Interplay between Nanoparticles and Phosphorus Dendrimers, and Their Properties
Abstract
1. Introduction
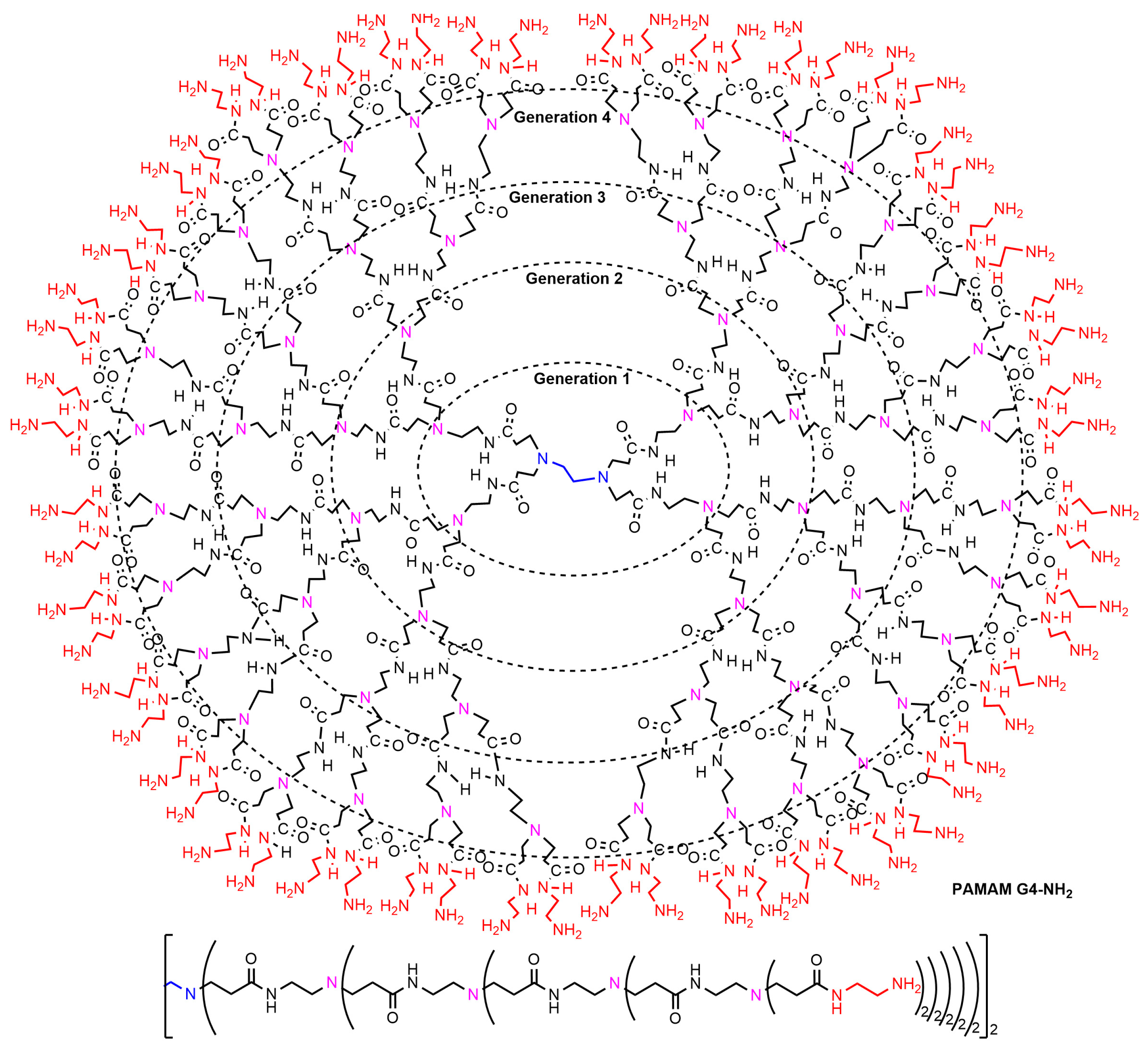
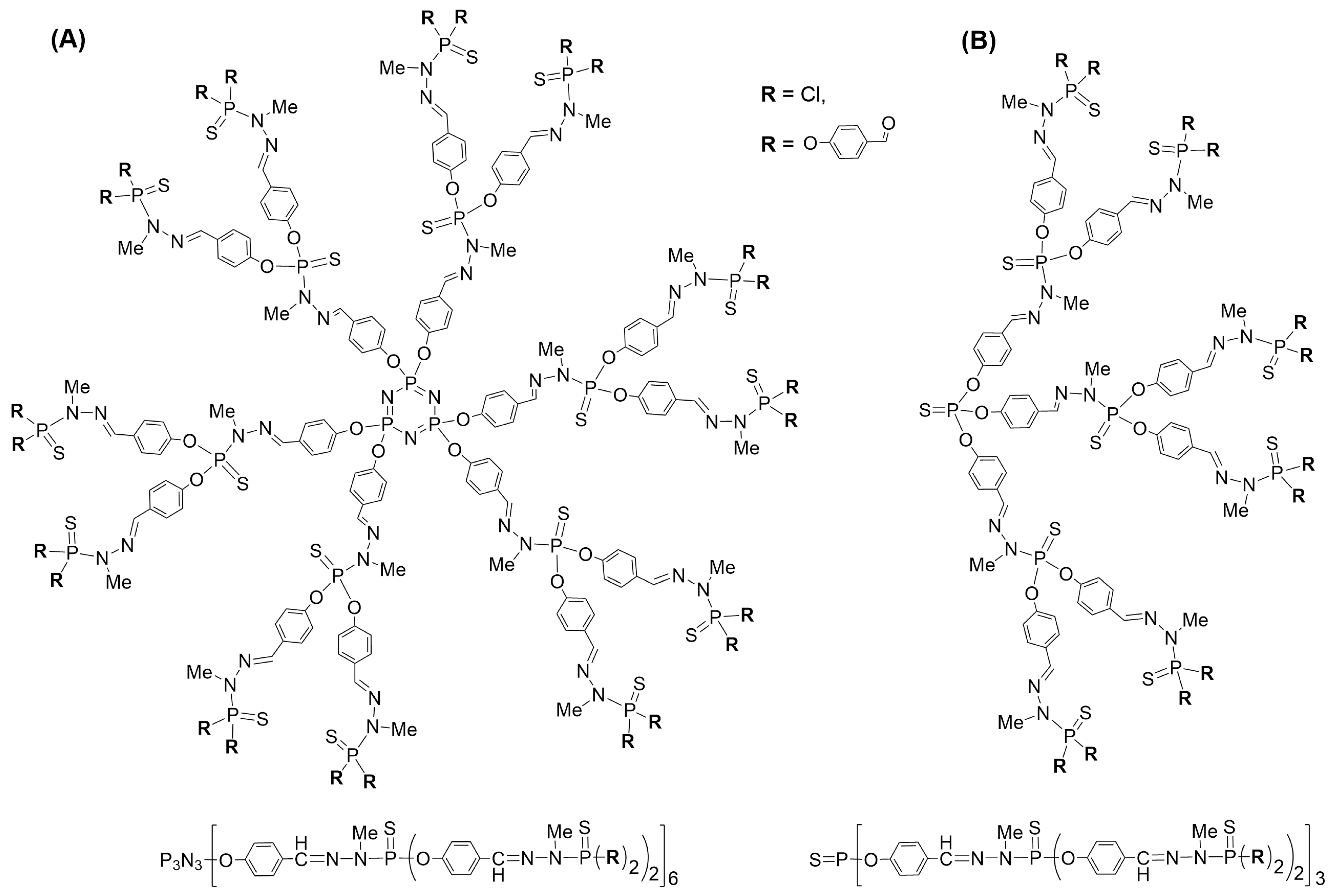
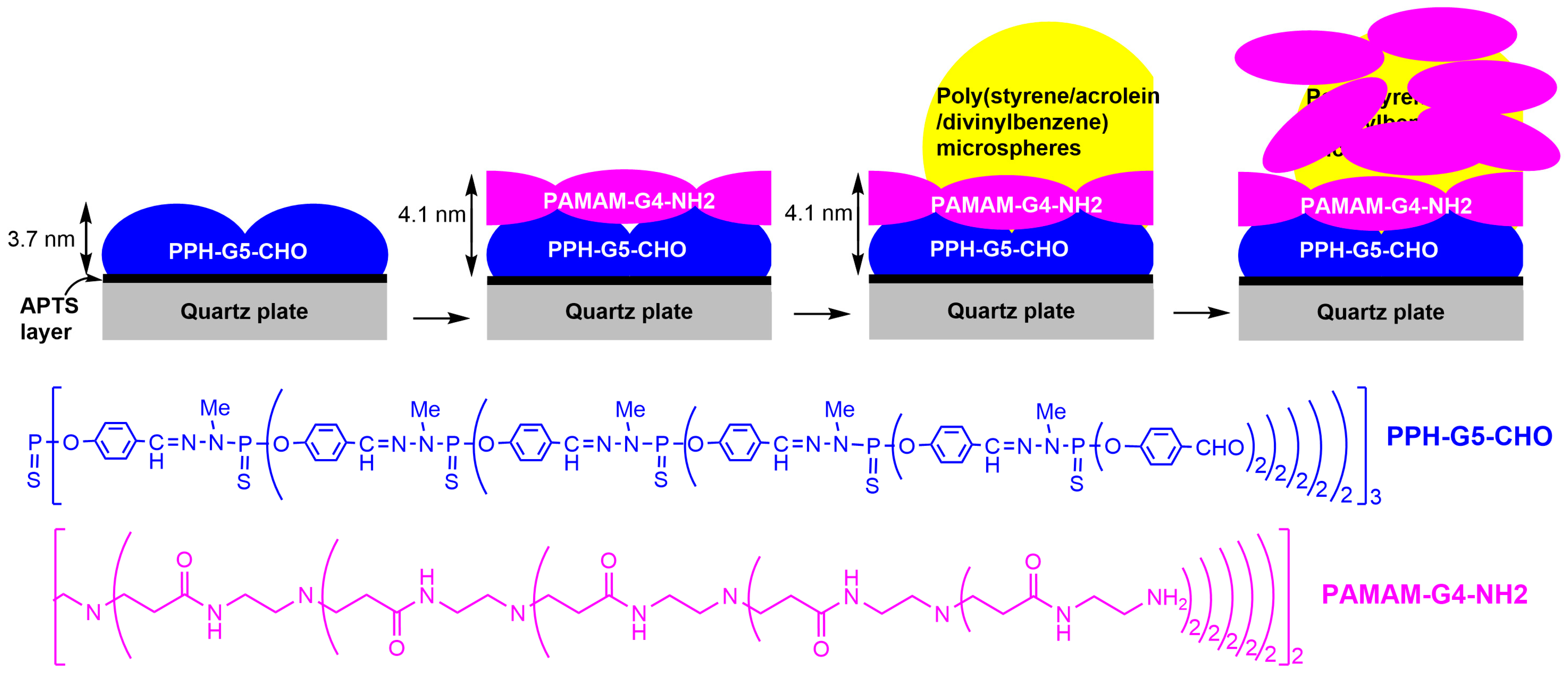
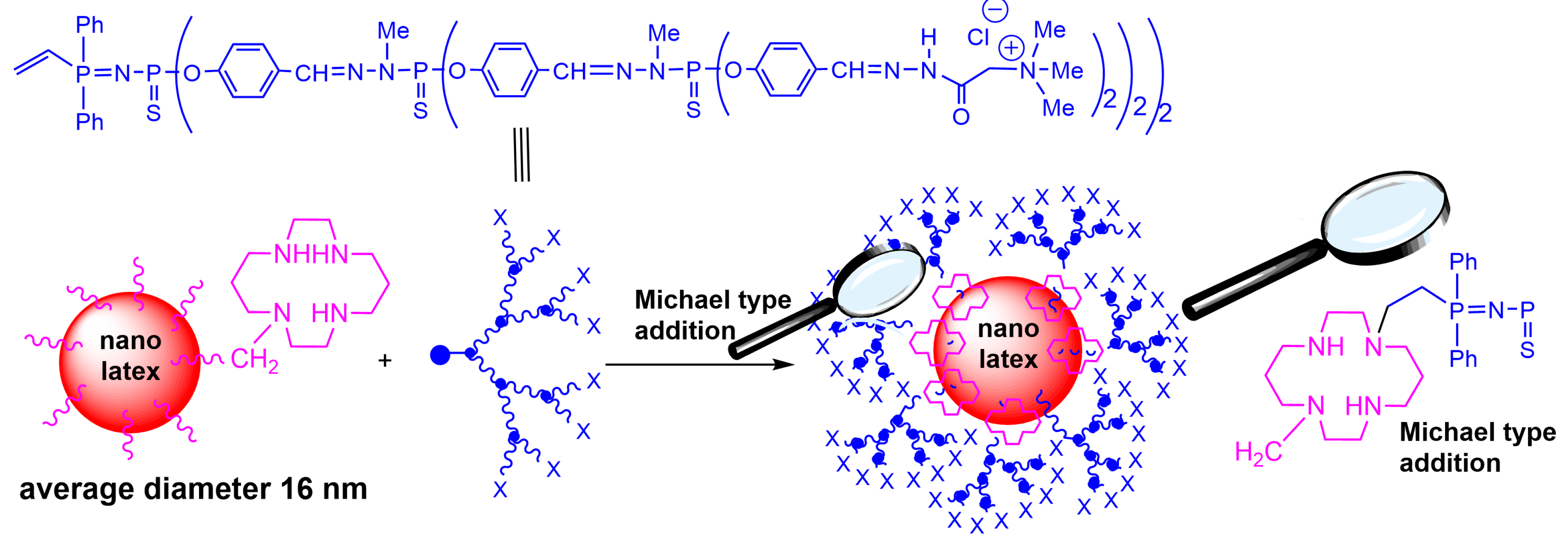
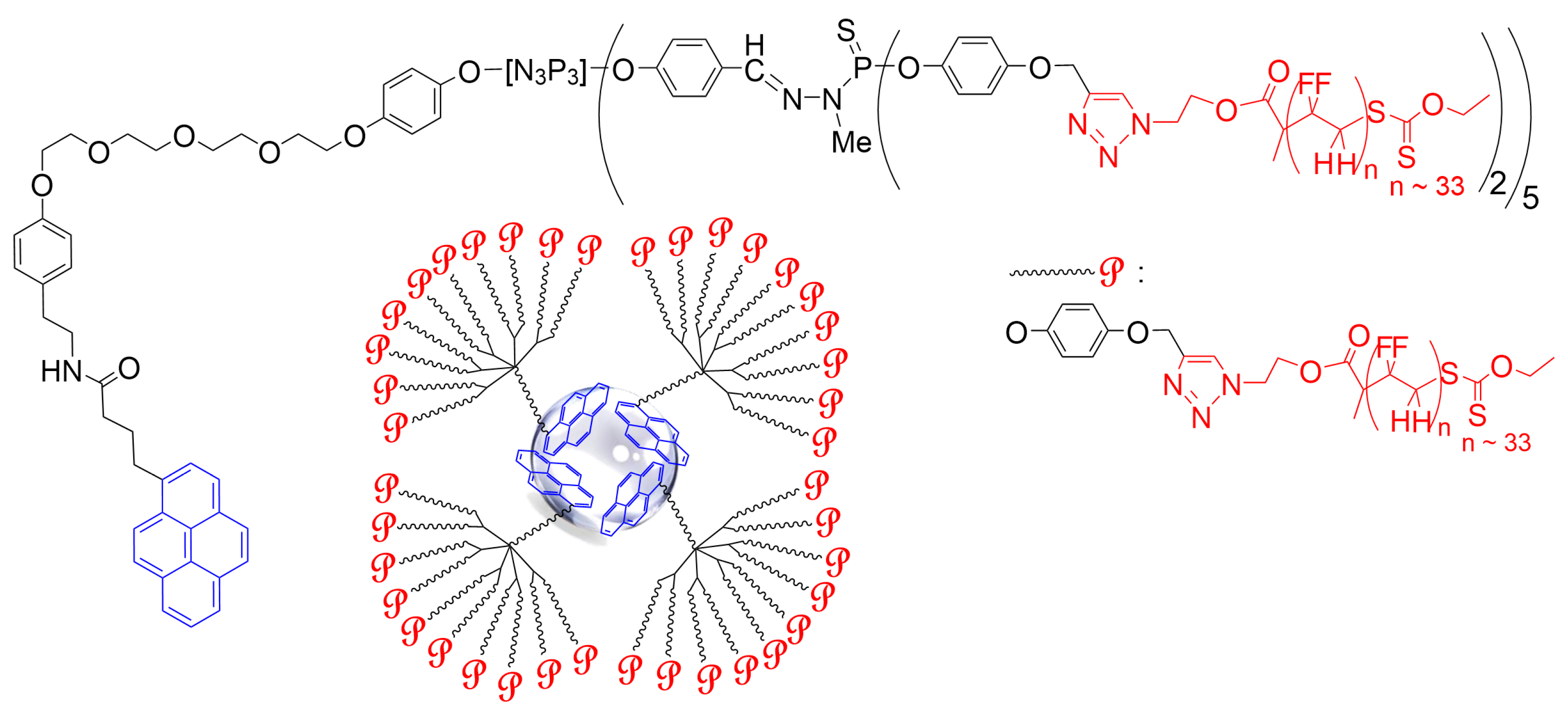
2. Interaction between Phosphorus Dendrimers and Organic Nanoparticles
3. Interaction of Dendrimers with Pre-Existing Metal Nanoparticles
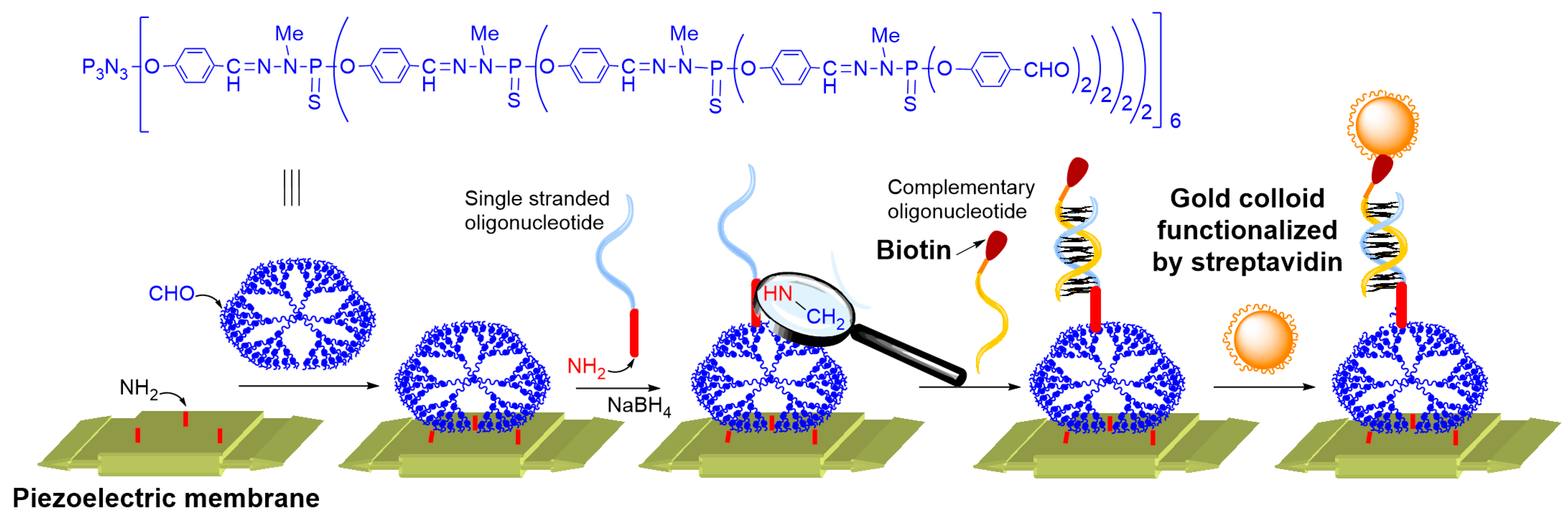
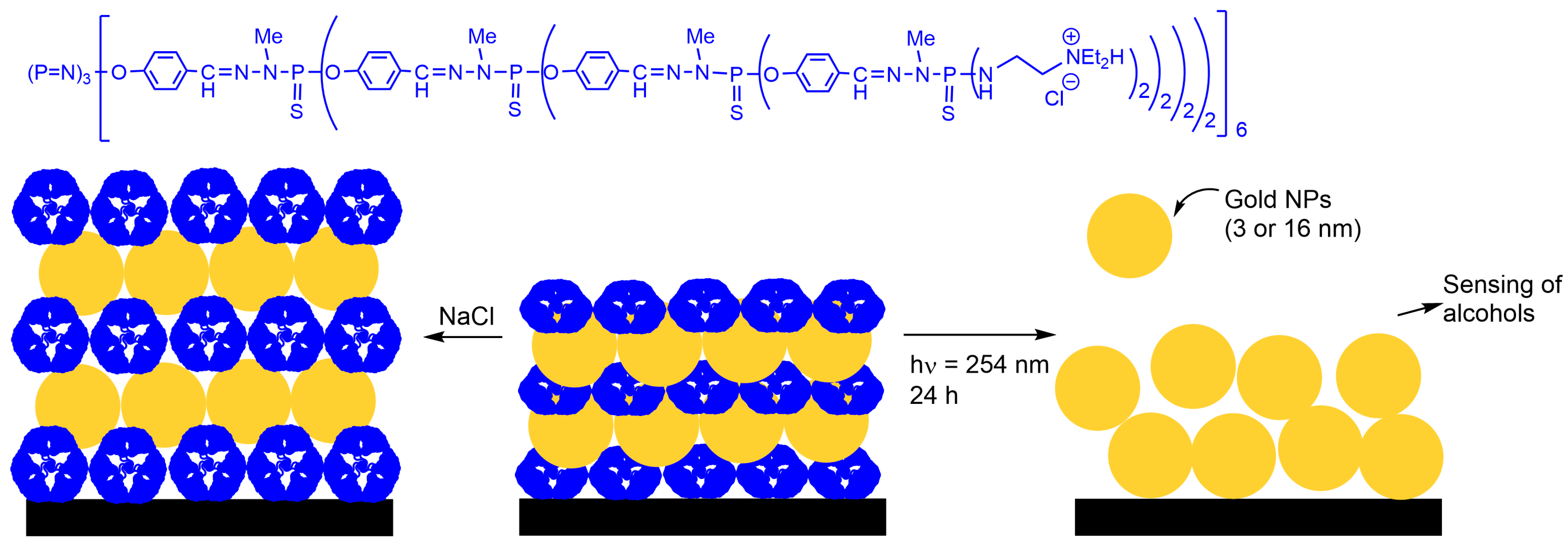
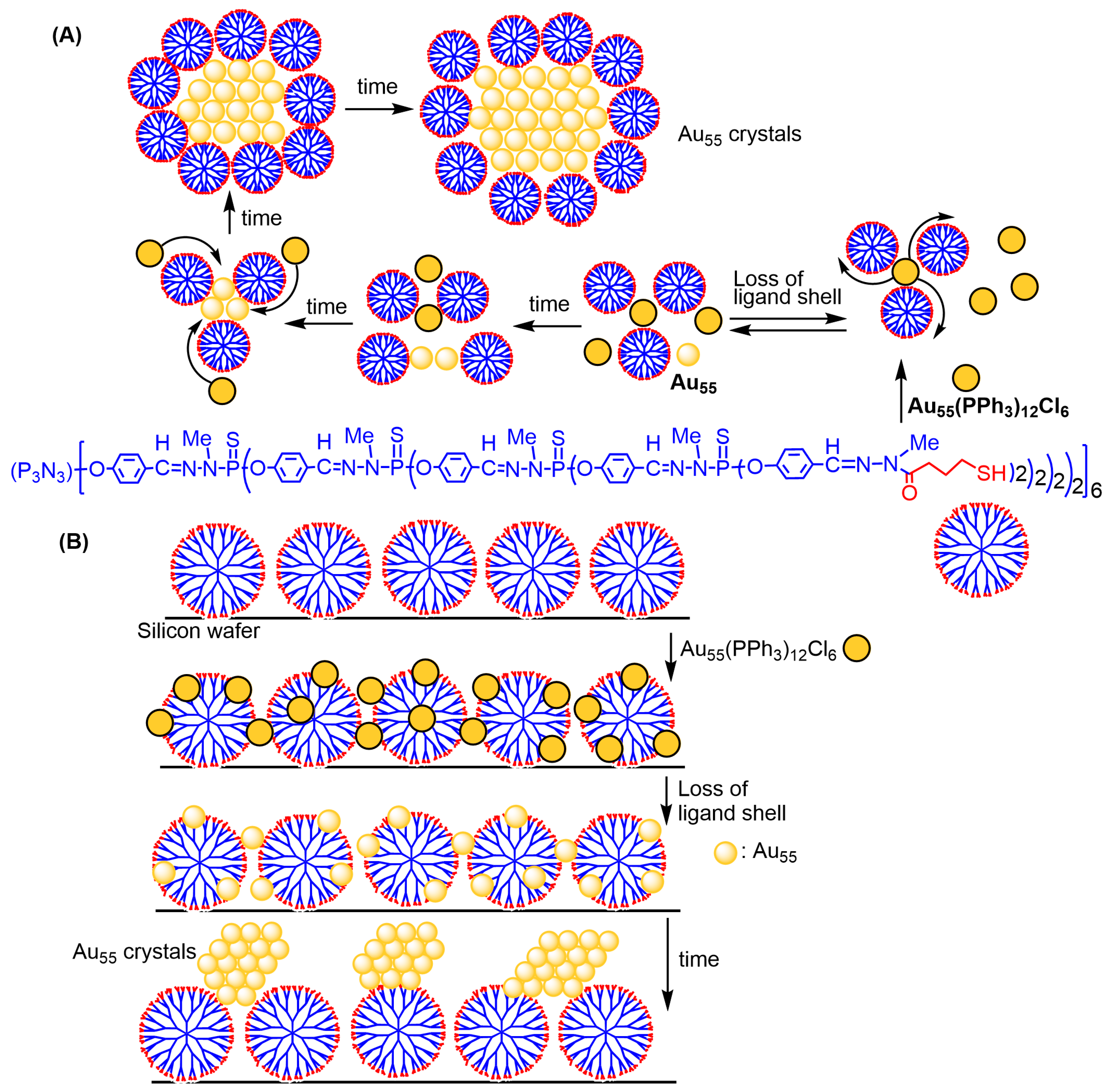
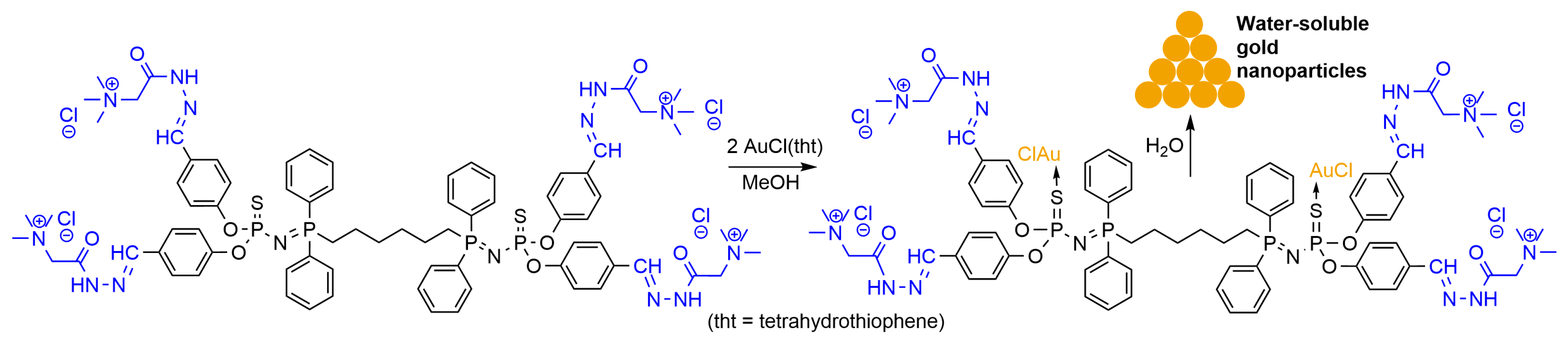
3.1. Gold Nanoparticles
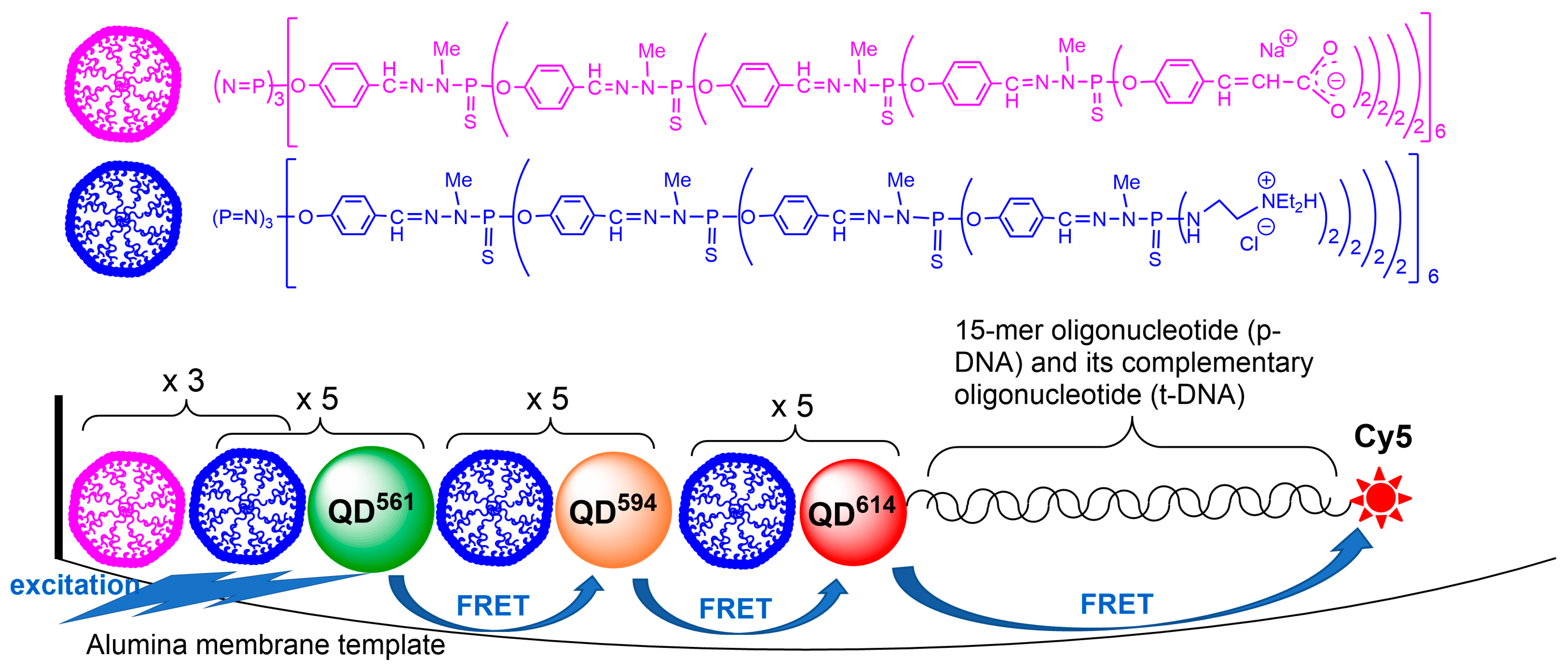
3.2. ZnCdSe Quantum Dots
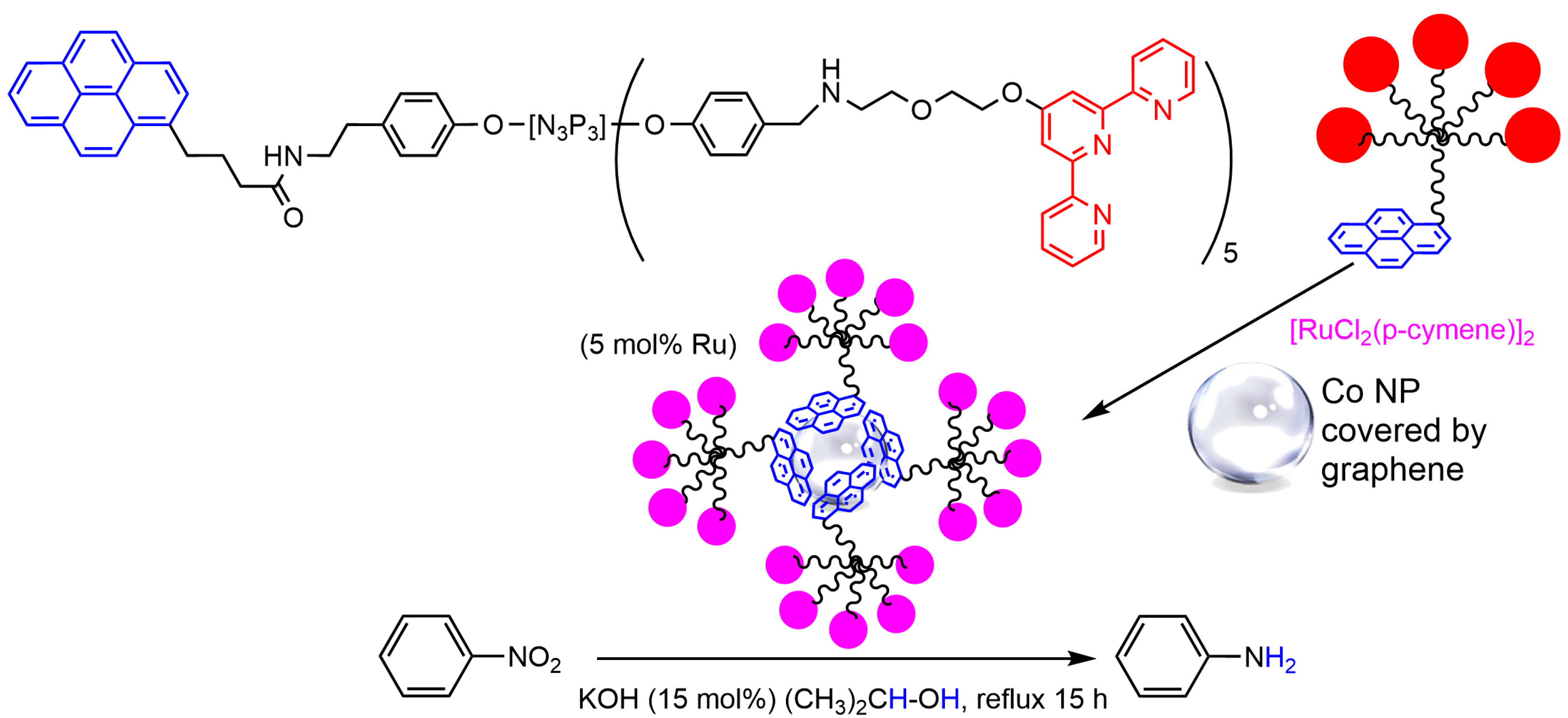
3.3. Cobalt Nanoparticles
4. Dendrimers for the Synthesis of Metal Nanoparticles
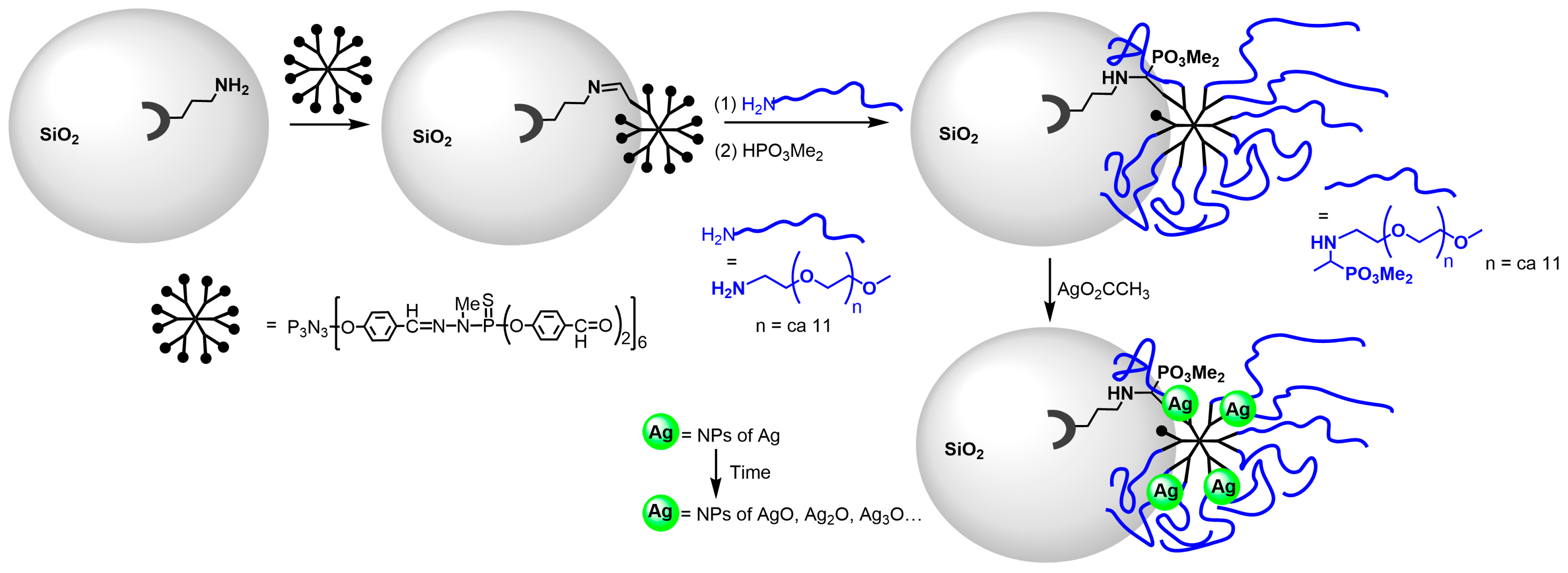
4.1. Synthesis of Gold and Silver Nanoparticles
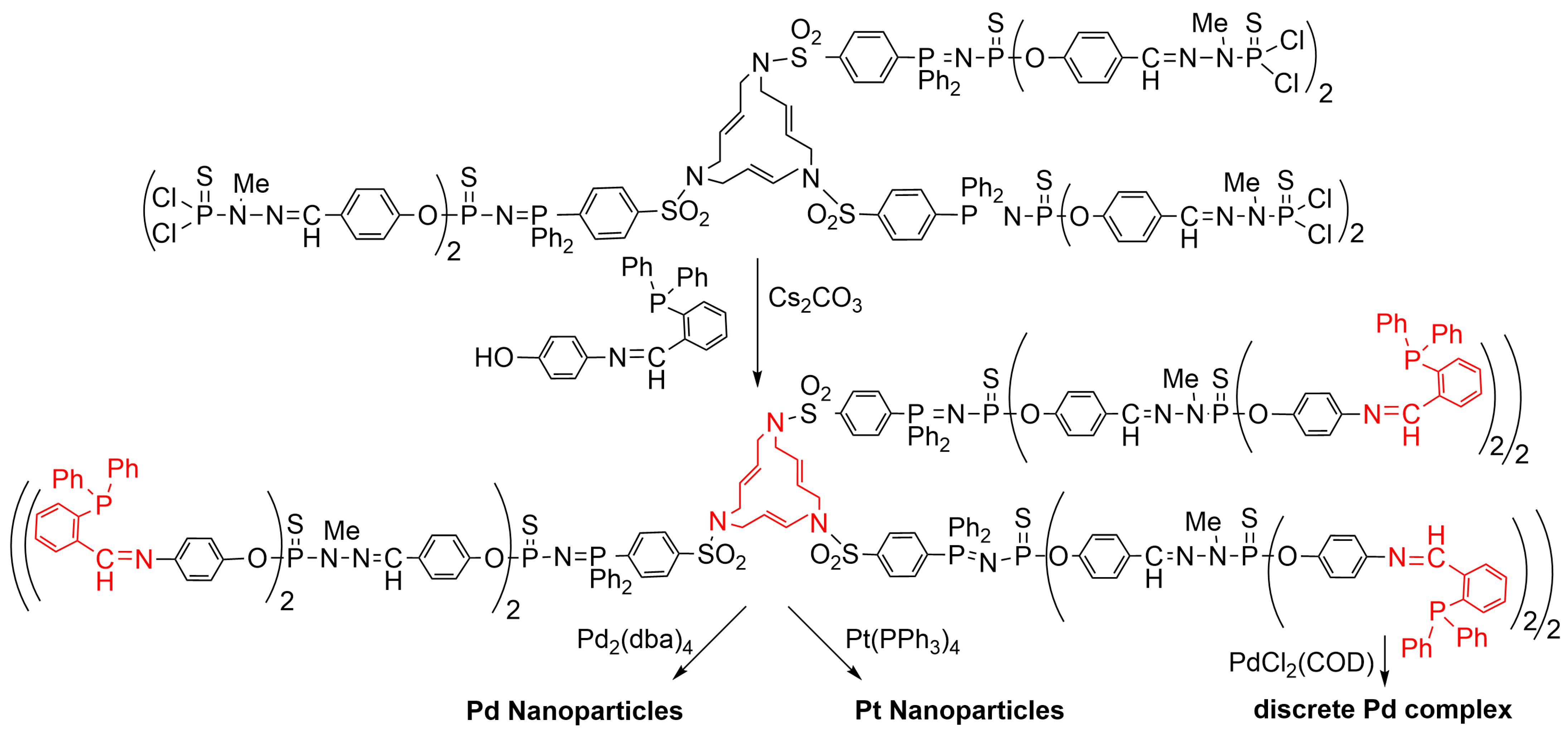
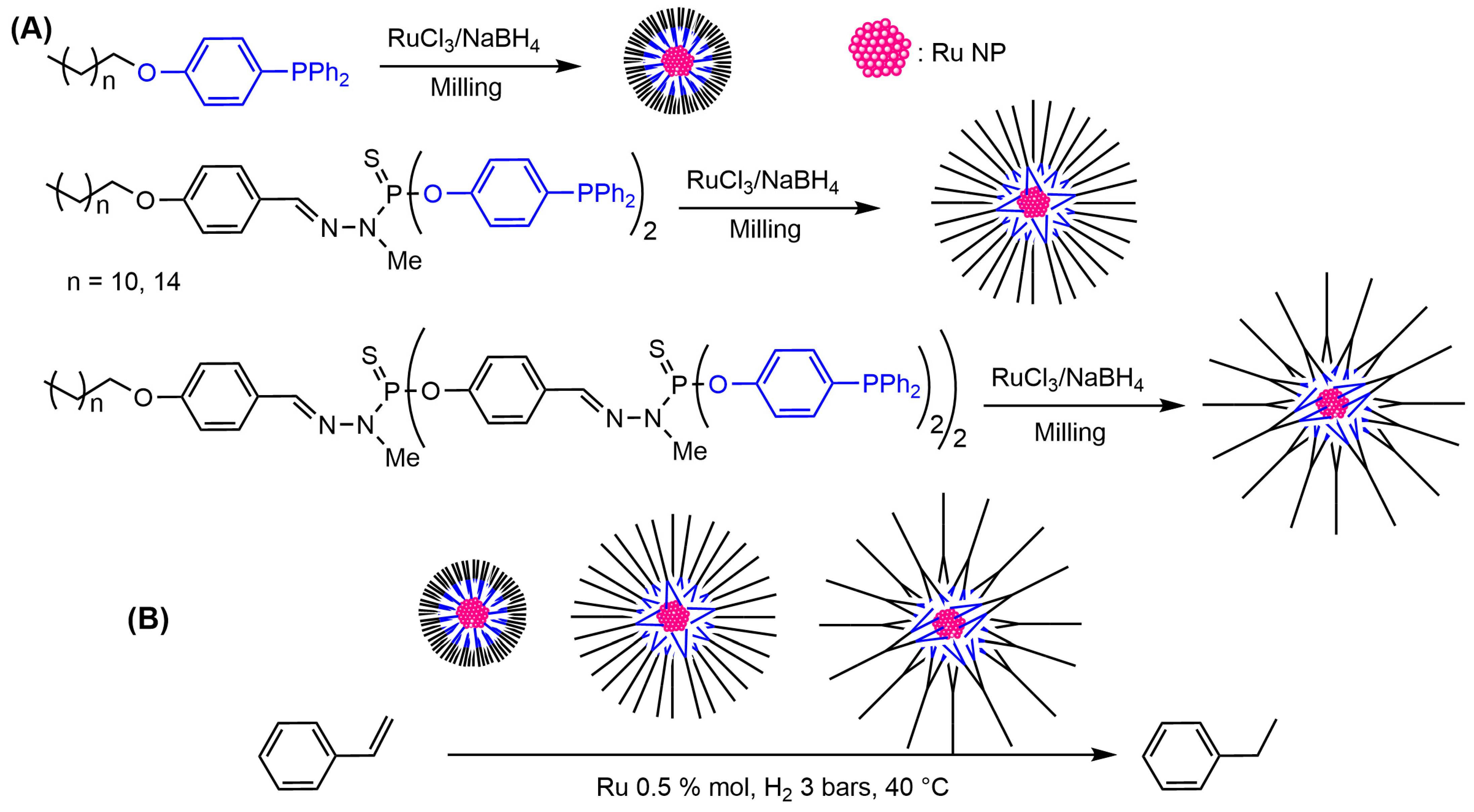
4.2. Synthesis of Palladium, Platinum, and Ruthenium Nanoparticles
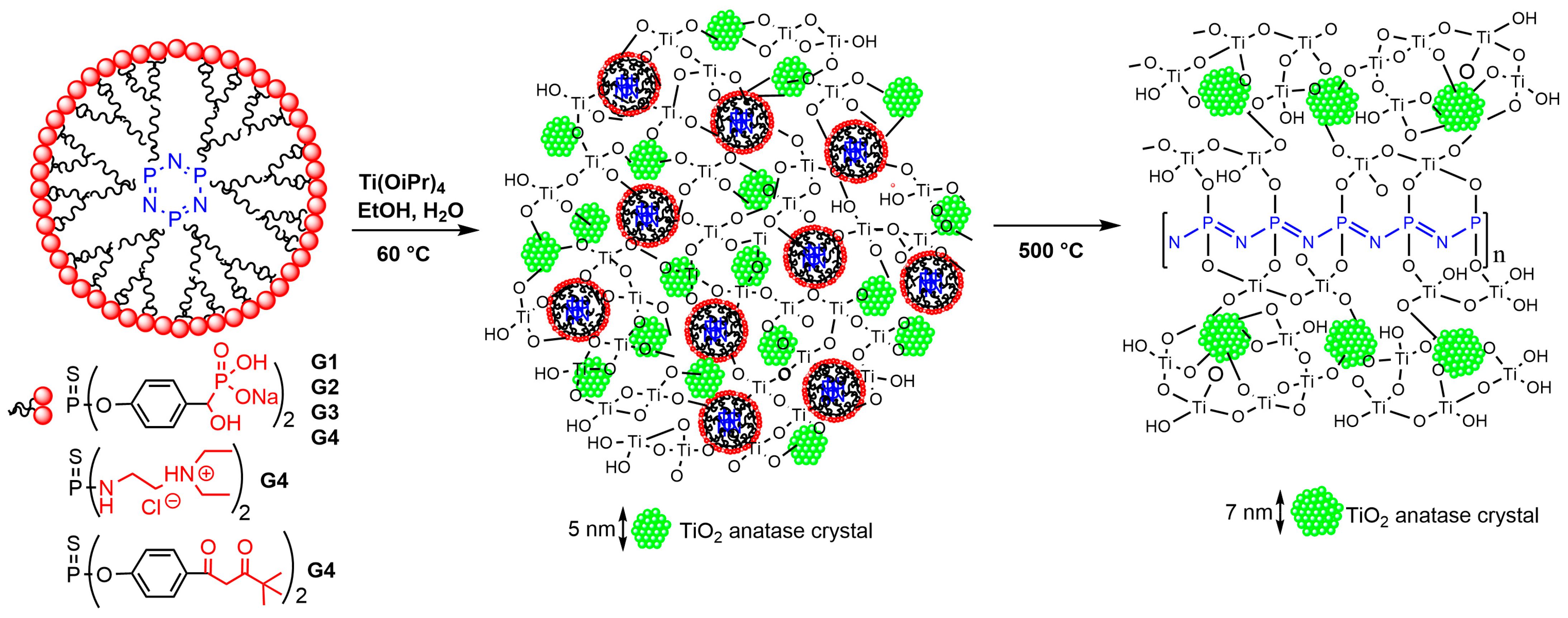
4.3. Synthesis of Titanium Nanoparticles
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys.-Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.X.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Frechet, J.M.J.; Hawker, C.J.; Gitsov, I.; Leon, J.W. Dendrimers and hyperbranched polymers: Two families of three-dimensional macromolecules with similar but clearly distinct properties. J. Macromol. Sci.-Pure Appl. Chem. 1996, A33, 1399–1425. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 -> 2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C. Dendrimers derived from 1 → 3 branching motifs. Chem. Rev. 2010, 110, 6338–6442. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Hervet, H. Statistics of Sarburst polymers. J. Phys. Lett. 1983, 44, L351–L360. [Google Scholar] [CrossRef]
- Maiti, P.K.; Cagin, T.; Wang, G.F.; Goddard, W.A. Structure of PAMAM dendrimers: Generations 1 through 11. Macromolecules 2004, 37, 6236–6254. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers–Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Lartigue, M.L.; Donnadieu, B.; Galliot, C.; Caminade, A.M.; Majoral, J.P.; Fayet, J.P. Large dipole moments of phosphorus-containing dendrimers. Macromolecules 1997, 30, 7335–7337. [Google Scholar] [CrossRef]
- Lim, J.; Kostiainen, M.; Maly, J.; da Costa, V.C.P.; Annunziata, O.; Pavan, G.M.; Simanek, E.E. Synthesis of large dendrimers with the dimensions of small viruses. J. Am. Chem. Soc. 2013, 135, 4660–4663. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture—A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Percec, V.; Won, B.C.; Peterca, M.; Heiney, P.A. Expanding the structural diversity of self-assembling dendrons and supramolecular dendrimers via complex building blocks. J. Am. Chem. Soc. 2007, 129, 11265–11278. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Khanna, S.N. A systematic framework and nanoperiodic concept for unifying nanoscience: Hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive Mendeleev-like nanoperiodic tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Sun, L.; Crooks, R.M. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Balogh, L.; Tomalia, D.A. Poly(amidoamine) dendrimer-templated nanocomposites. 1. Synthesis of zerovalent copper nanoclusters. J. Am. Chem. Soc. 1998, 120, 7355–7356. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usui, K.; Torigoe, K. Preparation of gold colloids with UV irradiation using dendrimers as stabilizer. Langmuir 1998, 14, 3157–3159. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.Q.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Peng, X.; Pan, Q.; Rempel, G.L. Bimetallic dendrimer-encapsulated nanoparticles as catalysts: A review of the research advances. Chem. Soc. Rev. 2008, 37, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New horizon of nanoparticle and cluster catalysis with dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, X. Dendrimer-based organic/inorganic hybrid nanoparticles in biomedical applications. Nanoscale 2010, 2, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.R.; Nain, A.; Jain, S.; Punjabi, N.; Mukherji, S.; Satija, J. Dendrimer as a multifunctional capping agent for metal nanoparticles for use in bioimaging, drug delivery and sensor applications. J. Mat. Chem. B 2018, 6, 2368–2384. [Google Scholar] [CrossRef] [PubMed]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem.-Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Galliot, C.; Larre, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R. Homogeneous catalysis with phosphorus dendrimer complexes. Coord. Chem. Rev. 2019, 389, 59–72. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournie, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed]
- Miksa, B.; Slomkowski, S.; Chehimi, M.M.; Delamar, M.; Majoral, J.P.; Caminade, A.M. Tailored modification of quartz surfaces by covalent immobilization of small molecules (gamma-aminopropyltriethoxysilane), monodisperse macromolecules (dendrimers), and poly(styrene/acrolein/divinylbenzene) microspheres with narrow diameter distribution. Colloid Polym. Sci. 1999, 277, 58–65. [Google Scholar] [CrossRef]
- Slomkowski, S.; Miksa, B.; Chehimi, M.M.; Delamar, M.; Cabet-Deliry, E.; Majoral, J.P.; Caminade, A.M. Inorganic-organic systems with tailored properties controlled on molecular, macromolecular and microscopic level. React. Funct. Polym. 1999, 41, 45–57. [Google Scholar] [CrossRef]
- Larpent, C.; Genies, C.; Delgado, A.P.D.; Caminade, A.M.; Majoral, J.P.; Sassi, J.F.; Leising, F. Giant dendrimer-like particles from nanolatexes. Chem. Commun. 2004, 16, 1816–1817. [Google Scholar] [CrossRef]
- Marmillon, C.; Gauffre, F.; Gulik-Krzywicki, T.; Loup, C.; Caminade, A.M.; Majoral, J.P.; Vors, J.P.; Rump, E. Organophosphorus dendrimers as new gelators for hydrogels. Angew. Chem. Int. Ed. 2001, 40, 2626–2629. [Google Scholar] [CrossRef]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold nanoparticles: Past, present, and future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Uni.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Delivery Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Majoral, J.P.; Zablocka, M.; Caminade, A.-M.; Balczewski, P.; Shi, X.; Mignani, S. Interactions gold/phosphorus dendrimers. Versatile ways to hybrid organic-metallic macromolecules. Coord. Chem. Rev. 2018, 358, 80–91. [Google Scholar] [CrossRef]
- Le Berre, V.; Trevisiol, E.; Dagkessamanskaia, A.; Sokol, S.; Caminade, A.M.; Majoral, J.P.; Meunier, B.; Francois, J. Dendrimeric coating of glass slides for sensitive DNA microarrays analysis. Nucleic Acids Res. 2003, 31, e88. [Google Scholar] [CrossRef] [PubMed]
- Trevisiol, E.; Le Berre-Anton, V.; Leclaire, J.; Pratviel, G.; Caminade, A.M.; Majoral, J.P.; Francois, J.M.; Meunier, B. Dendrislides, dendrichips: A simple chemical functionalization of glass slides with phosphorus dendrimers as an effective means for the preparation of biochips. New J. Chem. 2003, 27, 1713–1719. [Google Scholar] [CrossRef]
- Caminade, A.M.; Padie, C.; Laurent, R.; Maraval, A.; Majoral, J.P. Uses of dendrimers for DNA microarrays. Sensors 2006, 6, 901–914. [Google Scholar] [CrossRef]
- Nicu, L.; Guirardel, M.; Chambosse, F.; Rougerie, P.; Hinh, S.; Trevisiol, E.; Francois, J.M.; Majoral, J.P.; Caminade, A.M.; Cattan, E.; et al. Resonating piezoelectric membranes for microelectromechanically based bioassay: Detection of streptavidin-gold nanoparticles interaction with biotinylated DNA. Sens. Actuator B-Chem. 2005, 110, 125–136. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Christopoulos, T.K. The biotin (stept)avidin system-Principles and applications in biotechnology. Clin. Chem. 1991, 37, 625–636. [Google Scholar] [CrossRef]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin-biotin technology: Improvements and innovations in chemical and biological applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, T.; Ward, T.R. Artificial metalloenzymes based on the biotin-streptavidin technology: Challenges and opportunities. Acc. Chem. Res. 2016, 49, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Karan, P.; Goring, P.; Leclaire, J.; Caminade, A.M.; Majoral, J.P.; Gosele, U.; Steinhart, M.; Knoll, W. Formation of dendrimer nanotubes by layer-by-layer deposition. Small 2005, 1, 99–102. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 2001, 13, 1389–1393. [Google Scholar] [CrossRef]
- Zhao, W.B.; Park, J.; Caminade, A.M.; Jeong, S.J.; Jang, Y.H.; Kim, S.O.; Majoral, J.P.; Cho, J.; Kim, D.H. Localized surface plasmon resonance coupling in Au nanoparticles/phosphorus dendrimer multilayer thin films fabricated by layer-by-layer self-assembly method. J. Mater. Chem. 2009, 19, 2006–2012. [Google Scholar] [CrossRef]
- Lebedeva, O.V.; Kim, B.S.; Vasilev, K.; Vinogradova, O.I. Salt softening of polyelectrolyte multilayer microcapsules. J. Colloid Interface Sci. 2005, 284, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Blais, J.C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. MALDI TOF mass spectrometry for the characterization of phosphorus-containing dendrimers. Scope and limitations. Anal. Chem. 2000, 72, 5097–5105. [Google Scholar] [CrossRef]
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Graded-bandgap quantum-dot-modified nanotubes: A sensitive biosensor for enhanced detection of DNA hybridization. Adv. Mater. 2007, 19, 1933–1936. [Google Scholar] [CrossRef]
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Functional quantum-dot/dendrimer nanotubes for sensitive detection of DNA hybridization. Small 2008, 4, 566–571. [Google Scholar] [CrossRef]
- Knoll, W.; Caminade, A.M.; Char, K.; Duran, H.; Feng, C.L.; Gitsas, A.; Kim, D.H.; Lau, A.; Lazzara, T.D.; Majoral, J.P.; et al. Nanostructuring polymeric materials by templating strategies. Small 2011, 7, 1384–1391. [Google Scholar] [CrossRef]
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-tagged dendritic catalysts noncovalently grafted onto magnetic Co/C nanoparticles: An efficient and recyclable system for drug synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629. [Google Scholar] [CrossRef] [PubMed]
- Senapati, K.K.; Roy, S.; Borgohain, C.; Phukan, P. Palladium nanoparticle supported on cobalt ferrite: An efficient magnetically separable catalyst for ligand free Suzuki coupling. J. Mol. Cat. A-Chem. 2012, 352, 128–134. [Google Scholar] [CrossRef]
- Keller, M.; Hameau, A.; Spataro, G.; Ladeira, S.; Caminade, A.M.; Majoral, J.P.; Ouali, A. An efficient and recyclable dendritic catalyst able to dramatically decrease palladium leaching in Suzuki couplings. Green Chem. 2012, 14, 2807–2815. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Asri, H.; Dautel, O.; Ouali, A. Terpyridine-Ru complexes noncovalently supported on cobalt magnetic nanoparticles for nitroarene transfer hydrogenation. ACS Appl. Nano Mater. 2020, 3, 11811–11818. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Reiser, O. Polymer- and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef]
- Folgado, E.; Guerre, M.; Mimouni, N.; Colliere, V.; Bijani, C.; Ching, K.M.C.; Caminade, A.M.; Ladmiral, V.; Ameduri, B.; Ouali, A. pi-stacking interactions of graphene-coated cobalt magnetic nanoparticles with pyrene-tagged dendritic poly(vinylidene fluoride). ChemPlusChem 2019, 84, 78–84. [Google Scholar] [CrossRef]
- Vanstaveren, M.P.J.; Brom, H.B.; Dejongh, L.J.; Schmid, G. Large transition-metal clusters.6. Physical-properties of metal cluster compounds II - DC-conductivity of the high-nuclearity gold cluster compound Au55(PPh3)12Cl6. Solid State Commun. 1986, 60, 319–322. [Google Scholar] [CrossRef]
- Schmid, G.; Meyer-Zaika, W.; Pugin, R.; Sawitowski, T.; Majoral, J.P.; Caminade, A.M.; Turrin, C.O. Naked Au-55 clusters: Dramatic effect of a thiol-terminated dendrimer. Chem.-Eur. J. 2000, 6, 1693–1697. [Google Scholar] [CrossRef]
- Schmid, G.; Emmrich, E.; Majoral, J.P.; Caminade, A.M. The behavior of Au-55 nanoclusters on and in thiol-terminated dendrimer monolayers. Small 2005, 1, 73–75. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P. (Tetrahydrothiophene) gold(I) or gold(III) complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar] [CrossRef]
- Larre, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Regioselective gold complexation within the cascade structure of phosphorus-containing dendrimers. Chem.-Eur. J. 1998, 4, 2031–2036. [Google Scholar] [CrossRef]
- Gottis, S.; Laurent, R.; Colliere, V.; Caminade, A.-M. Straightforward synthesis of gold nanoparticles by adding water to an engineered small dendrimer. Beilstein J. Nanotechnol. 2020, 11, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, N.; Ichioka, H.; Satoh, T.; Hayashi, S.; Fujiki, K. Grafting of ‘dendrimer-like’ highly branched polymer onto ultrafine silica surface. React. Funct. Polym. 1998, 37, 75–82. [Google Scholar] [CrossRef]
- Hameau, A.; Colliere, V.; Grimoud, J.; Fau, P.; Roques, C.; Caminade, A.M.; Turrin, C.O. PPH dendrimers grafted on silica nanoparticles: Surface chemistry, characterization, silver colloids hosting and antibacterial activity. RSC Adv. 2013, 3, 19015–19026. [Google Scholar] [CrossRef]
- Dal Lago, V.; de Oliveira, L.F.; Goncalves, K.D.; Kobarg, J.; Cardoso, M.B. Size-selective silver nanoparticles: Future of biomedical devices with enhanced bactericidal properties. J. Mater. Chem. 2011, 21, 12267–12273. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Badetti, E.; Caminade, A.M.; Majoral, J.P.; Moreno-Manas, M.; Sebastian, R.M. Palladium(0) nanoparticles stabilized by phosphorus dendrimers containing coordinating 15-membered triolefinic macrocycles in periphery. Langmuir 2008, 24, 2090–2101. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki-Heck and Suzuki-Miyaura couplings - Homogeneous or heterogeneous catalysis, a critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Moseley, K.; Maitlis, P.M. Bis- and tris-(dibenzylideneacetone)platinum and the stabilization of zerovalent complexes by an unsaturated ketone. J. Chem. Soc. D 1971, 16, 982–983. [Google Scholar] [CrossRef]
- Franc, G.; Badetti, E.; Colliere, V.; Majoral, J.P.; Sebastian, R.M.; Caminade, A.M. Dendritic structures within dendritic structures: Dendrimer-induced formation and self-assembly of nanoparticle networks. Nanoscale 2009, 1, 233–237. [Google Scholar] [CrossRef]
- Franc, G.; Badetti, E.; Duhayon, C.; Coppel, Y.; Turrin, C.O.; Majoral, J.P.; Sebastian, R.M.; Caminade, A.M. An efficient synthesis combining phosphorus dendrimers and 15-membered triolefinic azamacrocycles: Towards the stabilization of platinum nanoparticles. New J. Chem. 2010, 34, 547–555. [Google Scholar] [CrossRef]
- Badetti, E.; Franc, G.; Majoral, J.P.; Caminade, A.M.; Sebastian, R.M.; Moreno-Manas, M. Macrocyclic core phosphorus dendrimers covered on the surface by N,P ligands. Eur. J. Org. Chem. 2011, 2011, 1256–1265. [Google Scholar] [CrossRef]
- Koprowski, M.; Sebastian, R.M.; Maraval, V.; Zablocka, M.; Cadierno, V.; Donnadieu, B.; Igau, A.; Caminade, A.M.; Majoral, J.P. Iminophosphine palladium complexes in catalytic stille coupling reactions: From monomers to dendrimers. Organometallics 2002, 21, 4680–4687. [Google Scholar] [CrossRef]
- Moreno-Manas, M.; Pleixats, R.; Sebastian, R.M.; Vallribera, A.; Roglans, A. Organometallic chemistry of 15-membered tri-olefinic macrocycles: Catalysis by palladium(0) complexes in carbon-carbon bond-forming reactions. J. Organomet. Chem. 2004, 689, 3669–3684. [Google Scholar] [CrossRef]
- Garcia-Pena, N.G.; Caminade, A.M.; Ouali, A.; Redon, R.; Turrin, C.O. Solventless synthesis of Ru(0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC Adv. 2016, 6, 64557–64567. [Google Scholar] [CrossRef]
- Soler-Illia, G.; Rozes, L.; Boggiano, M.K.; Sanchez, C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. New mesotextured hybrid materials made from assemblies of dendrimers and titanium(IV)-oxo-organo clusters. Angew. Chem. Int. Ed. 2000, 39, 4250–4254. [Google Scholar] [CrossRef]
- Bouchara, A.; Rozes, L.; Soler-Illia, G.J.D.; Sanchez, C.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. Use of functional dendritic macromolecules for the design of metal oxo based hybrid materials. J. Sol-Gel Sci. Technol. 2003, 26, 629–633. [Google Scholar] [CrossRef]
- Martinez-Ferrero, E.; Franc, G.; Mazeres, S.; Turrin, C.O.; Boissiere, U.; Caminade, A.M.; Majoral, J.P.; Sanchez, C. Optical properties of hybrid dendritic-mesoporous titania nanocomposite films. Chem.-Eur. J. 2008, 14, 7658–7669. [Google Scholar] [CrossRef]
- Brahmi, Y.; Katir, N.; Hameau, A.; Essoumhi, A.; Essassi, E.; Caminade, A.M.; Bousmina, M.; Majoral, J.P.; El Kadib, A. Hierarchically porous nanostructures through phosphonate-metal alkoxide condensation and growth using functionalized dendrimeric building blocks. Chem. Commun. 2011, 47, 8626–8628. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jaeckel, B.; Parkinson, B.A. Preparation and characterization of terraced surfaces of low-index faces of anatase, rutile, and brookite. Langmuir 2006, 22, 4472–4475. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, Y.; Katir, N.; Ianchuk, M.; Colliere, V.; Essassi, E.; Ouali, A.; Caminade, A.M.; Bousmina, M.; Majoral, J.P.; El Kadib, A. Low temperature synthesis of ordered mesoporous stable anatase nanocrystals: The phosphorus dendrimer approach. Nanoscale 2013, 5, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, Y.; Katir, N.; Agullo, J.A.M.; Primo, A.; Bousmina, M.; Majoral, J.P.; Garcia, H.; El Kadib, A. Organophosphonate bridged anatase mesocrystals: Low temperature crystallization, thermal growth and hydrogen photo-evolution. Dalton Trans. 2015, 44, 15544–15556. [Google Scholar] [CrossRef]
- Katir, N.; Marcotte, N.; Michlewska, S.; Ionov, M.; El Brahmi, N.; Bousmina, M.; Majoral, J.P.; Bryszewska, M.; El Kadib, A. Dendrimer for templating the growth of porous catechol-coordinated titanium dioxide frameworks: Toward hemocompatible nanomaterials. Acs Appl. Nano Mater. 2019, 2, 2979–2990. [Google Scholar] [CrossRef]
- Qiu, J.; Kong, L.; Cao, X.; Li, A.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Enhanced delivery of therapeutic siRNA into glioblastoma cells using dendrimer-entrapped gold nanoparticles conjugated with beta-cyclodextrin. Nanomaterials 2018, 8, 131. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef]



| Materials | MIC 1/MBC 1 of S. aureus CIP 4.83 (Gram+) | MIC 1/MBC 1 of E. hirae CIP 58.55 (Gram+) | MIC 1/MBC 1 of E. coli CIP 103571 (Gram−) | MIC 1/MBC 1 of P. aeruginosa CIP 104116 (Gram−) |
|---|---|---|---|---|
| Ag0@SidendriPEG | 500/>500 | 250/>500 | 250/500–1000 | 250/>500 |
| Ag0@SidendriPEG | 50–62.5/>500 | 25–31.25/>500 | 50–62.5/250 | 25–31.25/250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M. Interplay between Nanoparticles and Phosphorus Dendrimers, and Their Properties. Molecules 2023, 28, 5739. https://doi.org/10.3390/molecules28155739
Caminade A-M. Interplay between Nanoparticles and Phosphorus Dendrimers, and Their Properties. Molecules. 2023; 28(15):5739. https://doi.org/10.3390/molecules28155739
Chicago/Turabian StyleCaminade, Anne-Marie. 2023. "Interplay between Nanoparticles and Phosphorus Dendrimers, and Their Properties" Molecules 28, no. 15: 5739. https://doi.org/10.3390/molecules28155739
APA StyleCaminade, A.-M. (2023). Interplay between Nanoparticles and Phosphorus Dendrimers, and Their Properties. Molecules, 28(15), 5739. https://doi.org/10.3390/molecules28155739






