NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
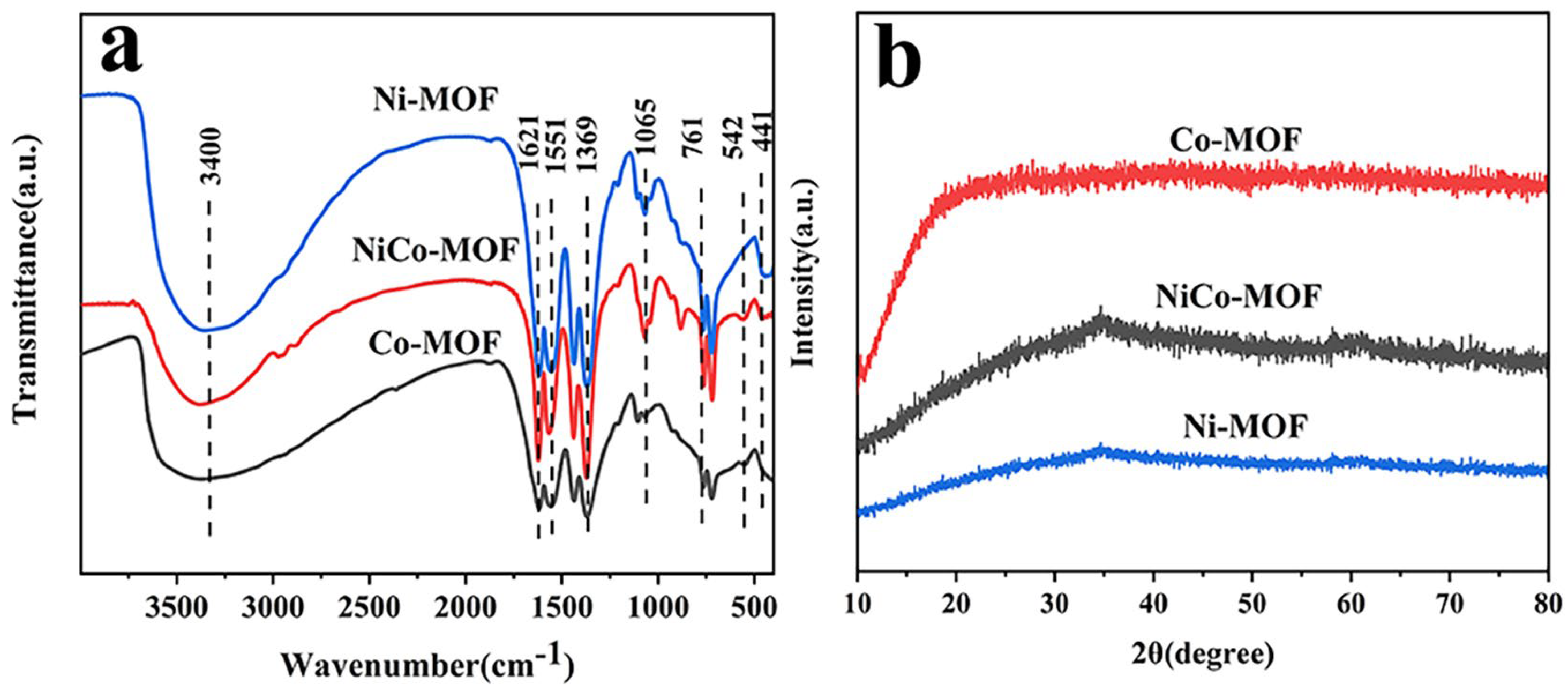
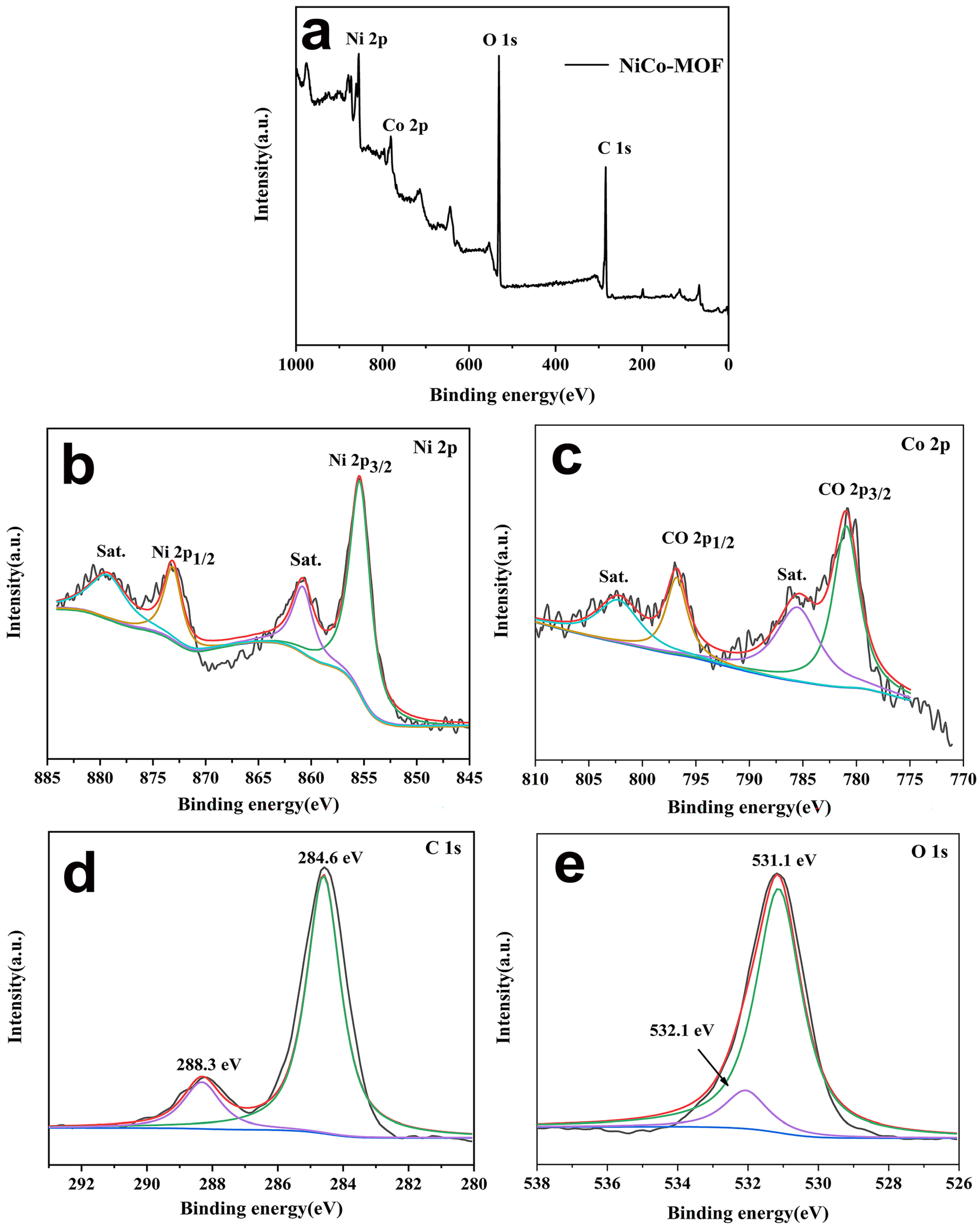
2.1. Characterization
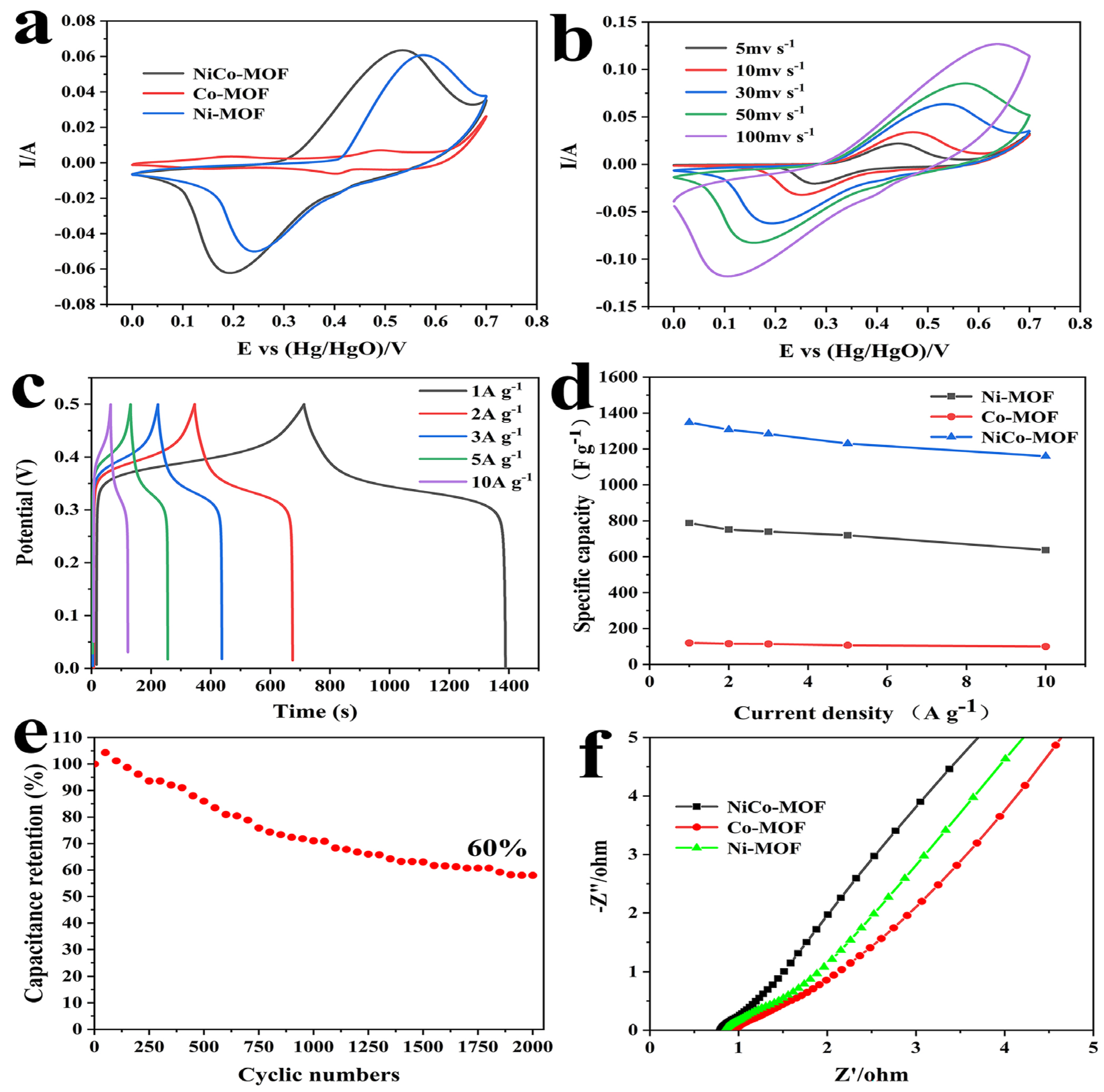
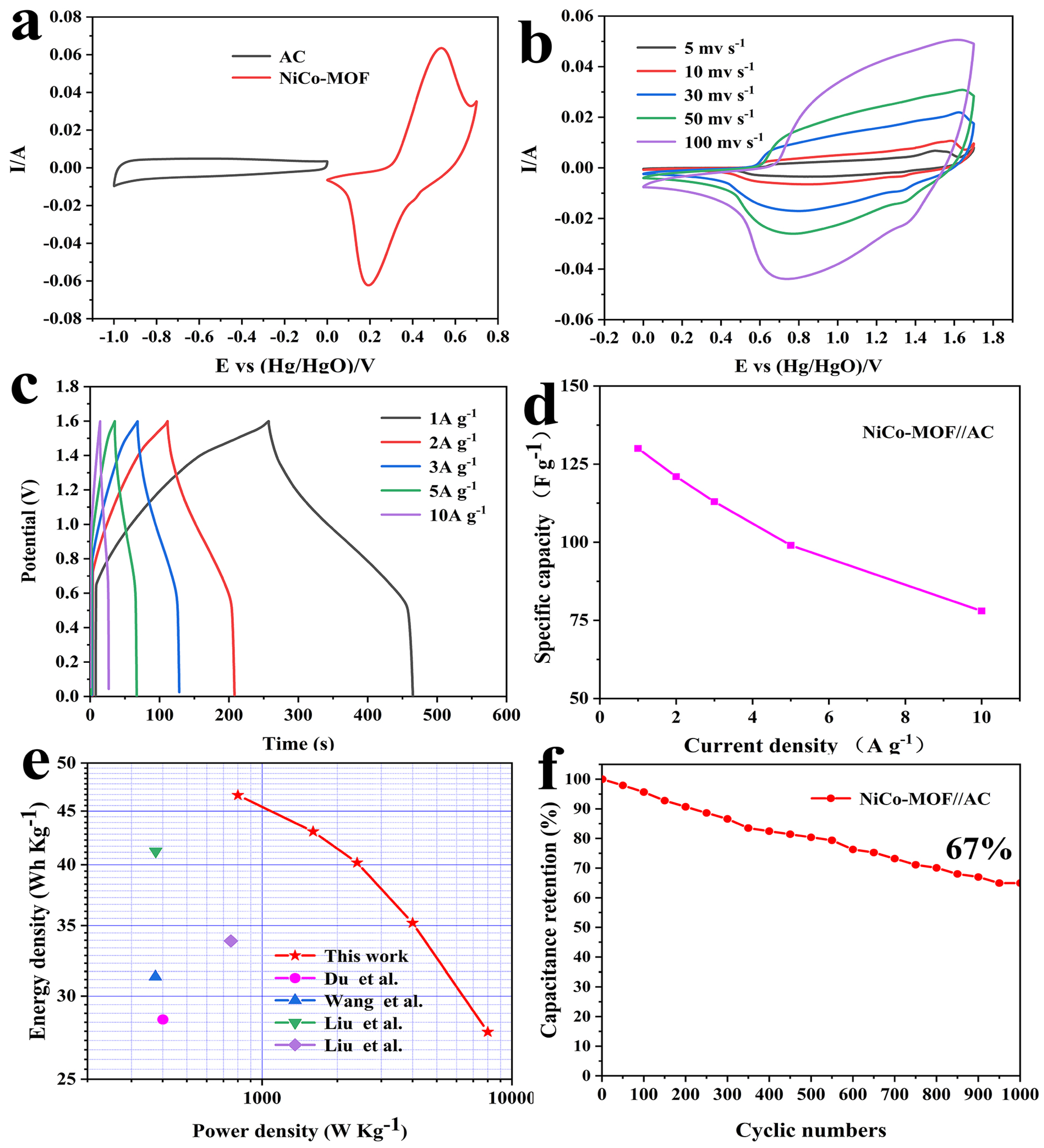
2.2. Electrochemical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation for NiCo-MOF Electrodes
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iqbal, M.F.; Ashiq, M.N.; Zhang, M. Design of Metals Sulfides with Carbon Materials for Supercapacitor Applications: A Review. Energy Technol. 2021, 9, 2000987. [Google Scholar] [CrossRef]
- Zhong, M.Z.; Zhang, M.; Li, X.F. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.B.; Peng, Y.M.; Yin, Y.H.; Li, Y.S.; Wu, Z.P. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Liang, R.B.; Du, Y.Q.; Xiao, P.; Cheng, J.Y.; Yuan, S.J.; Chen, Y.L.; Yuan, J.; Chen, J.W. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Guan, J.H.; Chen, Y.; Cao, L.T.; Liu, Y.; Lian, P.; Gao, Y.N.; Shi, X.C. Multicomponent design of Fe3O4 nanosheet-based binder-free anodes with a special substrate for supercapacitors. J. Power Sources 2020, 469, 228307. [Google Scholar] [CrossRef]
- Liu, J.C.; Huang, Z.H.; Ma, T.Y. Aqueous Supercapacitor with Ultrahigh Voltage Window Beyond 2.0 Volt. Small Struct. 2020, 1, 2000020. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, L.; Hou, H.Q.; Xu, W.H.; Duan, G.G.; He, S.J.; Liu, K.M.; Jiang, S.H. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.R.; Sarwar, S.; Zhang, M.M.; Zhang, H.P.; Luo, J.J.; Zhang, X.Y. Comparative evaluation of PPyNF/CoOx and PPyNT/CoOx nanocomposites as battery-type supercapacitor materials via a facile and low-cost microwave synthesis approach. Electrochim. Acta 2019, 311, 230–243. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Zadeh, M.H.R.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Li, Z.H.; Xu, K.; Pan, Y.S. Recent development of Supercapacitor Electrode Based on Carbon Materials. Nanotechnol. Rev. 2019, 8, 35–49. [Google Scholar] [CrossRef]
- Meng, Q.F.; Cai, K.F.; Chen, Y.X.; Chen, L.D. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Akhtar, M.; Rafiq, S.; Warsi, M.F.; El-Bahy, S.M.; Hessien, M.M.; Mersal, G.A.M.; Ibrahim, M.M.; Shahid, M. Hierarchically porous NiO microspheres and their nanocomposites with exfoliated carbon as electrode materials for supercapacitor applications. J. Taibah Univ. Sci. 2022, 16, 575–584. [Google Scholar] [CrossRef]
- Wu, Y.D.; Wang, Y.; Zhu, P.; Ye, X.; Liu, R.N.; Cai, W.F. Precisely controllable oxygen vacancy-manufactured Ov-Co3O4@PPy for high energy density supercapacitor. Appl. Surf. Sci. 2022, 606, 154863. [Google Scholar] [CrossRef]
- Trabelsi, A.B.; Essam, D.; Alkallas, F.H.H.; Ahmed, A.M.M.; Rabia, M. Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines 2022, 13, 2134. [Google Scholar] [CrossRef]
- Gao, R.X.; Lei, H.; Li, W.L.; He, M.; Ren, Z.Y. MOF-derived Co-Fe-P@NiCo-layered double hydroxides with high areal capacity for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2023, 34, 1136. [Google Scholar] [CrossRef]
- Dewan, A.; Narayanan, R.; Thotiyl, M.O. A multi-chromic supercapacitor of high coloration efficiency integrating a MOF-derived V2O5 electrode. Nanoscale 2022, 14, 17372–17384. [Google Scholar] [CrossRef]
- Dhiman, N.; Sharma, V.; Ghosh, S. Perspective on Biomass-Based Cotton-Derived Nanocarbon for Multifunctional Energy Storage and Harvesting Applications. ACS Appl. Electron. Mater. 2023, 5, 1970–1991. [Google Scholar] [CrossRef]
- Shen, W.X.; Guo, X.T.; Pang, H. Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF. Molecules 2022, 27, 8226. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, J.T.; Gao, W.; Jiang, S.X.; Guo, R.H. Preparation of flexible supercapacitor with RGO/Ni-MOF film on Ni-coated polyester fabric. Electrochim. Acta 2019, 318, 23–31. [Google Scholar] [CrossRef]
- Qu, C.; Jiao, Y.; Zhao, B.T.; Chen, D.C.; Zou, R.Q.; Walton, K.S.; Liu, M.L. Nickel-based pillared MOFs for high-performance supercapacitors: Design, synthesis and stability study. Nano Energy 2016, 26, 66–73. [Google Scholar] [CrossRef]
- Xuan, W.L.; Ramachandran, R.; Zhao, C.H.; Wang, F. Influence of synthesis temperature on cobalt metal-organic framework (Co-MOF) formation and its electrochemical performance towards supercapacitor electrodes. J. Solid State Electr. 2018, 22, 3873–3881. [Google Scholar] [CrossRef]
- Zheng, S.S.; Li, X.R.; Yan, B.Y.; Hu, Q.; Xu, Y.X.; Xiao, X.; Xue, H.G.; Pang, H. Transition-Metal (Fe, Co, Ni) Based Metal-Organic Frameworks for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 7, 1602733. [Google Scholar] [CrossRef]
- Yang, Q.J.; Liu, Y.; Xiao, L.S.; Yan, M.; Bai, H.Y.; Zhu, F.F.; Lei, Y.; Shi, W.D. Self-templated transformation of MOFs into layered double hydroxide nanoarrays with selectively formed Co9S8 for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 354, 716–726. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Liu, X.M.; Guan, C.; Wang, J. Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim. Acta 2018, 291, 177–187. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H.X. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.B.; Zhao, S.H.; Chen, H.M.; Tao, K.; Han, L. Solvent-Controlled Morphology of Amino-Functionalized Bimetal Metal-Organic Frameworks for Asymmetric Supercapacitors. Inorg. Chem. 2020, 59, 11385–11395. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liu, Y.X.; Wang, H.Q.; Liu, W.; Li, Y.; Zhang, J.F.; Hou, H.; Yang, J.L. Ultrathin NiCo-MOF Nanosheets for High-Performance Supercapacitor Electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Du, Y.Q.; Liang, R.B.; Wu, J.X.; Ye, Y.Y.; Chen, S.Y.; Yuan, J.; Chen, J.W.; Xiao, P. High-performance quasi-solid-state flexible supercapacitors based on a flower-like NiCo metal-organic framework. RSC Adv. 2022, 12, 5910–5918. [Google Scholar] [CrossRef]
- Gao, S.W.; Sui, Y.W.; Wei, F.X.; Qi, J.Q.; Meng, Q.K.; Ren, Y.J.; He, Y.Z. Dandelion-like nickel/cobalt metal-organic framework based electrode materials for high performance supercapacitors. J. Colloid Interface Sci. 2018, 531, 83–90. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Y.Z.; Chen, Y.J.; Wang, C.; Guo, L. NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-performance supercapacitors. Appl. Surf. Sci. 2020, 507, 145089. [Google Scholar] [CrossRef]
- Yan, W.J.; Guo, Z.Y.; Xu, H.S.; Lou, Y.B.; Chen, J.X.; Li, Q.W. Downsizing metal-organic frameworks with distinct morphologies as cathode materials for high-capacity Li-O2 batteries. Mater. Chem. Front. 2017, 1, 1324–1330. [Google Scholar] [CrossRef]
- Hounkanrin, S.R.J.E.; Guo, Z.; Luo, J.J. Microwave-synthesized Bismuth oxide/Activated Carbon felt composite as electrode for ultra-high supercapacitors performance. Int. J. Electrochem. Sci. 2023, 18, 100128. [Google Scholar] [CrossRef]
- Du, L.J.; Lv, N.; Li, J.S.; Zhang, J.Y.; Chen, Y.L.; Zhang, Y.L.; Li, Z.; Huang, X.Q.; Luo, J.J. NiCoSe4@CFF with excellent properties prepared by microwave method for flexible supercapacitors and oxygen evolution reaction. J. Ind. Eng. Chem. 2023, 120, 467–476. [Google Scholar] [CrossRef]
- Murgia, F.; Antitomaso, P.; Stievano, L.; Monconduit, L.; Berthelot, R. Express and low-cost microwave synthesis of the ternary Chevrel phase Cu2Mo6S8 for application in rechargeable magnesium batteries. J. Solid State Chem. 2016, 242, 151–154. [Google Scholar] [CrossRef]
- Levin, E.E.; Grebenkemper, J.H.; Pollock, T.M.; Seshadri, R. Protocols for High Temperature Assisted-Microwave Preparation of Inorganic Compounds. Chem. Mater. 2019, 31, 7151–7159. [Google Scholar] [CrossRef]
- Wu, V.C.; Evans, H.A.; Giovine, R.; Preefer, M.B.; Ong, J.; Yoshida, E.; Cabelguen, P.E.; Clement, R.J. Rapid and Energy-Efficient Synthesis of Disordered Rocksalt Cathodes. Adv. Energy Mater. 2023, 13, 2203860. [Google Scholar] [CrossRef]
- De Grenu, B.D.; Torres, J.; Garcia-Gonzalez, J.; Munoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amoros, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. Chemsuschem 2021, 14, 208–233. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, Q.Q.; Yang, N.N.; Yang, Y.F.; He, F.; Chu, J.; Gong, M.; Wu, B.H.; Zhang, R.L.; Xiong, S.X. Hydrothermal synthesis of NiCo-based bimetal-organic frameworks as electrode materials for supercapacitors. J. Solid State Chem. 2019, 270, 370–378. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, X.Q.; Yang, N.N.; He, F.; Yang, Y.F.; Wu, B.H.; Chu, J.; Zhou, A.N.; Xiong, S.X. Hydrangea-like NiCo-based Bimetal-organic Frameworks, and their Pros and Cons as Supercapacitor Electrode Materials in Aqueous Electrolytes. Z. Anorg. Allg. Chem. 2019, 645, 1022–1030. [Google Scholar] [CrossRef]
- Yao, J.N.; Ji, Y.J.; Lu, F.X.; Shi, D.; Pei, L.J. Facile route to high-mass-loading amorphous NiCo-MOFs as high-performance electrode materials for asymmetric supercapacitors. New J. Chem. 2023, 47, 4182–4186. [Google Scholar] [CrossRef]
- Wei, K.L.; Wang, X.; Jiao, X.L.; Li, C.; Chen, D.R. Self-supported three-dimensional macroporous amorphous NiFe bimetallic-organic frameworks for enhanced water oxidation. Appl. Surf. Sci. 2021, 550, 149323. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Tian, Y.R.; Sarwar, S.; Luo, J.J.; Zhang, X.Y. Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors. J. Power Sources 2020, 452, 227793. [Google Scholar] [CrossRef]
- Young, C.; Kim, J.; Kaneti, Y.V.; Yamauchi, Y. One-Step Synthetic Strategy of Hybrid Materials from Bimetallic Metal-Organic Frameworks for Supercapacitor Applications. ACS Appl. Energy Mater. 2018, 1, 2007–2015. [Google Scholar] [CrossRef]
- Wang, G.S.; Yan, Z.X.; Wang, N.H.; Xiang, M.; Xu, Z.H. NiO/Ni Metal-Organic Framework Nanostructures for Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2021, 4, 9034–9043. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Y.Z.; Wang, H.Q.; Zhao, P.H.; Hou, H.; Guo, L. Acetylene black enhancing the electrochemical performance of NiCo-MOF nanosheets for supercapacitor electrodes. Appl. Surf. Sci. 2019, 492, 455–463. [Google Scholar] [CrossRef]
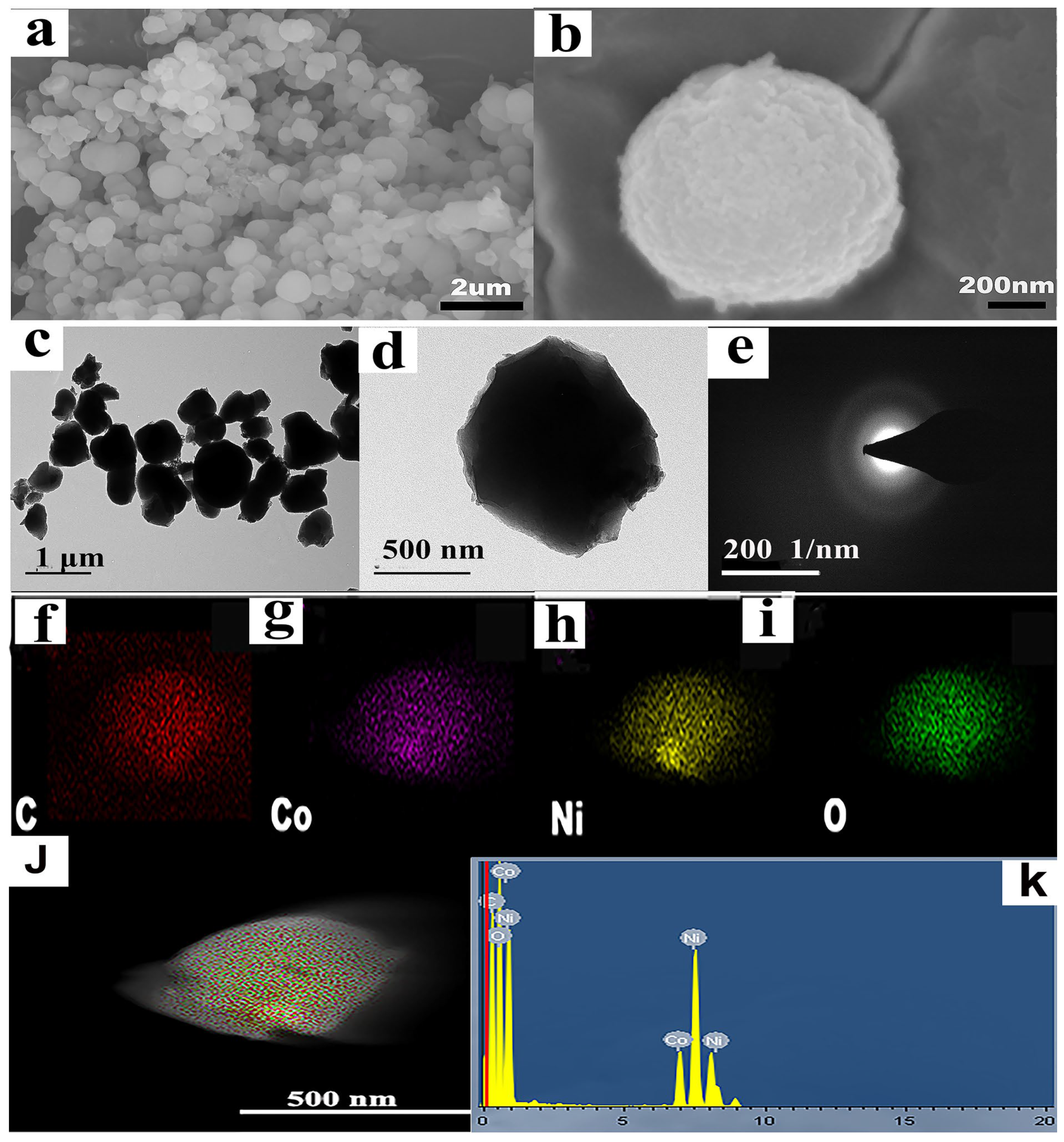


| Sample | Element Content (At %) | |||
|---|---|---|---|---|
| C | O | Ni | Co | |
| NiCo-MOF | 56.23% | 32.83% | 7.16% | 3.78% |
| Sample | Microwave Power (W) | Microwave Time (s) |
|---|---|---|
| NiCo-MOF | 600 | 210 |
| NiCo-MOF 150 s | 600 | 150 |
| NiCo-MOF 270 s | 600 | 270 |
| NiCo-MOF 400 w | 400 | 210 |
| NiCo-MOF 800 w | 800 | 210 |
| Sample | Electrolyte | Specific Capacitance | Ref |
|---|---|---|---|
| Flower-like NiCo MOF | 3 M KOH | 927 F/g at 1 A/g | Ref [29] |
| Dandelion-like NiCo MOF | 2 M KOH | 758 F/g at 1 A/g | Ref [30] |
| Ultrathin nanosheets NiCo MOF | 2 M KOH | 1202.2 F/g at 1 A/g | Ref [28] |
| Hydrangea-like NiCo MOF | 2 M KOH | 1056.6 F/g at 0.5 A/g | Ref [40] |
| Pillar Ni MOF | 2 M KOH | 552 F/g at 1 A/g | Ref [21] |
| Urchin-like Co-MOF | 3 M KOH | 952.5 F/g at 0.25 A/g | Ref [22] |
| Spherical NiCo-MOF | 6 M KOH | 715 F/g at 1 A/g | Ref [44] |
| Nanosphere-like NiCo-MOF | 2 M KOH | 1348 F/g at 1 A/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, X.; Yang, N.; Yang, L.; Wang, W.; Fang, X.; He, Q. NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors. Molecules 2023, 28, 5613. https://doi.org/10.3390/molecules28145613
Yang X, Zhang X, Yang N, Yang L, Wang W, Fang X, He Q. NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors. Molecules. 2023; 28(14):5613. https://doi.org/10.3390/molecules28145613
Chicago/Turabian StyleYang, Xing, Xin Zhang, Ning Yang, Lei Yang, Wanglong Wang, Xing Fang, and Qing He. 2023. "NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors" Molecules 28, no. 14: 5613. https://doi.org/10.3390/molecules28145613
APA StyleYang, X., Zhang, X., Yang, N., Yang, L., Wang, W., Fang, X., & He, Q. (2023). NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors. Molecules, 28(14), 5613. https://doi.org/10.3390/molecules28145613






