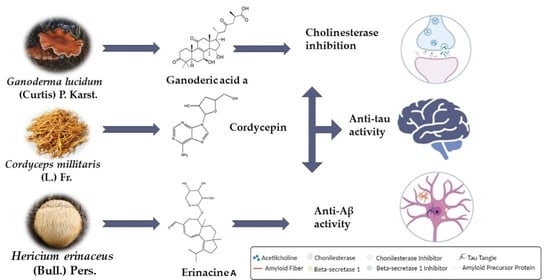
Tracing the Path between Mushrooms and Alzheimer’s Disease—A Literature Review
Abstract
1. Introduction
2. Mushrooms in Alzheimer’s Disease
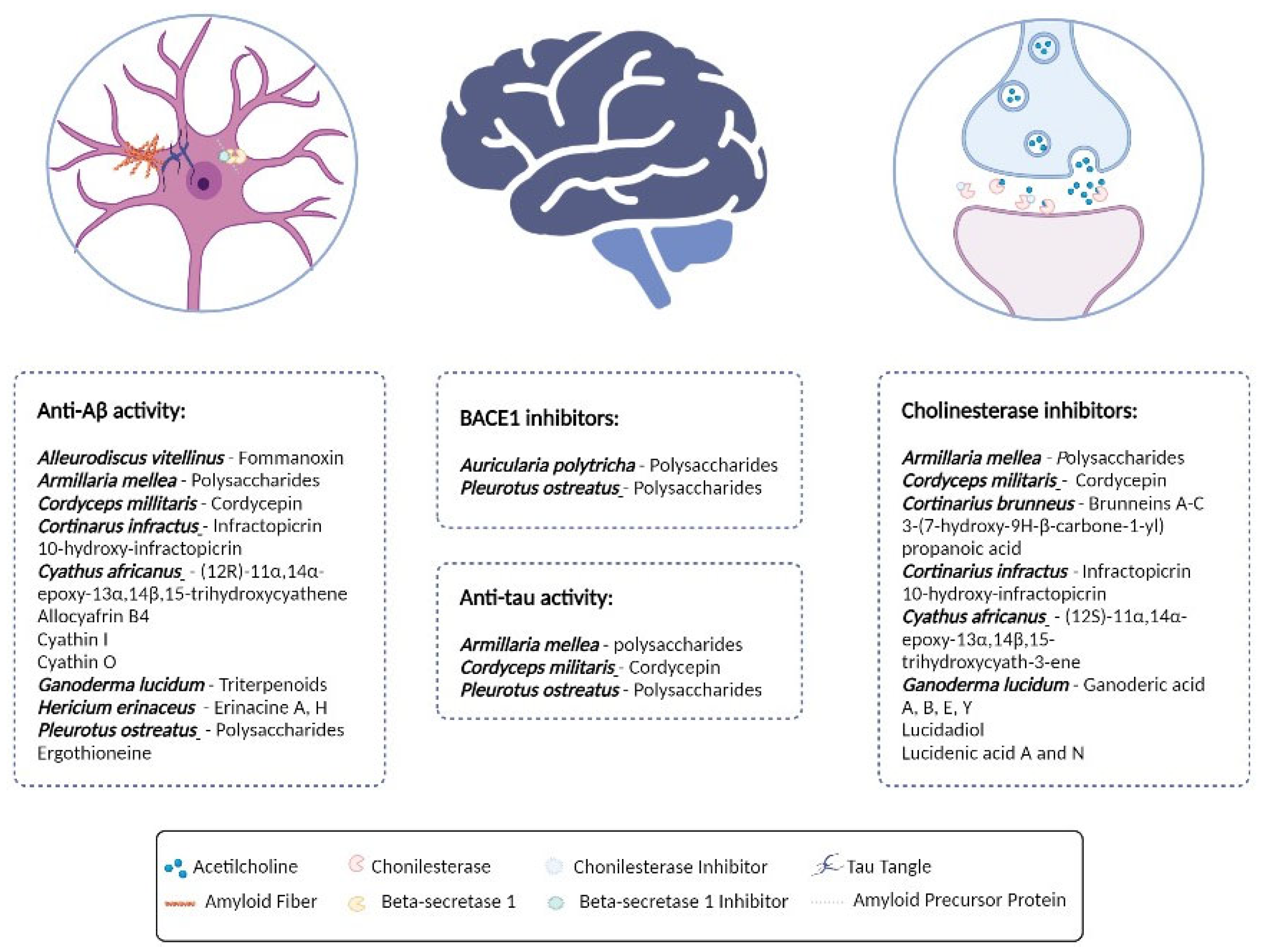
2.1. Inhibition of BACE1 and Prevention of Aβ Aggregation and Aβ Cytotoxicity
| Species | Body Part | Extract/ Compound | In Vitro Assays | In Vivo Assays | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| Aleurodiscus vitellinus (Lév.) Pat. | Cultured mycelium | Fomannoxin | Cytotoxicity by MTT; Microfluorimetry of cytosolic Ca2+; Immunofluorescence for hippocampal neurons; Thioflavin T binding assay for Aβ aggregation | ↓ Aβ-induced cytotoxicity; preservation of synaptic function in hippocampal neurons; ↓ Aβ binding to neurons | [54] | |
| Amanita caesarea (Scop.) Pers. | Sporocarp | Aqueous extract | HT22 neuron cells: Apoptosis (annexin V, propidium iodide); MTT; Intracellular ROS; Mitochondrial transmembrane potential (JC-1 staining); Aβ1–42 concentration, SOD, ROS levels by ELISA | AlCl3, D-galactose induced BALB/c mice: Morris water maze; Rotarod test; Locomotor activity test; Aβ1–42 in serum and brain of BALB/c mice measured by ELISA | ↓ Aβ1–42 in brain; ↑ Aβ1–42 in serum | [55] |
| Antrodia camphorate (M. Zang & C.H. Su) Sheng H. Wu, Ryvarden & T.T. Chang | Mycelium and Fruiting body | 95% ethanolic extract | PC12 cells: Cytotoxicity by MTT; oxidative stress by DPPH | Aβ40 induced Wistar rats: Morris water maze; Immunoblotting (Aβ and BACE1); IHC—Aβ40 in hippocampus | ↓ Aβ-induced cytotoxicity; ↓ Aβ-induced oxidative stress | [56] |
| Armillaria mellea (Vahl) P. Kumm. | Mycelium | Polysaccharides (precipitated in 70% ethanol) | D-galactose- (D-gal-) induced AD mouse model: Aβ in serum and hippocampus measured by ELISA | ↓ Aβ1–42 deposition | [57] | |
| Auricularia polytricha (Mont.) Sacc. | Whole | HPLC fractions—aqueous, 100% methanol and 10% ethanol | BACE1 inhibition | ↓ BACE1; | [46,58] | |
| Cordyceps militaris (L.) Fr. | Cordycepin (13) | Hippocampal neurons from rats: Acridine orange staining (Aβ25–35); Aβ cytotoxicity by MTT; ROS induction | ↓ Aβ25–35; ↓ Aβ-induced ROS; ↓ Aβ-induced cytotoxicity | [46] | ||
| Cortinarius infractus (Pers.) Fr. | Fruiting body | Infractopicrin (11) 10-hydroxy-infractopicrin (12) | HepG2 and SHSY5 cell lines: Cytotoxicity (CytoTox-One assay); AChE inhibition; Thioflavin T binding assay for Aβ aggregation | ↓ Aβ1–40 aggregation in the absence of AChE | [59] | |
| Cyathus africanus H.J. Brodie | Mycelium | (12R)-11α,14α-epoxy-13α,14β,15-trihydroxycyathene Allocyafrin B4 Cyathin I Cyathin O | BV2 microglial cells: Cytotoxicity by MTT; iNOS induction by Aβ1–42 | ↓ Aβ1–42 synthesis; ↓ Aβ-induced iNOS | [60] | |
| Ganoderma lucidum (Curtis) P. Karst. | Fruiting body | Aqueous | Neurons collected from embryonic Sprague Dawley rats: Caspase-like activity; TUNEL staining; Immunofluorescence staining for synaptophysin and Aβ-induced cytotoxicity | ↓ Aβ-induced synaptotoxicity by preserving the synaptic density protein, synaptophysin | [61] | |
| Whole | Biomass | SAMP8: Open field test Active avoidance test Aging scale | ↓ Aβ plaque formation | [48] | ||
| Triterpenoids | APP/PS1 mice: Hematoxylin–eosin staining; IHC; Morris water maze; Exploration test; Burrowing test; TUNEL staining for neuronal apoptosis; Western-blot for Bax, caspase-3, Nrf2, hemeoxidase, NQO1, ROCK-2, GAPDH; SOD; MDA; LDH; Flow cytometry for apoptosis; | ↓inhibitory effect of Aβ25–35 on neuron proliferation | [49] | |||
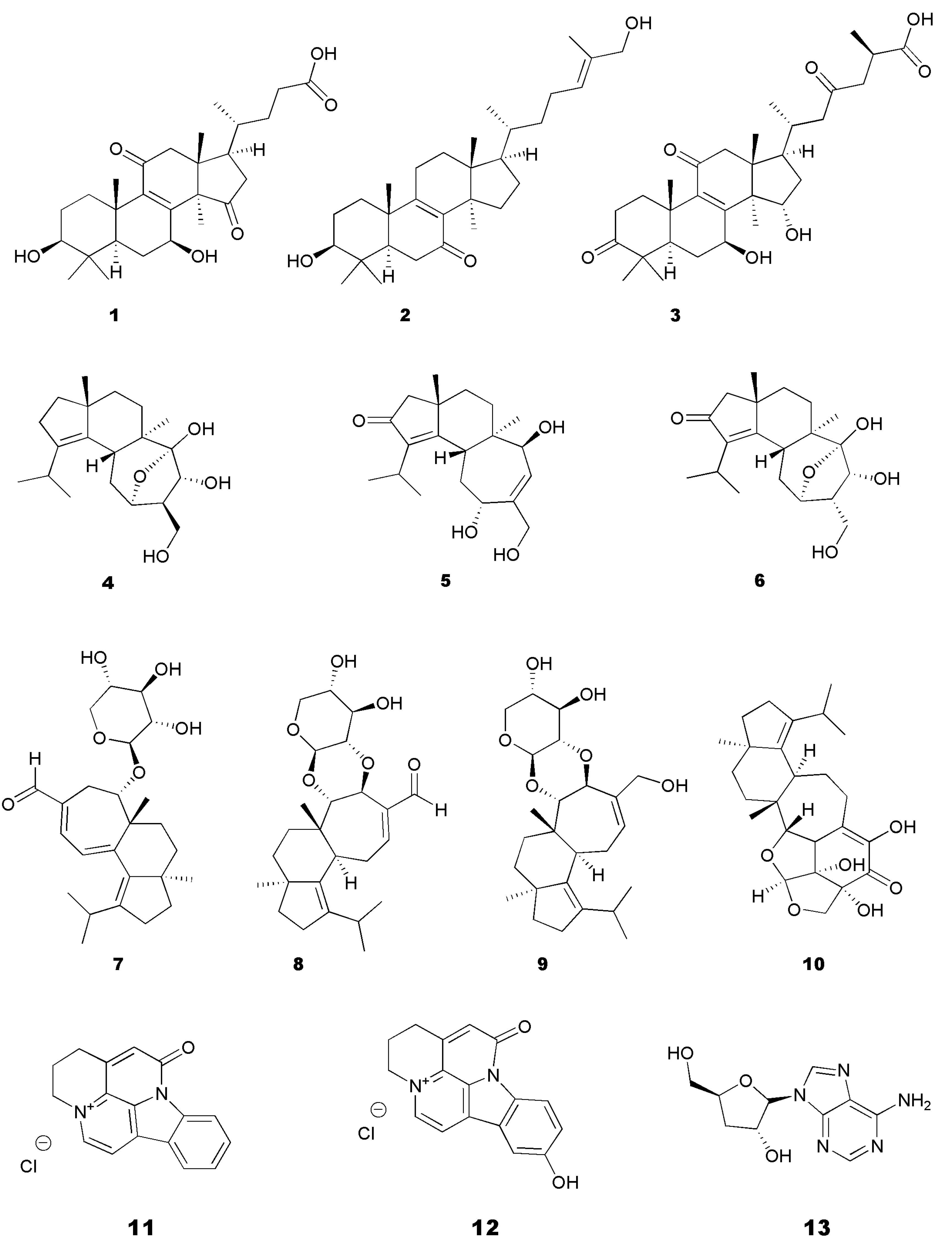
| Hericium erinaceus (Bull.) Pers. | Cultured mycelia | 90% Ethanolic extracts containing erinacine A (7), erinacine C (9), and erinacine S (10) | APPswe/PS1ΔE9 double transgenic mouse model (APP/PS1) of AD: Immunoblot; Thioflavin S and AB10 staining for plaque formation and size; ELISA for Aβ levels; Nesting test | ↓Aβ deposition; ↓ plaque size; ↑ Aβ-degrading enzymes IDE and neprilysin; ↑NGF | [52] | |
| Whole | Powdered | AlCl3-induced Wistar rats: Morris water maze; Elevated plus maze; Novel object recognition; IHC; Western blot | ↓ Aβ1–42; ↓ APP | [62] | ||
| Mycelia | Erinacine A (7) Erinacine H | APP/PS1 or SAMP 8 mice: Thioflavin S and AB10 staining for plaque formation and size; ELISA for Aβ; IHC; Immunostaining for Iba-1, AB10-P, and glial fibrillary acid protein; Nesting test; Burrowing test; Morris water maze | ↓Aβ deposition inhibition; ↓ microglial activation; ↑hippocampal neurogenesis ↑dendritic complexity; ↓ Aβ-induced iNOS | [53] | ||
| Mycelium | Erinacine A (7) enriched mycelium | SAMP8: Avoidance test; iNOS; TBARS; Aβ-aggregation; 8 OHdG for DNA damage | ↓ iNOS; ↓ Aβ aggregation | [63] | ||
| Pleurotus ostreatus (Jacq.) P. Kumm. | Whole | Polysaccharides | Wistar AlCl3/D-galactose induced: Morris water maze; Step-down memory test; Western blot for APP, BACE1, Aβ, and, PP2A in hippocampus tissue | ↓ Aβ formation; ↓ APP; ↓ BACE1; ↑PP2-A | [64] | |
| Ergothioneine (14) | HeLa cells: ROS measurement | 5XFAD mice Elevated zero maze; Locomotor activity; Rotarod; Novel object recognition; Fear conditioning; IHC; Dynamic PET imaging | ↓ Aβ formation | [65] |
2.2. AChE and BChE Inhibition
| Species | Body Part | Extract/ Compound | In Vitro Assays | In Vivo Assays | Refs. |
|---|---|---|---|---|---|
| Agaricus campestris L. | Fruiting body Whole | Methanol | AChE inhibition; BChE inhibition | [74] | |
| Amanita caesaria (Scop.) Pers. | Sporocarp | Aqueous | Enzyme inhibition of brain and blood ELISA for AChE, ACh, and ChAT | AlCl3, D-galactose BALB/c mice: Morris water maze; Rotarod test; Locomotor activity test | [55] |
| Amanita crocea (Quél.) Singer | Fruiting body | Methanolic | AChE inhibition; BChE inhibition | [75,76] | |
| Armillaria mellea (Vahl) P. Kumm. | Mycelium | Polysaccharides (precipitated in 70% ethanol) | AlCl3, D-galactose Balb/c mice: Morris water maze; Fatigue rotarod test; Autonomic activity test; Serum and hypothalamus ACh, AChE, and ChAT; Serum and hypothalamus oxidation status; TUNEL staining; Aβ in serum and hippocampus; IHC | [57] | |
| Cordyceps militaris (L.) Fr. | Cordycepin (13) | Rat hippocampal neurons: AChE activity induced by Aβ25–35 | [46] | ||
| Cortinarius brunneus (Pers.) Fr. | Fruiting body | Brunneins A-C 3-(7-hydroxy-9H-β-carbone-1-yl)propanoic acid | AChE inhibition; | [77] | |
| Cortinarius infractus (Pers.) Fr. | Fruiting body | Infractopicrin (11) 10-hydroxy-infractopicrin (12) | AChE inhibition; Molecular docking | [59] | |
| Cyathus africanus H.J. Brodie | Mycelium | (12S)-11α,14α-epoxy-13α,14β,15-trihydroxycyath-3-ene (4) Neocyathin B (5) Neocyathin J (6) | AChE inhibition; Molecular docking | [60,78] | |
| Cyclocybe cylindracea (DC.) Vizzini & Angelini | Fruiting body | Methanolic | AChE inhibition; BChE inhibition | [75] | |
| Ganoderma lucidum | Fruiting body | Aqueous | AChE inhibition | [72] | |
| (Curtis) P. Kumm. | Fruiting body | Ganoderic acid A (3), B, E, Y Ganodermadiol Ganodermanondiol Ganoderiol F Lucidadiol (2) Lucidenic acid A, N (1), Lucidumol B Methyl lucidenate E2 Methyl ganoderate A Methyl ganoderate A acetonide n-Butyl ganoderate H n-butyl lucidenate A n-butyl lucidenate N | AChE inhibition | [73] | |
| Hygrocybe acutoconica (Clem.) Singer | Fruiting body | Aqueous | AChE inhibition; BChE inhibition | [75] | |
| Inonotus obliquus (Ach. ex Pers.) Pilát | Lanostante type triterpenoids | AChE inhibition; BchE inhibition; Molecular docking | [79] | ||
| Morchella esculenta (L.) Pers. | Whole | Polysaccharides | AchE inhibition; BchE inhibition | [80,81] | |
| Neoboletus erythropus (Pers.) C. Hahn | Fruiting body | Aqueous | AChE inhibition | [75] | |
| Phellinus pini (Brot.) A. Ames | Fruiting body | 80% methanolic and hot water extracts | AChE inhibition; BChE inhibition | Sprague Dawley rat: Carrageenin-induced hind-paw edema | [82] |
| Pleurotus ostreatus (Jacq.) P. Kumm. | Fruiting body | Aqueous | AChE inhibition | [75] | |
| Russula aurea Pers. | Fruiting body | Aqueous | AChE inhibition | [75] | |
| Russula sanguinea (Bull.) Fr. | Fruiting body | Aqueous | AChE inhibition in solid and liquid | [75] | |
| Trametes versicolor (L.) Lloyd | Mycelia and fruiting body | DMSO, ethanol, or sodium phosphate solution extracts | AChE inhibition | [83] | |
| Trametes gibbosa (Pers.) Fr. | Mycelia and fruiting body | DMSO, ethanol, or sodium phosphate solution extracts | AChE inhibition | [83] | |
| Trametes Hirsute (Wulfen) Pilát | Mycelia and fruiting body | DMSO, ethanol, or sodium phosphate solution extracts | AChE inhibition | [83] | |
| Tremella fuciformis Berk. | Fruiting body | Aqueous | Scopolamine-treated Sprague Dawley rat: Morris water maze; IHC for ChAT | [84] | |
| Tricholoma imbricatum (Fr.) P. Kumm. | Whole | Methanol Hexane Ethyl acetate | AChE inhibition; BChE inhibition | [85] |
2.3. Tau Protein Expression and Aggregation
| Species | Body Part | Extract/ Compound | In Vitro Assays | In Vivo Assays | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| Antrodia camphorata (M. Zang & C.H. Su) Sheng H. Wu, Ryvarden & T.T. Chang | Mycelium Fruiting body | Aqueous Ethanolic | Aβ-induced Wistar rats: Immunoblotting; Morris water maze; | ↓ p-tau expression | [56] | |
| Armillaria mellea (Vahl) P. Kumm. | Polysaccharides | AlCl3, D-galactose-induced Balb/c mice: Morris water maze; Fatigue rotarod test; Autonomic activity test; TUNEL staining | ↓ p-tau aggregation; Antioxidant activity | [57] | ||
| Cordyceps millitaris (L.) Fr. | Cordycepin (13) | Rat hippocampal neurons: Western blotting for p-tau | ↓ Aβ25–35-induced p-tau expression | [46] | ||
| Hericium erinaceus (Bull.) Pers. | Whole | Powdered | AlCl3-induced Wistar rats: Morris water maze; Elevated plus maze; Novel object recognition; IHC; Western blot | ↓ p-tau | [62] | |
| Pleurotus Ostreatus (Jacq.) P. Kumm. | Whole | Polysaccharides | Wistar AlCl3/D-galactose-induced rats: Morris water maze; Step-down memory test; Serum, hippocampus, and liver levels of SOD, GSH-Px, CAT, and MDA; Western blot for p-tau in hippocampus tissue | ↓ p-tau | [64] |
2.4. Other Activities for General Neuronal Protection
| Species | Body Part | Extract/ Compound | In Vitro Assays | In Vivo Assays | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| Armillaria mellea (Vahl) P. Kumm. | Mycelium | Polysaccharides (precipitated in 70% ethanol) | HT22 apoptotic cells: ROS inhibition; MMP depolarization | AlCl3, D-galactose Balb/c mice: Morris water maze; Fatigue rotarod test; Autonomic activity test; IHC | ↑ Cell viability; ↑Behavior | [57] |
| Cordyceps millitaris (L.) Fr. | Cordycepin (13) | Aβ1–42 induced ICR mice: T maze test; Novel object recognition test; Morris water maze; NO scavenging activity | ↑Spatial memory ↑Behavior; ↑Memory | [45] | ||
| Coriolus versicolor (L.) Quél. | Whole | Biomass | C57BL/6 WT mice: Immunofluorescence; Microscopy for dendritic morphology assessment and β-catenin quantification; Mouse body conditions | ↑ Arborization of newly generated neurons; ↑ β-catenin | [102] | |
| Dictyophora indusitata (Vent.) Desv. | Dictyophorine A Dictyophorine B | Quiescent rat astroglial cells: NGF production | ↑NGF | [103] | ||
| Ganoderma lucidum (Curtis) P. Kumm. | Fruiting body | Aqueous | Neurons collected from embryonic Sprague Dawley rats: Aβ-induced cytotoxicity; Caspase-like activity | ↓ JNK phosphorylation; ↓ Caspase-3-like activity | [61] | |
| Basidiocarp | Aqueous | PC-12 cells: Cell viability by MTT; Neurite outgrowth stimulation; Immunofluorescence | ↑NGF | [93] | ||
| Ganodenic acid A (3) | C57 BL/6 APP/PS1 mice: IHC; Western blotting; Transcriptome sequencing; Metabolic analysis | ↑p-mTOR; ↓ apoptosis; ↑sphingolipid metabolism; ↑ iron function; ↑autophagy; Regulation of lipid metabolism; | [96] | |||
| Ganoderma neo-japonicum Imazeki | Basidiocarp | Aqueous | PC-12 cells: Cell viability by MTT Neurite outgrowth stimulation Immunofluorescence | ↑NGF | [93] | |
| Grifola frondosa (Dicks.) Gray | Lysophosphatidylethanolamine (17) | PC12 cells: MAPK activation; Protein phosphorylation; Signal transduction inhibition; Apoptotic DNA fragmentation | ↑ neurite outgrowth- GLPE; ↑ neurofilament M expression; ↑Ras/MAPK; ↑NGF | [95] | ||
| Basidiocarp | Aqueous | PC-12 cells: Cell viability by MTT; Neurite outgrowth stimulation; Immunofluorescence | ↑NGF | [93] | ||
| Hericium erinaceus (Bull.) Pers. | Basidiocarp | Hot water and 80% ethanolic extract | NG108-15 (neuroblastoma glial cell hybrid) and MRC-5 (human lung fibroblast) cell lines: Cell viability by MTT, trypan blue, tunnel assay; Neurite outgrowth stimulation assay; ELISA for NGF | ↑NGF | [104] | |
| Hericenone C (19), hericenone D (20), hericenone E (21) | PC12 cells: ELISA for NGF; Enzymatic inhibition for MAPK, PI3K, TrkA; Phospho-ERK levels; Phospho-Akt levels | ↑NGF (mediated by TrkA, MEK); ↑ Neurite outgrowth (mediated by Pi3K/Akt) | [99] | |||
| Mycelia | Erinacine A–C (7–9) | Mouse astroglial cells: Medium NGF | ↑NGF | [105] | ||
| Erinacine A (7) | Wistar rats: Catecholamine levels (tissue); Indoleamine levels (tissue); ELISA for NGF; | ↑NGF; ↑Catecholamine | [106] | |||
| dilinoleoyl phosphatidylethanolamine (22) | Neuro2a: Cell viability by MTT; PI staining; Caspase-12 activation; Protein kinase C activation | ↓ Endoplasmic reticulum stress-induced death; ↑PKC | [97] | |||
| Mycoleptodonoides aitchisonii Karasaki | Whole | Aqueous; Powder | Rat astrocytes: NGF | Newborn Wistar rats: Morris water maze; Monoamine concentration; Amino acid concentration; ELISA for NGF | ↑NGF; ↑L-serine; ↑spatial memory | [107] |
| Terpenoids: 3-(hydroxymethyl)-4-methylfuran-2(5H)-one (15) (3R,4S,10R)-3-(1′-hydroxy-ethyl)-4-methyldihydrofuran-2(3H)-one (16) 1-hydroxy-3-pentanone (18) | Neuro2a cells: Cell viability by MTT | ↓ Endoplasmic reticulum stress-induced death; ↑NGF | [100] | |||
| Paxillus panuidodes (Fr.) Fr. | p-terphenyl leucomentins 2–6 | Mouse cortical cells: Cell viability by MTT; NMDA cytotoxicity; Glutamate cytotoxicity; Iron chelation—DNA damage | ↓ NMDA cytotoxicity; ↓ Glutamate cytotoxicity; ↓DNA damage; ↓ Fenton reactions | [108] | ||
| Sarcodon cyrneus Maast Geest. | Cyrneine A Cyrneine B | PC12 and 1321N astrocytoma cells Medium NGF levels | ↑ Neurite growth | [109,110] | ||
| Sarcodon scabrosus (Fr.) P. Karst. | Scabronine A | 1321N astrocytoma cells: Medium NGF levels; RT-PCR | ↑ NFG synthesis | [111] | ||
| Scabronine B–F | Rat astroglial cells: Medium NGF levels | ↑ NFG synthesis | [101] | |||
| Termitomyces albuminosus (Berk.) R. Heim | Termitomycesphins A–D | PC-12 cells: Neurite growth | ↑ Neurite growth | [112] | ||
| Termitomycesphin E–H | PC12 cells Morphology monitoring—phase contrast microscopy | ↑ Neurogenesis | [113] | |||
| Termitomyces titanicus Pegler & Piearce | Termitomycamides B (23) and E (24) | Neuro2a cells: Tunicamycin-induced stress test | ↓ Endoplasmic reticulum stress-induced death | [114] | ||
| Tremella fuciformis Berk. | Fruiting body | Aqueous | PC12h cells: Morphology evaluation by imaging | Scopolamine-treated Sprague Dawley rat: Morris water maze | ↑ neuritogenesis; ↑ NGF; ↑ FGF | [84] |
2.5. Medicinal Mushrooms in AD Clinical Studies
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Alzheimer Europe. Dementia in Europe Yearbook 2019: Estimating the Prevalence of Dementia in Europe; Alzheimer Europe: Helsinki, Finland, 2019; ISBN 978-99959-995-9-9. [Google Scholar]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Maresova, P.; Klimova, B.; Novotny, M.; Kuca, K. Alzheimer’s and Parkinson’s Diseases: Expected Economic Impact on Europe-A Call for a Uniform European Strategy. J. Alzheimers Dis. 2016, 54, 1123–1133. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Bauzon, J.; Lee, G.; Cummings, J. Repurposed Agents in the Alzheimer’s Disease Drug Development Pipeline. Alzheimer’s Res. Ther. 2020, 12, 98. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Martins, R.N.; Villemagne, V.; Sohrabi, H.R.; Chatterjee, P.; Shah, T.M.; Verdile, G.; Fraser, P.; Taddei, K.; Gupta, V.B.; Rainey-Smith, S.R.; et al. Alzheimer’s Disease: A Journey from Amyloid Peptides and Oxidative Stress, to Biomarker Technologies and Disease Prevention Strategies-Gains from AIBL and DIAN Cohort Studies. J. Alzheimers Dis. 2018, 62, 965–992. [Google Scholar] [CrossRef]
- Metcalfe, M.J.; Figueiredo-Pereira, M.E. Relationship between Tau Pathology and Neuroinflammation in Alzheimer’s Disease. Mt. Sinai J. Med. 2010, 77, 50–58. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s Disease Drug Development Pipeline: 2022. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12295. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Kokras, N.; Stamouli, E.; Sotiropoulos, I.; Katirtzoglou, E.A.; Siarkos, K.T.; Dalagiorgou, G.; Alexandraki, K.I.; Coulocheri, S.; Piperi, C.; Politis, A.M. Acetyl Cholinesterase Inhibitors and Cell-Derived Peripheral Inflammatory Cytokines in Early Stages of Alzheimer’s Disease. J. Clin. Psychopharmacol. 2018, 38, 138–143. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2022, 20, 195–206. [Google Scholar] [CrossRef]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in Patients with Early Alzheimer’s Disease: Detailed Results on Biomarker, Cognitive, and Clinical Effects from the Randomized and Open-Label Extension of the Phase 2 Proof-of-Concept Study. Alzheimer’s Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef]
- Withington, C.G.; Turner, R.S. Amyloid-Related Imaging Abnormalities with Anti-Amyloid Antibodies for the Treatment of Dementia Due to Alzheimer’s Disease. Front. Neurol. 2022, 13, 862369. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hirai, T.; Kumazawa, R.; Nakagawa, S.; Yonezawa, A.; Matsubara, K.; Nakao, H. Prevalence of and Risk Factors for Adverse Events in Alzheimer’s Patients Receiving Anti-Dementia Drugs in at-Home Care. PLoS ONE 2020, 15, e0231226. [Google Scholar] [CrossRef] [PubMed]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2023, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen Suppresses Plaque Pathology and Inflammation in a Mouse Model for Alzheimer’s Disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Solfrizzi, V.; Panza, F. Are NSAIDs Useful to Treat Alzheimer’s Disease or Mild Cognitive Impairment? Front. Aging Neurosci. 2010, 2, 1517. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, I.S.; Park, M.H.; Lee, Y.M.; Song, J.K.; Kim, Y.H.; Kim, K.H.; Hwang, D.Y.; Jeong, J.H.; Yun, Y.P.; et al. 4-O-Methylhonokiol Attenuates Memory Impairment in Presenilin 2 Mutant Mice through Reduction of Oxidative Damage and Inactivation of Astrocytes and the ERK Pathway. Free Radic. Biol. Med. 2011, 50, 66–77. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Dukie, S.A.; Grant, G.; Rao, C.M.; Arora, D. Caffeic Acid Attenuates Lipopolysaccharide-Induced Sickness Behaviour and Neuroinflammation in Mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, β-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Wang, X.; Yang, Z.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. 2,2’,4’-Trihydroxychalcone from Glycyrrhiza Glabra as a New Specific BACE1 Inhibitor Efficiently Ameliorates Memory Impairment in Mice. J. Neurochem. 2010, 114, 374–385. [Google Scholar] [CrossRef]
- ‘t Hart, B.A.; Copray, S.; Philippens, I. Apocynin, a Low Molecular Oral Treatment for Neurodegenerative Disease. Biomed. Res. Int. 2014, 2014, 298020. [Google Scholar] [CrossRef]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as Acetylcholinesterase Inhibitors: Current Therapeutic Standing and Future Prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Lakshmi, S.; Prakash, P.; M Essa, M.; Qoronfleh, W.; Akbar, M.; Song, B.-J.; Kumar, S.; Elumalai, P. Marine Derived Bioactive Compounds for Treatment of Alzheimer’s Disease. Front. Biosci. 2018, 10, 537–548. [Google Scholar]
- Wang, S.; Kong, X.; Chen, Z.; Wang, G.; Zhang, J.; Wang, J. Role of Natural Compounds and Target Enzymes in the Treatment of Alzheimer’s Disease. Molecules 2022, 27, 4175. [Google Scholar] [CrossRef]
- Abitbol, A.; Mallard, B.; Tiralongo, E.; Tiralongo, J. Mushroom Natural Products in Neurodegenerative Disease Drug Discovery. Cells 2022, 11, 3938. [Google Scholar] [CrossRef]
- Lee, W.; Fujihashi, A.; Govindarajulu, M.; Ramesh, S.; Deruiter, J.; Majrashi, M.; Almaghrabi, M.; Nadar, R.M.; Moore, T.; Agrawal, D.C.; et al. Role of Mushrooms in Neurodegenerative Diseases. In Medicinal Mushrooms: Recent Progress in Research and Development; Springer: Singapore, 2019; pp. 223–249. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Chami, L.; Checler, F. BACE1 Is at the Crossroad of a Toxic Vicious Cycle Involving Cellular Stress and β-Amyloid Production in Alzheimer’s Disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef]
- Jędrejko, K.J.; Lazur, J.; Muszyńska, B. Cordyceps Militaris: An Overview of Its Chemical Constituents in Relation to Biological Activity. Foods 2021, 10, 2634. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a Metabolic Product Isolated from Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps Militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- He, M.T.; Lee, A.Y.; Kim, J.H.; Park, C.H.; Shin, Y.S.; Cho, E.J. Protective Role of Cordyceps Militaris in Aβ 1–42 -Induced Alzheimer’s Disease in Vivo. Food Sci. Biotechnol. 2019, 28, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, L.P.; Li, Y.; Liu, C.; Wang, S.; Meng, W.; Wei, S.; Liu, X.P.; Gong, Y.; Yao, L.H. Neuroprotective Effects of Cordycepin Inhibit Aβ-Induced Apoptosis in Hippocampal Neurons. Neurotoxicology 2018, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cör Andrejč, D.; Knez, Ž.; Knez Marevci, M. Antioxidant, Antibacterial, Antitumor, Antifungal, Antiviral, Anti-Inflammatory, and Nevro-Protective Activity of Ganoderma Lucidum: An Overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef]
- Wang, M.-F.; Chan, Y.-C.; Wu, C.-L.; Wong, Y.-C.; Hosoda, K.; Yamamoto, S. Effects of Ganoderma on Aging and Learning and Memory Ability in Senescence Accelerated Mice. Int. Congr. Ser. 2004, 1260, 399–404. [Google Scholar] [CrossRef]
- Yu, N.; Huang, Y.; Jiang, Y.; Zou, L.; Liu, X.; Liu, S.; Chen, F.; Luo, J.; Zhu, Y. Ganoderma Lucidum Triterpenoids (GLTs) Reduce Neuronal Apoptosis via Inhibition of ROCK Signal Pathway in APP/PS1 Transgenic Alzheimer’s Disease Mice. Oxid. Med. Cell. Longev. 2020, 2020, 9894037. [Google Scholar] [CrossRef]
- Ghosh, S.; Nandi, S.; Banerjee, A.; Sarkar, S.; Chakraborty, N.; Acharya, K. Prospecting Medicinal Properties of Lion’s Mane Mushroom. J. Food Biochem. 2021, 45, e13833. [Google Scholar] [CrossRef]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine A-Enriched Hericium erinaceus Mycelium Ameliorates Alzheimer’s Disease-Related Pathologies in APPswe/PS1dE9 Transgenic Mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef]
- González-Ramírez, M.; Gavilán, J.; Silva-Grecchi, T.; Cajas-Madriaga, D.; Triviño, S.; Becerra, J.; Saez-Orellana, F.; Pérez, C.; Fuentealba, J. A Natural Benzofuran from the Patagonic Aleurodiscus Vitellinus Fungus Has Potent Neuroprotective Properties on a Cellular Model of Amyloid-β Peptide Toxicity. J. Alzheimers Dis. 2018, 61, 1463–1475. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Lu, W.; Zhang, S.; Guan, X.; Li, Z.; Wang, D. Anti-Oxidative Stress Activity Is Essential for Amanita Caesarea Mediated Neuroprotection on Glutamate-Induced Apoptotic HT22 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2017, 18, 1623. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In Vitro and in Vivo Comparisons of the Effects of the Fruiting Body and Mycelium of Antrodia Camphorata against Amyloid β-Protein-Induced Neurotoxicity and Memory Impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef]
- An, S.; Lu, W.; Zhang, Y.; Yuan, Q.; Wang, D. Pharmacological Basis for Use of Armillaria Mellea Polysaccharides in Alzheimer’s Disease: Antiapoptosis and Antioxidation. Oxid. Med. Cell Longev. 2017, 2017, 4184562. [Google Scholar] [CrossRef]
- Bennett, L.; Sheean, P.; Zabaras, D.; Head, R. Heat-Stable Components of Wood Ear Mushroom, Auricularia Polytricha (Higher Basidiomycetes), Inhibit in Vitro Activity of Beta Secretase (BACE1). Int. J. Med. Mushrooms 2013, 15, 233–249. [Google Scholar] [CrossRef]
- Geissler, T.; Brandt, W.; Porzel, A.; Schlenzig, D.; Kehlen, A.; Wessjohann, L.; Arnold, N. Acetylcholinesterase Inhibitors from the Toadstool Cortinarius Infractus. Bioorg. Med. Chem. 2010, 18, 2173–2177. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, Y.; Guo, W.H.; Wang, D.C.; Zhang, Q.; Li, D.; Rong, J.; Gao, J.M. Molecular Diversity and Potential Anti-Neuroinflammatory Activities of Cyathane Diterpenoids from the Basidiomycete Cyathus Africanus. Sci. Rep. 2017, 7, 8883. [Google Scholar] [CrossRef]
- Lai, C.S.W.; Yu, M.S.; Yuen, W.H.; So, K.F.; Zee, S.Y.; Chang, R.C.C. Antagonizing β-Amyloid Peptide Neurotoxicity of the Anti-Aging Fungus Ganoderma lucidum. Brain Res. 2008, 1190, 215–224. [Google Scholar] [CrossRef]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium Erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Lee, L.Y.; Chou, W.; Chen, W.P.; Wang, M.F.; Chen, Y.J.; Chen, C.C.; Tung, K.C. Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (Samp8) Mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus Ostreatus Alleviate Cognitive Impairment in a Rat Model of Alzheimer’s Disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2022, 15, 742. [Google Scholar] [CrossRef] [PubMed]
- Lesa, K.N.; Khandaker, M.U.; Mohammad Rashed Iqbal, F.; Sharma, R.; Islam, F.; Mitra, S.; Emran, T.B. Nutritional Value, Medicinal Importance, and Health-Promoting Effects of Dietary Mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Oblak, A.L.; Lin, P.B.; Kotredes, K.P.; Pandey, R.S.; Garceau, D.; Williams, H.M.; Uyar, A.; O’Rourke, R.; O’Rourke, S.; Ingraham, C.; et al. Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Front. Aging Neurosci. 2021, 13, 713726. [Google Scholar] [CrossRef]
- Jasiecki, J.; Wasąg, B. Butyrylcholinesterase Protein Ends in the Pathogenesis of Alzheimer’s Disease—Could BCHE Genotyping Be Helpful in Alzheimer’s Therapy? Biomolecules 2019, 9, 592. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.F.; Yu, Y. Galantamine Improves Cognition, Hippocampal Inflammation, and Synaptic Plasticity Impairments Induced by Lipopolysaccharide in Mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Haertel, C.; Maelicke, A.; Montag, D. Galantamine Slows down Plaque Formation and Behavioral Decline in the 5XFAD Mouse Model of Alzheimer’s Disease. PLoS ONE 2014, 9, e89454. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. Acetylcholinesterase Inhibition and in Vitro and in Vivo Antioxidant Activities of Ganoderma Lucidum Grown on Germinated Brown Rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef]
- Lee, I.S.; Ahn, B.R.; Choi, J.S.; Hattori, M.; Min, B.; Bae, K.H. Selective Cholinesterase Inhibition by Lanostane Triterpenes from Fruiting Bodies of Ganoderma Lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Akata, I.; Zengin, G.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme Inhibitory and Antioxidant Properties of Six Mushroom Species from the Agaricaceae Family. S. Afr. J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- Raquel Leal, A.; Barros, L.; Barreira, J.C.; João Sousa, M.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Portuguese Wild Mushrooms at the “Pharma-Nutrition” Interface: Nutritional Characterization and Antioxidant Properties. Food Res. Int. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Teichert, A.; Schmidt, J.; Porzel, A.; Arnold, N.; Wessjohann, L. Brunneins A-C, β-Carboline Alkaloids from Cortinarius Brunneus. J. Nat. Prod 2007, 70, 1529–1531. [Google Scholar] [CrossRef]
- Wei, J.; Guo, W.H.; Cao, C.Y.; Kou, R.W.; Xu, Y.Z.; Górecki, M.; Di Bari, L.; Pescitelli, G.; Gao, J.M. Polyoxygenated Cyathane Diterpenoids from the Mushroom Cyathus Africanus, and Their Neurotrophic and Anti-Neuroinflammatory Activities. Sci. Rep. 2018, 8, 2175. [Google Scholar] [CrossRef]
- Wei, Y.M.; Yang, L.; Wang, H.; Cai, C.H.; Chen, Z.B.; Chen, H.Q.; Mei, W.L.; Dai, H.F. Triterpenoids as Bivalent and Dual Inhibitors of Acetylcholinesterase/Butyrylcholinesterase from the Fruiting Bodies of Inonotus obliquus. Phytochemistry 2022, 200, 113182. [Google Scholar] [CrossRef]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Çayan, G.T.; Çayan, F.; Duru, M.E.; Ahmad, N.; Emwas, A.H.; Jaremko, M. Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef]
- Heleno, S.A.; Stojković, D.; Barros, L.; Glamočlija, J.; Soković, M.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. A Comparative Study of Chemical Composition, Antioxidant and Antimicrobial Properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res. Int. 2013, 51, 236–243. [Google Scholar] [CrossRef]
- Im, K.H.; Nguyen, K.; Kim, J.K.; Choi, J.-H.; Lee, T.S. Evaluation of Anticholinesterase and Inflammation Inhibitory Activity of Medicinal Mushroom Phellinus pini (Basidiomycetes) Fruiting Bodies. Int. J. Med. Mushrooms 2016, 18, 1011–1022. [Google Scholar] [CrossRef]
- Knežević, A.; Stajić, M.; Sofrenić, I.; Stanojković, T.; Milovanović, I.; Tešević, V.; Vukojević, J. Antioxidative, Antifungal, Cytotoxic and Antineurodegenerative Activity of Selected Trametes Species from Serbia. PLoS ONE 2018, 13, e0203064. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ha, H.-C.; Lee, M.-S.; Kang, J.-I.; Kim, H.-S.; Lee, S.-Y.; Pyun, K.-H.; Shim, I. Effect of Tremella fuciformis on the Neurite Outgrowth of PC12h Cells and the Improvement of Memory in Rats. Biol. Pharm. Bull. 2007, 30, 708–714. [Google Scholar] [CrossRef]
- Tel, G.; Apaydın, M.; Duru, M.E.; Öztürk, M. Antioxidant and Cholinesterase Inhibition Activities of Three Tricholoma Species with Total Phenolic and Flavonoid Contents: The Edible Mushrooms from Anatolia. Food Anal. Methods 2012, 5, 495–504. [Google Scholar] [CrossRef]
- Doǧan, H.H.; Akbaş, G. Biological Activity and Fatty Acid Composition of Caesar’s Mushroom. Pharm. Biol. 2013, 51, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Yu, J.; Bernays, J.; Wan, S.S.; Christophidis, L.J.; Sberna, G.; Tait, R.M.; Wildman, H.G.; Lebeller, D.; Platel, D.; et al. Trichomycins A and B: Antibacterial Triterpenes from the New Species Tricholoma sp. AU1. J. Nat. Prod. 2005, 68, 409–412. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Sardar, R.; Bukhari, S.; Akrem, A.; Mustafa, S.; Manan, A.; Sajid, M.; Talha Rafiq, M. Edible and Medicinal Mushrooms: That Mitigate the Threatening Diseases. Med. Mycol. 2021, 7, 1–6. [Google Scholar]
- Brondz, I.; Høiland, K. Biogenesis of Infractine Alkaloids in Cortinarius Infractus: Importance of 5-Hydroxytriptophane Pathway in Biogenesis of Alkaloids in Mushrooms. In Proceedings of the 5th Conference Nordic Separation Science Society, Tallinn, Estonia, 26–29 January 2010. [Google Scholar]
- Muszynska, B.; Sulkowska-Ziaja, K.; Wolkowska, M.; Ekiert, H. Chemical, Pharmacological, and Biological Characterization of the Culinary-Medinical Honey Mushroom, Armillaria mellea (Vahl) P. Kumm. (Agaricomycetideae): A Review. Int. J. Med. Mushrooms 2011, 13, 167–175. [Google Scholar] [CrossRef]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Tan, W.C.; Kuppusamy, U.R.; Phan, C.W.; Tan, Y.S.; Raman, J.; Anuar, A.M.; Sabaratnam, V. Ganoderma Neo-Japonicum Imazeki Revisited: Domestication Study and Antioxidant Properties of Its Basidiocarps and Mycelia. Sci. Rep. 2015, 5, 12515. [Google Scholar] [CrossRef]
- Ling-Sing Seow, S.; Naidu, M.; David, P.; Wong, K.H.; Sabaratnam, V. Potentiation of Neuritogenic Activity of Medicinal Mushrooms in Rat Pheochromocytoma Cells. BMC Complement. Altern. Med. 2013, 13, 157. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Nishina, A.; Kimura, H.; Sekiguchi, A.; Fukumoto, R.H.; Nakajima, S.; Furukawa, S. Lysophosphatidylethanolamine in Grifola Frondosa as a Neurotrophic Activator via Activation of MAPK. J. Lipid. Res. 2006, 47, 1434–1443. [Google Scholar] [CrossRef]
- Zeng, M.; Qi, L.; Guo, Y.; Zhu, X.; Tang, X.; Yong, T.; Xie, Y.; Wu, Q.; Zhang, M.; Chen, D. Long-Term Administration of Triterpenoids from Ganoderma lucidum Mitigates Age-Associated Brain Physiological Decline via Regulating Sphingolipid Metabolism and Enhancing Autophagy in Mice. Front. Aging Neurosci. 2021, 13, 628860. [Google Scholar] [CrossRef]
- Nagai, K.; Chiba, A.; Nishino, T.; Kubota, T.; Kawagishi, H. Dilinoleoyl-Phosphatidylethanolamine from Hericium Erinaceum Protects against ER Stress-Dependent Neuro2a Cell Death via Protein Kinase C Pathway. J. Nutr. Biochem. 2006, 17, 525–530. [Google Scholar] [CrossRef]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and Erinacines: Stimulators of Nerve Growth Factor (NGF) Biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, Stimulators of Nerve Growth Fact (NGF)-Synthesis, from the Mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Choi, J.-H.; Horikawa, M.; Okumura, H.; Kodani, S.; Nagai, K.; Hashizume, D.; Koshino, H.; Kawagishi, H. Endoplasmic Reticulum (ER) Stress Protecting Compounds from the Mushroom Mycoleptodonoides aitchisonii. Tetrahedron 2009, 65, 221–224. [Google Scholar] [CrossRef]
- Kita, T.; Takaya, Y.; Oshima, Y.; Ohta, T.; Aizawa, K.; Hirano, T.; Inakuma, T. Scabromines B, C, C, D, E and F, Novel Diterpenoids Showing Stimulating Activity of Nerve Growth Factor-Synthesis, from the Mushroom Sarcodon scabrosus. Pergamon Tetrahedron 1998, 54, 11877–11886. [Google Scholar] [CrossRef]
- Ferreiro, E.; Pita, I.R.; Mota, S.I.; Valero, J.; Ferreira, N.R.; Fernandes, T.; Calabrese, V.; Fontes-Ribeiro, C.A.; Pereira, F.C.; Rego, A.C. Coriolus Versicolor Biomass Increases Dendritic Arborization of Newly-Generated Neurons in Mouse Hippocampal Dentate Gyrus. Oncotarget 2018, 9, 32929. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ishiyama, D.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S.; Li, J. Dictyophorines A and B, Two Stimulators of NGF-Synthesis from the Mushroom Dictyophora indusitata. Pergamon 1997, 45, 1203–1205. [Google Scholar]
- Lai, P.-L.; Naidu, M.; Sabaratnam, V.; Wong, K.H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N.A. Neurotrophic Properties of the Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Kawagishia, H.; Shimadaa, A.; Shiraia, R.; Okamotob, K.; Ojimab, F.; Sakamotob, H.; Ishigurob, Y.; Furukawac, S. Erinacines A, B and C, Strong Stimulators of Nerve Growth Factor (NGF)-Synthesis, from the Mycelia of Hericium erinaceum. Pergamon 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A Increases Catecholamine and Nerve Growth Factor Content in the Central Nervous System of Rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Okuyama, S.; Lam, N.V.; Hatakeyama, T.; Terashima, T.; Yamagata, K.; Yokogoshi, H. Mycoleptodonoides aitchisonii Affects Brain Nerve Growth Factor Concentration in Newborn Rats. Nutr. Neurosci. 2004, 7, 341–349. [Google Scholar] [CrossRef]
- Lee, I.-K.; Yun, B.-S.; Kim, J.-P.; Ryoo, I.-J.; Kim, Y.-H.; Yoo, I.-D. Neuroprotective Activity of P-Terphenyl Leucomentins from the Mushroom Paxillus panuoides. Biosci. Biotechnol. Biochem. 2003, 67, 1813–1816. [Google Scholar] [CrossRef]
- Obara, Y.; Hoshino, T.; Marcotullio, M.C.; Pagiotti, R.; Nakahata, N. A Novel Cyathane Diterpene, Cyrneine A, Induces Neurite Outgrowth in a Rac1-Dependent Mechanism in PC12 Cells. Life Sci. 2007, 80, 1669–1677. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Pagiotti, R.; Maltese, F.; Oball-Mond Mwankie, G.N.; Hoshino, T.; Obara, Y.; Nakahata, N. Cyathane Diterpenes from Sarcodon cyrneus and Evaluation of Their Activities of Neuritegenesis and Nerve Growth Factor Production. Bioorg. Med. Chem. 2007, 15, 2878–2882. [Google Scholar] [CrossRef]
- Ohta, T.; Kita, T.; Kobayashi, N.; Obara, Y.; Nakahata, N.; Ohizumi, Y.; Takaya, Y.; Oshima, Y. Scabronine A, a Novel Diterpenoid Having Potent Inductive Activity of the Nerve Growth Factor Synthesis, Isolated from the Mushroom, Sarcodon scabrosus. Tetrahedron Lett. 1998, 39, 6229–6232. [Google Scholar] [CrossRef]
- Qi, J.; Ojika, M.; Sakagami, Y. Termitomycesphins A-D, Novel Neuritogenic Cerebrosides from the Edible Chinese Mushroom Termitomyces albuminosus. Tetrahedron 2000, 56, 5835–5841. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, K.; Gao, L.; Sakagami, Y.; Kawagishi, H.; Ojika, M.; Qi, J. Termitomycesphins G and H, Additional Cerebrosides from the Edible Chinese Mushroom Termitomyces albuminosus. Biosci. Biotechnol. Biochem. 2012, 76, 791–793. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite Mushrooms (Termitomyces), a Potential Source of Nutrients and Bioactive Compounds Exhibiting Human Health Benefits: A Review. J. Fungi 2023, 90, 112. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A Potential Natural Source of Anti-Inflammatory Compounds for Medical Applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Gabr, M.T. Multitarget Therapeutic Strategies for Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Medica 2020, 86, 1161–1175. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Sabaratnam, V. Edible and Medicinal Mushrooms: Emerging Brain Food for the Mitigation of Neurodegenerative Diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving Effects of the Mushroom Yamabushitake (Hericium erinaceus) on Mild Cognitive Impairment: A Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P.C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Chan, Y.C.; Lin, T.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, Y.Z.; Hwang, J.H. Effects of Erinacine A-Enriched Hericium Erinaceus on Elderly Hearing-Impaired Patients: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Funct. Foods 2022, 97, 105220. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Preto, M.; Grosso, C.; Vieira, M.; Delerue-Matos, C.; Vasconcelos, V.; Reis, M.; Barros, L.; Martins, R. Tracing the Path between Mushrooms and Alzheimer’s Disease—A Literature Review. Molecules 2023, 28, 5614. https://doi.org/10.3390/molecules28145614
Silva AM, Preto M, Grosso C, Vieira M, Delerue-Matos C, Vasconcelos V, Reis M, Barros L, Martins R. Tracing the Path between Mushrooms and Alzheimer’s Disease—A Literature Review. Molecules. 2023; 28(14):5614. https://doi.org/10.3390/molecules28145614
Chicago/Turabian StyleSilva, Ana Margarida, Marco Preto, Clara Grosso, Mónica Vieira, Cristina Delerue-Matos, Vitor Vasconcelos, Mariana Reis, Lillian Barros, and Rosário Martins. 2023. "Tracing the Path between Mushrooms and Alzheimer’s Disease—A Literature Review" Molecules 28, no. 14: 5614. https://doi.org/10.3390/molecules28145614
APA StyleSilva, A. M., Preto, M., Grosso, C., Vieira, M., Delerue-Matos, C., Vasconcelos, V., Reis, M., Barros, L., & Martins, R. (2023). Tracing the Path between Mushrooms and Alzheimer’s Disease—A Literature Review. Molecules, 28(14), 5614. https://doi.org/10.3390/molecules28145614