DNA-Interactive and Damage Study with meso-Tetra(2-thienyl)porphyrins Coordinated with Polypyridyl Pd(II) and Pt(II) Complexes
Abstract
1. Introduction
2. Results
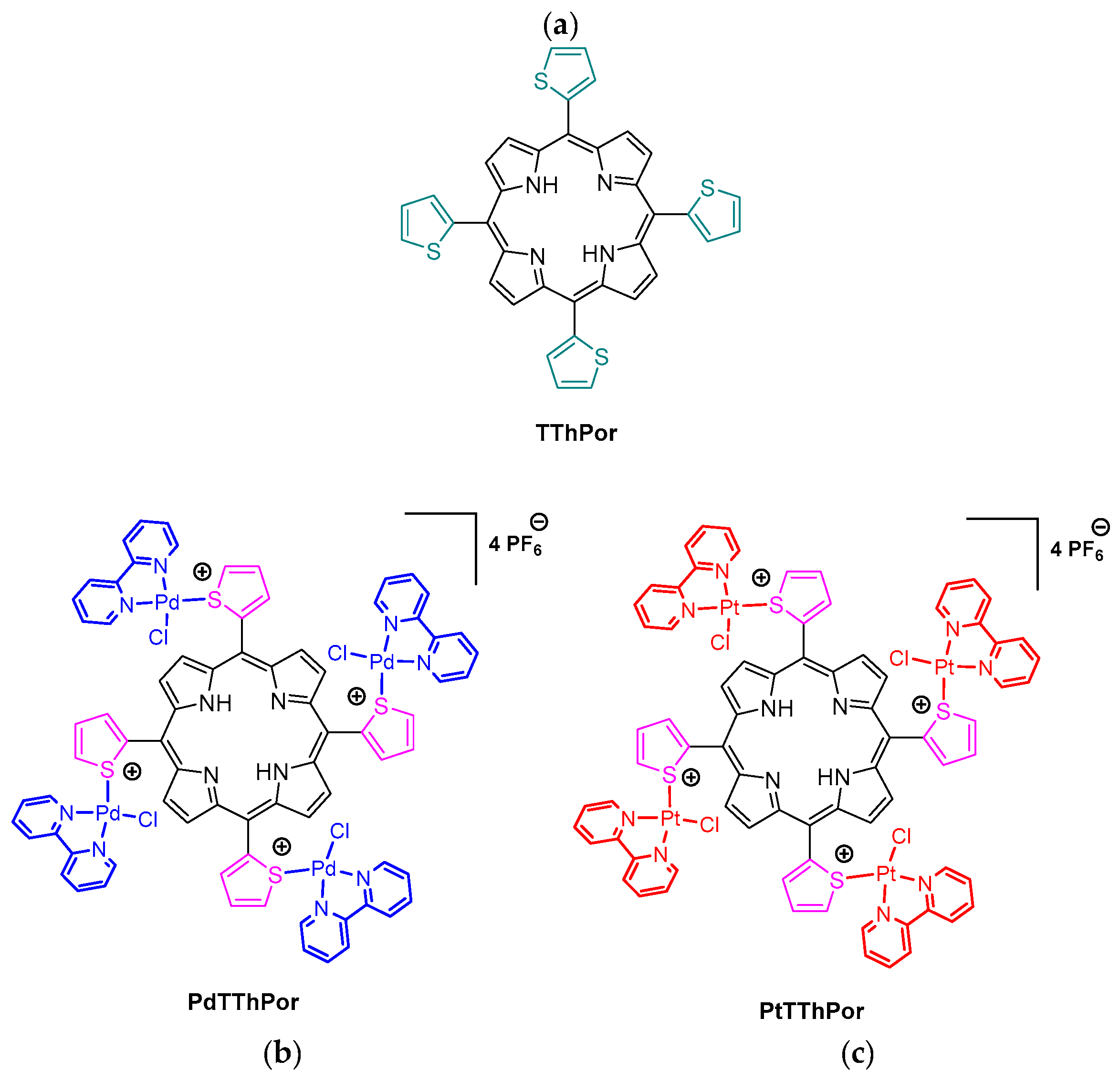
2.1. Thienyl-Porphyrins
2.2. DNA-Binding Assays
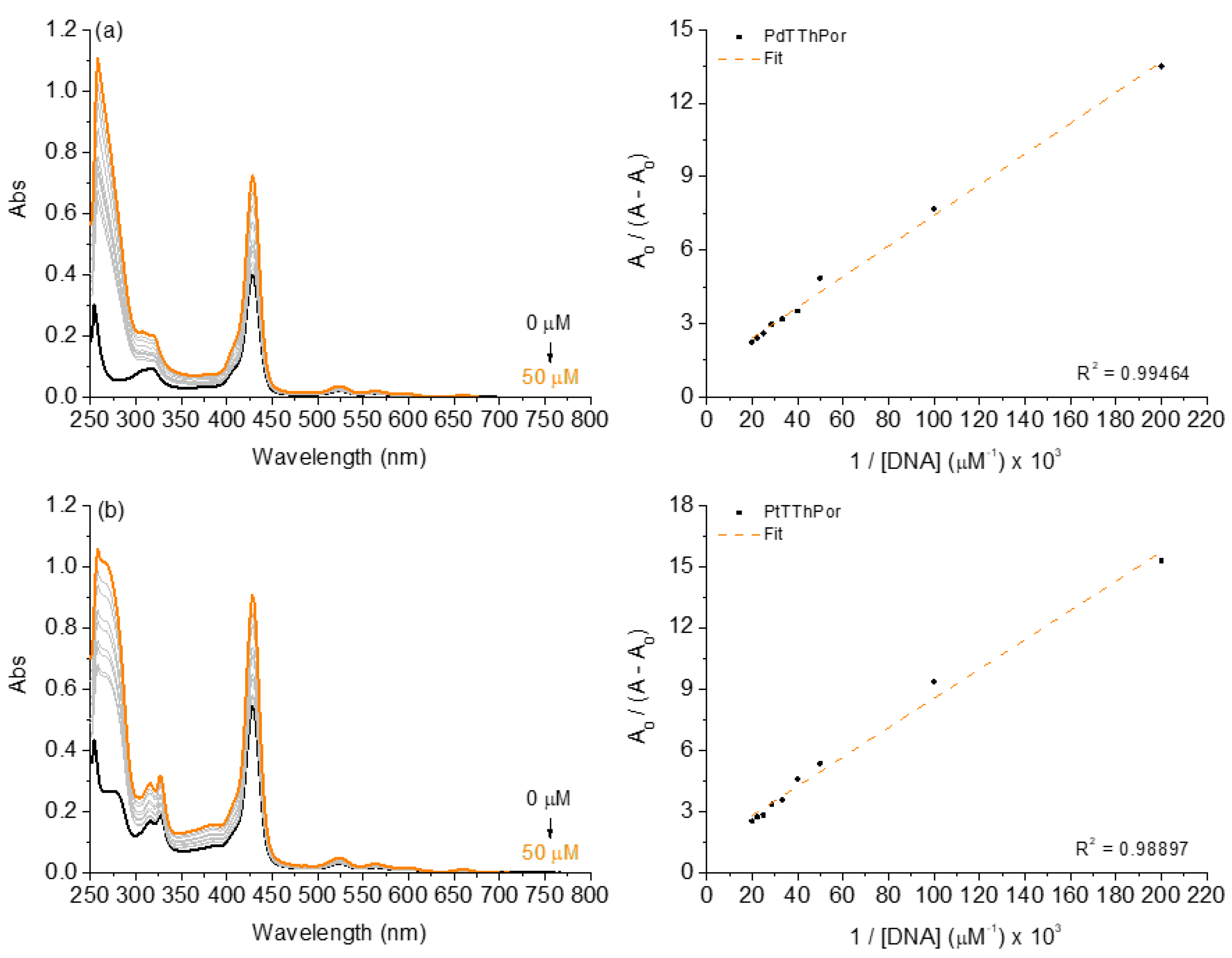
2.2.1. Binding Properties of DNA by UV-Vis Analysis
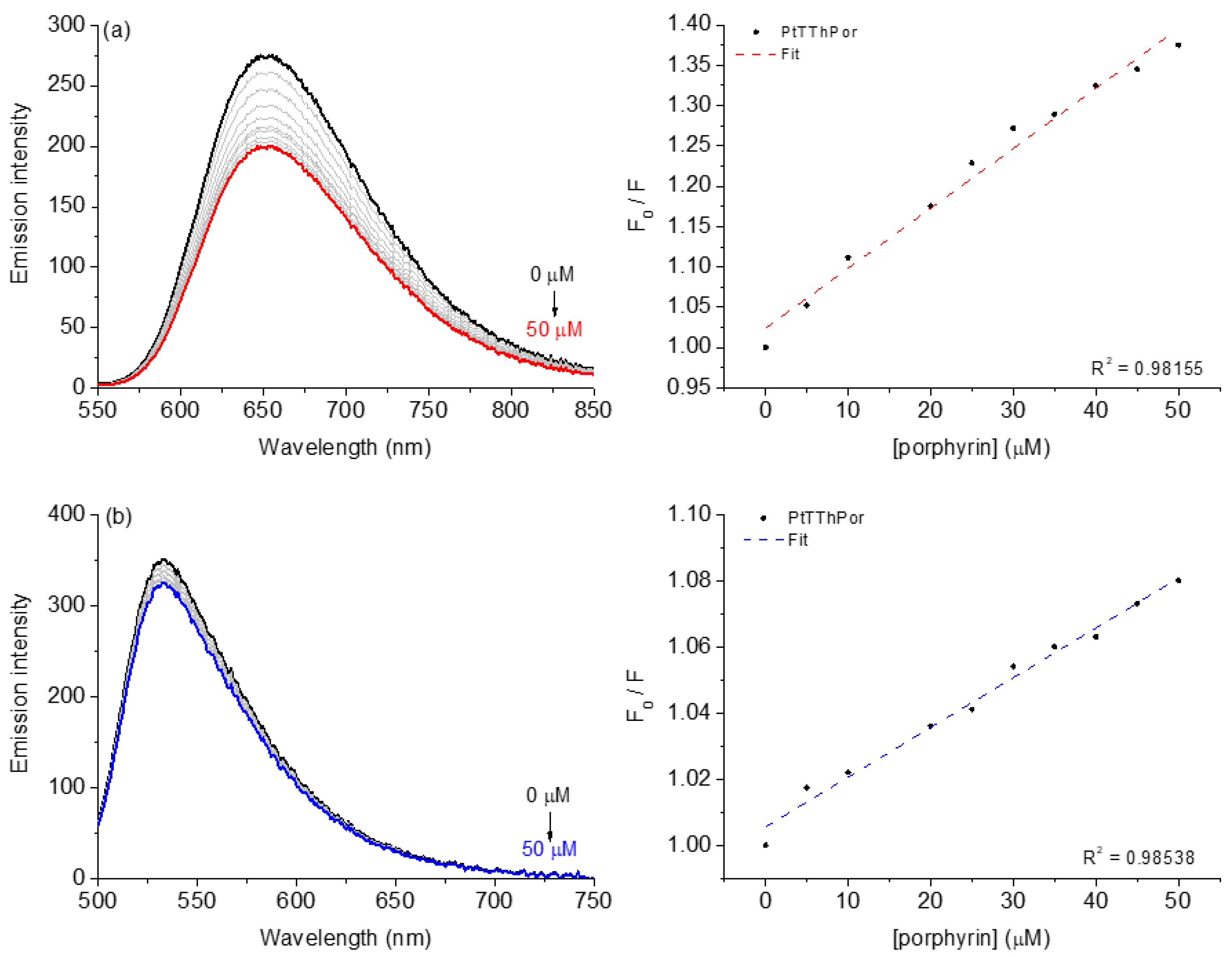
2.2.2. Competitive Binding Assays with DNA by Steady-State Fluorescence Emission
| UV-Vis Analysis | |||||
| Porphyrin | H (%) a | Δλ (nm) b | Kb (×104; M−1) c | ΔG° (kcal mol −1) d | |
| TThPor | 63.0 | 0.0 | 0.92 ± 0.09 | −5.40 | |
| PdTThPor | 44.5 | 0.0 | 1.59 ± 0.13 | −5.75 | |
| PtTThPor | 40.0 | 0.0 | 1.39 ± 0.21 | −5.65 | |
| Steady-State Fluorescence Emission Analysis | |||||
| EB:DNA | |||||
| Q (%) e | KSV (×103; M−1) f | kq (×1011; M−1s−1) g | Kb (×103; M−1) h | ΔG° (kcal mol−1) d | |
| TThPor | 20.0 | 4.86 ± 0.04 | 2.11 ± 0.08 | 7.44 ± 0.47 | −5.30 |
| PdTThPor | 27.0 | 6.14 ± 0.08 | 2.67 ± 0.15 | 14.3 ± 0.23 | −5.65 |
| PtTThPor | 28.0 | 7.43 ± 0.01 | 3.23 ± 0.02 | 11.1 ± 0.18 | −5.50 |
| AO:DNA | |||||
| Q (%) e | KSV (×103; M−1) f | kq (×1012; M−1s−1) i | Kb (×103; M−1) h | ΔG° (kcal mol−1) d | |
| TThPor | 23.0 | 5.27 ± 0.01 | 3.10 ± 0.02 | 5.35 ± 0.55 | −5.10 |
| PdTThPor | 8.0 | 1.72 ± 0.01 | 1.01 ± 0.02 | 2.01 ± 0.83 | −4.50 |
| PtTThPor | 7.5 | 1.50 ± 0.02 | 0.88 ± 0.04 | 1.63 ± 0.79 | −4.40 |
| DAPI:DNA | |||||
| Q (%) e | KSV (×104; M−1) f | kq (×1012; M−1s−1) j | Kb (×104; M−1) h | ΔG° (kcal mol−1) d | |
| TThPor | 54.0 | 2.31 ± 0.05 | 10.5 ± 0.10 | 1.42 ± 0.13 | −5.65 |
| PdTThPor | 36.0 | 8.98 ± 0.02 | 40.8 ± 0.04 | 0.96 ± 0.37 | −5.45 |
| PtTThPor | 54.0 | 2.29 ± 0.03 | 10.4 ± 0.06 | 2.36 ± 0.14 | −5.95 |
| MG:DNA | |||||
| Q (%) e | KSV (×104; M−1) f | kq (×1012; M−1s−1) k | Kb (×104; M−1) h | ΔG° (kcal mol−1) d | |
| TThPor | 38.5 | 1.25 ± 0.02 | 4.46 ± 0.04 | 3.58 ± 0.28 | −6.20 |
| PdTThPor | 63.0 | 3.50 ± 0.02 | 12.5 ± 0.04 | 3.74 ± 0.03 | −6.25 |
| PtTThPor | 62.0 | 3.42 ± 0.04 | 12.2 ± 0.08 | 2.77 ± 0.17 | −6.05 |
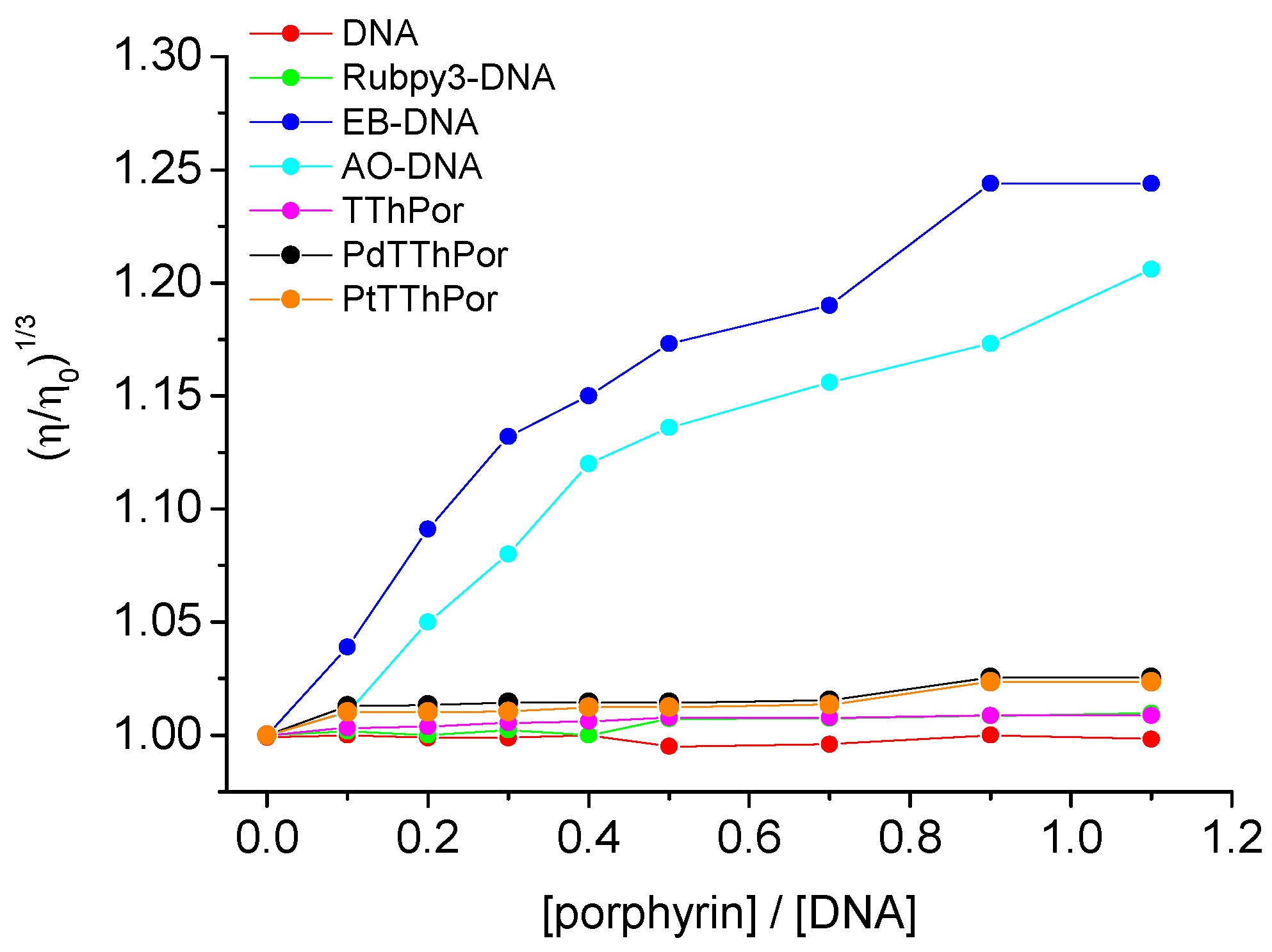
2.2.3. Viscosity Measurements with DNA and Porphyrins
2.2.4. Circular Dichroism (CD) Analysis with DNA
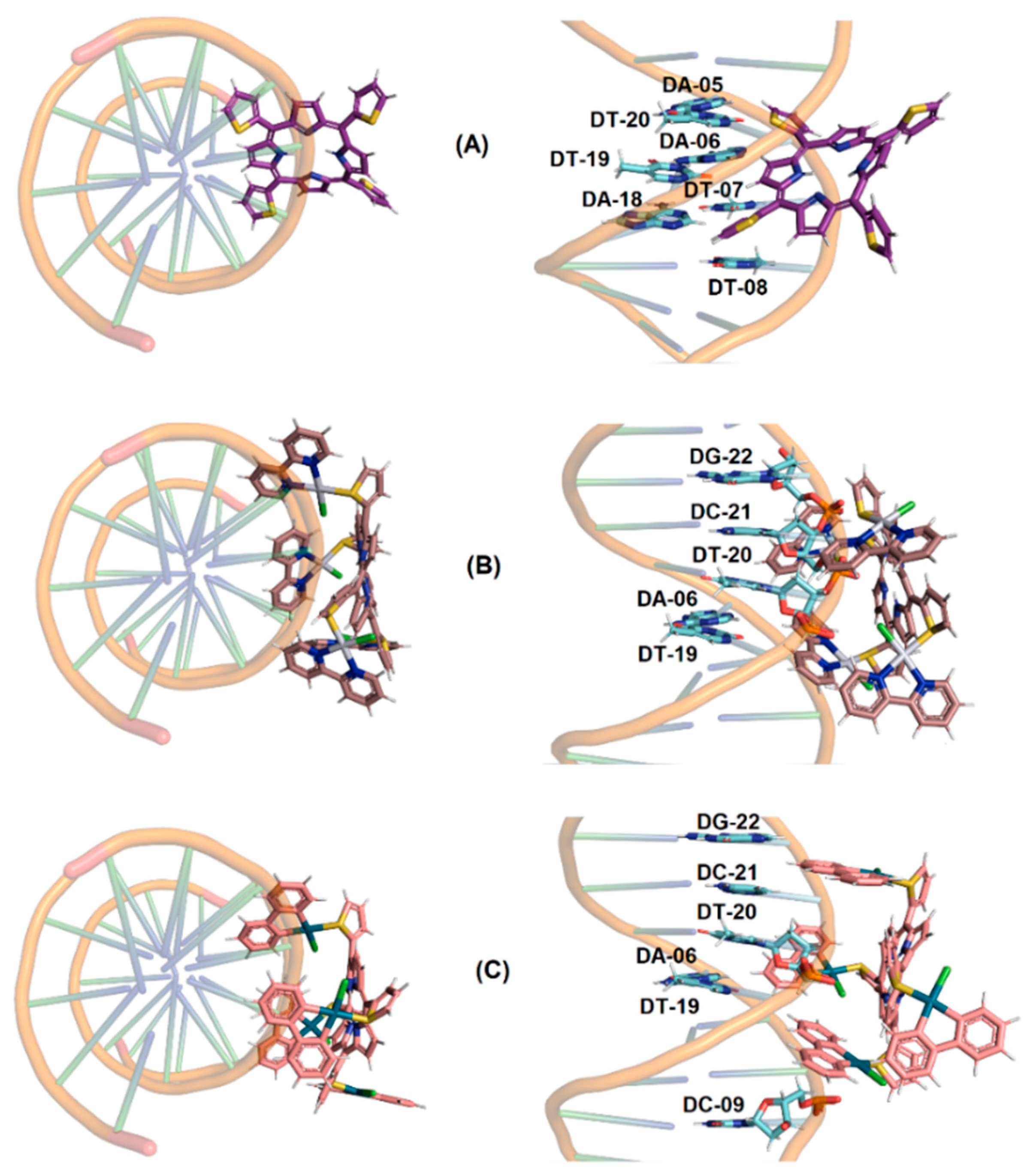
2.2.5. Molecular Docking Analysis with DNA
2.3. DNA Photo-Oxidation and Damage
2.3.1. DNA Photo-Oxidation by Absorption Analysis
2.3.2. DNA Oxidative Damage by Electrophoresis
3. Materials and Methods
3.1. General
3.2. Photobiological Parameters of Porphyrins
3.3. DNA Interactive Studies
3.4. Molecular Docking Procedure with DNA
3.5. DNA Photo-Oxidation by UV-Vis Analysis
3.6. Electrophoresis and Detection of DNA Oxidative Damage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Urbani, M.; Gra, M.; Nazeeruddin, M.K. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Baglia, R.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic Reactivity of Oxygen-Derived Manganese and Iron Porphyrinoid Complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lindstrom, A.; Li, Y. Porphyrin-Based Nanomedicines for Cancer Treatment. Bioconjugate Chem. 2019, 30, 1585–1603. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Ravikumar, M.; Raghav, D.; Rathinasamy, K.; Kathiravan, A.; Mothi, E.M. DNA Targeting Long-Chain Alkoxy Appended Tin(IV) Porphyrin Scaffolds: Photophysical and Antimicrobial PDT Investigations. ACS Appl. Bio Mater. 2018, 1, 1705–1716. [Google Scholar] [CrossRef]
- Pandey, V.; Raza, M.K.; Joshi, P.; Gupta, I. Synthesis of Water-Soluble Thioglycosylated Trans-A2B2Type Porphyrins: Cellular Uptake Studies and Photodynamic Efficiency. J. Org. Chem. 2020, 85, 6309–6322. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef]
- Betoni Momo, P.; Pavani, C.; Baptista, M.S.; Brocksom, T.J.; Thiago De Oliveira, K. Chemical Transformations and Photophysical Properties of meso-Tetrathienyl-Substituted Porphyrin Derivatives. Eur. J. Org. Chem. 2014, 2014, 4536–4547. [Google Scholar] [CrossRef]
- Bansal, A.; Kaushik, S.; Kukreti, S. Non-Canonical DNA Structures: Diversity and Disease Association. Front. Genet. 2022, 13, 959258. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. Int. J. Mol. Sci. 2019, 20, 4258. [Google Scholar] [CrossRef]
- Kloster, M.; Kostrhunova, H.; Zaludova, R.; Malina, J.; Kasparkova, J.; Brabec, V.; Farrell, N. Trifunctional Dinuclear Platinum Complexes as DNA-Protein Cross-Linking Agents. Biochemistry 2004, 43, 7776–7786. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Sullivan, M.P.; Tong, K.K.H.; Goldstone, D.C.; Hanif, M.; Jamieson, S.M.F.; Hartinger, C.G. Mustards-Derived Terpyridine-Platinum Complexes as Anticancer Agents: DNA Alkylation vs Coordination. Inorg. Chem. 2021, 60, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Zhu, M.; Liu, L.; Huang, Y.; Wang, L.; Shi, C.; Zhang, W.; Sun, Y. Impact of the Carbon Chain Length of Novel Palladium(Ll) Complexes on Interaction with DNA and Cytotoxic Activity. Inorg. Chem. 2010, 49, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Lorenzo, J.; Sanglas, L.; Cutillas, N.; Vicente, C.; Villa, M.D.; Avilés, F.X.; López, G.; Moreno, V.; Pérez, J.; et al. Palladium(II) and Platinum(II) Organometallic Complexes with the Model Nucleobase Anions of Thymine, Uracil, and Cytosine: Antitumor Activity and Interactions with DNA of the Platinum Compounds. Inorg. Chem. 2006, 45, 6347–6360. [Google Scholar] [CrossRef] [PubMed]
- Ruhayel, R.A.; Langner, J.S.; Oke, M.J.; Berners-Price, S.J.; Zgani, I.; Farrell, N.P. Chimeric Platinum-Polyamines and DNA Binding. Kinetics of DNA Interstrand Cross-Link Formation by Dinuclear Platinum Complexes with Polyamine Linkers. J. Am. Chem. Soc. 2012, 134, 7135–7146. [Google Scholar] [CrossRef]
- Micklitz, W.; Sheldrick, W.S.; Lippert, B. Mono- and Dinuclear Palladium (I1) Complexes of Uracil and Thymine Model A. Inorg. Chem. 1990, 29, 211–216. [Google Scholar] [CrossRef]
- Tisoco, I.; Donatoni, M.C.; Victória, H.F.V.; de Toledo, J.R.; Krambrock, K.; Chaves, O.A.; de Oliveira, K.T.; Iglesias, B.A. Photophysical, photooxidation, and biomolecule-interaction of meso-tetra(thienyl)porphyrins containing peripheral Pt(II) and Pd(II) complexes. Insights for photodynamic therapy applications. Dalton Trans. 2022, 51, 1646–1657. [Google Scholar] [CrossRef]
- Lourenço, L.M.O.; Iglesias, B.A.; Pereira, P.M.R.; Girão, H.; Fernandes, R.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Tomé, J.P.C. Synthesis, characterization and biomolecule-binding properties of novel tetra-platinum(II)-thiopyridylporphyrins. Dalton Trans. 2015, 44, 530–538. [Google Scholar] [CrossRef]
- Iglesias, B.A.; Barata, J.F.B.; Pereira, P.M.R.; Girão, H.; Fernandes, R.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. New Platinum(II)-Bipyridyl Corrole Complexes: Synthesis, Characterization and Binding Studies with DNA and HSA. J. Inorg. Biochem. 2015, 153, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Auras, B.L.; Oliveira, V.A.; Terenzi, H.; Neves, A.; Iglesias, B.A. meso-Mono-[4-(1,4,7-triazacyclononanyl)]-tri(phenyl)]porphyrin and the respective zinc(II)-complex: Complete characterization and biomolecules binding abilities. Photochem. Photobiol. Sci. 2016, 15, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Terenzi, H.; Menezes, L.B.; Chaves, O.A.; Iglesias, B.A. Evaluation of DNA-binding and DNA-photocleavage ability of tetra-cationic porphyrins containing peripheral [Ru(Bpy)2Cl]+ complexes: Insights for photodynamic therapy agents. J. Photochem. Photobiol. B Biol. 2020, 211, 111991. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Iglesias, B.A.; Auras, B.L.; Neves, A.; Terenzi, H. Photoactive: meso-tetra(4-pyridyl)porphyrin-tetrakis-[chloro(2,2′bipyridine)platinum(II) derivatives recognize and cleave DNA upon irradiation. Dalton Trans. 2017, 46, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Acunha, T.V.; Chaves, O.A.; Iglesias, B.A. Fluorescent Pyrene Moiety in Fluorinated C6F5-Corroles Increases the Interaction with HSA and CT-DNA. J. Porphyr. Phthalocyanines 2021, 25, 75–94. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Teresa Gandolfi, M. Handbook of Photochemistry, 3rd ed.; Press, C., Ed.; Taylor & Francis: Singapore, 2006. [Google Scholar]
- Heller, D.P.; Greenstock, C.L. Fluorescence Lifetime Analysis of DNA Intercalated Ethidium Bromide and Quenching by Free Dye. Biophys. Chem. 1994, 50, 305–312. [Google Scholar] [CrossRef]
- Sayed, M.; Krishnamurthy, B.; Pal, H. Unraveling Multiple Binding Modes of Acridine Orange to DNA Using a Multispectroscopic Approach. Phys. Chem. Chem. Phys. 2016, 18, 24642–24653. [Google Scholar] [CrossRef]
- Estandarte, A.K.; Botchway, S.; Lynch, C.; Yusuf, M.; Robinson, I. The Use of DAPI Fluorescence Lifetime Imaging for Investigating Chromatin Condensation in Human Chromosomes. Sci. Rep. 2016, 6, 31417. [Google Scholar] [CrossRef]
- Sahar, E.; Latt, S.A. Energy Transfer and Binding Competition between Dyes Used to Enhance Staining Differentiation in Metaphase Chromosomes. Chromosoma 1980, 79, 1–28. [Google Scholar] [CrossRef]
- Fioravanço, L.P.; Pôrto, J.B.; Martins, F.M.; Siqueira, J.D.; Iglesias, B.A.; Rodrigues, B.M.; Chaves, O.A.; Back, D.F. A Vanadium(V) Complexes Derived from Pyridoxal/Salicylaldehyde. Interaction with CT-DNA/HSA, and Molecular Docking Assessments. J. Inorg. Biochem. 2023, 239, 112070. [Google Scholar] [CrossRef]
- Dodero, V.I.; Quirolo, Z.B.; Sequeira, M.A. Biomolecular studies by circular dichroism. Front. Biosci. (Landmark Ed) 2011, 16, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kypr, J.; Kejnovská, I.; Renčiuk, D.; Vorlíčková, M. Circular Dichroism and Conformational Polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef]
- Haeubl, M.; Reith, L.M.; Gruber, B.; Karner, U.; Müller, N.; Knör, G.; Schoefberger, W. DNA Interactions and Photocatalytic Strand Cleavage by Artificial Nucleases Based on Water-Soluble Gold(III) Porphyrins. J. Biol. Inorg. Chem. 2009, 14, 1037–1052. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.; Kukreti, S. Porphyrin Induced Structural Destabilization of a Parallel DNA G-Quadruplex in Human MRP1 Gene Promoter. J. Mol. Recognit. JMR 2022, 35, e2950. [Google Scholar] [CrossRef]
- da Silveira, C.H.; Vieceli, V.; Clerici, D.J.; Santos, R.C.V.; Iglesias, B.A. Investigation of isomeric tetra-cationic porphyrin activity with peripheral [Pd(bpy)Cl]+ units by antimicrobial photodynamic therapy. Photodiag. Photodyn.Ther. 2020, 31, 101920. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA Dodecamer: Conformation and Dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Krah, A.; Wien, F.; Garman, E.; Mckenna, R.; Sanderson, M.; Neidle, S. A DNA-Porphyrin Minor-Groove Complex at Atomic Resolution: The Structural Consequences of Porphyrin Ruffling. Proc. Natl. Acad. Sci. USA 2000, 97, 9476–9481. [Google Scholar] [CrossRef]
- Romera, C.; Sabater, L.; Garofalo, A.; Dixon, I.M.; Pratviel, G. Interaction of Cationic Nickel and Manganese Porphyrins with the Minor Groove of DNA. Inorg. Chem. 2010, 49, 8558–8567. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.A.; Acunha, T.V.; Iglesias, B.A.; Jesus, C.S.H.; Serpa, C. Effect of Peripheral Platinum(II) Bipyridyl Complexes on the Interaction of Tetra-Cationic Porphyrins with Human Serum Albumin. J. Mol. Liq. 2020, 301, 112466. [Google Scholar] [CrossRef]
- Rodrigues, B.M.; Victória, H.F.V.; Leite, G.; Krambrock, K.; Chaves, O.A.; de Oliveira, D.F.; de Q. Garcia, R.; De Boni, L.; Costa, L.A.S.; Iglesias, B.A. Photophysical, photobiological, and biomolecule-binding properties of new tri-cationic meso-tri(2-thienyl)corroles with Pt(II) and Pd(II) polypyridyl derivatives. J. Inorg. Biochem. 2023, 242, 112149. [Google Scholar] [CrossRef]
- Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction, Inc.: Irvine, CA, USA, 2003; ISBN 189066118X. [Google Scholar]
- CCDC Solutions—CSD-Discovery Components. Available online: http://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/ (accessed on 1 May 2023).
- Bessega, T.; Chaves, O.A.; Martins, F.M.; Acunha, T.V.; Back, D.F.; Iglesias, B.A.; de Oliveira, G.M. Coordination of Zn(II), Pd(II) and Pt(II) with Ligands Derived from Diformylpyridine and Thiosemicarbazide: Synthesis, Structural Characterization, DNA/BSA Binding Properties and Molecular Docking Analysis. Inorg. Chim. Acta 2019, 496, 119049. [Google Scholar] [CrossRef]
- DeLano, W.; Bromberg, S. PyMOL User’s Guide (Original); DeLano Scientific LLC: San Carlos, CA, USA, 2004; pp. 1–66. [Google Scholar]
- Chang, D.-K.; Cheng, S.-F. On the Importance of van Der Waals Interaction in the Groove Binding of DNA with Ligands: Restrained Molecular Dynamics Study. Int. J. Biol. Macromol. 1996, 19, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Da Silva Galhardo, R.; De Lima-Bessa, K.M.; Schuch, N.J.; Menck, C.F.M. Development of a DNA-Dosimeter System for Monitoring the Effects of Solar-Ultraviolet Radiation. Photochem. Photobiol. Sci. 2009, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]




| Compound | Minor Groove | Major Groove |
|---|---|---|
| TThPor | 69.0 | 44.5 |
| PdTThPor | 66.3 | 43.5 |
| PdTThPor | 62.3 | 42.2 |
| Porphyrin | Q (%) a | kpo (min−1) | t½ (h) |
|---|---|---|---|
| TThPor | 18.0 | 6.18 × 10−3 ± 0.03 | 1.87 |
| PdTThPor | 25.5 | 9.41 × 10−3 ± 0.05 | 1.23 |
| PtTThPor | 27.5 | 1.12 × 10−2 ± 0.04 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, B.A.; Peranzoni, N.P.; Faria, S.I.; Trentin, L.B.; Schuch, A.P.; Chaves, O.A.; Bertoloni, R.R.; Nikolaou, S.; de Oliveira, K.T. DNA-Interactive and Damage Study with meso-Tetra(2-thienyl)porphyrins Coordinated with Polypyridyl Pd(II) and Pt(II) Complexes. Molecules 2023, 28, 5217. https://doi.org/10.3390/molecules28135217
Iglesias BA, Peranzoni NP, Faria SI, Trentin LB, Schuch AP, Chaves OA, Bertoloni RR, Nikolaou S, de Oliveira KT. DNA-Interactive and Damage Study with meso-Tetra(2-thienyl)porphyrins Coordinated with Polypyridyl Pd(II) and Pt(II) Complexes. Molecules. 2023; 28(13):5217. https://doi.org/10.3390/molecules28135217
Chicago/Turabian StyleIglesias, Bernardo Almeida, Níckolas Pippi Peranzoni, Sophia Iwersen Faria, Luana Belo Trentin, André Passaglia Schuch, Otávio Augusto Chaves, Renan Ribeiro Bertoloni, Sofia Nikolaou, and Kleber Thiago de Oliveira. 2023. "DNA-Interactive and Damage Study with meso-Tetra(2-thienyl)porphyrins Coordinated with Polypyridyl Pd(II) and Pt(II) Complexes" Molecules 28, no. 13: 5217. https://doi.org/10.3390/molecules28135217
APA StyleIglesias, B. A., Peranzoni, N. P., Faria, S. I., Trentin, L. B., Schuch, A. P., Chaves, O. A., Bertoloni, R. R., Nikolaou, S., & de Oliveira, K. T. (2023). DNA-Interactive and Damage Study with meso-Tetra(2-thienyl)porphyrins Coordinated with Polypyridyl Pd(II) and Pt(II) Complexes. Molecules, 28(13), 5217. https://doi.org/10.3390/molecules28135217