Quercetin Antagonizes the Sedative Effects of Linalool, Possibly through the GABAergic Interaction Pathway
Abstract
1. Introduction
2. Results
2.1. In Vivo Study
2.2. In Silico Study
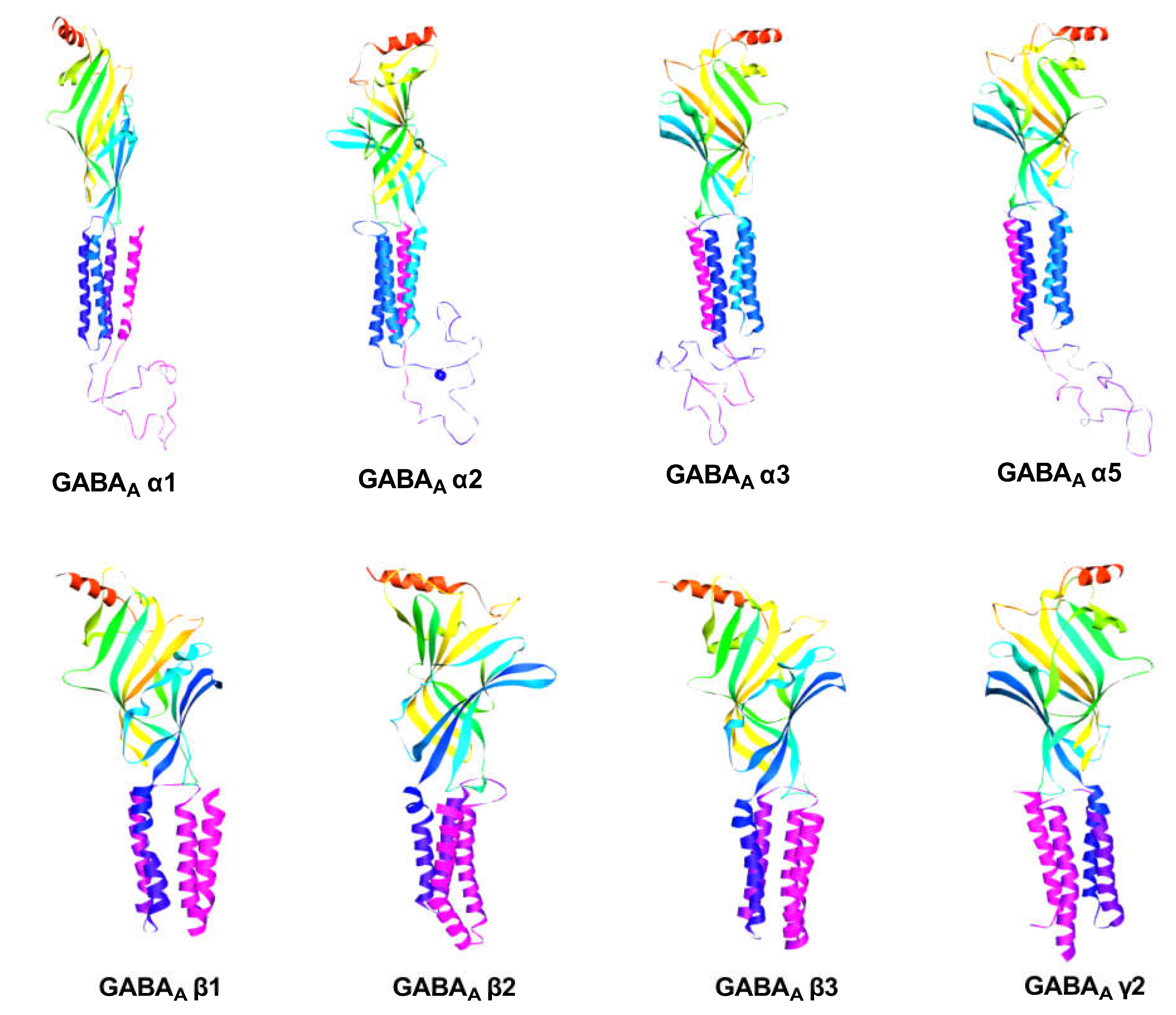
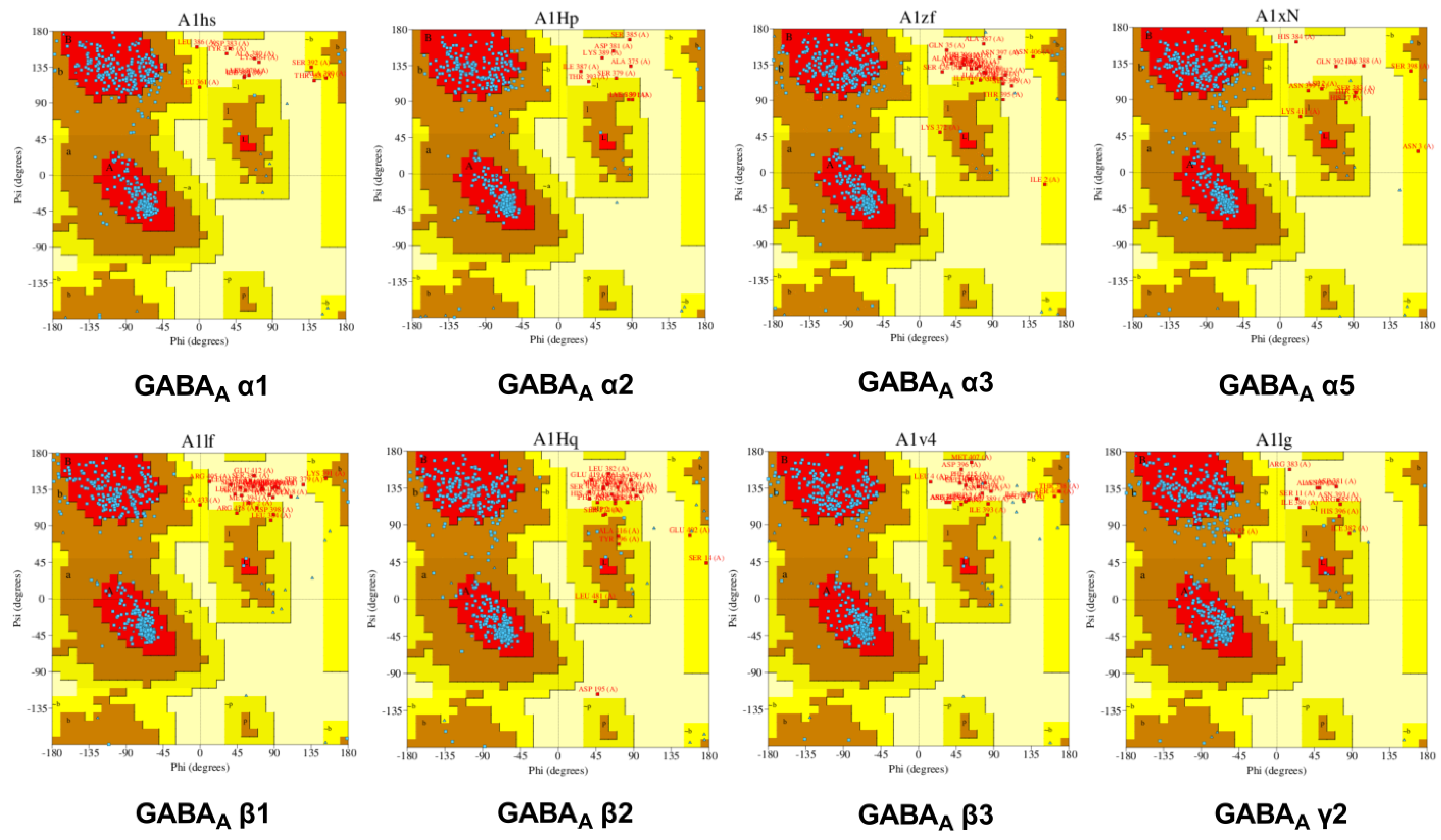
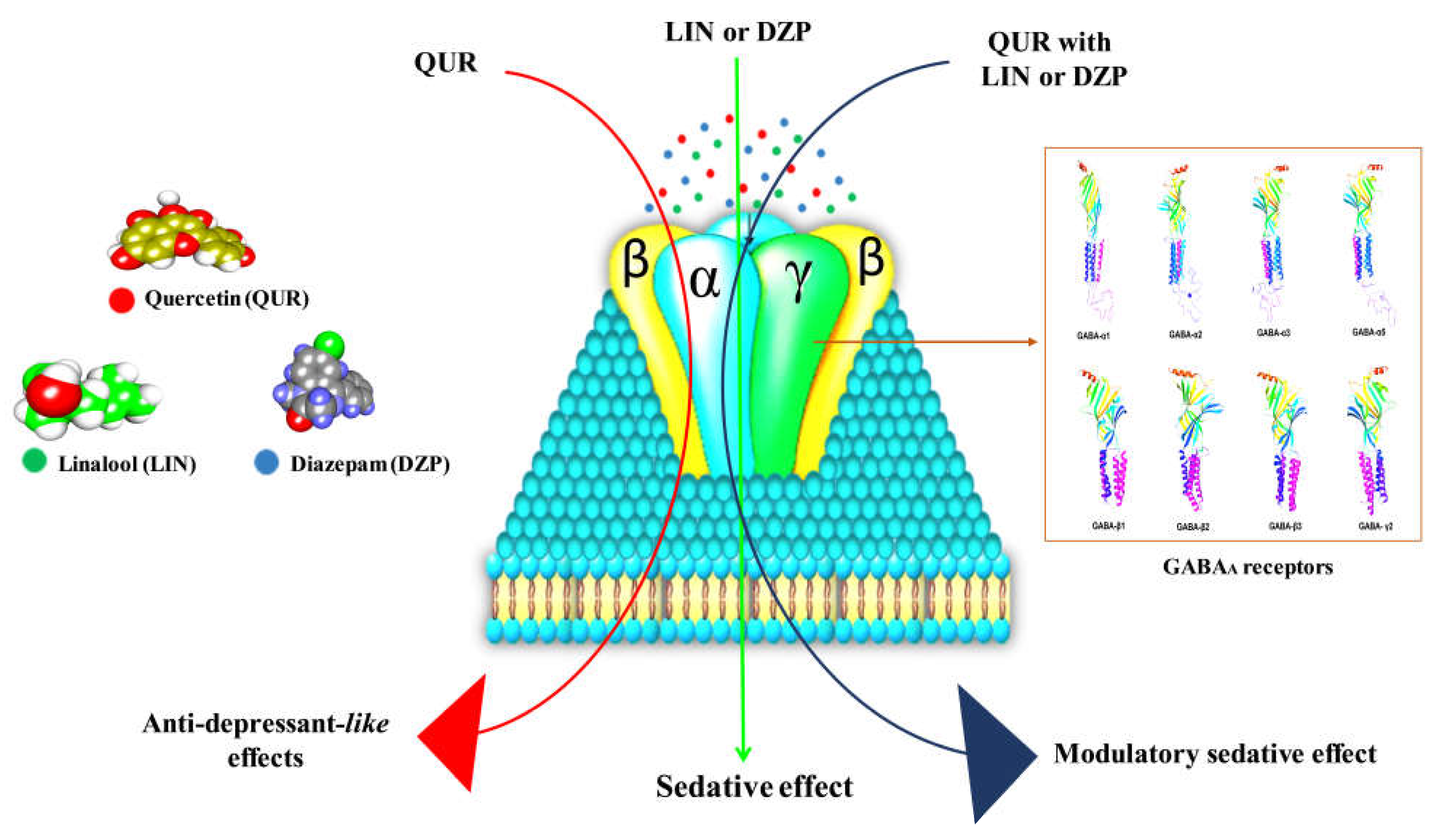
2.2.1. GABA Homology Model
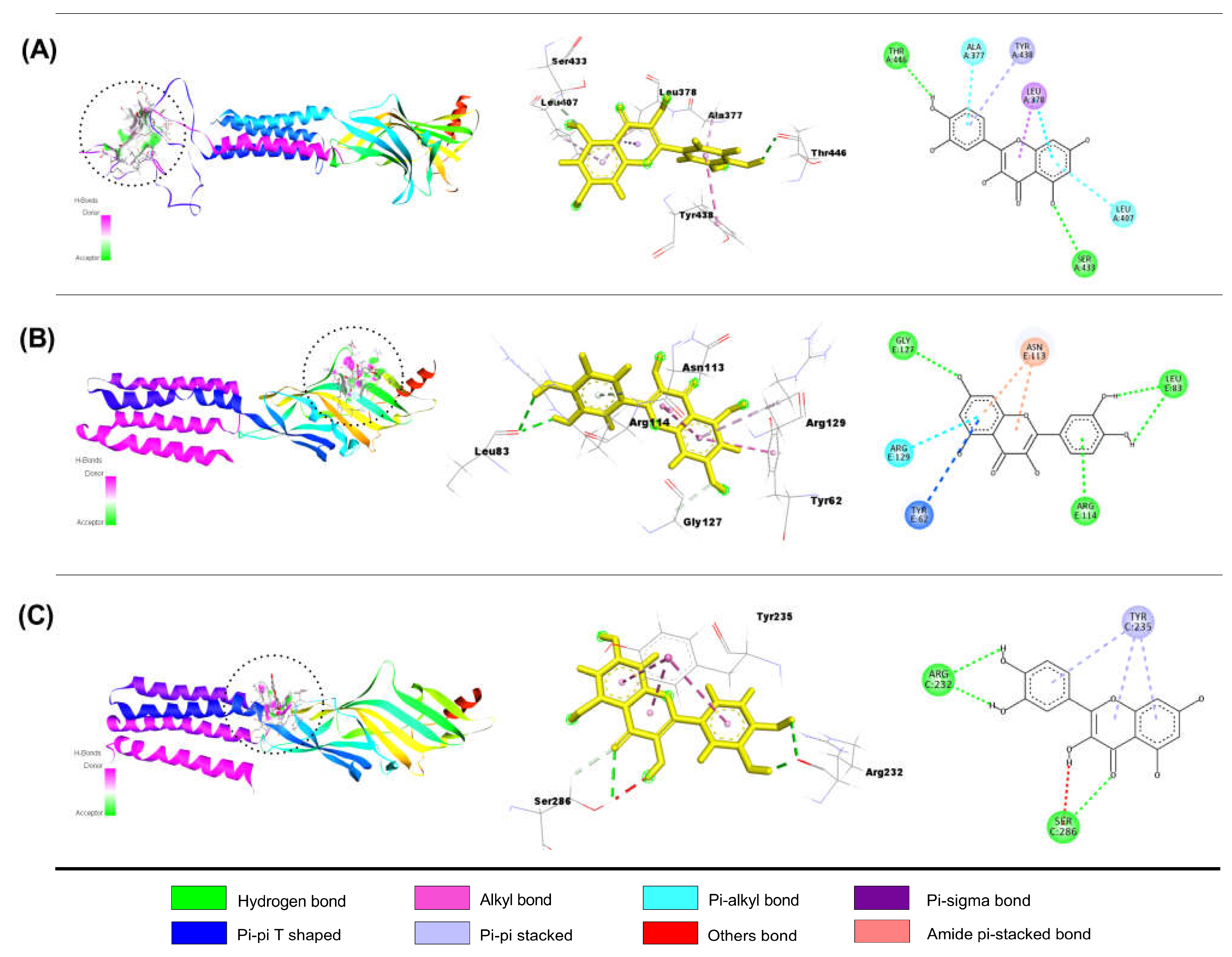
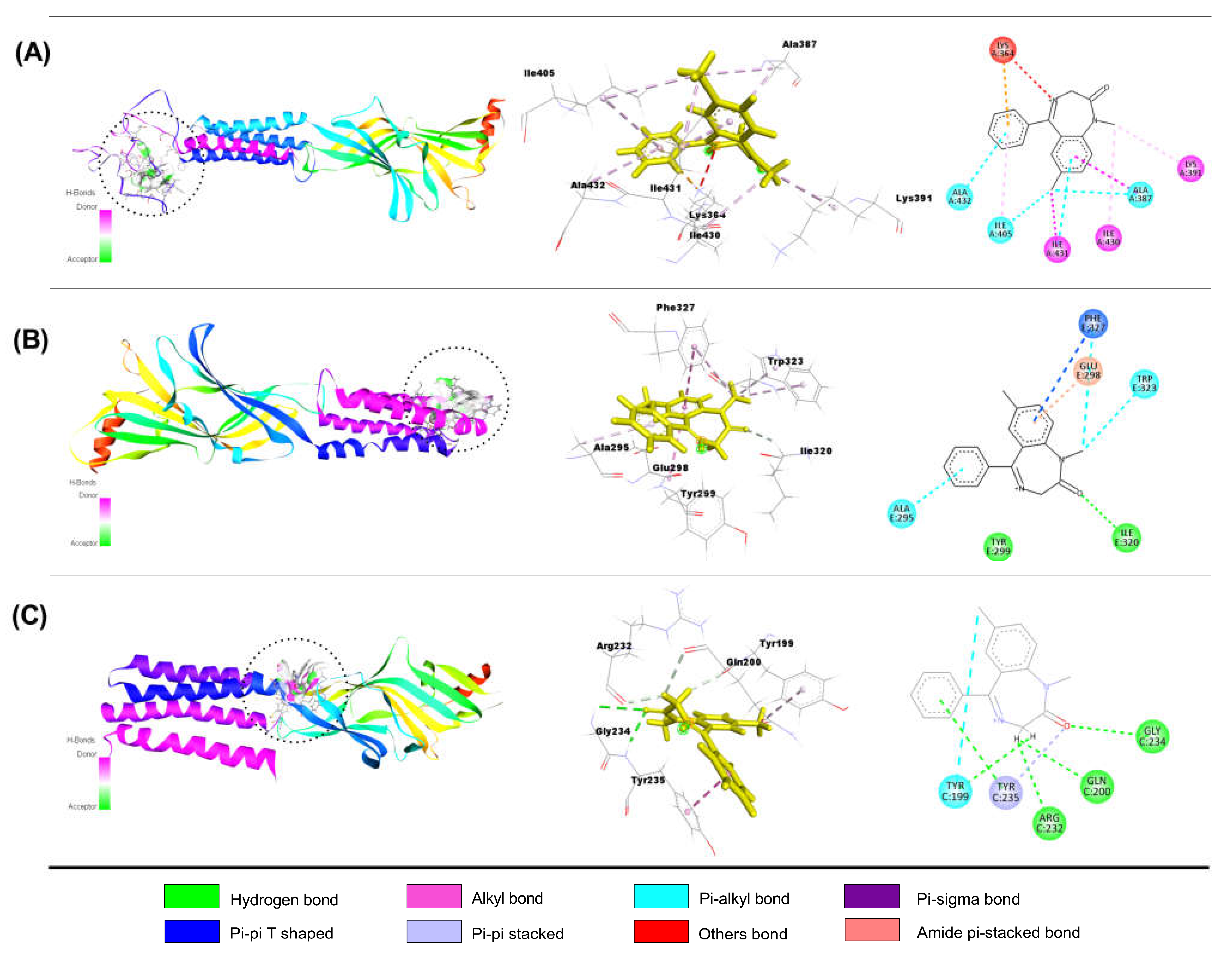
Interaction of QUR with GABAA Receptor Subunits
Interaction of LIN with GABAA Receptor Subunits
Interaction of DZP with GABAA Receptor Subunits
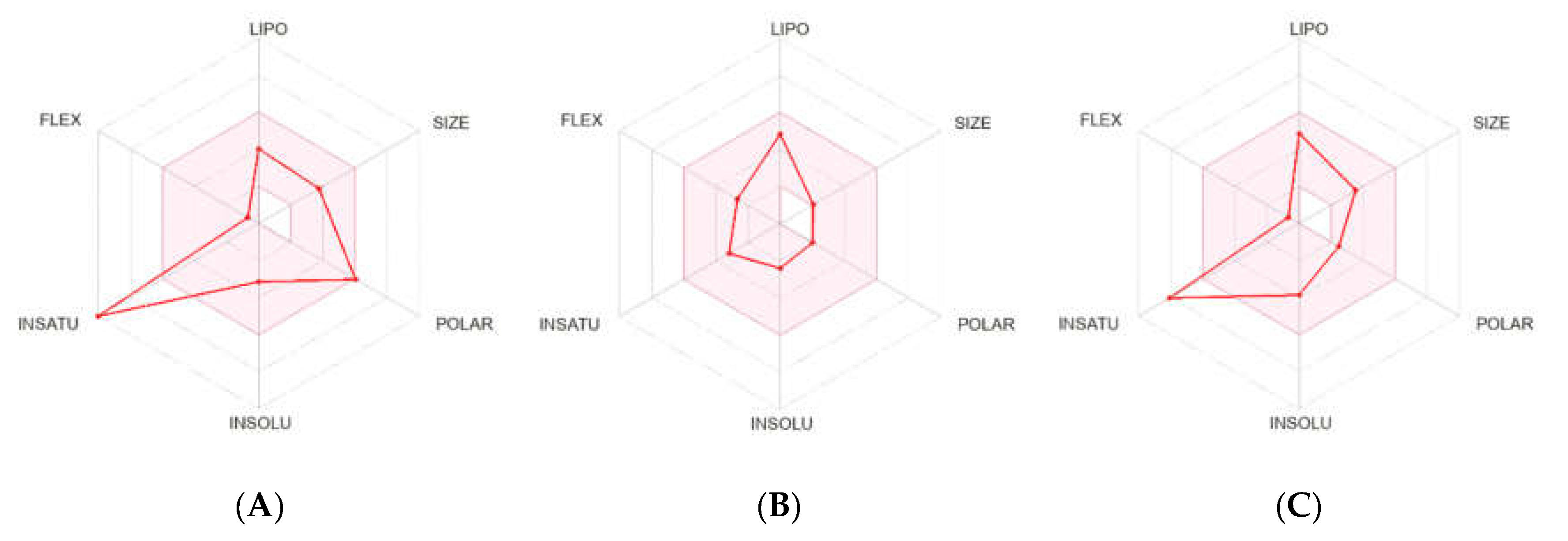
2.2.2. Pharmacokinetics and Drug-Likeness Properties
3. Discussion
4. Materials and Methods
4.1. In Vivo Study
4.1.1. Chemicals and Reagents
4.1.2. Experimental Animals
4.1.3. Selection of Test Doses for Quercetin and Linalool
4.1.4. Study Design (Thiopental Sodium-Induced Sleeping Test in Mice)
4.1.5. Statistical Analysis
4.2. Molecular Docking (In Silico) Study
4.2.1. GABA Homology Model
Retrieval of Sequence
Model Building and Evaluation
4.2.2. Protein Preparation
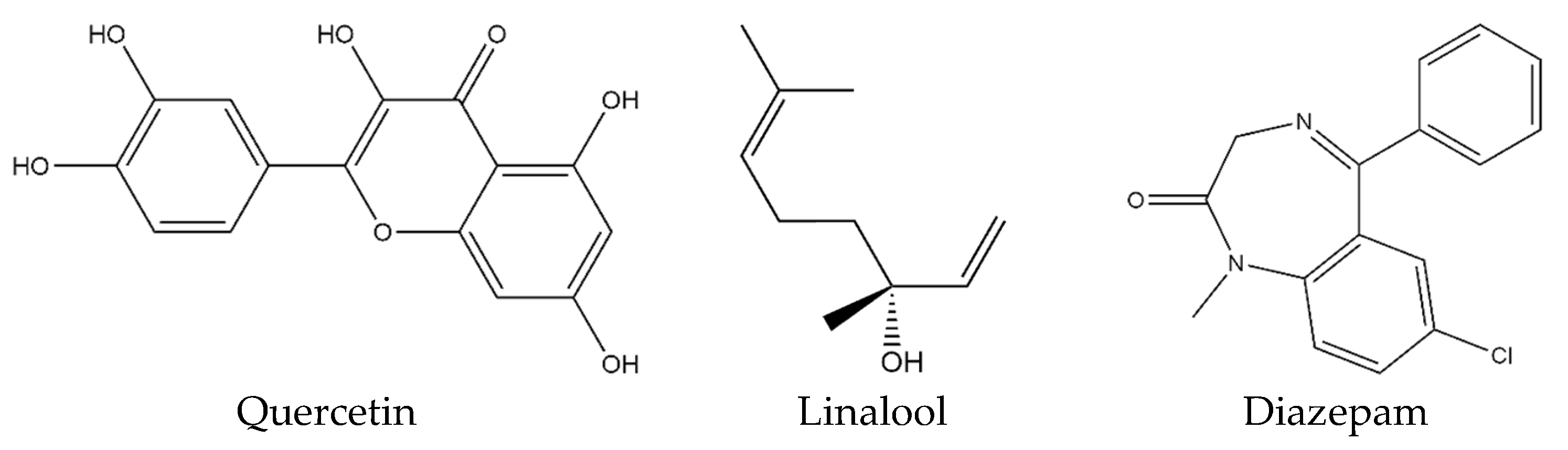
4.2.3. Ligand Preparation
4.2.4. Docking Protocol and Non-Bond Interactions
4.2.5. Pharmacokinetics and Drug-Likeness Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Ghrouz, A.K.; Noohu, M.M.; Dilshad Manzar, M.; Warren Spence, D.; BaHammam, A.S.; Pandi-Perumal, S.R. Physical activity and sleep quality in relation to mental health among college students. Sleep Breath. 2019, 23, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Sharifi-Rad, J.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Ormel, J.; Hollon, S.D.; Kessler, R.C.; Cuijpers, P.; Monroe, S.M. More treatment but no less depression: The treatment-prevalence paradox. Clin. Psychol. Rev. 2022, 91, 102111. [Google Scholar] [CrossRef]
- Perez-Pozuelo, I.; Zhai, B.; Palotti, J.; Mall, R.; Aupetit, M.; Garcia-Gomez, J.M.; Taheri, S.; Guan, Y.; Fernandez-Luque, L. The future of sleep health: A data-driven revolution in sleep science and medicine. NPJ Digit. Med. 2020, 3, 42. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Bogan, R.K.; Arnulf, I.; Scammell, T.E.; Louis, E.K.S.; Thorpy, M.J. Clinical considerations for the diagnosis of idiopathic hypersomnia. Sleep Med. Rev. 2022, 66, 101709. [Google Scholar] [CrossRef]
- Alabd, S.M.; Emara, T.A.Z.; Gad, N.H.; Rabea, M.M. Pharyngoplasty and Other Options for Treatment of Obstructive Sleep Apnea. Egypt. J. Hosp. Med. 2023, 90, 2145–2148. [Google Scholar] [CrossRef]
- Parikh, R.M.; Lebowitz, B.D. Current perspectives in the management of treatment-resistant depression. Dialogues Clin. Neurosci. 2004, 6, 53–60. [Google Scholar] [CrossRef]
- Dumas, E. Sedative-, Hypnotic-, or Anxiolytic-Related Disorders. Addict. Med. A Case Evid. -Based Guide 2022, 2, 81–90. [Google Scholar]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mohler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Patri, M. Synaptic transmission and amino acid neurotransmitters. In Neurochemical Basis of Brain Function and Dysfunction; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Djebaili, R.; Kenouche, S.; Daoud, I.; Melkemi, N.; Belkadi, A.; Mesli, F. Investigation of [3H] diazepam derivatives as allosteric modulators of GABAA receptor α1β2γ2 subtypes: Combination of molecular docking/dynamic simulations, pharmacokinetics/drug-likeness prediction, and QSAR analysis. Struct. Chem. 2023, 34, 791–823. [Google Scholar] [CrossRef]
- Jazvinscak Jembrek, M.; Vlainic, J. GABA receptors: Pharmacological potential and pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.; He, J.; Xie, Z.; Xia, S.; Lu, G. The roles of GABA in ischemia-reperfusion injury in the central nervous system and peripheral organs. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef]
- Phulera, S.; Zhu, H.; Yu, J.; Claxton, D.P.; Yoder, N.; Yoshioka, C.; Gouaux, E. Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA. Elife 2018, 7, e39383. [Google Scholar] [CrossRef]
- Derry, J.M.; Dunn, S.M.; Davies, M. Identification of a residue in the γ-aminobutyric acid type A receptor α subunit that differentially affects diazepam-sensitive and-insensitive benzodiazepine site binding. J. Neurochem. 2004, 88, 1431–1438. [Google Scholar] [CrossRef]
- Engin, E. GABAA receptor subtypes and benzodiazepine use, misuse, and abuse. Front. Psychiatry 2023, 13, 1060949. [Google Scholar] [CrossRef]
- Lee, P.C.; Yang, Y.Y.; Lin, M.W.; Hou, M.C.; Huang, C.S.; Lee, K.C.; Wang, Y.W.; Hsieh, Y.C.; Huang, Y.H.; Lin, H.C.; et al. Benzodiazepine-associated hepatic encephalopathy significantly increased healthcare utilization and medical costs of Chinese cirrhotic patients: 7-year experience. Dig. Dis. Sci. 2014, 59, 1603–1616. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflammation 2014, 11, 1–9. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, Y.; Ruan, Y.; Chen, Z.; Xu, C. Secondary Epileptogenesis: Common to See, but Possible to Treat? Front. Neurol. 2021, 12, 747372. [Google Scholar] [CrossRef]
- Braat, S.; Kooy, R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef]
- Frere, S.; Slutsky, I. Alzheimer’s disease: From firing instability to homeostasis network collapse. Neuron 2018, 97, 32–58. [Google Scholar] [CrossRef]
- Selkoe, D.J. Early network dysfunction in Alzheimer’s disease. Science 2019, 365, 540–541. [Google Scholar] [CrossRef]
- Groth, C.L.; Brown, M.; Honce, J.M.; Shelton, E.; Sillau, S.H.; Berman, B.D. Cervical dystonia is associated with aberrant inhibitory signaling within the thalamus. Front. Neurol. 2021, 11, 575879. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Bellocchio, L.; Bouzón-Arnáiz, I.; Yee, B.K. The role of the endocannabinoid system in autism spectrum disorders: Evidence from mouse studies. Prog Mol Biol Transl Sci 2020, 173, 183–208. [Google Scholar]
- Pizzarelli, R.; Cherubini, E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011, 2011, 12. [Google Scholar] [CrossRef]
- Mohapatra, A.N.; Wagner, S. The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front. Psychiatry 2023, 14, 1205199. [Google Scholar] [CrossRef]
- Ulenberg, S.; Ciura, K.; Georgiev, P.; Pastewska, M.; Ślifirski, G.; Król, M.; Herold, F.; Bączek, T. Use of biomimetic chromatography and in vitro assay to develop predictive GA-MLR model for use in drug-property prediction among anti-depressant drug candidates. Microchem. J. 2022, 175, 107183. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and Alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.K.; Oyouni, A.A.W.A.; Aljohani, M.M.; Ahmad, M.A. Polyphenols more than an antioxidant: Role and scope. J. Pure Appl. Microbiol. 2020, 14, 47–61. [Google Scholar] [CrossRef]
- Bhimanwar, R.; Kothapalli, L.; Khawshi, A. Quercetin as Natural Bioavailability Modulator: An Overview. Res. J. Pharm. Technol. 2020, 13, 2045–2052. [Google Scholar] [CrossRef]
- Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar]
- Barreca, D.; Bellocco, E.; DOnofrio, G.; Fazel Nabavi, S.; Daglia, M.; Rastrelli, L.; Mohammad Nabavi, S. Neuroprotective effects of quercetin: From chemistry to medicine. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2016, 15, 964–975. [Google Scholar] [CrossRef]
- Fideles, S.O.M.; de Cássia Ortiz, A.; Buchaim, D.V.; de Souza Bastos Mazuqueli Pereira, E.; Parreira, M.J.B.M.; de Oliveira Rossi, J.; da Cunha, M.R.; de Souza, A.T.; Soares, W.C.; Buchaim, R.L. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. [Google Scholar] [CrossRef]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1934578X0900400928. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Verdin, A.; Mistrulli, L.; Laruelle, F.; Fourmentin, S.; Lounès-Hadj Sahraoui, A. Chemical composition, antioxidant and anti-inflammatory activities of clary sage and coriander essential oils produced on polluted and amended soils-phytomanagement approach. Molecules 2021, 26, 5321. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Almeida, E.R.D.; Rafael, K.R.D.O.; Couto, G.B.L.; Ishigami, A.B.M. Anxiolytic and anticonvulsant effects on mice of flavonoids, linalool, and 𝛼-tocopherol presents in the extract of leaves of Cissus sicyoides L. (Vitaceae). J. Biomed. Biotechnol. 2009, 2009, 274740. [Google Scholar] [CrossRef]
- Cheng, B.H.; Sheen, L.Y.; Chang, S.T. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2015, 5, 27–34. [Google Scholar] [CrossRef][Green Version]
- Park, H.; Seol, G.H.; Ryu, S.; Choi, I.Y. Neuroprotective effects of (−)-linalool against oxygen-glucose deprivation-induced neuronal injury. Arch. Pharmacal Res. 2016, 39, 555–564. [Google Scholar] [CrossRef]
- Singh, V.; Chauhan, G.; Shri, R. Antidepressant-like effects of quercetin 4′-O-glucoside from Allium cepa via regulation of brain oxidative stress and monoamine levels in mice subjected to unpredictable chronic mild stress. Nutr. Neurosci. 2021, 24, 35–44. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- dos Santos, É.R.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a therapeutic and medicinal tool in depression treatment: A review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- Lopes Campêlo, L.M.; Gonçalves e Sá, C.; De Almeida, A.A.C.; Pereira da Costa, J.; Costa Marques, T.H.; Mendes Feitosa, C.; Saldanha, G.B.; Mendes de Freitas, R. Sedative, anxiolytic and antidepressant activities of Citrus limon (Burn) essential oil in mice. Die Pharm. -Int. J. Pharm. Sci. 2011, 66, 623–627. [Google Scholar]
- Wardakhan, W.A.G.N.A.T.; Abdel-Salam, O.M.; Elmegeed, G.A. Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharm. 2008, 58, 1–14. [Google Scholar] [CrossRef]
- Moraes, W.A.D.S.; Burke, P.R.; Coutinho, P.L.; Guilleminault, C.; Bittencourt, A.G.; Tufik, S.; Poyares, D. Sedative antidepressants and insomnia. Braz. J. Psychiatry 2011, 33, 91–95. [Google Scholar] [CrossRef]
- Misra, A.K.; Sharma, P.K. Sedative and Hypnotic Drugs. In Advances in Neuropharmacology; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 259–282. [Google Scholar]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Wu, M.; Sirota, M.; Butte, A.J.; Chen, B.I.N. Characteristics of drug combination therapy in oncology by analyzing clinical trial data on ClinicalTrials. gov. Pac. Symp. Biocomput. Co-Chairs 2014, 20, 68–79. [Google Scholar]
- Shao, Y.; Yu, H.; Yang, Y.; Li, M.; Hang, L.; Xu, X. A Solid Dispersion of Quercetin Shows Enhanced Nrf2 Activation and Protective Effects against Oxidative Injury in a Mouse Model of Dry Age-Related Macular Degeneration. Oxidative Med. Cell. Longev. 2019, 2019, 1479571. [Google Scholar] [CrossRef] [PubMed]
- Blaskó, Á.; Gazdag, Z.; Gróf, P.; Máté, G.; Sárosi, S.; Krisch, J.; Vágvölgyi, C.; Makszin, L.; Pesti, M. Effects of clary sage oil and its main components, linalool and linalyl acetate, on the plasma membrane of Candida albicans: An in vivo EPR study. Apoptosis 2017, 22, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug Des. 2019, 93, 12–20. [Google Scholar] [CrossRef]
- Haredi Abdelmonsef, A. Computer-aided identification of lung cancer inhibitors through homology modeling and virtual screening. Egypt. J. Med. Hum. Genet. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ranjith, D.; Ravikumar, C. SwissADME predictions of pharmacokinetics and drug-likeness properties of small molecules present in Ipomoea mauritiana Jacq. J. Pharmacogn. Phytochem. 2019, 8, 2063–2073. [Google Scholar]
- Chen, X.; Li, H.; Tian, L.; Li, Q.; Luo, J.; Zhang, Y. Analysis of the physicochemical properties of acaricides based on Lipinski’s rule of five. J. Comput. Biol. 2020, 27, 1397–1406. [Google Scholar] [CrossRef]
- Chandran, A.; Merlin, N.J.; Ammu, L.; Dharan, S.S. Fennel treatment to PCOS: An in silico evaluation to explore the therapeutic efficacy of anethole. Res. J. Pharm. Technol. 2019, 12, 4958–4962. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, B.R.; Zhang, L.Q.; Huang, X.; Yuan, X.; Tian, Y.K.; Tian, X.B. The role of the GABAergic system in diseases of the central nervous system. Neuroscience 2021, 470, 88–99. [Google Scholar] [CrossRef]
- Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 2011, 69, 628–649. [Google Scholar] [CrossRef]
- Engin, E.; Benham, R.S.; Rudolph, U. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol. Sci. 2018, 39, 710–732. [Google Scholar] [CrossRef]
- Godfrey, K.E.; Gardner, A.C.; Kwon, S.; Chea, W.; Muthukumaraswamy, S.D. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 105, 33–44. [Google Scholar] [CrossRef]
- Knudsen, M.K.; Near, J.; Blicher, A.B.; Videbech, P.; Blicher, J.U. Magnetic resonance (MR) spectroscopic measurement of γ-aminobutyric acid (GABA) in major depression before and after electroconvulsive therapy. Acta Neuropsychiatr. 2019, 31, 17–26. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Johnston, G.A.; Hanrahan, J.R.; Chebib, M.; Duke, R.K.; Mewett, K.N. Modulation of ionotropic GABA receptors by natural products of plant origin. Adv. Pharmacol. 2006, 54, 285–316. [Google Scholar]
- Tan, K.R.; Rudolph, U.; Lüscher, C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011, 34, 188–197. [Google Scholar] [CrossRef]
- McKillop, L.E.; Fisher, S.P.; Milinski, L.; Krone, L.B.; Vyazovskiy, V.V. Diazepam effects on local cortical neural activity during sleep in mice. Biochem. Pharmacol. 2021, 191, 114515. [Google Scholar] [CrossRef]
- Tobler, I.; Kopp, C.; Deboer, T.; Rudolph, U. Diazepam-induced changes in sleep: Role of the α1 GABAA receptor subtype. Proc. Natl. Acad. Sci. USA 2001, 98, 6464–6469. [Google Scholar] [CrossRef]
- Lee, S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018, 51, 167. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, K.B.; Jo, K.; Suh, H.J. Quercetin-3-O-glucuronide in the ethanol extract of lotus leaf (Nelumbo nucifera) enhances sleep quantity and quality in a rodent model via a GABAergic mechanism. Molecules 2021, 26, 3023. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Bianchini, A.E.; Garlet, Q.I.; Da Cunha, J.A.; Bandeira, G.; Brusque, I.C.M.; Salbego, J.; Heinzmann, B.M.; Baldisserotto, B. Monoterpenoids (thymol, carvacrol and S-(+)-linalool) with anesthetic activity in silver catfish (Rhamdia quelen): Evaluation of acetylcholinesterase and GABAergic activity. Braz. J. Med. Biol. Res. 2017, 50, e6346. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, Y.; Firempong, C.K.; Xu, X. Preparation, characterization and pharmacokinetic studies of linalool-loaded nanostructured lipid carriers. Pharm. Biol. 2016, 54, 2320–2328. [Google Scholar] [CrossRef]
- Parke, D.V.; Rahman, K.M.Q.; Walker, R. The absorption, distribution and excretion of linalool in the rat. Biochem. Soc. Trans. 1974, 2, 612–615. [Google Scholar] [CrossRef]
- Shah, K.; Tankersley, W.; Mekala, H. Kratom: An Emerging Issue and Need for Regulations in the United States. Prim. Care Companion CNS Disord. 2021, 23, 20r02770. [Google Scholar] [CrossRef]
- Calver, L.; Drinkwater, V.; Isbister, G.K. A prospective study of high dose sedation for rapid tranquilisation of acute behavioural disturbance in an acute mental health unit. BMC Psychiatry 2013, 13, 225. [Google Scholar] [CrossRef]
- Manathunga, M.; Götz, A.W.; Merz, K.M., Jr. Computer-aided drug design, quantum-mechanical methods for biological problems. Curr. Opin. Struct. Biol. 2022, 75, 102417. [Google Scholar] [CrossRef]
- Crofton, K.M.; Bassan, A.; Behl, M.; Chushak, Y.G.; Fritsche, E.; Gearhart, J.M.; Marty, M.S.; Mumtaz, M.; Pavan, M.; Myatt, G.J.; et al. Current status and future directions for a neurotoxicity hazard assessment framework that integrates in silico approaches. Comput. Toxicol. 2022, 22, 100223. [Google Scholar] [CrossRef]
- Khare, N.; Maheshwari, S.K.; Rizvi, S.M.D.; Albadrani, H.M.; Alsagaby, S.A.; Alturaiki, W.; Iqbal, D.; Zia, Q.; Villa, C.; Jha, A.K.; et al. Homology Modelling, Molecular Docking and Molecular Dynamics Simulation Studies of CALMH1 against Secondary Metabolites of Bauhinia variegata to Treat Alzheimer’s Disease. Brain Sci. 2022, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Paudel, Y.N.; Polat, A.K.; Dundar, H.E.; Angelopoulou, E. Enlightening the neuroprotective effect of quercetin in epilepsy: From mechanism to therapeutic opportunities. Epilepsy Behav. 2021, 115, 107701. [Google Scholar] [CrossRef]
- Bhat, I.U.H.; Bhat, R. Quercetin: A bioactive compound imparting cardiovascular and neuroprotective benefits: Scope for exploring fresh produce, their wastes, and by-products. Biology 2021, 10, 586. [Google Scholar] [CrossRef]
- Hossain, R.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Islam, M.S.; Dey, D.; Jain, D.; Faria, F.; Akbor, R.; Islam, M.T.; et al. Quercetin and/or ascorbic acid modulatory effect on phenobarbital-induced sleeping mice possibly through gabaa and gabab receptor interaction pathway. Pharmaceuticals 2021, 14, 721. [Google Scholar] [CrossRef]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Agrawal, K.; Chakraborty, P.; Dewanjee, S.; Arfin, S.; Das, S.S.; Dey, A.; Moustafa, M.; Mishra, P.C.; Jafari, S.M.; Kumar, D.; et al. Neuropharmacological Interventions of Quercetin and Its Derivatives in Neurological and Psychological Disorders. Neurosci. Biobehav. Rev. 2022, 144, 104955. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef]
- Milanos, S.; Elsharif, S.A.; Janzen, D.; Buettner, A.; Villmann, C. Metabolic products of linalool and modulation of GABAA receptors. Front. Chem. 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Prottay, A.A.S.; Sultana, I.; Al Faruq, A.; Bappi, M.H.; Akbor, M.S.; Asha, A.I.; Hossen, M.M.; Machado, P.E.M.; Islam, M.T.; et al. Phytochemical screening and evaluation of antioxidant, anti-inflammatory, antimicrobial, and membrane-stabilizing activities of different fractional extracts of Grewia nervosa (Lour.) Panigrahi. Food Biosci. 2023, 54, 102933. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A flavonoid on the brain: Quercetin as a potential therapeutic agent in central nervous system disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.; Mazzardo-Martins, L.; Martins, D.F.; Santos, A.R.S.; da Silva Brum, L.F.; Picada, J.N.; Pereira, P. Neurobehavioral and genotoxic evaluation of (−)-linalool in mice. J. Nat. Med. 2013, 67, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandah, H.; Hosseini, M.; Dolati, K. Hypnotic effect of Rosa damascena in mice. Iran. J. Pharm. Res. 2022, 3, 181–185. [Google Scholar]
- Meneses, C.; Valdes-Gonzalez, M.; Garrido-Suárez, B.B.; Garrido, G. Systematic review on the anxiolytic and hypnotic effects of flower extracts in in vivo pre-clinical studies published from 2010 to 2020. Phytother. Res. 2023, 37, 2144–2167. [Google Scholar] [CrossRef]
- Iwashita, H.; Sano, M.; Chiba, A. Diazepam induces retrograde facilitation of object recognition and object location memory in male mice. NeuroReport 2023, 34, 137–143. [Google Scholar] [CrossRef]
- Amalia, E.; Diantini, A.; Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020, 20, 274. [Google Scholar] [CrossRef]
- McGarvey, P.B.; Nightingale, A.; Luo, J.; Huang, H.; Martin, M.J.; Wu, C.; Consortium, U. UniProt genomic mapping for deciphering functional effects of missense variants. Hum. Mutat. 2019, 40, 694–705. [Google Scholar] [CrossRef]
- Sahay, A.; Piprodhe, A.; Pise, M. In silico analysis and homology modeling of strictosidine synthase involved in alkaloid biosynthesis in Catharanthus roseus. J. Genet. Eng. Biotechnol. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Shah, N.; Nute, M.G.; Warnow, T.; Pop, M. Misunderstood parameter of NCBI BLAST impacts the correctness of bioinformatics workflows. Bioinformatics 2019, 35, 1613–1614. [Google Scholar] [CrossRef]
- Prajapat, R.; Jain, S.; Vaishnav, M.K.; Sogani, S. Structural Modeling and Validation of Growth/Differentiation Factor 15 [NP_004855] Associated with Pregnancy Complication-Hyperemesis Gravidarum. J. Krishna Inst. Med. Sci. (JKIMSU) 2020, 9, 40–47. [Google Scholar]
- Yadav, D.K.; Shukla, D.; Tuteja, N. Rice heterotrimeric G-protein alpha subunit (RGA1): In silico analysis of the gene and promoter and its upregulation under abiotic stress. Plant Physiol. Biochem. 2013, 63, 262–271. [Google Scholar] [CrossRef]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020, 2020, 2020030226. [Google Scholar]
- Junaid, M.; Islam, N.; Hossain, M.K.; Ullah, M.O.; Halim, M.A. Metal based donepezil analogues designed to inhibit human acetylcholinesterase for Alzheimer’s disease. PLoS ONE 2019, 14, e0211935. [Google Scholar] [CrossRef]
- Ali, S.M.; Shamim, S. Analysis of computational models of β-cyclodextrin complexes: Structural studies of morniflumate hydrochloride and β-cyclodextrin complex in aqueous solution by quantitative ROESY analysis. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 19–26. [Google Scholar] [CrossRef]
- de Sousa, A.C.C.; Combrinck, J.M.; Maepa, K.; Egan, T.J. Virtual screening as a tool to discover new β-haematin inhibitors with activity against malaria parasites. Sci. Rep. 2020, 10, 3374. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wahyuni, D.K.; Wacharasindhu, S.; Bankeeree, W.; Punnapayak, H.; Parikesit, A.A.; Kharisma, V.D. Molecular simulation of compounds from n-hexane fraction of Sonchus arvensis L. leaves as SARS-CoV-2 antiviral through inhibitor activity targeting strategic viral protein. J. Pharm. Pharmacogn. Res. 2022, 10, 1126–1138. [Google Scholar] [CrossRef]
- Lombardo, F.; Desai, P.V.; Arimoto, R.; Desino, K.E.; Fischer, H.; Keefer, C.E.; Petersson, C.; Winiwarter, S.; Broccatelli, F. In Silico absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME-PK): Utility and best practices. An industry perspective from the international consortium for innovation through quality in pharmaceutical development: Miniperspective. J. Med. Chem. 2017, 60, 9097–9113. [Google Scholar] [CrossRef]
- Mahanthesh, M.T.; Ranjith, D.; Yaligar, R.; Jyothi, R.; Narappa, G.; Ravi, M.V. Swiss ADME prediction of phytochemicals present in Butea monosperma (Lam.) Taub. J. Pharmacogn. Phytochem. 2020, 9, 1799–1809. [Google Scholar]

| First Squad | |||
|---|---|---|---|
| Treatment Group | Latency (min) | Sleeping Time (min) | Sleep Incidence (%) |
| Gr-I | 5.40 ± 0.84 | 41.20 ± 5.02 | 100 |
| Gr-II | 3.20 ± 0.65 * | 99.60 ± 5.73 * | 100 |
| Gr-III | 29.40 ± 2.08 | 12.40 ± 2.21 | 100 |
| Gr-IV | 42.60 ± 2.01 | 15.60 ± 1.23 | 100 |
| Gr-V | 53.40 ± 2.56 | 24.00 ± 5.24 | 100 |
| Gr-VI | 5.80 ± 0.73 | 44.60 ± 2.01 * | 100 |
| Gr-VII | 6.80 ± 0.97 | 46.40 ± 2.01 * | 100 |
| Gr-VIII | 8.40 ± 0.76 | 53.20 ± 4.04 * | 100 |
| Second Squad | |||
|---|---|---|---|
| Treatment Group | Latency (min) | Sleeping Time (min) | Sleep Incidence (%) |
| Gr-CI * | 5.40 ± 0.84 | 41.20 ± 5.02 | 100 |
| Gr-CII a | 53.40 ± 2.56 | 24.00 ± 5.24 | 100 |
| Gr-CIII b | 8.40 ± 0.76 a | 53.20 ± 4.04 *a | 100 |
| Gr-CIV | 3.20 ± 0.65 *ab | 99.60 ± 5.73 *ab | 100 |
| Gr-CV | 16.80 ± 2.43 a | 34.00 ± 5.46 a | 100 |
| Gr-CVI | 43.00 ± 4.15 a | 46.20 ± 1.30 *a | 100 |
| Treatment Group | Latency Decrease (%) | Sleeping Time Increase (%) |
|---|---|---|
| Gr-CII | - | - |
| Gr-CIII | - | 22.56 |
| Gr-CIV | 92.23 | 58.63 |
| Gr-CV | - | - |
| Gr-CVI | - | 10.82 |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | Number of Hydrogen Bond | Number of Hydrophobic Bond | Number of Others Bond |
|---|---|---|---|---|
| GABAA α1 | −7.1 | 2 | 4 | - |
| GABAA α2 | −7.9 | 5 | 5 | 1 |
| GABAA α3 | −8.2 | 2 | 5 | - |
| GABAA α5 | −7.5 | 2 | 2 | 1 |
| GABAA β1 | −8.0 | 4 | 4 | - |
| GABAA β2 | −7.8 | 4 | 2 | - |
| GABAA β3 | −7.0 | 5 | 2 | - |
| GABAA γ2 | −7.0 | 4 | 3 | - |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | Number of Hydrogen Bond | Number of Hydrophobic Bond | Number of Others Bond |
|---|---|---|---|---|
| GABAA α1 | −4.8 | 1 | 11 | - |
| GABAA α2 | −4.5 | - | 6 | - |
| GABAA α3 | −5.2 | 1 | 9 | - |
| GABAA α5 | −4.8 | 1 | 7 | - |
| GABAA β1 | −5.8 | - | 12 | - |
| GABAA β2 | −4.8 | 1 | 6 | - |
| GABAA β3 | −4.8 | 1 | 6 | - |
| GABAA γ2 | −4.3 | - | 7 | - |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | Number of Hydrogen Bond | Number of Hydrophobic Bond | Number of Others Bond |
|---|---|---|---|---|
| GABAA α1 | −6.4 | 1 | 7 | 1 |
| GABAA α2 | −6.7 | 1 | 7 | 1 |
| GABAA α3 | −6.8 | - | 9 | - |
| GABAA α5 | −6.5 | 3 | 4 | 3 |
| GABAA β1 | −7.8 | 1 | 6 | - |
| GABAA β2 | −7.0 | 2 | 2 | 2 |
| GABAA β3 | −6.3 | 2 | 2 | - |
| GABAA γ2 | −7.7 | 5 | 2 | - |
| Properties | Factors | Quercetin | Linalool | Diazepam |
|---|---|---|---|---|
| Physico-chemical properties | Formula | C15H10O7 | C10H18O | C16H13ClN2O |
| MW (g mol−1) | 302.24 | 154.25 | 284.74 | |
| Heavy atoms | 22 | 11 | 20 | |
| Arom. heavy atoms | 16 | 0 | 12 | |
| H-Bond acceptors (HBAs) | 7 | 1 | 2 | |
| H-Bond donors (HBDs) | 5 | 1 | 0 | |
| Molar refractivity | 78.03 | 50.44 | 87.95 | |
| TPSA (Å2) | 131.36 | 20.23 | 32.67 | |
| Lipophilicity | log Po/w (XLOGP3) | 1.54 | 2.97 | 2.99 |
| Water solubility | log S (ESOL) | Soluble | Soluble | Soluble |
| Pharmacokinetics | GI absorption | High | High | High |
| Drug likeness | Lipinski | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 | |
| Medicinal chemistry | Synthetic accessibility | 3.23 | 2.74 | 3.00 |
| First Squad | ||
|---|---|---|
| Treatments | Composition | Dose |
| Gr-I | Vehicle (0.5% tween 80 dissolved in normal saline) | 10 mL/kg |
| Gr-II | Diazepam (DZP) | 3 mg/kg |
| Gr-III | Quercetin (QUR) | 10 mg/kg |
| Gr-IV | QUR | 25 mg/kg |
| Gr-V | QUR | 50 mg/kg |
| Gr-VI | Linalool (LIN) | 10 mg/kg |
| Gr-VI | LIN | 25 mg/kg |
| Gr-VI | LIN | 50 mg/kg |
| Second squad | ||
| Treatments | Composition | Dose |
| Gr-CI | Vehicle | 10 mL/kg |
| Gr-CII | QUR | 50 mg/kg |
| Gr-CIII | LIN | 50 mg/kg |
| Gr-CIV | DZP | 3 mg/kg |
| Gr-CV | QUR-50 + LIN-50 | 50 mg/kg + 50 mg/kg |
| Gr-CVI | QUR-50 + DZP-3 + LIN-50 | 50 mg/kg + 3 mg/kg + 50 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bappi, M.H.; Prottay, A.A.S.; Kamli, H.; Sonia, F.A.; Mia, M.N.; Akbor, M.S.; Hossen, M.M.; Awadallah, S.; Mubarak, M.S.; Islam, M.T. Quercetin Antagonizes the Sedative Effects of Linalool, Possibly through the GABAergic Interaction Pathway. Molecules 2023, 28, 5616. https://doi.org/10.3390/molecules28145616
Bappi MH, Prottay AAS, Kamli H, Sonia FA, Mia MN, Akbor MS, Hossen MM, Awadallah S, Mubarak MS, Islam MT. Quercetin Antagonizes the Sedative Effects of Linalool, Possibly through the GABAergic Interaction Pathway. Molecules. 2023; 28(14):5616. https://doi.org/10.3390/molecules28145616
Chicago/Turabian StyleBappi, Mehedi Hasan, Abdullah Al Shamsh Prottay, Hossam Kamli, Fatema Akter Sonia, Md. Nayem Mia, Md. Showkoth Akbor, Md. Munnaf Hossen, Samir Awadallah, Mohammad S. Mubarak, and Muhammad Torequl Islam. 2023. "Quercetin Antagonizes the Sedative Effects of Linalool, Possibly through the GABAergic Interaction Pathway" Molecules 28, no. 14: 5616. https://doi.org/10.3390/molecules28145616
APA StyleBappi, M. H., Prottay, A. A. S., Kamli, H., Sonia, F. A., Mia, M. N., Akbor, M. S., Hossen, M. M., Awadallah, S., Mubarak, M. S., & Islam, M. T. (2023). Quercetin Antagonizes the Sedative Effects of Linalool, Possibly through the GABAergic Interaction Pathway. Molecules, 28(14), 5616. https://doi.org/10.3390/molecules28145616









