Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings
Abstract
1. Introduction
2. Results and Discussion
2.1. Selecting the Sources of Information
2.2. Ethnobotanical Studies
| Author, Year | Place | Country | Cited Species | Use in Inflammation | Use in Pain | Pharmaceutical Form | Part Used | Preparation | Administration | Total of Informants |
|---|---|---|---|---|---|---|---|---|---|---|
| Ignacimuthu et al., 2006 [31] | Madurai, Tamil Nadu | India | P. coleoides Benth. | Wound healing | Labor pain (during pregnancy) | Juice, paste of leaves | Leaves | NR | Oral (drink), local administration | 12 |
| Maregesi et al., 2007 [32] | Bunda | Tanzânia | P. kilimandschari Gurke | Chest pain | Infusion | Leaves | NR | NR | 10 | |
| Ferreira, 2009 [25] | Marudá, Pará | Brazil | P. barbatus (Andrews) Benth. | Fever | Nonspecific pain, toothache | Fresh infusion, | Leaves | NR | Oral | 37 |
| Pereira et al., 2009 [28] | Ponta Porã, Mato Grosso | Brazil | P. barbatus (Andrews) Benth. | - | Recurrent pain | NR | NR | NR | NR | 137 |
| Cartaxo et al., 2010 [17] | Riacho Catingueira, Aiuaba, Ceará | Brazil | P. amboinicus (Lour.) Spreng | Bronchitis, uterine inflammation, inflammation of internal organs, nonspecific inflammation | Headache | Decoction, syrup, juice | NR | NR | Oral (drink) or bathing | 91 |
| Rahmatullah et al., 2010 [29] | Khulna | Bangladesh | P. barbatus (Andrews) Benth. | - | Cramps | NR | Whole plant | NR | NR | NR |
| Waruruai et al., 2011 [35] | Bougainville | Papua New Guinea | P. scutellarioides (L.) R. Br. | - | Headache | NR | NR | NR | Oral | 21 |
| Bieski et al., 2012 [15] | Pantanal, Mato Grosso | Brazil | P. amboinicus (Lour.) Spreng | Bronchitis, uterine inflammation | - | Infusion | NR | NR | NR | 262 |
| P. barbatus (Andrews) Benth. | - | Pain | Maceration | NR | NR | NR | ||||
| P. neochilus Schtr. | Labyrinthitis | Pain | Maceration | NR | NR | NR | ||||
| Furlanetto et al., 2012 [18] | Mandaguaçu, Paraná | Brazil | P. amboinicus (Lour.) Spreng | Gastritis | Headache | Maceration, decoction | Leaves | NR | NR | 220 |
| P. barbatus (Andrews) Benth. | Gastritis | Headache | Maceration, decoction | Leaves | NR | NR | ||||
| P. ornatos Codd. | Gastritis | Headache | Maceration, decoction | Leaves | NR | NR | ||||
| Ong and Kim, 2014 [20] | Ati Negrito, Guimaras | Filipinas | P. amboinicus (Lour.) Spreng | Asthma | - | Decoction | Leaves | NR | Oral | 65 |
| Bieski et al., 2015 [16] | Vale do Juruena, Legal Amazon, Mato Grosso | Brazil | P. amboinicus (Lour.) Spreng | Wound healing, fever, gastritis | Local pain | NR | NR | NR | NR | 383 |
| P. barbatus (Andrews) Benth. | Fever, labyrinthitis | Heartburn, pain, local pain, menstrual cramps | NR | NR | NR | NR | ||||
| Oliveira et al., 2015 [26] | Oriximiná, Pará | Brazil | P. barbatus (Andrews) Benth. | Migraine | NR | Leaves | NR | NR | 35 | |
| Lemos et al., 2016 [19] | Barbalha, Ceará | Brazil | P. amboinicus (Lour.) Spreng | Bronchitis | Sore throat | Infusion, juice, syrup | Leaves | NR | NR | 54 |
| Li and Xing, 2016 [23] | Hainan | China | P. amboinicus (Lour.) Spreng | Abscess | Pain | Decoction | Leaves | NR | NR | 27 |
| Pedrollo et al., 2016 [21] | Jauaperi, Roraima | Brazil | P. amboinicus (Lour.) Spreng | - | Headache | NR | Leaves | NR | Oral | 62 |
| P. ornatus Codd. | - | Bellyache | NR | Leaves | NR | Oral | ||||
| Santana et al., 2016 [30] | Quilombo Salamina Putumujumar, Bahia | Brazil | P. neochilus Schtr. | - | Cramps | NR | NR | NR | NR | 74 |
| Penido et al., 2016 [27] | Imperatriz, Maranhão | Brazil | P. barbatus (Andrews) Benth. | Hepatite | Stomachache | Infusion | Leaves | NR | NR | 205 |
| Rajalakshmi et al., 2019 [22] | Thanjavur, Tamil Nadu | Índia | P. amboinicus (Lour.) Spreng | – | Headache | Juice | Leaves | 10 g of leaves with sesame oil | Topical use | 137 |
| Napagoda et al., 2018 [36] | Gampaha | Sri Lanka | P. zeylanicus Benth. | Fever | - | Decoction, infusion | Roots | NR | NR | 458 |
| Kidane et al., 2018 [33] | Ganta Afeshum, Tigray | Ethiopia | P. lanuginosus | Tonsillitis | - | NR | NR | NR | NR | 78 |
2.3. Pharmacological Studies
2.4. Methodological Quality/Risk of Bias Analysis
3. Materials and Methods
3.1. Review Outline and Data Selection, Procedure, and Analysis
3.2. Review Outline and Data Selection Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sales, M.D.C.; Sartor, E.B.; Gentilli, R.M. Ethnobotany and ethnopharmacology: Traditional medicine and the bioprospection of phytotherapics. Salus J. Health Sci. 2015, 1, 17–25. [Google Scholar] [CrossRef]
- Saggar, S.; Mir, P.A.; Kumar, N.; Chawla, A.; Uppal, J.; Shilpa, S.; Kaur, A. Traditional and Herbal Medicines: Opportunities and Challenges. Pharmacogn. Res. 2022, 14, 107–114. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Berdigaliyev, N.; Aljofan, M. An overview of drug discovery and development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Marmitt, D.J.; Rempel, C.; Goettert, M.I.; Silva, A.D.C.E. Plantas Medicinais da RENISUS Com Potencial Anti-inflamatório: Revisão Sistemática Em Três Bases de Dados Científicas. Rev. Fitos 2015, 9, 129–144. [Google Scholar] [CrossRef][Green Version]
- Braun, J.; Baraliakos, X.; Westhoff, T. Nonsteroidal anti-inflammatory drugs and cardiovascular risk–a matter of indication. Semin. Arthritis Rheum. 2020, 50, 285–288. [Google Scholar] [CrossRef]
- Figueiredo, W.L.M.; Alves, T.C.A. Uso dos anti-inflamatórios não esteroides no controle da dor aguda. Rev. Neurociênc. 2015, 23, 463–467. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Conceição, L.D.O.D.; Oyama, E.M.; Furlan, M.R. Boldo verdadeiro x boldo falso: Caracterização morfoanatômica foliar. Visão Acad. 2017, 18. [Google Scholar] [CrossRef]
- Bandeira, J.; Barbosa, F.; Barbosa, L.; Rodrigues, I.; Bacarin, M.; Peters, J.; Braga, E. Composição do óleo essencial de quatro espécies do gênero Plectranthus. Rev. Bras. Plantas Med. 2011, 13, 157–164. [Google Scholar] [CrossRef]
- Mauro, C.; Silva, C.d.P.; Missima, J.; Ohnuki, T.; Rinaldi, R.B.; Frota, M. Estudo anatômico comparado de órgãos vegetativos de boldo miúdo, Plectranthus ornatus Codd. e malvariço, Plectranthus amboinicus (Lour.) Spreng.—Lamiaceae. Rev. Bras. Farm. 2008, 18, 608–613. [Google Scholar] [CrossRef]
- Santos, L.A.; Menezes, J.D.S.; Rufino, L.R.A.; Oliveira, N.D.M.S.; Fiorini, J.E. Determination of the antimicrobial activity in the hydroalcoholic extract of the plant Plectranthus ornatus Codd (Bilberry Chinese). Rev. Méd. Minas Gerais 2014, 24. [Google Scholar] [CrossRef]
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.-L.; Cuendet, M.; Christen, P. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46. [Google Scholar] [CrossRef]
- Freitas, M.; Amorim, A.; Bezerra, A.; Pereira, M.; Bessa, M.; Filho, F.N.; Lacerda, C. Crescimento e tolerância à salinidade em três espécies medicinais do gênero Plectranthus expostas a diferentes níveis de radiação. Rev. Bras. Plantas Med. 2014, 16, 839–849. [Google Scholar] [CrossRef]
- Mesquita, L.S.F.; Matos, T.S.; Ávila, F.D.N.; Batista, A.d.S.; Moura, A.F.; de Moraes, M.O.; da Silva, M.C.M.; Ferreira, T.L.A.; Nascimento, N.R.F.; Monteiro, N.K.V.; et al. Diterpenoids from Leaves of Cultivated Plectranthus ornatus. Planta Med. 2020, 87, 124–135. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Rios-Santos, F.; de Oliveira, R.M.; Espinosa, M.M.; Macedo, M.L.; Albuquerque, U.P.; de Oliveira Martins, D.T. Ethnopharmacology of Medicinal Plants of the Pantanal Region (Mato Grosso, Brazil). Evid. Based Complement. Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Leonti, M.; Arnason, J.T.; Ferrier, J.; Rapinski, M.; Violante, I.M.P.; Balogun, S.O.; Pereira, J.F.C.A.; Figueiredo, R.d.C.F.; Lopes, C.R.A.S.; et al. Ethnobotanical study of medicinal plants by population of Valley of Juruena Region, Legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 2015, 173, 383–423. [Google Scholar] [CrossRef]
- Cartaxo, S.L.; Souza, M.M.d.A.; de Albuquerque, U.P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. [Google Scholar] [CrossRef]
- Furlanetto, P.D.N.C.; Novakowski, G.C.; Correa, E.A. Folk medicine in Mandaguaçu municipality, Paraná State: An ethnobotanical approach. Acta Sci. Biol. Sci. 2012, 34, 463–471. [Google Scholar] [CrossRef][Green Version]
- Lemos, I.C.S.; Delmondes, G.d.A.; dos Santos, A.D.F.; Santos, E.S.; De Oliveira, D.R.; de Figueiredo, P.R.L.; Alves, D.d.A.; Barbosa, R.; de Menezes, I.R.A.; Coutinho, H.D.M.; et al. Ethnobiological survey of plants and animals used for the treatment of acute respiratory infections in children of a traditional community in the municipality of barbalha, ceará, Brazil. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 166–175. [Google Scholar] [CrossRef]
- Ong, H.G.; Kim, Y.-D. Quantitative ethnobotanical study of the medicinal plants used by the Ati Negrito indigenous group in Guimaras island, Philippines. J. Ethnopharmacol. 2014, 157, 228–242. [Google Scholar] [CrossRef]
- Pedrollo, C.T.; Kinupp, V.F.; Shepard, G.; Heinrich, M. Medicinal plants at Rio Jauaperi, Brazilian Amazon: Ethnobotanical survey and environmental conservation. J. Ethnopharmacol. 2016, 186, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, S.; Vijayakumar, S.; Arulmozhi, P. Ethnobotanical survey of medicinal plants in Thanjavur and its surrounding (Tamil Nadu—India). Acta Ecol. Sin. 2019, 39, 380–397. [Google Scholar] [CrossRef]
- Li, D.-L.; Xing, F.-W. Ethnobotanical study on medicinal plants used by local Hoklos people on Hainan Island, China. J. Ethnopharmacol. 2016, 194, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, T.M.G.; de Carvalho Correia, A.C.; dos Santos, R.V.; Lemos, R.P.L.; da Silva, S.A.S.; Barreto, E. The Use of Medicinal Plants in Maceió, Northeastern Brazil: An Ethnobotanical Survey. Medicines 2020, 7, 7. [Google Scholar] [CrossRef]
- Coelho-Ferreira, M. Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil). J. Ethnopharmacol. 2009, 126, 159–175. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Krettli, A.U.; Aguiar, A.C.C.; Leitão, G.G.; Vieira, M.N.; Martins, K.S.; Leitão, S.G. Ethnopharmacological evaluation of medicinal plants used against malaria by quilombola communities from Oriximiná, Brazil. J. Ethnopharmacol. 2015, 173, 424–434. [Google Scholar] [CrossRef]
- Penido, A.B.; De Morais, S.M.; Ribeiro, A.B.; Silva, A.Z. Ethnobotanical study of medicinal plants in Imperatriz, State of Maranhão, Northeastern Brazil. Acta Amaz. 2016, 46, 345–354. [Google Scholar] [CrossRef]
- Pereira, Z.V.; Mussury, R.M.; de Almeida, A.B.; Sangalli, A. Medicinal Plants Used by Ponta Porã Community, Mato Grosso Do Sul State. Acta Sci. Biol. sci 2009, 31, 293–299. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Ferdausi, D.; Mollik, A.H.; Jahan, R.; Chowdhury, M.H.; Haque, W.M. A Survey of Medicinal Plants Used by Kavirajes of Chalna Area, Khulna District, Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 91–97. [Google Scholar] [CrossRef]
- de Santana, B.F.; Voeks, R.A.; Funch, L.S. Ethnomedicinal survey of a maroon community in Brazil’s Atlantic tropical forest. J. Ethnopharmacol. 2016, 181, 37–49. [Google Scholar] [CrossRef]
- Ignacimuthu, S.; Ayyanar, M.; Sivaraman, K.S. Ethnobotanical investigations among tribes in Madurai District of Tamil Nadu (India). J. Ethnobiol. Ethnomed. 2006, 2, 25. [Google Scholar] [CrossRef]
- Maregesi, S.M.; Ngassapa, O.D.; Pieters, L.; Vlietinck, A.J. Ethnopharmacological survey of the Bunda district, Tanzania: Plants used to treat infectious diseases. J. Ethnopharmacol. 2007, 113, 457–470. [Google Scholar] [CrossRef]
- Kidane, L.; Gebremedhin, G.; Beyene, T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern Zone of Tigray, Northern Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 64. [Google Scholar] [CrossRef]
- Parkash, V.; Kumar, D. Ethnomedicinal Uses of Some Plants of Kanag Hill in Shimla, Himachal Pradesh, India. Int. J. Res. Ayurveda Pharm. 2012, 3, 319–322. [Google Scholar]
- Waruruai, J.; Sipana, B.; Koch, M.; Barrows, L.R.; Matainaho, T.K.; Rai, P.P. An ethnobotanical survey of medicinal plants used in the Siwai and Buin districts of the Autonomous Region of Bougainville. J. Ethnopharmacol. 2011, 138, 564–577. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Sundarapperuma, T.; Fonseka, D.; Amarasiri, S.; Gunaratna, P. An Ethnobotanical Study of the Medicinal Plants Used as Anti-Inflammatory Remedies in Gampaha District, Western Province, Sri Lanka. Scientifica 2018, 2018, 9395052. [Google Scholar] [CrossRef]
- Chuchuca, C.C.; Quinche, A.R.S.; González, O.N.V.; Flores, L.S.H.; Guerrero, J.N.Q. Uso de Infusión de oreganón Plectranthus amboinicus (Lour.) Spreng y del vinagre en la crianza de pollos “Acriollados” (Gallus gallus domesticus) mejorados. Acta Agron. 2016, 65, 298–303. [Google Scholar] [CrossRef]
- Oliveira, R.d.A.G.d.; Lima, E.d.O.; de Souza, E.L.; Vieira, W.L.; Freire, K.R.L.; Trajano, V.N.; Lima, I.O.; Silva-Filho, R.N. Interference of Plectranthus amboinicus (Lour.) Spreng essential oil on the anti-Candida activity of some clinically used antifungals. Rev. Bras. Farm. 2007, 17, 186–190. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yu, H.-M.; Shie, J.-J.; Cheng, T.-J.R.; Wu, C.-Y.; Fang, J.-M.; Wong, C.-H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorganic Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef]
- Kumar, P.; Sangam; Kumar, N. Plectranthus amboinicus: A review on its pharmacological and pharmacognostical studies. A. J. Physiol. Biochem. Pharmacol. 2020, 10, 55–62. [Google Scholar] [CrossRef]
- Rice, L.; Brits, G.; Potgieter, C.; Van Staden, J. Plectranthus: A plant for the future? S. Afr. J. Bot. 2011, 77, 947–959. [Google Scholar] [CrossRef]
- Cruz, M.G.; Lopes Junior, M.L.; Freitas, M.C.C.; Freitas, A.C.G.d.A.; Santos, L.d.S.; Corrêa, M.J.C.; Araújo, R.N.M.; Silva, L.O.; Pinheiro, W.B.d.S. Levantamento etnobotânico de plantas medicinais na comunidade Guajará de Carapajó, Cametá-pa. Open Sci. Res. VII 2022, 7, 1408–1431. [Google Scholar]
- Farias, O.T.; Nascimento, L.C.; Oliveira, F.S.; Santos, M.D.R.; Bruno, R.L.A. Essential Oil of Andiroba (Carapa guianensis Aubl.) and Copaiba (Copaifera langsdorffi Desf) on Health and Physiology of Seeds Macassar Bean (Vigna unguiculata L. Walp). Rev. Bras. Plantas Med. 2016, 18, 629–635. [Google Scholar]
- Pasa, M.C. Local knowledge and folk medicine: Ethnobotany in Cuiabá, Mato Grosso, Brazil. Bol. Mus. Para. Emílio Goeldi. Ciênc. hum. 2011, 6, 179–196. [Google Scholar] [CrossRef][Green Version]
- Dos Santos, A.G.S.; Dos Santos, A.B.S. Uso popular de plantas medicinais para tratamento de distúrbios gastrointestinais. Res. Soc. Dev. 2020, 9, e91891110560. [Google Scholar] [CrossRef]
- Junior, A.A.d.S.; Tavares, R.M.; Lara, R.P.; Faleiros, B.E.; Gomez, R.S.; Teixeira, A.L. Frequência dos tipos de cefaleia no centro de atendimento terciário do Hospital das Clínicas da Universidade Federal de Minas Gerais. Rev. Assoc. Med. Bras. 2012, 58, 709–713. [Google Scholar] [CrossRef]
- Silva, D.M.d.; Mocelin, K.R. O Cuidado de Enfermagem Ao Cliente Portador de Feridas Sob a Ótica Do Cuidado Transcultural. Nursing 2007, 9, 81–88. [Google Scholar]
- Souza, D.R.; Rodrigues, E.C.A.M.D.S. Plantas medicinais: Indicação de raizeiros para o tratamento de feridas. Rev. Bras. Em Promoção Saúde 2016, 29, 197–203. [Google Scholar] [CrossRef]
- Rodrigues, F.F.G.; Costa, J.G.M.; Campos, A.R. Study of the Interference between Plectranthus Species Essential Oils from Brazil and Aminoglycosides. Evid.-Based Complement. Altern. Med. 2013, 2013, 724161. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; Thipe, V.C.; Katti, K.V.; Mandiwana, V.; Kalombo, M.L.; Ray, S.S.; Rikhotso, R.; Janse van Vuuren, A.; Esmear, T.; Lall, N. Targeting Acne Bacteria and Wound Healing In Vitro Using Plectranthus Aliciae, Rosmarinic Acid, and Tetracycline Gold Nanoparticles. Pharmaceuticals 2022, 15, 933. [Google Scholar] [CrossRef]
- Chang, J.-M.; Cheng, C.-M.; Hung, L.-M.; Chung, Y.-S.; Wu, R.-Y. Potential Use of Plectranthus amboinicus in the Treatment of Rheumatoid Arthritis. Evid.-Based Complement. Altern. Med. 2010, 7, 115–120. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Huang, T.-H.; Chiu, C.-S.; Lu, T.-C.; Chen, Y.-W.; Peng, W.-H.; Chen, C.-Y. Analgesic and Antiinflammatory Activities of the Aqueous Extract from Plectranthus amboinicus (Lour.) Spreng. Both In Vitro and In Vivo. Evid. -Based Complement. Altern. Med. 2012, 2012, 508137. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S.; Sleem, A.A. Polyphenolics content and biological activity of Plectranthus amboinicus (Lour.) spreng growing in Egypt (Lamiaceae). Pharmacogn. J. 2012, 4, 45–54. [Google Scholar] [CrossRef]
- Gurgel, A.P.A.D.; da Silva, J.G.; Grangeiro, A.R.S.; Oliveira, D.C.; Lima, C.M.; da Silva, A.C.; Oliveira, R.A.; Souza, I.A. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J. Ethnopharmacol. 2009, 125, 361–363. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Cheng, C.-P.; Chang, D.-M. Plectranthus amboinicus Attenuates Inflammatory Bone Erosion in Mice with Collagen-induced Arthritis by Downregulation of RANKL-induced NFATc1 Expression. J. Rheumatol. 2011, 38, 1844–1857. [Google Scholar] [CrossRef]
- Duraisamy, P.; Manikandan, B.; Koodalingam, A.; Munusamy, A.; Ramar, M. Anti-inflammatory, anti-nociceptive and anti-oxidant activities of carvacrol containing leaf extracts of edible Indian borage plant Plectranthus amboinicus: An in vivo and in vitro approach. Comp. Clin. Pathol. 2021, 30, 397–413. [Google Scholar] [CrossRef]
- Harefa, K.; Sulastri, D.; Nasrul, E.; Ilyas, S. Analysis of Several Inflammatory Markers Expression in Obese Rats given Plectranthus amboinicus (Lour.) Spreng Ethanol Extract. Pharmacogn. J. 2021, 13, 172–178. [Google Scholar] [CrossRef]
- Leu, W.J.; Chen, J.C.; Guh, J.H. Extract from Plectranthus amboinicus Inhibit Maturation and Release of Interleukin 1β through Inhibition of NF-ΚB Nuclear Translocation and NLRP3 Inflammasome Activation. Front. Pharmacol. 2019, 10, 446300. [Google Scholar] [CrossRef]
- Manjamalai, A.; Alexander, T.; Grace, V.B. Bioactive evaluation of the essential oil of plectranthus amboinicus by gc-ms analysis and its role as a drug for microbial infections and inflammation. Int. J. Pharm. Pharm. Sci. 2012, 4, 205–211. [Google Scholar]
- Ravikumar, V.R.; Dhanamani, M.; Sudhamani, T. In-vitro anti- inflammatory activity of aqueous extract of leaves of Plectranthus amboinicus (Lour.) Spreng. Anc. Sci. Life 2009, 28, 7–9. [Google Scholar] [PubMed]
- Kapewangolo, P.; Hussein, A.A.; Meyer, D. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. J. Ethnopharmacol. 2013, 149, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Mazumder, A.; Bucar, F.; Veeresham, C.; Asres, K. Chemical Composition and Biological Activities of the Essential Oil of Plectranthus caninus Roth. Pharmacogn. J. 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Nicolas, M.; Lasalo, M.; Chow, S.; Antheaume, C.; Huet, K.; Hnawia, E.; Guillemin, G.J.; Nour, M.; Matsui, M. Anti-inflammatory activities of Coleus forsteri (formerly Plectranthus forsteri) extracts on human macrophages and chemical characterization. Front. Pharmacol. 2023, 13, 1081310. [Google Scholar] [CrossRef]
- Menon, D.B.; Sasikumar, J.M. Anti Inflammtory and Cytotoxic Activity of Methanolic Extract of Plectranthus Hadiensis Stem. Antioxidants from Ethiopian Plants View Project Antioxidant Activities of Under-Exploited Fruits from the Nilgiris. View Project. Available online: https://www.researchgate.net/publication/216675059_Anti_inflammtory_and_cytotoxic_activity_of_methanolic_extract_of_Plectranthus_hadiensis_stem (accessed on 19 July 2023).
- Menon, D.; Sasikumar, J.; Gopalakrishnan, V.K. Antioxidant and Anti-Inflammatory Properties of Terpenoid Fraction Isolated from the Shoot of Plectranthus Hadiensis. Int. J. Pharma. Bio. Sci. 2014, 5, B197–B205. [Google Scholar]
- Schultz, F.; Osuji, O.F.; Wack, B.; Anywar, G.; Garbe, L.-A. Antiinflammatory Medicinal Plants from the Ugandan Greater Mpigi Region Act as Potent Inhibitors in the COX-2/PGH2 Pathway. Plants 2021, 10, 351. [Google Scholar] [CrossRef]
- Rêgo, M.; Franco, E.; Oliveira, R.; Linden, L.; Silva, V.; Maia, C.; Teixeira, M.; Marinho, M.; Lima, E. Evaluation of tissue repair using phytotherapeutic gel from Plectranthus neochilus, Schlechter (boldo-gambá) and Cnidoscolus quercifolius Pohl (favela) in Wistar rats. Arq. Bras. Med. Vet. Zootec. 2021, 73, 395–405. [Google Scholar] [CrossRef]
- Fakhriati, S.G.; Ida, M.; Moektiwardoyo, M.; Ayu, A.C. Inhibition of Nitric Oxide Production in Lipopolysaccharide-Induced Macrophages Cell by Plectranthus scutellarioides (L.) R.Br Leaves. Res. J. Chem. Environ. 2018, 22, 38–42. [Google Scholar]
- Napagoda, M.; Gerstmeier, J.; Wesely, S.; Popella, S.; Lorenz, S.; Scheubert, K.; Svatoš, A.; Werz, O. Inhibition of 5-lipoxygenase as anti-inflammatory mode of action of Plectranthus zeylanicus Benth and chemical characterization of ingredients by a mass spectrometric approach. J. Ethnopharmacol. 2014, 151, 800–809. [Google Scholar] [CrossRef]
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; Lorenz, S.; De Soyza, S.; Qader, M.; Nagahawatte, A.; Wijayaratne, G.B.; Schneider, B.; Svatoš, A.; et al. Plectranthus zeylanicus: A Rich Source of Secondary Metabolites with Antimicrobial, Disinfectant and Anti-Inflammatory Activities. Pharmaceuticals 2022, 15, 436. [Google Scholar] [CrossRef]
- Arcila-Lozano, C.C.; Loarca-Piña, G.; Lecona-Uribe, S.; González de Mejía, E. El Orégano: Propiedades, Composición y Actividad Biológica de Sus Componentes. Arch. Latinoam. Nutr. 2004, 54, 100–111. [Google Scholar]
- Galvão, T.F.; Pansani, T.D.S.A.; Harrad, D. Principais itens para relatar Revisões sistemáticas e Meta-análises: A recomendação PRISMA. Epidemiol. Serv. Saude. 2015, 24, 335–342. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Delmondes, G.; Bezerra, D.S.; Dias, D.D.Q.; Borges, A.D.S.; Araújo, I.M.; da Cunha, G.L.; Bandeira, P.F.R.; Barbosa, R.; Coutinho, H.D.M.; Felipe, C.F.B.; et al. Toxicological and pharmacologic effects of farnesol (C15H26O): A descriptive systematic review. Food Chem. Toxicol. 2019, 129, 169–200. [Google Scholar] [CrossRef]


| Species | Number of Citations | RFC | Use in Inflammation | Use in Pain Conditions |
|---|---|---|---|---|
| Plectranthus amboinicus (Lour.) Spreng. | 9 | 0.23 | Wound healing, fever, gastritis, bronchitis, uterine inflammation, inflammation of internal organs, nonspecified inflammation, abscess, asthma | Headache, sore throat, local pain, nonspecified pain |
| Plectranthus barbatus (Andrews) Benth. | 8 | 0.20 | Fever, gastritis, hepatitis, labyrinthitis | Heartburn, menstrual cramps, headache, toothache, stomachache, nonspecific pain, local pain, recurrent pain, migraine |
| Plectranthus neochilus Schltr. Schtr. | 2 | 0.05 | Labyrinthitis | Cramps, pain |
| Plectranthus coleoides Benth. | 1 | 0.03 | Wound healing | Labor pain |
| Plectranthus kilimandschari (Gürke ex Engl.) H.I.Maass Gurke | 1 | 0.03 | - | Chest pain |
| Plectranthus lanuginosus (Hochst. ex Benth.) Agnew | 1 | 0.03 | Tonsillitis | - |
| Plectranthus ornatus Codd. | 2 | 0.05 | Gastritis | Bellyache, headache |
| Plectranthus rugosus Wall. ex Benth. | 1 | 0.03 | Wound healing | - |
| Plectranthus scutellarioides (L.) R. Br. | 1 | 0.03 | - | Headache |
| Plectranthus zeylanicus Benth. | 1 | 0.03 | Fever | - |
| Species | Author Year | Objective of the Study | Type of Study | Protocol Used | Type of Extract | Part of the Plant Used | Chemical Component | Findings |
|---|---|---|---|---|---|---|---|---|
| P. amboinicus (Lour.) Spreng | Gurgel et al., 2009 [55] | Evaluate the anti-inflammatory and antitumor activities. | In vivo | Carrageenan-induced paw edema, sarcoma-180 and Erlich’s ascites carcinoma cancer models. | Hydroalcoholic extract | Leaves | NR | Animals treated with the extract showed a significant decrease in edema at 150, 250, and 350 mg/kg (i.p.). The extract also inhibited the growth of sarcoma-180 and Ehrlich’s ascites tumors. |
| P. amboinicus (Lour.) Spreng | Ravikumar et al., 2009 [61] | Evaluate the anti-inflammatory activity. | In vitro | Human red blood cell (HRBC) membrane stabilization assay. | Aqueous extract | Leaves | NR | The extract (500 µg/mL) showed significant anti-inflammatory activity, comparable to hydrocortisone sodium. |
| P. amboinicus (Lour.) Spreng | Chang et al., 2010 [52] | Investigate the anti-inflammatory activity in a rheumatoid arthritis (RA) model. | In vivo | In collagen-induced arthritis (ASD) model, the following parameters were evaluated: serum levels of anti-collagen IgG, IgM, and C-reactive protein (CRP), concentrations of TNF-α, IL-6, and IL-1β production in peritoneal exudate (PEC) cells. | Aqueous extract | Whole plant | NR | The plant extract treatment significantly inhibited paw swelling and arthritis symptoms. Rats treated with the highest extract dose presented significantly reduced IgM, CRP, TNF-α, IL-6, and IL-1β levels. |
| P. amboinicus (Lour.) Spreng | Hsu et al., 2011 [56] | Investigate the effects on osteoclastogenesis and inflammatory bone erosion in mice in a collagen-induced arthritis model. Identify the active component of the plant involved in the regulation of osteoclastogenesis. | In vitro and in vivo | Cell viability was determined in an MTT assay. Bone marrow macrophages and RAW 264.7 had the expression transcriptional factors analyzed by immunofluorescence, collagen-induced arthritis in mice was assessed through the quantification of IL-1ß and TNF-α, analysis of arthritic index, paw thickness, and histopathological evaluation. | Crude extract | Leaves | Rosmarinic acid | P. amboinicus significantly inhibited bone resorption by mature osteoclasts. Rosmarinic acid showed potent inhibition of NF-κB NFATc1 in RANKL-stimulated BMM and inhibited RANKL-induced formation of TRAP-positive multinucleated cells. |
| P. amboinicus (Lour.) Spreng | Chiu et al., 2012 [53] | Investigate the analgesic and anti-inflammatory properties of the aqueous extract and essential oil. | In vivo and in vitro | The analgesic effect was evaluated in acetic acid and formalin models. The anti-inflammatory activity was assessed in carrageenan-induced paw edema by evaluating oxidative stress, cytokine production, and protein expression in tissue homogenates and cultures of LPS-stimulated RAW 264.7 cells. | Aqueous extract and essential oil | Whole plant | Carvacrol, thymol, α-humulene, undecanal, C-terpinene, R-cymene, caryophyllene oxide, α-terpineol, and β-seline | The reduced abdominal contortions and paw-licking behavior demonstrated analgesic activity. The anti-inflammatory effect was due to the modulation of antioxidant enzymes in the liver and decreased levels of malondialdehyde (MDA), TNF-α, and cyclooxygenase 2 (COX-2). In vitro, the treatment inhibited cytokine production and prevented NF-κB activation. |
| P. amboinicus (Lour.) Spreng | El-Hawary et al., 2012 [54] | Investigate the phenolic content and evaluate the antioxidant, anti-inflammatory, analgesic, diuretic, cytotoxic, and antimicrobial activities. | In vivo and in vitro | Chemical analysis was performed through UPLC–MS. In vivo testing evaluated LD50, blood glutathione levels in diabetic animals, carrageenan-induced paw edema, contortions induced by acetic acid, and diuretic effect. The antimicrobial effect was determined through the agar diffusion method. | Alcoholic, aqueous, and hydroalcoholic extracts | Stem, leaves, and roots | Caffeic acid, rosmarinic acid, coumaric acid, crosseriol, stem, luteolin, quercithin, and eryodithiol | The different extracts showed antioxidant, anti-inflammatory, analgesic, diuretic, cytotoxic, and antimicrobial activities, although their potency varied significantly. |
| P. amboinicus (Lour.) Spreng | Manjamalai et al., 2012 [60] | Evaluate the antimicrobial and anti-inflammatory activity. | In vivo and in vitro | The antimicrobial activity was evaluated through the determination of the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC), the anti-inflammatory activity was assessed in xylene-induced ear edema, carrageenan-induced paw edema, and ovalbumin-induced allergic inflammation. | Essential oil | Leaves | Carvacrol, thymol, cys-caryophyllene, t-caryophyllene, and p-cymene | The essential oil had promising antimicrobial effects against bacteria and fungi and inhibited the inflammatory response triggered by different harmful stimuli. |
| P. amboinicus (Lour.) Spreng | Chen et al., 2014 [40] | Identify the constituents and evaluate the anti-inflammatory effect. Prepare analogs to maximize the anti-inflammatory effect. | In vitro | The AP-1 binding affinity in TPA-treated HeLa cells and TNF-α expression by LPS-stimulated human histiocytic lymphoma U-937 cells were evaluated through an isolation-guided bioassay. The cytotoxicity of the human fibroblast cell line Detroit551 cells was determined in an MTT assay. | Hexane and aqueous extracts | Leaves and stem | 1-2-(3,4-dihydroxybenzylidenyl)-3-(3,4-dihydroxyphenyl)-4-hydroxypentanedioic acid, shimobashiric acid, salvianolic acid, rosmarinic acid (2-alkylidenenyl-4-cyclopentene-1), 3-diones), thymoquinone | P. amboinicus extract inhibited the binding of AP-1 to its consensual DNA sequence. Tymquinone, isolated from the hexane extract, suppressed TNF-α expression, indicating in vitro anti-inflammatory activity. |
| P. amboinicus (Lour.) Spreng | Leu et al., 2019 [59] | Analyze the anti-inflammatory mechanism of compounds extracted from P. amboinicus in the NLRP3 inflammasome signaling pathway. | In vitro | Phorbol-12-myristate with 13-acetate (PMA)-differentiated and LPS-stimulated THP-1 monocytic leukemia cells were used to examine the effect of PA-F4, a P. amboinicus extract, on the inflammasome signaling pathway. | NR | NR | Rosmarinic acid, cirsimaritin, salvigenin, carvacrol | PA-F4 inhibited ASC oligomerization, KC efflux, the caspase-1/ IL-1b/ IL-18 release reaction, and NF-kB p65 activation, demonstrating an interference with NLRP3- NF-kB signaling pathway in LPS-activated macrophages. |
| P. amboinicus (Lour.) Spreng | Duraisamy et al., 2021 [57] | Evaluate the anti-inflammatory activities. | In vivo and in vitro | The analgesic and anti-inflammatory effect was analyzed in the formalin test and correlated with the analysis of inflammatory mediator production and protein expression in vitro. | Aqueous and ethyl acetate extracts | Leaves | Carbohydrates, steroids, flavonoids, saponins, glycosides, terpenoids | The extracts inhibited nociceptive responses and the paw edema through the modulation of the inflammatory reaction, which was associated with a decrease in oxidative stress markers and inhibition of gene expression of iNOS, COX-2, IL-1β, histamine receptor 1, and NF-κB. In addition, P. amboinicus inhibited NO production by in vitro-stimulated macrophages. |
| P. amboinicus (Lour.) Spreng | Harefa et al., 2021 [58] | Analyze the effects of the treatment on ICAM-1, VCAM-1, and CD40 expression in obese rats. | In vivo | Obesity was induced in Wistar rats through a standard diet of CP511 with the addition of a high-fat diet for 21 weeks. The expression of ICAM-1 and VCAM-1 in the plasma was analyzed by ELISA, while Immunohistochemistry was used to analyze CD40 expression in the aorta. | Ethanol extract | Leaves | NR | The treatment showed a mild decrease in ICAM-1 and VCAM-1 levels in the blood plasma. The same occurred with the expression of CD40 in the intimal layer of the aorta of treated rats. |
| P. barbatus Andrews | Kapewangolo et al., 2013 [62] | Investigate the antioxidant, anti-inflammatory, and anti-HIV-1 activities of the species. | In vitro | The anti-HIV-1 activity was assessed through inhibition of protease (PR) and reverse transcriptase (RT), cytotoxicity was evaluated in peripheral blood mononuclear cells (SPMC) and TZM cells, free radical-scavenging activity was used to assess antioxidant activity, while cytometric matrix Th1/Th2/Th17 cytokine production was used to determine the anti-inflammatory activity. | Ethanol extract | Leaves | NR | The extract inhibited HIV-1 PR with a CI50 of 62.0 μg/mL and induced cell proliferation in HIV-positive and HIV-negative cells. Finally, the extract showed a relevant antioxidant effect (CI50 = 16 μg/mL) and reduced the production of pro-inflammatory cytokines. |
| P. caninus Roth | Tadesse et al., 2011 [63] | Characterize the chemical composition of P. caninus essential oil and investigate its antioxidant and anti-inflammatory activities. | In vivo and in vitro | Gas chromatography coupled to mass spectrometer (GC–MS), determination of the minimum inhibitory concentration (MIC), antioxidant activity by the 2,2-diphenyl-1-picrylhydrazi DPPH method, carrageenan-induced paw edema. | Essential oil | NR | Camphor and α-thurjene | The essential oil showed significant activity against a broad spectrum of pathogens, including Gram-positive and Gram-negative bacteria and some fungal strains. The extract showed a concentration-dependent DPPH-scavenging activity and inhibited paw edema in the late phase of inflammation. |
| P. forsteri Benth. | Nicolas et al., 2023 [64] | Investigate the chemical composition and anti-inflammatory potential of P. forsteri. | In vitro | Primary culture of human monocyte-derived macrophages THP-1 cells were assessed for cytotoxicity and production of TNF-α, IL-6, IL-10, and IL-1β. | Ethanolic (ePE) and cyclohexane (cPE) extract of C. forsteri | Whole plant | Coleon U (1), coleon U-quinone (2), 8α,9α-epoxycoleon U-uinone (3), 7α- hydroxyroyleanone (4), 6β,7α-dihydroxyroyleanone (5), 7α-acetoxy-6β- hydroxyroyleanone (6) and 7α-formyloxy-6β-hydroxyroyleanone (7) | Both extracts significantly inhibited cytokine production in LPS-stimulated THP-1 cells and human macrophages. |
| P. hadiensis (Forssk.) Schweinf. ex Sprenger | Menon et al., 2011 [65] | Investigate possible anti-inflammatory and cytotoxic activities. | In vitro | Analysis of ADP-induced platelet aggregation, human red blood cell (HRBC) membrane stabilization assay, MTT cytotoxicity test. | Methanolic extract | NR | NR | The extract significantly inhibited platelet aggregation and promoted membrane stabilization of HRBC, which was comparable in magnitude to the standard drug diclofenac. |
| P. hadiensis (Forssk.) Schweinf. ex Sprenger | Menon et al., 2014 [66] | Investigate the antioxidant and anti-inflammatory activities of the terpenoid fraction isolated from P. hadiensis. | In vitro | DPPH assay for antioxidant activity, reduction capacity by the potassium ferricyanide reduction method, nitric oxide elimination capacity, evaluation of bovine serum albumin, stabilization of the erythroblast membrane of red blood cells. | Aqueous and ethanol extract | NR | NR | The terpenoid fraction exhibited significant free radical-scavenging activity. |
| P. hadiensis (Forssk.) Schweinf. ex Sprenger | Schultz et al., 2021 [67] | Investigate the anti-inflammatory, antioxidant, and antibacterial activities. | In vitro | In vitro screening for the selective inhibition of COX-2, COX-1, and 15-LOX. Evaluation of the growth of multidrug-resistant Staphylococcus aureus, Listeria innocua, Listeria monocytogenes, and Escherichia coli K12, DPPH assay for antioxidant activity, and determination of total phenolic content (TCT). | Diethyl ether extract | Leaves | NR | Nine extracts, including the ethyl ether extract of P. hadiensis, were active as COX-2 inhibitors (IC50 < 20 g/mL). There was no counteractivity between the inhibition of COX-2 and 15-LOX in these extracts. No relevant activity was observed regarding the other analysis. |
| Plectranthus madagascariensis var. aliciae Codd | Lambrechts et al., 2022 [51] | Investigate the antibacterial effects and healing potential of gold nanoparticle-encapsulated P. aliciae extract, compound rosmarinic acid (AuNPRA), and tetracycline (AuNPTET). | In vitro | The antibacterial activity of nanoparticles was tested against C. Acnes (ATCC® 6919), S. epidermidis (ATCC® 35984), and a combination of C. acnes and S. epidermidis under anaerobic and aerobic growth conditions. The cytotoxicity and wound healing potential were also evaluated using human keratinocytes (HaCaT). | Ethanolic | Leaves | Rosmarinic acid + gold nanoparticles | None of the nanoparticles presented antibacterial or antibiofilm against C. acnes and S. epidermis. However, they showed significant wound healing potential. Rosmarinic acid showed effectiveness at the highest concentration (500 g/mL). |
| P. neochilus Schltr. | Rêgo et al., 2021 [68] | Investigate the effects of a gel formulation containing the combination of P. neochilus and Cnidoscolus quercifolius in tissue repair in rat skin wounds. | In vivo | Tissue repair in skin wounds of rats. | Hydroalcoholic extract | Slum Bark | NR | The macroscopic evaluation revealed angiogenic potential. The histomorphometry of the skin revealed reepithelialization of the epidermis and superficial dermis with longitudinal collagen fibers, fibroblasts, and blood vessels. The deeper dermis was marked with transverse and longitudinal collagen fibers, blood vessels, and inflammatory cells. |
| P. scutellarioides (L.) R.Br. | Fakhriati et al., 2018 [69] | Determine the anti-inflammatory activity. | In vitro | Nitrite quantification through the Griess method. | Ethanolic, ethyl acetate, and aqueous extract | Leaves | NR | The ethanol extract showed the most potent inhibitory effect on nitrite production by macrophages. |
| P. zeylanicus Benth. | Napagoda et al., 2014 [70] | Investigate the effects on 5-LOX activity and free radical scavenging by P. zeylanicus extracts and analyze their chemical constituents. | In vitro | Evaluation of bioactivity: 5-lipoxygenase (5-LOX) activity in intact neutrophils and whole blood, activity of 5-LOX in cell-free assays using semipurified 5-LOX, DPPH assay for antioxidant activity, measurement of reactive oxygen species in neutrophils. Phytochemical screening: Bioassay-guided fractionation, Liquid chromatography coupled to mass spectrometry analysis, gas chromatography coupled to mass spectrometry analysis. | Hexane, dichloromethane, ethyl, and methanolic extracts | Whole plant | Coleone P, cinasassiol A and C and caloric acid | Regarding the pharmacological activities, the hexane and dichloromethane extracts of P. zeylanicus showed a suppressive effect of 5-LOX in stimulated human neutrophils and the recombinant human isolate of 5-LOX. |
| P. zeylanicus Benth. | Napagoda et al., 2022 [71] | Evaluate the antimicrobial activity of different extracts of P. zeylanicus and characterize bioactive secondary metabolites. | In vitro | The antibacterial activity of the purified compound against S. aureus, S. saprophyticus, E. faecalis, S. typhi, P. aeruginosa, and nine clinical isolates of methicillin-resistant S. aureus was determined by the broth microdilution method, cell-based 5-LOX activity assay | n-hexane, dichloromethane (DCM), ethyl acetate (EtOAc), and methanolic | NR | 7-acetoxy-6-hydroxyroyleanone | The dichloromethane extract (DCM) showed a potent effect against several bacterial species. The isolated compound showed strong antibacterial activity against methicillin-resistant Staphylococcus aureus and inhibited 5-LOX in free and cell-based assays. |
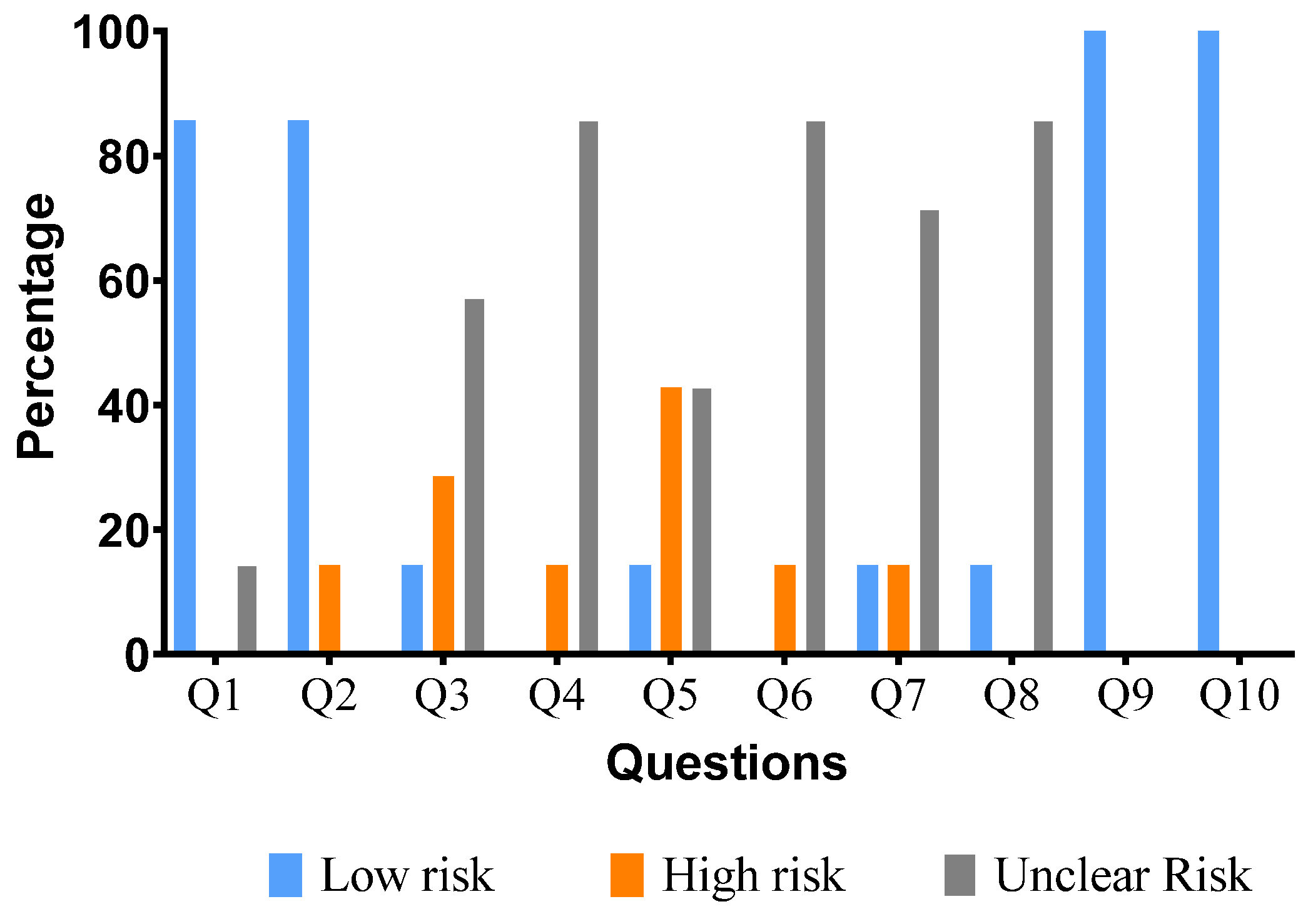
| Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Gurgel et al., 2009 [55] | + | + | ? | ? | - | ? | - | ? | + | + |
| Chang et al., 2010 [52] | + | + | - | ? | + | ? | + | ? | + | + |
| Hsu et al., 2011 [56] | + | + | + | ? | ? | ? | ? | + | + | + |
| Tadesse et al., 2011 [63] | + | + | ? | ? | ? | ? | ? | ? | + | + |
| Chiu et al., 2012 [53] | ? | - | ? | - | - | - | ? | ? | + | + |
| El-Hawary et al., 2012 [54] | + | + | - | ? | - | ? | ? | ? | + | + |
| Manjamalai et al., 2012 [60] | + | + | ? | ? | ? | ? | ? | ? | + | + |
| Duraisamy et al., 2021 [57] | + | + | ? | ? | ? | ? | ? | ? | + | + |
| Label: | ||||||||||
| Yes | + | Low risk of bias | ||||||||
| No | - | high risk of bias | ||||||||
| Not clear | ? | The risk of bias is not clear | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.d.O.; Wilairatana, P.; Leite, G.M.d.L.; Delmondes, G.d.A.; Silva, L.Y.S.d.; Júnior, S.C.A.; Dantas, L.B.R.; Bezerra, D.S.; Beltrão, I.C.S.L.d.; Dias, D.d.Q.; et al. Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings. Molecules 2023, 28, 5653. https://doi.org/10.3390/molecules28155653
Barbosa MdO, Wilairatana P, Leite GMdL, Delmondes GdA, Silva LYSd, Júnior SCA, Dantas LBR, Bezerra DS, Beltrão ICSLd, Dias DdQ, et al. Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings. Molecules. 2023; 28(15):5653. https://doi.org/10.3390/molecules28155653
Chicago/Turabian StyleBarbosa, Maysa de Oliveira, Polrat Wilairatana, Giovana Mendes de Lacerda Leite, Gyllyandeson de Araújo Delmondes, Lucas Yure Santos da Silva, Silvio Caetano Alves Júnior, Lindaiane Bezerra Rodrigues Dantas, Daniel Souza Bezerra, Izabel Cristina Santiago Lemos de Beltrão, Diógenes de Queiroz Dias, and et al. 2023. "Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings" Molecules 28, no. 15: 5653. https://doi.org/10.3390/molecules28155653
APA StyleBarbosa, M. d. O., Wilairatana, P., Leite, G. M. d. L., Delmondes, G. d. A., Silva, L. Y. S. d., Júnior, S. C. A., Dantas, L. B. R., Bezerra, D. S., Beltrão, I. C. S. L. d., Dias, D. d. Q., Ribeiro-Filho, J., Felipe, C. F. B., Coutinho, H. D. M., Menezes, I. R. A. d., & Kerntopf Mendonça, M. R. (2023). Plectranthus Species with Anti-Inflammatory and Analgesic Potential: A Systematic Review on Ethnobotanical and Pharmacological Findings. Molecules, 28(15), 5653. https://doi.org/10.3390/molecules28155653










