianionic States of Trithiadodecaazahexaphyrin Complexes with Homotrinuclear MII3O Clusters (M = Ni and Cu): Crystal Structures, Metal- Or Macrocycle-Centered Reduction, and Doublet-Quartet Transitions in the Dianions. Inorg. Chem. 2021, 60, 9857–9868. [Google Scholar] [CrossRef]
- Filatov, M.S.; Trukhina, O.N.; Rodríguez-Morgade, M.S.; Islyaikin, M.K.; Koifman, O.I.; Torres, T. Synthesis and spectroscopic properties of chiral bornane[2,3-b]pyrazino-fused [30]trithiadodecaazahexaphyrins. J. Porphyr. Phthalocyanines 2014, 18, 1014–1020. [Google Scholar] [CrossRef]
- Filatov, M.S.; Trukhina, O.N.; Islyaikin, M.K. Synthesis and Properties of Hexa(4-tert-butylphenoxy) Substituted [30]Trithiadodecaazahexaphyrin and its Metal Complexes. Macroheterocycles 2014, 7, 281–286. [Google Scholar] [CrossRef][Green Version]
- Kibireva, Y.E.; Islyaikin, M.K.; Rodríguez-Morgade, M.S.; Torres, T. Synthesis and Characterization of a Soluble Hemihexaphyrazine Derivative. Macroheterocycles 2023, 16, 19–23. [Google Scholar] [CrossRef]
- Toriumi, N.; Muranaka, A.; Hirano, K.; Yoshida, K.; Hashizume, D.; Uchiyama, M. 18π-Electron tautomeric benziphthalocyanine: A functional near-infrared dye with tunable aromaticity. Angew. Chem. Int. Ed. 2014, 53, 7814–7818. [Google Scholar] [CrossRef]
- Muranaka, A.; Ohira, S.; Hashizume, D.; Koshino, H.; Kyotani, F.; Hirayama, M.; Uchiyama, M. 18/20π hemiporphyrazine: A redox-switchable near-infrared dye. J. Am. Chem. Soc. 2012, 134, 190–193. [Google Scholar] [CrossRef]
- Tuhl, A.; Chidawanayika, W.; Ibrahim, H.M.; Al-Awadi, N.; Litwinski, C.; Nyokong, T.; Behbehani, H.; Manaa, H.; Makhseed, S. Tetra and octa(2,6-di- iso-propylphenoxy)-substituted phthalocyanines: A comparative study among their photophysicochemical properties. J. Porphyr. Phthalocyanines 2012, 16, 163–174. [Google Scholar] [CrossRef]
- Makhseed, S.; Samuel, J. The synthesis and characterization of zincphthalocyanines bearing functionalized bulky phenoxy substituents. Dyes Pigm. 2009, 82, 1–5. [Google Scholar] [CrossRef]
- McKeown, N.B.; Makhseed, S.; Msayib, K.J.; Ooi, L.-L.; Helliwell, M.; Warren, J.E. A Phthalocyanine Clathrate of Cubic Symmetry Containing Interconnected Solvent-Filled Voids of Nanometer Dimensions. Angew. Chem. Int. Ed. 2005, 44, 7546–7549. [Google Scholar] [CrossRef]
- Burt, L.A.; Bezzu, C.G.; McMonagle, C.J.; Moggach, S.A.; McKeown, N.B. A hindered subphthalocyanine that forms crystals withincluded aromatic solvent but will not play ball with C60. J. Porphyr. Phthalocyanines 2016, 20, 1034–1040. [Google Scholar] [CrossRef]
- Danilova, E.A.; Melenchuk, T.V.; Trukhina, O.N.; Islyaikin, M.K. Diaminothiadiazoles—Building Blocks for Macroheterocycles. Macroheterocycles 2010, 3, 68–81. [Google Scholar] [CrossRef]
- Islyaikin, M.K.; Koifman, O.I.; Torres, T. Hemihexaphyrazines. Synthesis and perspectives of their applications as new functional materials. In Functional Materials on the Base of the Tetrapyrrol Macroheterocyclic Compounds; Koifman, O.I., Ageeva, T.A., Bazanov, M.I., Berezin, D.B., Berezina, N.M., Borisov, A.V., Eds.; Lenand: Moscow, Russia, 2019; pp. 270–301. ISBN 978-5-9710-6952-2. (In Russian) [Google Scholar]
- Fery-Forgues, S.; Lavabre, D. Are Fluorescence Quantum Yields So Tricky to Measure? A Demonstration Using Familiar Stationery Products. J. Chem. Educ. 1999, 76, 1260. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Pellegrinet, S.C. A Multi-standard Approach for GIAO 13C NMR Calculations. J. Org. Chem. 2009, 74, 7254–7260. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]

 | ||
|---|---|---|
| Centers | NICS, ppm | |
| 4 | H3Hhp | |
| A | 1.52 | 1.48 |
| B | −2.08–−2.10 | −2.06 |
| C | 1.15 | 1.25 |
| D | −9.73 | −8.72 |
| E | −8.81 | −8.81 |
| F | −10.55 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, E.N.; Almeida-Marrero, V.; Koifman, O.I.; Aleksandriiskii, V.V.; Torres, T.; Islyaikin, M.K. Synthesis and Characterization of Bulky Substituted Hemihexaphyrazines Bearing 2,6-Diisopropylphenoxy Groups. Molecules 2023, 28, 5740. https://doi.org/10.3390/molecules28155740
Ivanov EN, Almeida-Marrero V, Koifman OI, Aleksandriiskii VV, Torres T, Islyaikin MK. Synthesis and Characterization of Bulky Substituted Hemihexaphyrazines Bearing 2,6-Diisopropylphenoxy Groups. Molecules. 2023; 28(15):5740. https://doi.org/10.3390/molecules28155740
Chicago/Turabian StyleIvanov, Evgenii N., Verónica Almeida-Marrero, Oskar I. Koifman, Viktor V. Aleksandriiskii, Tomas Torres, and Mikhail K. Islyaikin. 2023. "Synthesis and Characterization of Bulky Substituted Hemihexaphyrazines Bearing 2,6-Diisopropylphenoxy Groups" Molecules 28, no. 15: 5740. https://doi.org/10.3390/molecules28155740
APA StyleIvanov, E. N., Almeida-Marrero, V., Koifman, O. I., Aleksandriiskii, V. V., Torres, T., & Islyaikin, M. K. (2023). Synthesis and Characterization of Bulky Substituted Hemihexaphyrazines Bearing 2,6-Diisopropylphenoxy Groups. Molecules, 28(15), 5740. https://doi.org/10.3390/molecules28155740






