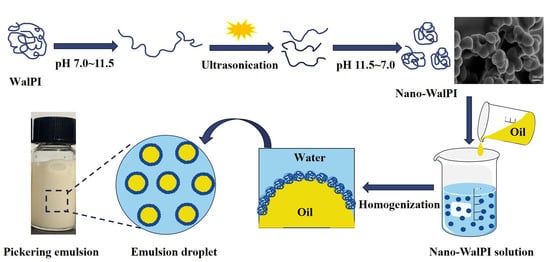
Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Nano-WalPI Characterization
2.1.1. Analysis of the Physicochemical Properties of WalPI and Nano-WalPI
2.1.2. Scanning Electron Microscopy
2.1.3. FTIR Analysis
2.1.4. Emulsion Type Analysis
2.1.5. Emulsifying Properties
2.2. Effects of Nano-WalPI Concentrations on PEs
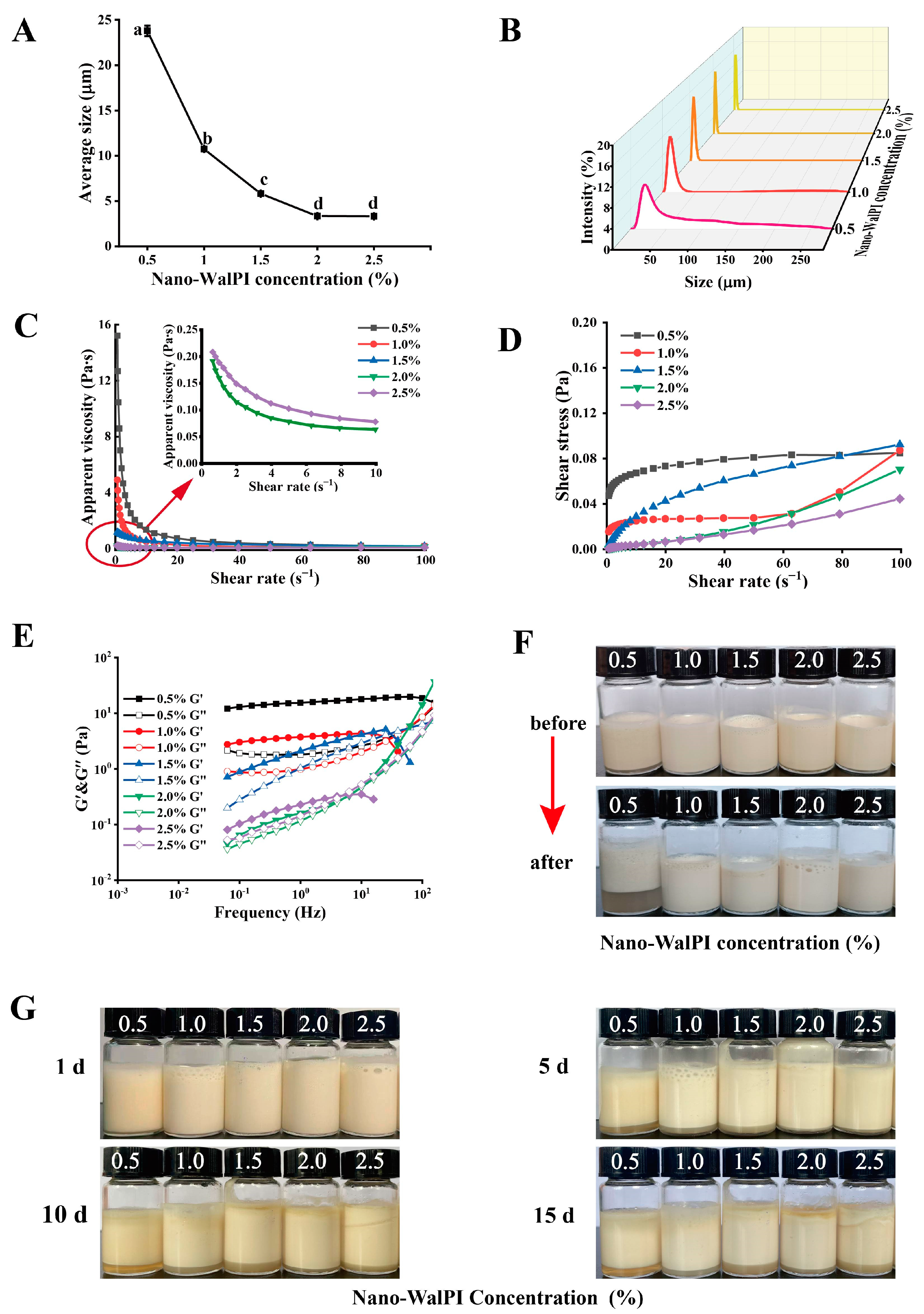
2.2.1. PE Droplets
2.2.2. Rheological Properties
2.2.3. Thermal Stability
2.2.4. Storage Stability
2.3. Effects of Oil Volume Fraction on PEs
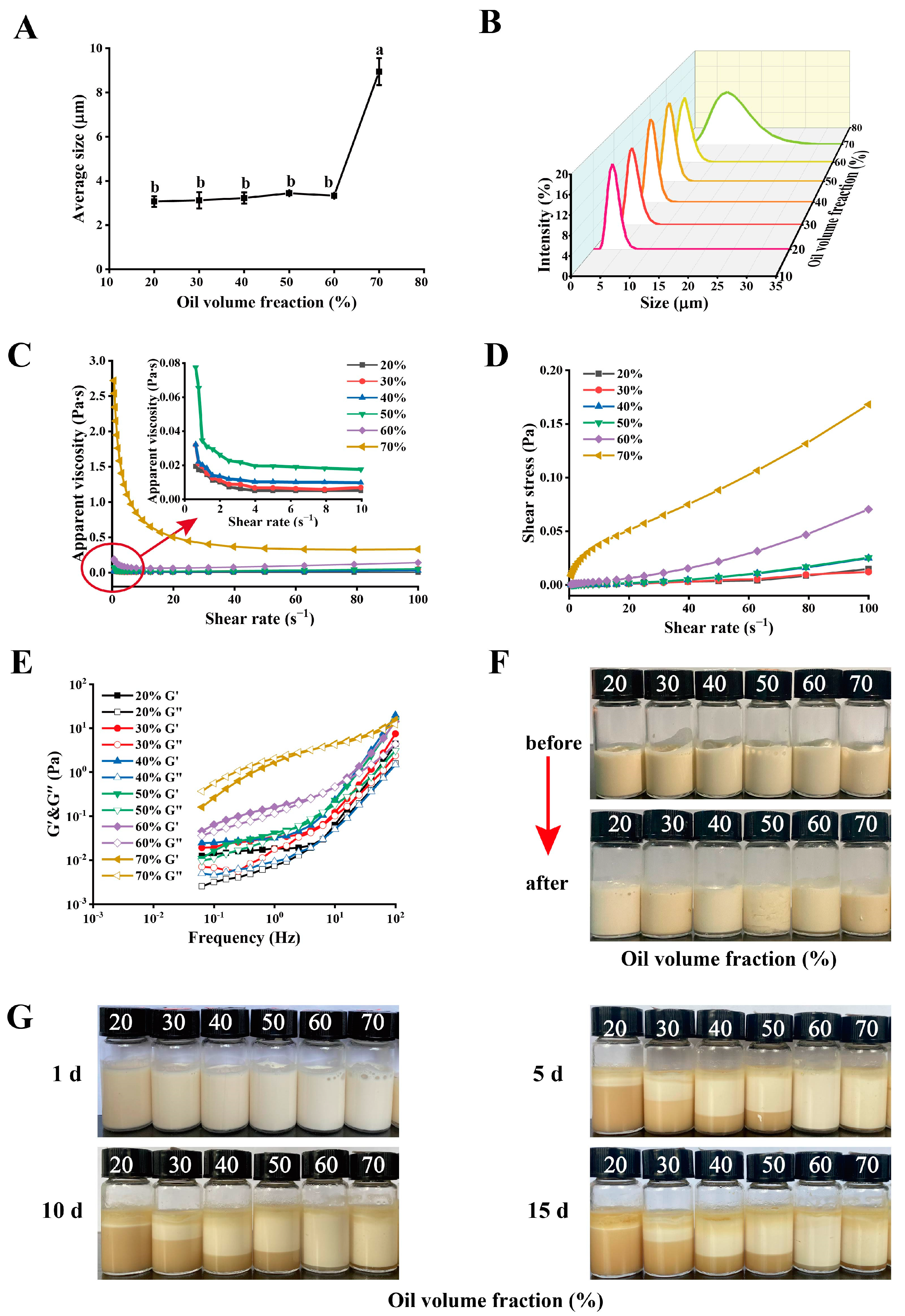
2.3.1. PE Droplets
2.3.2. Rheological Properties
2.3.3. Thermal Stability
2.3.4. Storage Stability
3. Materials and Methods
3.1. Materials
3.2. Walnut Protein Isolate Preparation
3.3. Nano-WalPI Preparation
3.4. Nano-WalPI Characterization
3.4.1. Particle Size and PDI
3.4.2. Surface Hydrophobicity
3.4.3. SEM Analysis
3.4.4. FTIR Analysis
3.4.5. Analysis of Emulsion Types
3.4.6. Emulsifying Properties
3.5. PE Preparation with Different Nano-WalPI Concentrations and Oil Volume Fractions
3.6. Characterization of PEs
3.6.1. Droplet Size
3.6.2. Rheological Properties
3.6.3. Thermal Stability
3.6.4. Storage Stability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Inam, M.; Jones, J.R.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P.; O’Reilly, R.K. Controlling the size of two-dimensional polymer platelets for water-in-water emulsifiers. ACS Cent. Sci. 2018, 4, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Harman, C.L.G.; Patel, M.A.; Guldin, S.; Davies, G.L. Recent developments in Pickering emulsions for biomedical applications. Curr. Opin. Colloid Interface Sci. 2019, 39, 173–189. [Google Scholar] [CrossRef]
- Benetti, J.V.M.; do Prado Silva, J.T.; Nicoletti, V.R. SPI microgels applied to Pickering stabilization of O/W emulsions by ultrasound and high-pressure homogenization: Rheology and spray drying. Food Res. Int. 2019, 122, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, J.; Ma, L.; Yan, B.; Shi, L.; Ran, R. Self-assembling graphene oxide/modified amphipathic hydroxyethyl cellulose hybrid stabilized Pickering emulsion polymerization for functional hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125742. [Google Scholar] [CrossRef]
- Low, L.E.; Ooi, C.W.; Chan, E.S.; Ong, B.H.; Tey, B.T. Dual (magnetic and pH) stimuli-reversible Pickering emulsions based on poly (2-(dimethylamino) ethyl methacrylate)-bonded Fe3O4 nanocomposites for oil recovery application. J. Environ. Chem. Eng. 2020, 8, 103715. [Google Scholar] [CrossRef]
- Lu, S.; Yang, D.; Wang, M.; Yan, M.; Qian, Y.; Zheng, D.; Qiu, X. Pickering emulsions synergistic-stabilized by amphoteric lignin and SiO2 nanoparticles: Stability and pH-responsive mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124158. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, H.; Zhu, J.; Zhang, M.; Yin, L. Development and characterization of Pickering emulsion stabilized by zein/corn fiber gum (CFG) complex colloidal particles. Food Hydrocoll. 2019, 91, 204–213. [Google Scholar] [CrossRef]
- Koop, J.; Merz, J.; Schembecker, G. Hydrophobicity, amphilicity, and flexibility: Relation between molecular protein properties and the macroscopic effects of surface activity. J. Biotechnol. 2021, 334, 11–25. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Q.; Xiong, Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]
- Sun, C.; Gao, Y.; Zhong, Q. Properties of ternary biopolymer nanocomplexes of zein, sodium caseinate, and propylene glycol alginate and their functions of stabilizing high internal phase Pickering emulsions. Langmuir 2018, 34, 9215–9227. [Google Scholar] [CrossRef]
- Lee, H.; Yildiz, G.; Dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016, 55, 200–209. [Google Scholar] [CrossRef]
- Yildiz, G.; Andrade, J.; Engeseth, N.E.; Feng, H. Functionalizing soy protein nano-aggregates with pH-shifting and mano-thermo-sonication. J. Colloid Interface Sci. 2017, 505, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, L.; Fan, F.; Su, J.; Zhou, C.; Kan, H. Preparation and characterization of soy protein isolate/SiO2 nanocomposite films and their walnut oil microcapsules. J. Appl. Polym. Sci. 2021, 138, 50695. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Li, Y.; Wang, Z.; He, X.; Sun, J. Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry. Foods 2022, 11, 385. [Google Scholar] [CrossRef]
- Tan, Y.; Deng, X.; Liu, T.; Yang, B.; Zhao, M.; Zhao, Q. Influence of NaCl on the oil/water interfacial and emulsifying properties of walnut protein-xanthan gum. Food Hydrocoll. 2017, 72, 73–80. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef]
- Ng, S.P.; Khor, Y.P.; Lim, H.K.; Lai, O.M.; Wang, Y.; Wang, Y.; Cheong, L.; Nehdi, I.A.; Mansour, L.; Ping Tan, C. Fabrication of concentrated palm olein-based diacylglycerol oil–soybean oil blend oil-in-water emulsion: In-depth study of the rheological properties and storage stability. Foods 2020, 9, 877. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Lu, Z.; Hu, W.; Wang, L. Zein/gum Arabic nanoparticle-stabilized Pickering emulsion with thymol as an antibacterial delivery system. Carbohydr. Polym. 2018, 200, 416–426. [Google Scholar] [CrossRef]
- Hazt, B.; Bassani, H.P.; Elias-Machado, J.P.; Buzzo, J.L.A.; Silveira, J.L.; de Freitas, R.A. Effect of pH and protein particle shape on the stability of amylopectin–xyloglucan water-in-water emulsions. Food Hydrocoll. 2020, 104, 105769. [Google Scholar] [CrossRef]
- Yu, C.; Wu, F.; Cha, Y.; Zou, H.; Guo, Y.; Piao, H.; Du, M. Structural and Functional Changes in Ultrasonicated Oyster Protein Isolates. Int. J. Food Eng. 2019, 15, 20180190. [Google Scholar] [CrossRef]
- Tang, L.; Yongsawatdigul, J. Physicochemical properties of tilapia (Oreochromis niloticus) actomyosin subjected to high intensity ultrasound in low NaCl concentrations. Ultrason. Sonochem. 2020, 63, 104922. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, Y.; Zhong, Q. Effects of acidification by glucono-delta-lactone or hydrochloric acid on structures of zein-caseinate nanocomplexes self-assembled during a pH cycle. Food Hydrocoll. 2018, 82, 173–185. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019, 53, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, F.; Wu, X. Effects of rice bran rancidity on oxidation, structural characteristics and interfacial properties of rice bran globulin. Food Hydrocoll. 2021, 110, 106123. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE 2019, 14, e0213189. [Google Scholar] [CrossRef]
- Li, R.; He, Q.; Guo, M.; Yuan, J.; Wu, Y.; Wang, S.; Rong, L.; Li, J. Universal and simple method for facile fabrication of sustainable high internal phase emulsions solely using meat protein particles with various pH values. Food Hydrocoll. 2020, 100, 105444. [Google Scholar] [CrossRef]
- Liang, L.; Chen, F.; Wang, X.; Jin, Q.; Decker, E.A.; McClements, D.J. Physical and oxidative stability of flaxseed oil-in-water emulsions fabricated from sunflower lecithins: Impact of blending lecithins with different phospholipid profiles. J. Agric. Food Chem. 2017, 65, 4755–4765. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Li, Y.; Tian, J.; Qin, X.; Shabani, K.I.; Liao, C.; Liu, X. Characterization of Pickering emulsions stabilized by OSA-modified sweet potato residue cellulose: Effect of degree of substitute and concentration. Food Hydrocoll. 2020, 108, 105915. [Google Scholar] [CrossRef]
- Tang, C.H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 105664. [Google Scholar] [CrossRef]
- Henriet, P.; Jessen, F.; Vall-Llosera, M.; Marie, R.; Jahromi, M.; Mohammadifar, M.A.; Stampe-Villadsen, H.L.; Petersen, H.O.; Sloth, J.J.; Eybye, K.L.; et al. Physical Stability of Oil-In-Water Emulsion Stabilized by Gelatin from Saithe (Pollachius virens) Skin. Foods 2020, 9, 1718. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Roberts, E.G.; Ebrahimi, D.; Dinjaski, N.; Jacobsen, M.M.; Martín-Moldes, Z.; Buehler, M.J.; Kaplan, D.L.; Wong, J.Y. Predicting silk fiber mechanical properties through multiscale simulation and protein design. ACS Biomater. Sci. Eng. 2017, 3, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; He, M.; Xu, J.; Chen, F.; Wu, C.; Wang, Z.; Li, Y. Effect of ultrasonication on the stability and storage of a soy protein isolate-phosphatidylcholine nanoemulsions. Sci. Rep. 2020, 10, 14010. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.A.; Shahabuddin, S.; Mohd Sabri, M.F.; Mohd Salleh, M.F.; Mohd Said, S.; Khedher, K.M.; Sridewi, N. Two-Dimensional Tungsten Disulfide-Based Ethylene Glycol Nanofluids: Stability, Thermal Conductivity, and Rheological Properties. Nanomaterials 2020, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, S.; Pahlavan, S.; Ghanian, M.H.; Rabbani, S.; Tavakol, S.; Barekat, M.; Yakhkeshi, S.; Mahmoudi, E.; Soleymani, M.; Baharvand, H. A cell-free SDKP-conjugated self-assembling peptide hydrogel sufficient for improvement of myocardial infarction. Biomolecules 2020, 10, 205. [Google Scholar] [CrossRef]
- Tsuboyama, K.; Osaki, T.; Matsuura-Suzuki, E.; Kozuka-Hata, H.; Okada, Y.; Oyama, M.; Lkeuchi, Y.; Lwasaki, S.; Tomari, Y. A widespread family of heat-resistant obscure (Hero) proteins protect against protein instability and aggregation. PLoS Biol. 2020, 18, e3000632. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Gao, J. Nanoparticles modulate autophagic effect in a dispersity-dependent manner. Sci. Rep. 2015, 5, 14361. [Google Scholar] [CrossRef]
- Heyse, A.; Kraume, M.; Drews, A. The impact of lipases on the rheological behavior of colloidal silica nanoparticle stabilized Pickering emulsions for biocatalytical applications. Colloids Surf. B. 2020, 185, 110580. [Google Scholar] [CrossRef]
- Hollenbach, R.; Ochsenreither, K.; Syldatk, C. Enzymatic synthesis of glucose monodecanoate in a hydrophobic deep eutectic solvent. Int. J. Mol. Sci. 2020, 21, 4342. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, G.; Fan, F. Preparation and Characteristics of Starch-Based Pickering Emulsions: Effects of Rosin Acid and Starch Size. Starch-Starke 2023, 75, 2200108. [Google Scholar] [CrossRef]
- Aziz, A.; Khan, N.M.; Ali, F.; Khan, Z.U.; Ahmad, S.; Jan, A.K.; Rehman, N.; Muhammad, N. Effect of protein and oil volume concentrations on emulsifying properties of acorn protein isolate. Food Chem. 2020, 324, 126894. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P. Silane treatment as an effective way of improving the reinforcing activity of carbon nanofibers in nitrile rubber composites. Materials 2020, 13, 3481. [Google Scholar] [CrossRef] [PubMed]
- Delahaije, R.J.; Hilgers, R.J.; Wierenga, P.A.; Gruppen, H. Relative contributions of charge and surface coverage on pH-induced flocculation of protein-stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 153–160. [Google Scholar] [CrossRef]
- Oliver, L.; Wieck, L.; Scholten, E. Influence of matrix inhomogeneity on the rheological properties of emulsion-filled gels. Food Hydrocoll. 2016, 52, 116–125. [Google Scholar] [CrossRef]
- Futami, J.; Miyamoto, A.; Hagimoto, A.; Suzuki, S.; Futami, M.; Tada, H. Evaluation of irreversible protein thermal inactivation caused by breakage of disulphide bonds using methanethiosulphonate. Sci. Rep. 2017, 7, 12471. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Tang, C.H.; Liu, T.X.; Liu, R. Ovalbumin as an outstanding Pickering Nano-stabilizer for high internal phase emulsions. J. Agric. Food Chem. 2018, 66, 8795–8804. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Yang, M.; Xu, D.; Chen, J.; Feng, H.; Lu, Y.; Zhang, L.; Yu, Y.; Kang, W. Stability mechanism of O/W Pickering emulsions stabilized with regenerated cellulose. Carbohydr. Polym. 2018, 181, 224–233. [Google Scholar] [CrossRef]
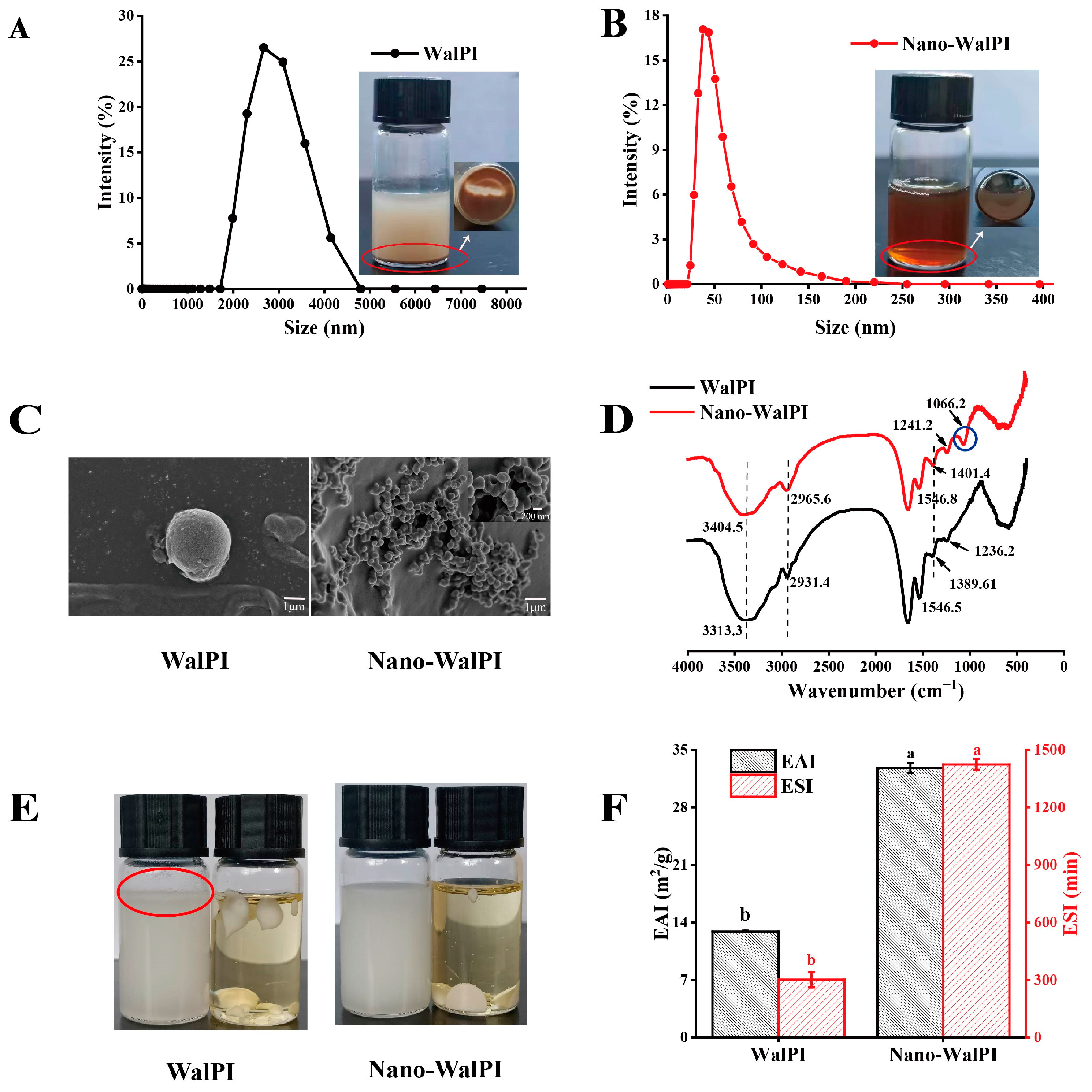
| Sample | Average Size/nm | PDI | H0 |
|---|---|---|---|
| WalPI | 3368.83 ± 119.19 a | 0.56 ± 0.02 a | 875.21 ± 2.66 b |
| Nano-WalPI | 108.20 ± 2.89 b | 0.40 ± 0.09 b | 1027.43 ± 4.16 a |
| Sample | α-Helix/% | β-Sheet/% | β-Turn/% | Random Coil/% |
|---|---|---|---|---|
| WalPI | 18.08 ± 0.05 b | 53.67 ± 0.32 a | 13.97 ± 0.39 b | 14.34 ± 0.02 b |
| Nano-WalPI | 26.22 ± 0.36 a | 39.67 ± 0.35 b | 17.42 ± 0.25 a | 16.68 ± 0.20 a |
| Nano-WalPI Concentration/% | CI/% | |||
|---|---|---|---|---|
| 1 d (1 h) | 5 d (120 h) | 10 d (240 h) | 15 d (360 h) | |
| 0.5 | 0 Ca | 11.75 ± 1.33 Ba | 13.16 ± 0.53 Ba | 18.07 ± 1.10 Aa |
| 1.0 | 0 Ba | 6.85 ± 1.40 Ab | 9.44 ± 0.55 Ac | 12.11 ± 1.39 Ac |
| 1.5 | 0 Ca | 4.60 ± 0.73 Bc | 11.29 ± 0.95 Ab | 15.35 ± 1.93 Ab |
| 2.0 | 0 Da | 1.00 ± 0. 25 Cd | 2.67 ± 0.29 Be | 4.67 ± 0.58 Ae |
| 2.5 | 0 Ca | 1.58 ± 0.53 Cd | 4.91 ± 0.61 Bd | 9.07 ± 0.85 Ad |
| Oil Volume Fraction/% | CI/% | |||
|---|---|---|---|---|
| 1 d (1 h) | 5 d (120 h) | 10 d (240 h) | 15 d (360 h) | |
| 20 | 0 Ca | 84.91 ± 0.80 Ba | 90.88 ± 1.61 Aa | 91.05 ± 1.39 Aa |
| 30 | 0 Db | 59.65 ± 1.61 Cb | 64.39 ± 1.69 Bb | 73.16 ± 1.90 Ab |
| 40 | 0 Db | 24.92 ± 1.20 Bc | 30.00 ± 1.26 Ac | 30.48 ± 0.82 Ac |
| 50 | 0 Cb | 23.86 ± 0.30 Cc | 27.89 ± 1.82 Bc | 32.46 ± 1.85 Ac |
| 60 | 0 Db | 1.00 ± 0. 25 Cd | 2.67 ± 0.29 Bd | 4.67 ± 0.58 Ad |
| 70 | 0 Cb | 2.33 ± 0.29 Bd | 2.50 ± 0.50 Bd | 7.17 ± 1.04 Ad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, H.; Sun, X.; Fan, F. Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles. Molecules 2023, 28, 5434. https://doi.org/10.3390/molecules28145434
Liu J, Zhang H, Sun X, Fan F. Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles. Molecules. 2023; 28(14):5434. https://doi.org/10.3390/molecules28145434
Chicago/Turabian StyleLiu, Jiongna, Hengxuan Zhang, Xue Sun, and Fangyu Fan. 2023. "Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles" Molecules 28, no. 14: 5434. https://doi.org/10.3390/molecules28145434
APA StyleLiu, J., Zhang, H., Sun, X., & Fan, F. (2023). Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles. Molecules, 28(14), 5434. https://doi.org/10.3390/molecules28145434