Effects of 2,2′-Azobis(2-methylpropionamidine) Dihydrochloride Stress on the Gel Properties of Duck Myofibrillar Protein Isolate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Carbonyl Content
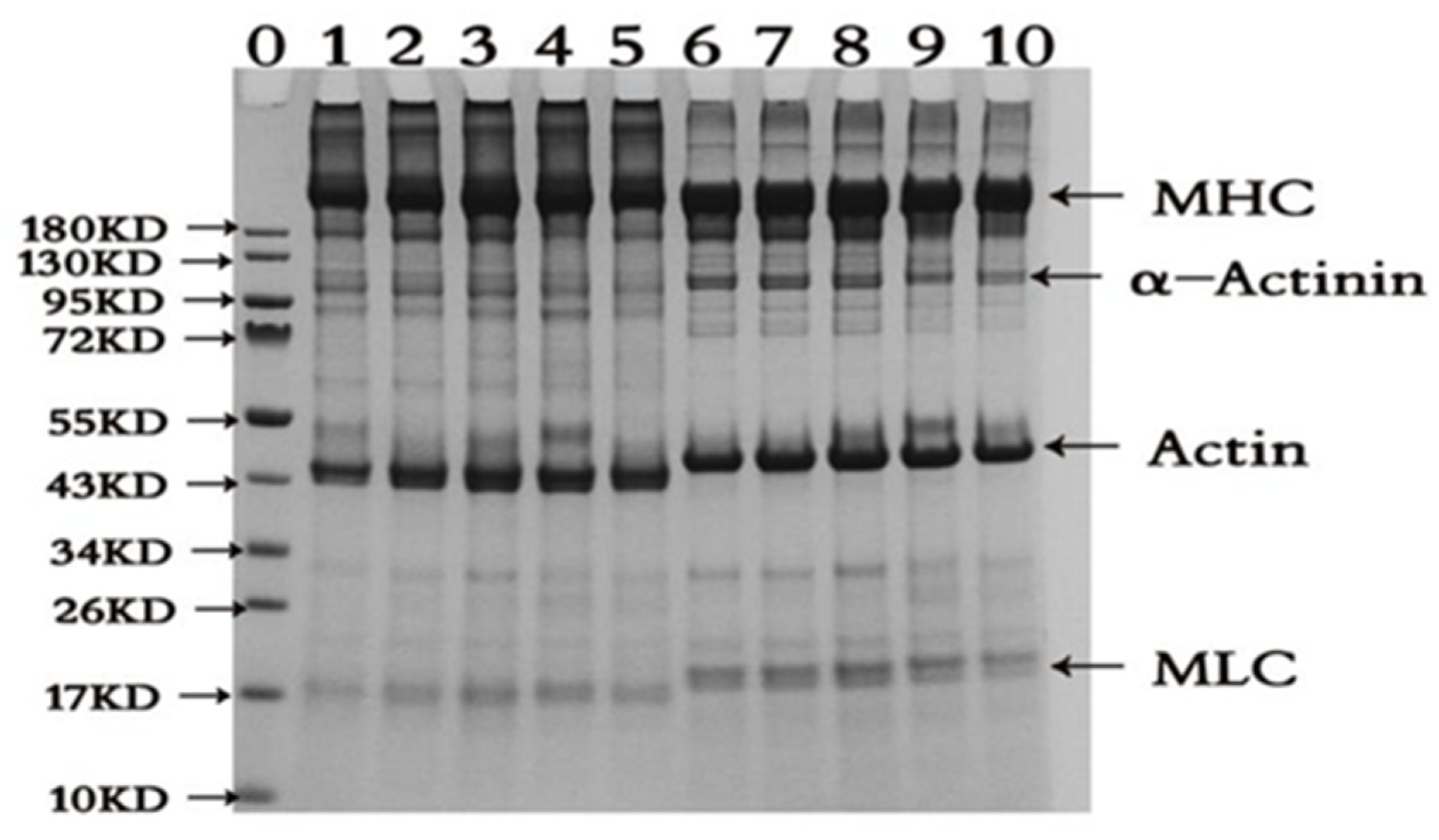
2.2. SDS-PAGE Pattern
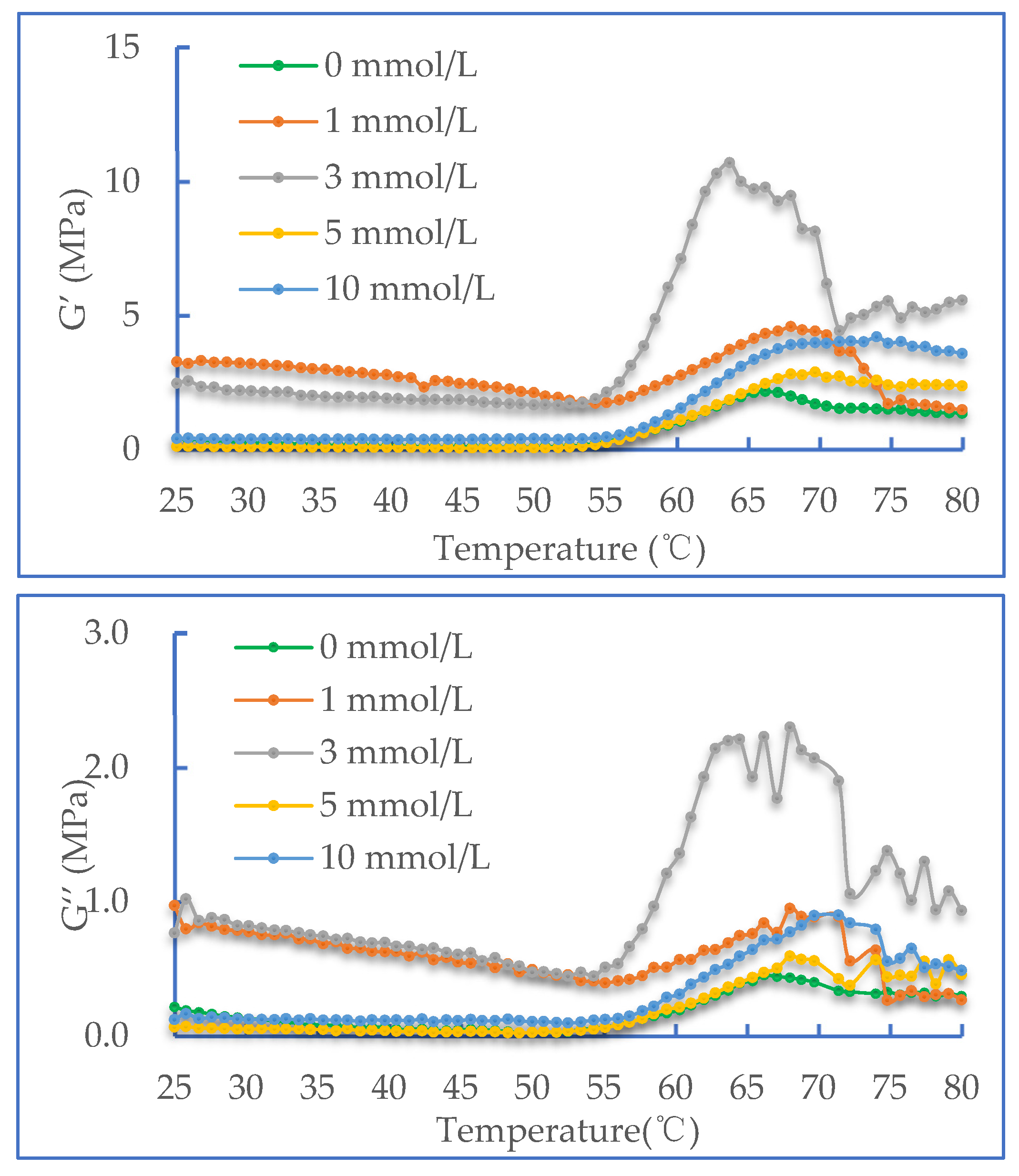
2.3. Dynamic Rheological Properties
2.4. Gel Strength
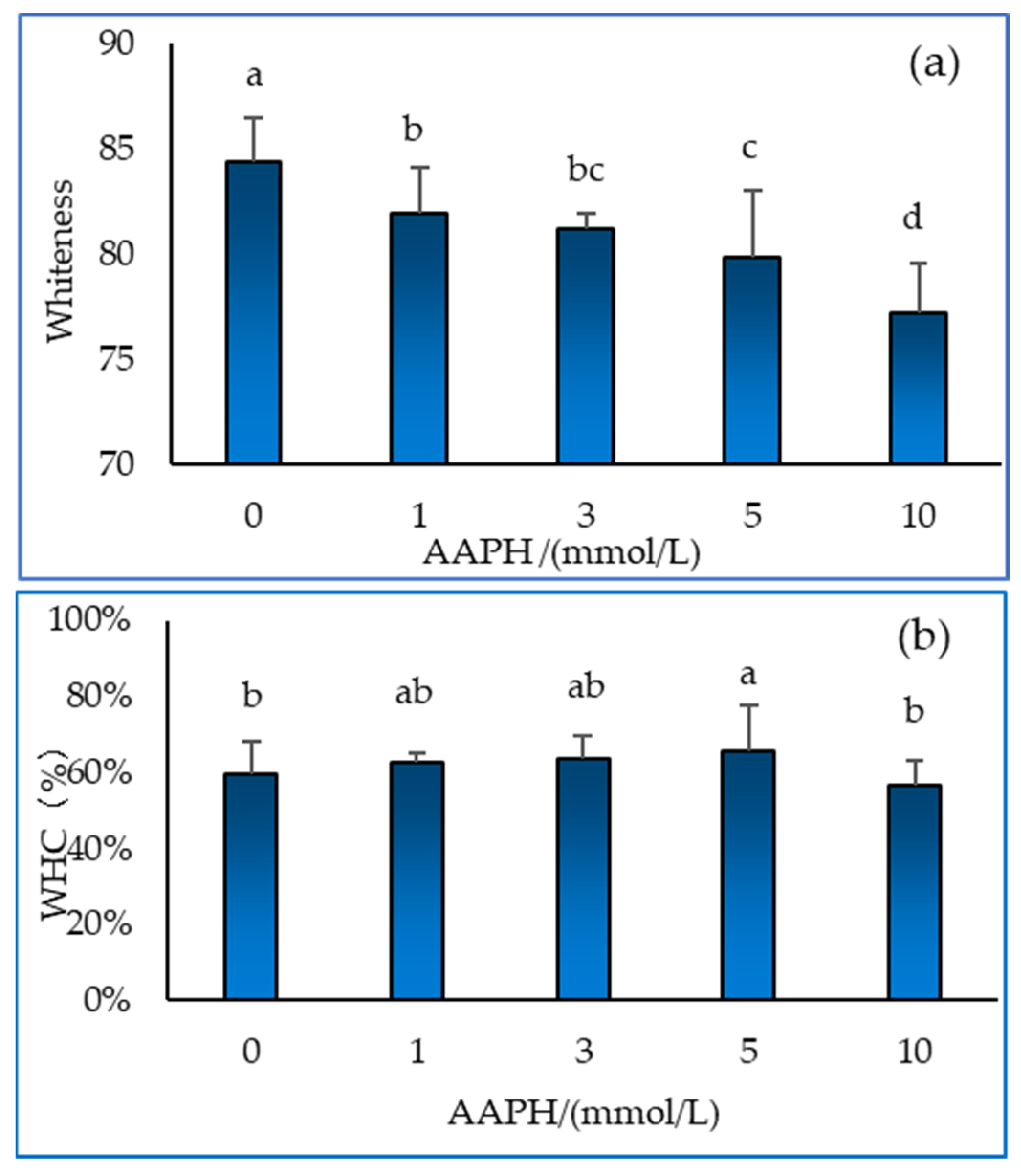
2.5. Whiteness and Water Holding Capacity of Gel
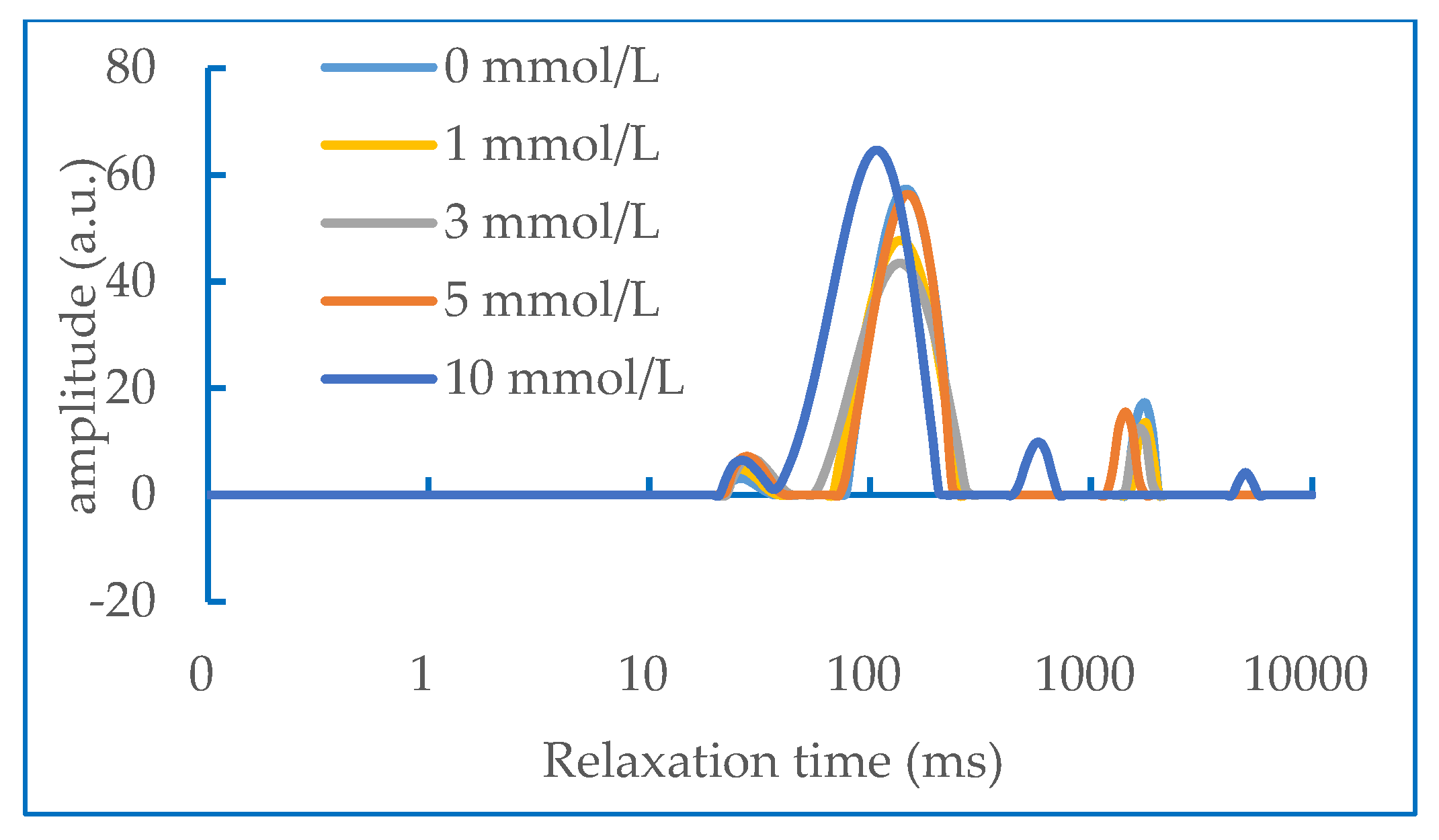
2.6. Water Status in Gel
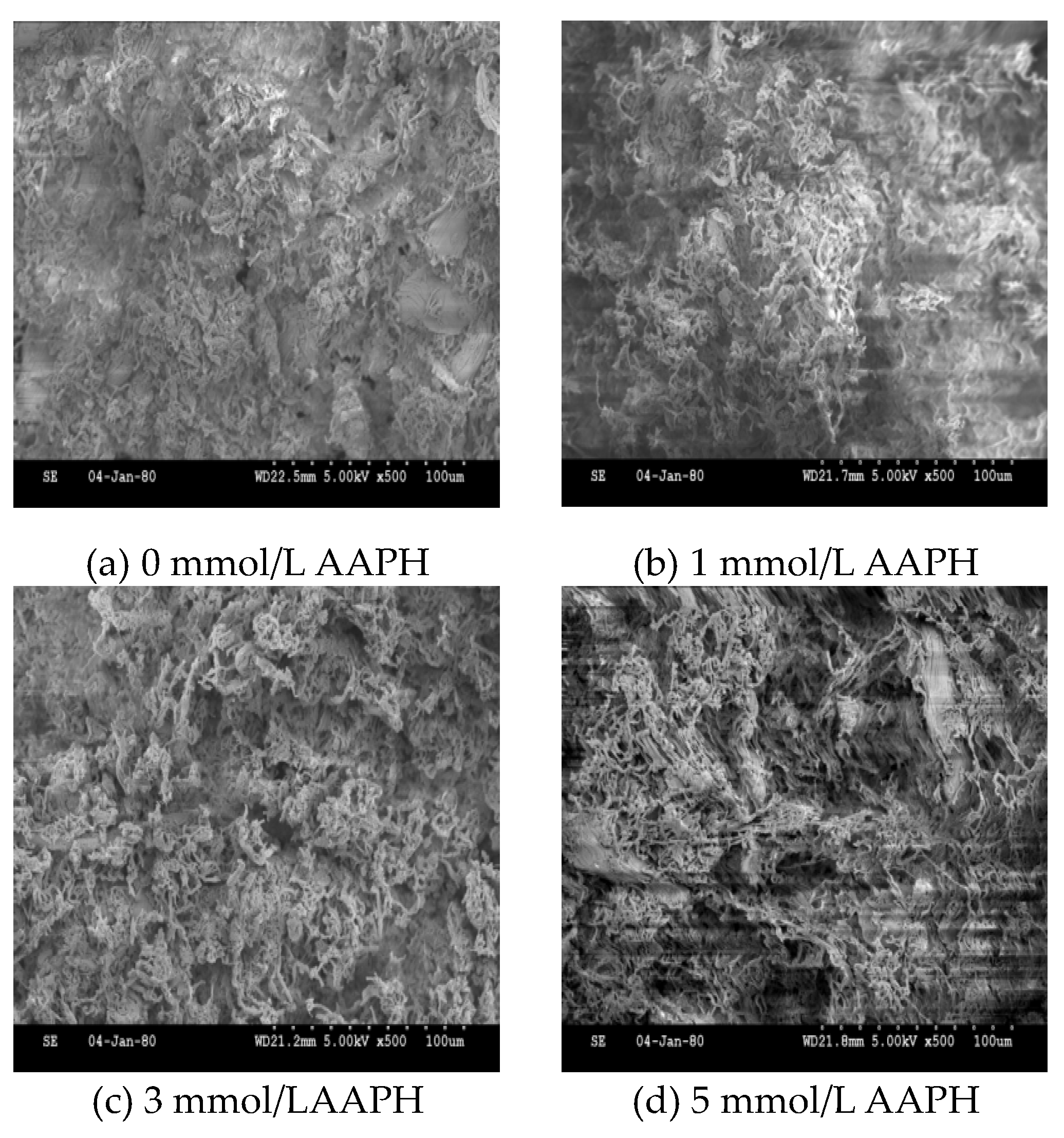
2.7. Gel Microstructure
3. Materials and Methods
3.1. Sample Preparation and Reagents
3.2. Extraction of Duck Myofibrillar Proteins (DMPs)
3.3. Oxidation of Duck Myofibrillar Proteins
3.4. Carbonyl Content
3.5. Sodium Dodecyl Sulfonate Polyacrylamide Gel Electrophoresis Analysis (SDS-PAGE)
3.6. Dynamic Rheological Test
3.7. Gel Preparation
3.8. Gel Strength Measurement
3.9. Gel Whiteness and Water Holding Capacity
3.10. Water Status in Gel
3.11. Gel Microstructure
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Johns, A.M.; Birkinshaw, L.H.; Ledward, D.A. Catalysts of lipid oxidation in meat-products. Meat Sci. 1989, 25, 209–220. [Google Scholar] [CrossRef]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.G.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estevez, M.; Yuan, L.; Gao, R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5548–5569. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.L.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, Y.L.; Chen, J. Protein Oxidation Enhances Hydration but Suppresses Water-Holding Capacity in Porcine Longissimus Muscle. J. Agric. Food Chem. 2010, 58, 10697–10704. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, X.; Liu, S.; Gu, Y.; Liu, J.; Fu, Q.; Wang, R. Physicochemical properties and gel-forming ability changes of duck myofibrillar protein induced by hydroxyl radical oxidizing systems. Front. Nutr. 2022, 9, 1029116. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, Z.; Zhang, X.; Huang, X.; Liu, J.; Zhuang, X. Effect of Malondialdehyde-Induced Oxidation Modification on Physicochemical Changes and Gel Characteristics of Duck Myofibrillar Proteins. Gels 2022, 8, 633. [Google Scholar] [CrossRef]
- Dion, M.Z.; Wang, Y.J.; Bregante, D.; Chan, W.M.; Andersen, N.; Hilderbrand, A.; Leiske, D.; Salisbury, C.M. The Use of a 2,2′-Azobis (2-Amidinopropane) Dihydrochloride Stress Model as an Indicator of Oxidation Susceptibility for Monoclonal Antibodies. J. Pharm. Sci. 2018, 107, 550–558. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Bridi, R.; Giordano, A.; Peñailillo, M.F.; Montenegro, G. Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules 2019, 24, 3264. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Silva, E.; Barrias, P.; Aspee, A.; Escobar, E.; Lorentzen, L.G.; Carroll, L.; Leinisch, F.; Davies, M.J.; López-Alarcón, C. Aggregation of α- and β-caseins induced by peroxyl radicals involves secondary reactions of carbonyl compounds as well as di-tyrosine and di-tryptophan formation. Free Radic. Biol. Med. 2018, 124, 176–188. [Google Scholar] [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2′-Azobis (2-Amidinopropane) Dihydrochloride Degradation and Hydrolysis in Aqueous Solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.M.; Kong, X.Z.; Hua, Y.F. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J.A. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [CrossRef]
- Hofmann, K.; Hamm, R. Sulfhydryl and Disulfide Groups in Meats**Dedicated to Professor Dr. Alfons Schoberl, Hannover (Germany), a pioneer in the chemistry of organic sulfur compounds. In Advances in Food Research; Chichester, C.O., Ed.; Academic Press: New York, NY, USA, 1978; Volume 24, pp. 1–111. [Google Scholar]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef]
- Dorta, E.; Avila, F.; Fuentes-Lemus, E.; Fuentealba, D.; Lopez-Alarcon, C. Oxidation of myofibrillar proteins induced by peroxyl radicals: Role of oxidizable amino acids. Food Res. Int. 2019, 126, 108580. [Google Scholar] [CrossRef] [PubMed]
- Relkin, P.; Fabre, M.; Guichard, E. Effect of Fat Nature and Aroma Compound Hydrophobicity on Flavor Release from Complex Food Emulsions. J. Agric. Food Chem. 2004, 52, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Silva, E.; Leinisch, F.; Dorta, E.; Lorentzen, L.G.; Davies, M.J.; López-Alarcón, C. α- and β-casein aggregation induced by riboflavin-sensitized photo-oxidation occurs via di-tyrosine cross-links and is oxygen concentration dependent. Food Chem. 2018, 256, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiong, Y.L. Interaction and Functionality of Mixed Myofibrillar and Enzyme-hydrolyzed Soy Proteins. J. Food Sci. 2003, 68, 803–809. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, J.; Zhou, X.; Khan, I.A.; Bassey, A.P.; Huang, M. Comparison of two kinds of peroxyl radical pretreatment at chicken myofibrillar proteins glycation on the formation of N-epsilon-carboxymethyllysine and N-epsilon-carboxyethyllysine. Food Chem. 2021, 353, 129487. [Google Scholar] [CrossRef]
- Mehta, N.K.; Rout, B.; Balange, A.K.; Nayak, B.B. Dynamic viscoelastic behaviour, gelling properties of myofibrillar proteins and histological changes in shrimp (L. vannamei) muscles during ice storage. Aquac. Fish. 2023, 8, 180–189. [Google Scholar] [CrossRef]
- Mehta, N.K.; Balange, A.K.; Lekshmi, M.; Nayak, B.B. Changes in Dynamic Viscoelastic and Functional Properties of Indian Squid Mantle During Ice Storage. J. Food Process. Preserv. 2017, 41, e12891. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y.Y. Hydroxyl Radical and Ferryl-Generating Systems Promote Gel Network Formation of Myofibrillar Protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef]
- Utrera, M.; Estevez, M. Oxidation of Myofibrillar Proteins and Impaired Functionality: Underlying Mechanisms of the Carbonylation Pathway. J. Agric. Food Chem. 2012, 60, 8002–8011. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lai, K.-M.; Hsu, K.-C. Changes in textural and rheological properties of gels from tilapia muscle proteins induced by high pressure and setting. Food Chem. 2007, 104, 746–753. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, J.-Z.; Zhang, C.-H.; Sun, H.-M.; Wang, C.-Q.; Xie, X.-L. Effects of Oxidation on Water Distribution and Physicochemical Properties of Porcine Myofibrillar Protein Gel. Food Biophys. 2014, 9, 169–178. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Zhu, X.S.; Ruusunen, M.; Gusella, M.; Zhou, G.H.; Puolanne, E. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Sci. 2011, 89, 181–188. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197, 670–675. [Google Scholar] [CrossRef]
- Jia, N.; Zhang, F.X.; Liu, Q.; Wang, L.T.; Lin, S.W.; Liu, D.Y. The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food Chem. 2019, 301, 125206. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. Insight into the mechanism of myofibrillar protein gel influenced by konjac glucomannan: Moisture stability and phase separation behavior. Food Chem. 2021, 339, 127941. [Google Scholar] [CrossRef]
- Xia, T.; Xu, Y.; Zhang, Y.; Xu, L.; Kong, Y.; Song, S.; Huang, M.; Bai, Y.; Luan, Y.; Han, M.; et al. Effect of oxidation on the process of thermal gelation of chicken breast myofibrillar protein. Food Chem. 2022, 384, 132368. [Google Scholar] [CrossRef]
- Salvador, P.; Toldra, M.; Saguer, E.; Carretero, C.; Pares, D. Microstructure-function relationships of heat-induced gels of porcine haemoglobin. Food Hydrocoll. 2009, 23, 1654–1659. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Liu, S.; Gu, Y.; Yu, X.; Gao, F.; Wang, R. Relationship between Molecular Structure and Heat-Induced Gel Properties of Duck Myofibrillar Proteins Affected by the Addition of Pea Protein Isolate. Foods 2022, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.L.; Cao, Y.Y.; Chen, X.; Zhang, Y.L.; Xue, X.W.; Han, M.Y.; Li, L.; Zhou, G.H.; Xu, X.L. Effects of chicken myofibrillar protein concentration on protein oxidation and water holding capacity of its heat-induced gels. J. Food Meas. Charact. 2018, 12, 2302–2312. [Google Scholar] [CrossRef]



| AAPH Concentraton (mmol/L) | 0 | 1 | 3 | 5 | 10 |
|---|---|---|---|---|---|
| DMPs solution (mL) | 6 | ||||
| AAPH stock solution (mL) | 0 | 0.2 | 0.6 | 1 | 2 |
| 20 mmol/L, pH 6.5 PBS (mL) | 4 | 3.8 | 3.4 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhang, J.; Zhang, X.; Dai, Q.; Fu, Q. Effects of 2,2′-Azobis(2-methylpropionamidine) Dihydrochloride Stress on the Gel Properties of Duck Myofibrillar Protein Isolate. Molecules 2023, 28, 6721. https://doi.org/10.3390/molecules28186721
Zhu X, Zhang J, Zhang X, Dai Q, Fu Q. Effects of 2,2′-Azobis(2-methylpropionamidine) Dihydrochloride Stress on the Gel Properties of Duck Myofibrillar Protein Isolate. Molecules. 2023; 28(18):6721. https://doi.org/10.3390/molecules28186721
Chicago/Turabian StyleZhu, Xueshen, Jin Zhang, Xinyu Zhang, Qun Dai, and Qingquan Fu. 2023. "Effects of 2,2′-Azobis(2-methylpropionamidine) Dihydrochloride Stress on the Gel Properties of Duck Myofibrillar Protein Isolate" Molecules 28, no. 18: 6721. https://doi.org/10.3390/molecules28186721
APA StyleZhu, X., Zhang, J., Zhang, X., Dai, Q., & Fu, Q. (2023). Effects of 2,2′-Azobis(2-methylpropionamidine) Dihydrochloride Stress on the Gel Properties of Duck Myofibrillar Protein Isolate. Molecules, 28(18), 6721. https://doi.org/10.3390/molecules28186721






