Bilobalide Induces Apoptosis in 3T3-L1 Mature Adipocytes through ROS-Mediated Mitochondria Pathway
Abstract
:1. Introduction
2. Results
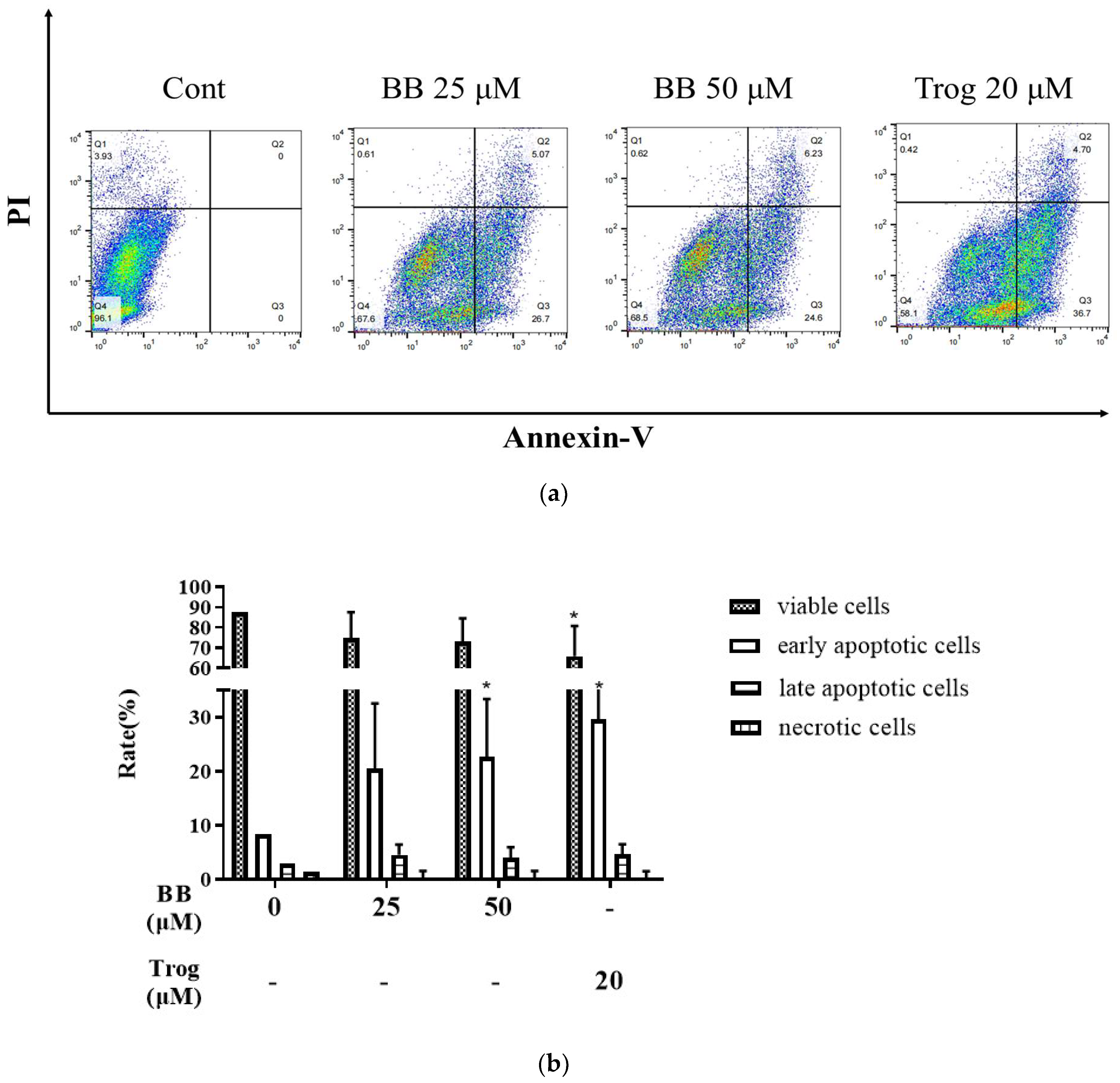
2.1. Bilobalide Induces Apoptosis in 3T3-L1 Adipocytes
2.2. Effect of Bilobalide on Reactive Oxygen Species (ROS) Generation in Adipocytes
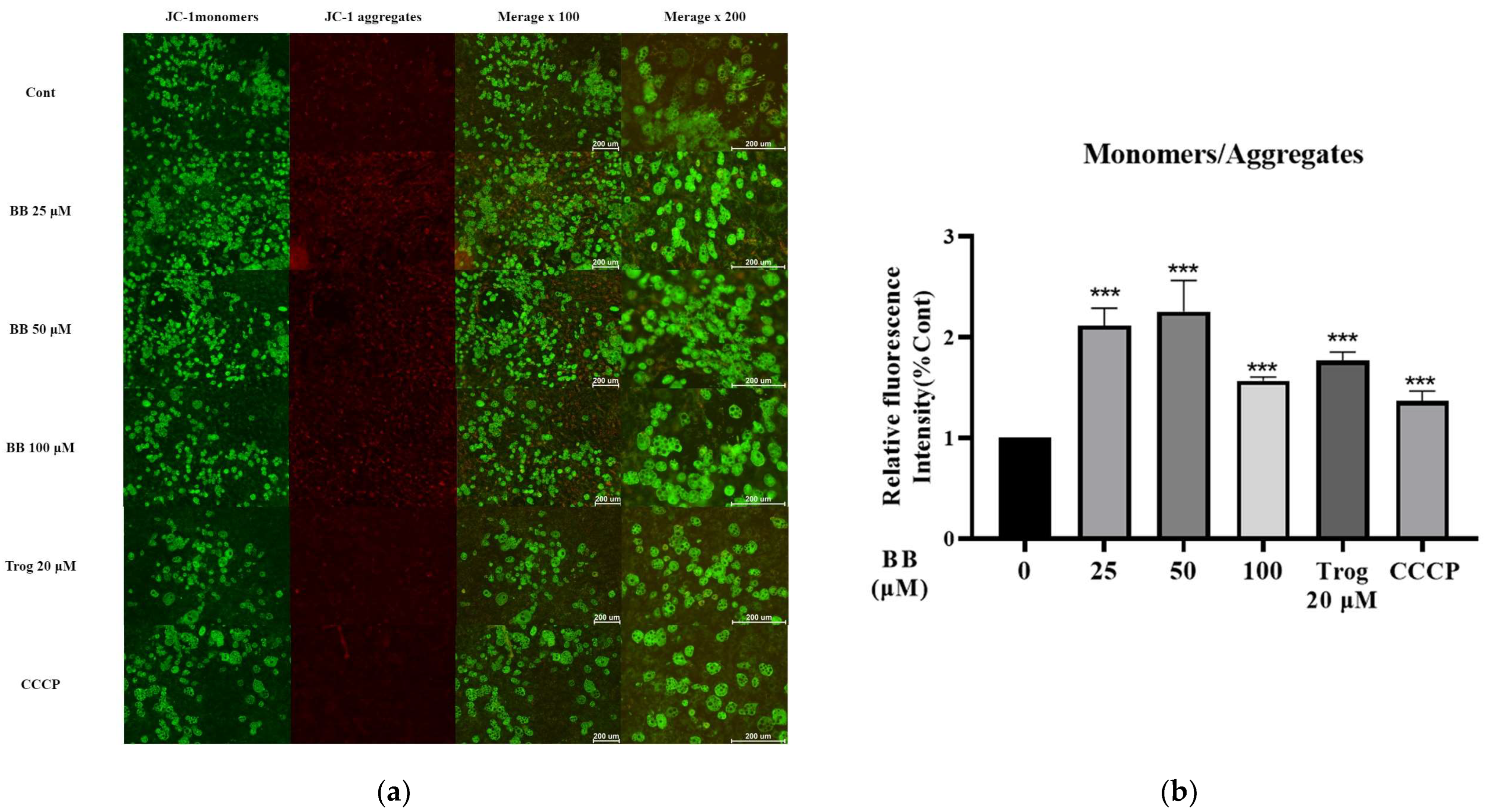
2.3. Bilobalide Treatment Reduced Mitochondrial Membrane Potential (MMP) in 3T3-L1 Mature Adipocytes
2.4. Effects of Bilobalide on Caspase 3/9/8 Activities in Apoptosis
2.5. The Effect of Bilobalide on the Expression of Apoptotic Genes in Adipocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Assessment of Apoptosis by Flow Cytometry
4.4. Measurement of Intracellular ROS Generation
4.5. TUNEL Analysis
4.6. Measurement of MMP
4.7. Quantification of the Expression of Apoptotic Genes in 3T3-L1 Adipocytes
4.8. Western Blot Analysis
4.9. Caspase 3/9/8 Assay and Their Specific Inhibitor Activities
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, T.; Gu, J.; Li, C.; Guo, X.; Tu, J.; Zhang, D.; Sun, W.; Kong, X. Low-intensity pulsed ultrasound suppresses proliferation and promotes apoptosis via p38 MAPK signaling in rat visceral preadipocytes. Am. J. Transl. Res. 2018, 10, 948–956. [Google Scholar] [PubMed]
- Yao, A.; Shen, Y.; Zhang, Z.; Zou, Z.; Wang, A.; Chen, S.; Zhang, H.; Chen, F.; Zhao, J.; Chen, Z.; et al. Sulforaphane and myricetin act synergistically to induce apoptosis in 3T3-L1 adipocytes. Mol. Med. Rep. 2018, 17, 2945–2951. [Google Scholar] [CrossRef]
- Gregg, E.W.; Shaw, J.E. Global Health Effects of Overweight and Obesity. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Yuan, C.Y.; Xue, Q.; Chen, Y.; Cao, F. Bilobalide Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Molecules 2019, 24, 3503. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yu, Z.; Tovar, J.; Nilsson, A.; Xu, B. Critical review on anti-obesity effects of phytochemicals through Wnt/beta-catenin signaling pathway. Pharmacol. Res. 2022, 184, 106461. [Google Scholar] [CrossRef]
- Lv, X.; Qiu, J.; Hao, T.; Zhang, H.; Jiang, H.; Tan, Y. HDAC inhibitor Trichostatin A suppresses adipogenesis in 3T3-L1 preadipocytes. Aging 2021, 13, 17489–17498. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Kim, B.; Choi, K. Torreya nucifera seed oil improves 3T3-L1 adipocyte differentiation. BMC Complement. Med. Ther. 2021, 21, 255. [Google Scholar] [CrossRef]
- Sorisky, A.; Magun, R.; Gagnon, A.M. Adipose cell apoptosis: Death in the energy depot. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. S4), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Papineau, D.; Gagnon, A.; Sorisky, A. Apoptosis of human abdominal preadipocytes before and after differentiation into adipocytes in culture. Metabolism 2003, 52, 987–992. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.-Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; A Baile, C. Genistein Decreases Food Intake, Body Weight, and Fat Pad Weight and Causes Adipose Tissue Apoptosis in Ovariectomized Female Mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef]
- Loftus, T.M.; Kuhajda, F.P.; Lane, M.D. Insulin depletion leads to adipose-specific cell death in obese but not lean mice. Proc. Natl. Acad. Sci. USA 1998, 95, 14168–14172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, C. Targeting adipocyte apoptosis: A novel strategy for obesity therapy. Biochem. Biophys. Res. Commun. 2012, 417, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; You, M.-K.; Lee, Y.-H.; Kim, H.-J.; Adhikari, D.; Kim, H.-A. Red pepper seed water extract inhibits preadipocyte differentiation and induces mature adipocyte apoptosis in 3T3-L1 cells. Nutr. Res. Pract. 2018, 12, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Yun, J.W. Honokiol exerts dual effects on browning and apoptosis of adipocytes. Pharmacol. Rep. 2017, 69, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xie, L.; Liu, K.; Zhang, X.; Wang, X.; Dai, X.; Liang, Y.; Cao, Y.; Li, X. Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity, and safety. Phytother. Res. 2021, 35, 6114–6130. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, P.; Sun, Q.; Li, Y.; Acharya, R.; Li, X.; Sun, C. FTO inhibits UPRmt-induced apoptosis by activating JAK2/STAT3 pathway and reducing m6A level in adipocytes. Apoptosis 2021, 26, 474–487. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tian, M.; Huang, Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, R239–R253. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, S.T.; Lee, J.H.; Chae, S.I.; Hwang, K.W.; Chung, Y.H.; Kim, H.-C.; El-Aty, A.A.; Lee, T.J.; Park, E.S.; et al. Phosphatidylcholine Causes Lipolysis and Apoptosis in Adipocytes through the Tumor Necrosis Factor Alpha-Dependent Pathway. Pharmacology 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Priyanka, A.; Sindhu, G.; Shyni, G.; Rani, P.; Nisha, V.; Raghu, K. Bilobalide abates inflammation, insulin resistance and secretion of angiogenic factors induced by hypoxia in 3T3-L1 adipocytes by controlling NF-kappaB and JNK activation. Int. Immunopharmacol. 2017, 42, 209–217. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Gonçalves, I.O.; Beleza, J.; Ascensão, A.; Magalhães, J. Effects of endurance training on autophagy and apoptotic signaling in visceral adipose tissue of prolonged high fat diet-fed rats. Eur. J. Nutr. 2018, 57, 2237–2247. [Google Scholar] [CrossRef]
- Shi, C.; Wu, F.; Yew, D.T.; Xu, J.; Zhu, Y. Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells. Apoptosis 2010, 15, 715–727. [Google Scholar] [CrossRef]
- Hua, J.; Yin, N.; Yang, B.; Zhang, J.; Ding, J.; Fan, Y.; Hu, G. Ginkgolide B and bilobalide ameliorate neural cell apoptosis in alpha-synuclein aggregates. Biomed. Pharmacother. 2017, 96, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Z.; Zhang, Y.; Alharbi, S.A.; Shi, Y. Sesquiterpenoid bilobalide inhibits gastric carcinoma cell growth and induces apoptosis both in vitro and in vivo models. J. Biochem. Mol. Toxicol. 2021, 35, e22723. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Cho, B.J.; Kim, M.; Park, H.S.; Cho, H.J.; Shin, H.; Kim, Y.-B.; et al. EGb761, a Ginkgo Biloba Extract, Is Effective Against Atherosclerosis In Vitro, and in a Rat Model of Type 2 Diabetes. PLoS ONE 2011, 6, e20301. [Google Scholar] [CrossRef]
- Lu, L.; Wang, S.; Fu, L.; Liu, D.; Zhu, Y.; Xu, A. Bilobalide protection of normal human melanocytes from hydrogen peroxide-induced oxidative damage via promotion of antioxidase expression and inhibition of endoplasmic reticulum stress. Clin. Exp. Dermatol. 2016, 41, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Nisoli, E.; Cardile, A.; Bulbarelli, A.; Tedesco, L.; Bracale, R.; Cozzi, V.; Morroni, M.; Cinti, S.; Valerio, A.; O Carruba, M. White adipocytes are less prone to apoptotic stimuli than brown adipocytes in rodent. Cell Death Differ. 2006, 13, 2154–2156. [Google Scholar] [CrossRef]
- Sarantopoulos, C.N.; Banyard, D.A.; Ziegler, M.E.; Sun, B.; Shaterian, A.; Widgerow, A.D. Elucidating the Preadipocyte and Its Role in Adipocyte Formation: A Comprehensive Review. Stem Cell Rev. Rep. 2018, 14, 27–42. [Google Scholar] [CrossRef]
- Rhyu, J.; Kim, M.S.; You, M.-K.; Bang, M.-A.; Kim, H.-A. Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Della-Fera, M.A.; Hartzell, D.L.; Nelson-Dooley, C.; Hausman, D.B.; Baile, C.A. Esculetin Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Cells. Obesity 2006, 14, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.-Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020, 131, 110769. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Veerappan, K.; Ranjan, A.; Kim, Y.-J.; Chellappan, D.K.; Dua, K.; Lee, J.; Perumalsamy, H. Phyllanthus emblica fruit extract attenuates lipid metabolism in 3T3-L1 adipocytes via activating apoptosis mediated cell death. Phytomedicine 2020, 66, 153129. [Google Scholar] [CrossRef]
- Goodman, G.J.; Ho, W.W.S.; Chang, K.-J.; Ling, Y.-F.; Sheu, A.-Y. Efficacy of a Novel Injection Lipolysis to Induce Targeted Adipocyte Apoptosis: A Randomized, Phase IIa Study of CBL-514 Injection on Abdominal Subcutaneous Fat Reduction. Aesthetic Surg. J. 2022, 42, NP662–NP674. [Google Scholar] [CrossRef]
- Byun, K.-A.; Park, H.J.; Oh, S.; Batsukh, S.; Sun, H.J.; Kim, T.; Kim, S.; Kang, D.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Decreases Subcutaneous Fat Tissue Thickness by Increasing Apoptosis and Autophagy. Biomolecules 2023, 13, 392. [Google Scholar] [CrossRef]
- Eguchi, A.; Feldstein, A.E. Adipocyte Cell Death, Fatty Liver Disease and Associated Metabolic Disorders. Dig. Dis. 2014, 32, 579–585. [Google Scholar] [CrossRef]
- Röszer, T. Adipose Tissue Immunometabolism and Apoptotic Cell Clearance. Cells 2021, 10, 2288. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- D’arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Batandier, C.; Leverve, X.; Fontaine, E. Opening of the Mitochondrial Permeability Transition Pore Induces Reactive Oxygen Species Production at the Level of the Respiratory Chain Complex I. J. Biol. Chem. 2004, 279, 17197–17204. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Loveless, J.; Shay, C.; Teng, Y. Targeting ROS-Mediated Crosstalk Between Autophagy and Apoptosis in Cancer. Adv. Exp. Med. Biol. 2020, 1260, 1–12. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Cui, Y.; Liu, P.; Xiao, B.; Zhang, X.; Zhang, J.; Sun, Z.; Song, M.; Shao, B.; et al. Mitophagy and apoptosis mediated by ROS participate in AlCl(3)-induced MC3T3-E1 cell dysfunction. Food Chem. Toxicol. 2021, 155, 112388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Zhu, R.; Zhang, K.; Li, S.; Chen, Z.; Li, L. Betulinic Acid Induces Apoptosis in Differentiated PC12 Cells Via ROS-Mediated Mitochondrial Pathway. Neurochem. Res. 2017, 42, 1130–1140. [Google Scholar] [CrossRef]
- Cui, L.; Bu, W.; Song, J.; Feng, L.; Xu, T.; Liu, D.; Ding, W.; Wang, J.; Li, C.; Ma, B.; et al. Apoptosis induction by alantolactone in breast cancer MDA-MB-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Arch. Pharmacal Res. 2018, 41, 299–313. [Google Scholar] [CrossRef]
- Herold, C.; Rennekampff, H.O.; Engeli, S. Apoptotic pathways in adipose tissue. Apoptosis 2013, 18, 911–916. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Fujisaka, S.; Cai, W.; Winnay, J.N.; Konishi, M.; O’Neill, B.T.; Li, M.; García-Martín, R.; Takahashi, H.; Hu, J.; et al. Adipocyte Dynamics and Reversible Metabolic Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab. 2017, 25, 448–462. [Google Scholar] [CrossRef]
- Yi, Y.; Hu, W.; Zhao, C.; Wu, M.; Zeng, H.; Xiong, M.; Lv, W.; Wu, Y.; Zhang, Q. Deciphering the Emerging Roles of Adipocytes and Adipose-Derived Stem Cells in Fat Transplantation. Cell Transplant. 2021, 30, 963689721997799. [Google Scholar] [CrossRef]
- Lagerquist, M.K.; Gustafsson, K.L.; Henning, P.; Farman, H.; Wu, J.; Sjögren, K.; Koskela, A.; Tuukkanen, J.; Ohlsson, C.; Asterholm, I.W.; et al. Acute fat loss does not affect bone mass. Sci. Rep. 2021, 11, 14177. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, A.; Gheibihayat, S.M.; Karami, N.; Savardashtaki, A.; Butler, A.E.; Rizzo, M.; Sahebkar, A. The regulation of efferocytosis signaling pathways and adipose tissue homeostasis in physiological conditions and obesity: Current understanding and treatment options. Obes. Rev. 2022, 23, e13487. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yuan, T.; Yao, W.; Liao, K. 3T3-L1 adipocyte apoptosis induced by thiazolidinediones is peroxisome proliferator-activated receptor-gamma-dependent and mediated by the caspase-3-dependent apoptotic pathway. FEBS J. 2010, 277, 687–696. [Google Scholar] [CrossRef]






| Name | Primer Sequence (5′–3′) | GenBank No. | Size |
|---|---|---|---|
| Bax | 5‘-TGCTAGCAAACTGGTGCTCA-3′ | XM_011250780.3 | 183 |
| 5′-GGCCTTCCTAATGCCAACCTG-3′ | |||
| Bcl-2 | 5’-CTTTGAGTTCGGTGGGGTCA-3’ | NM_177410.3 | 168 |
| 5’-GCCCAGACTCATTCAACCAGA-3’ | |||
| GAPDH | 5’-TTTGTGGTGGGTGTGAACCA-3’ | XM_029535298.1 | 469 |
| 5’-TGAAGTCGCAGGAGACAACC-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, S.; Xiong, A.; Yang, Z.; Aissa-Brahim, F.; Chen, Y.; Zhang, Y.; Zhou, X.; Cao, F. Bilobalide Induces Apoptosis in 3T3-L1 Mature Adipocytes through ROS-Mediated Mitochondria Pathway. Molecules 2023, 28, 6410. https://doi.org/10.3390/molecules28176410
Bu S, Xiong A, Yang Z, Aissa-Brahim F, Chen Y, Zhang Y, Zhou X, Cao F. Bilobalide Induces Apoptosis in 3T3-L1 Mature Adipocytes through ROS-Mediated Mitochondria Pathway. Molecules. 2023; 28(17):6410. https://doi.org/10.3390/molecules28176410
Chicago/Turabian StyleBu, Su, Anran Xiong, Zhiying Yang, Faycal Aissa-Brahim, Ying Chen, Yichun Zhang, Xunyong Zhou, and Fuliang Cao. 2023. "Bilobalide Induces Apoptosis in 3T3-L1 Mature Adipocytes through ROS-Mediated Mitochondria Pathway" Molecules 28, no. 17: 6410. https://doi.org/10.3390/molecules28176410
APA StyleBu, S., Xiong, A., Yang, Z., Aissa-Brahim, F., Chen, Y., Zhang, Y., Zhou, X., & Cao, F. (2023). Bilobalide Induces Apoptosis in 3T3-L1 Mature Adipocytes through ROS-Mediated Mitochondria Pathway. Molecules, 28(17), 6410. https://doi.org/10.3390/molecules28176410






