Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties
Abstract
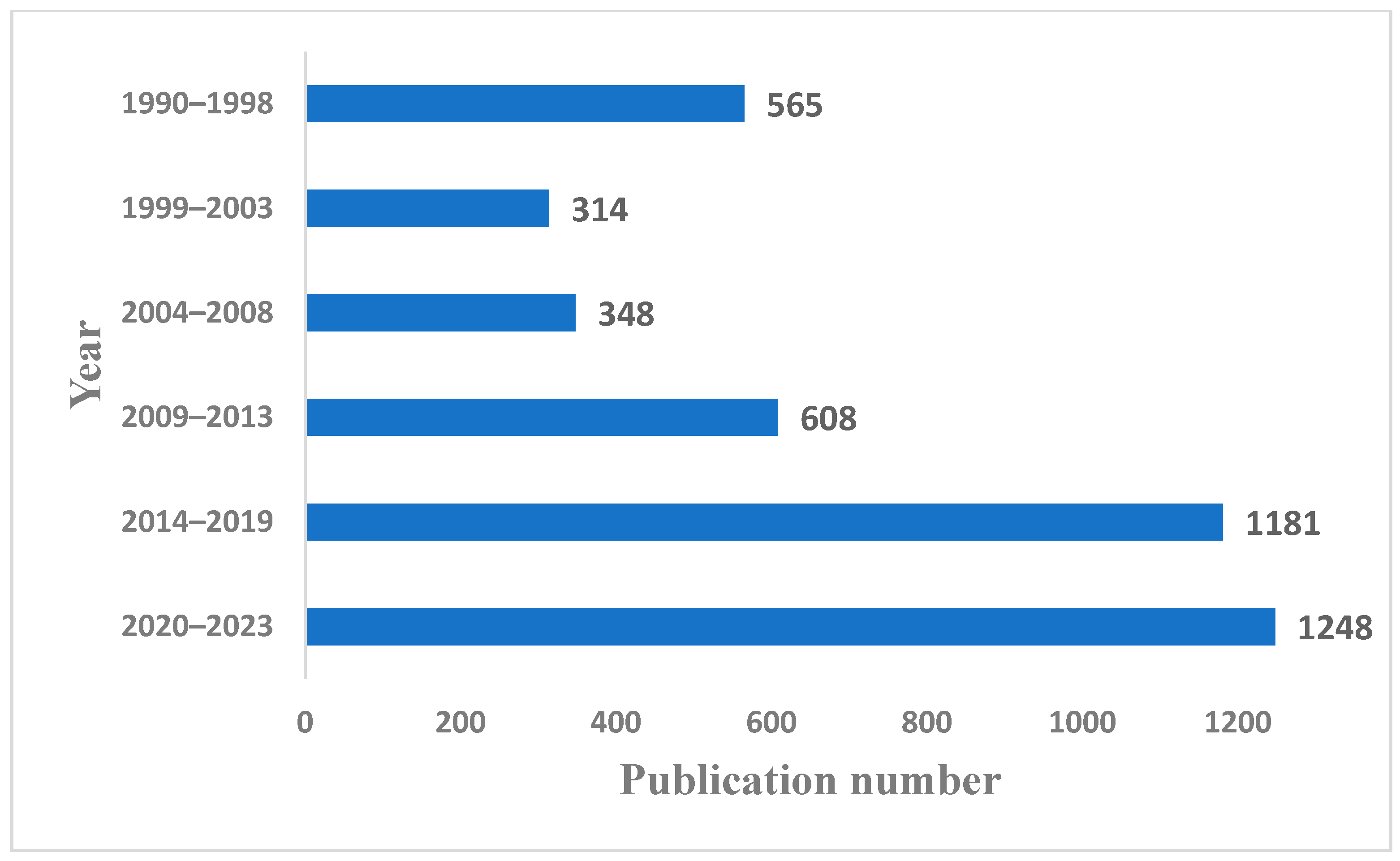
1. Introduction

2. Faba Bean Seeds’ Constituents and Properties
2.1. Proteins
2.2. Lipids
2.3. Carbohydrates
2.4. Minerals
3. Nutritional Quality
3.1. Amino Acid Composition
3.2. Digestibility
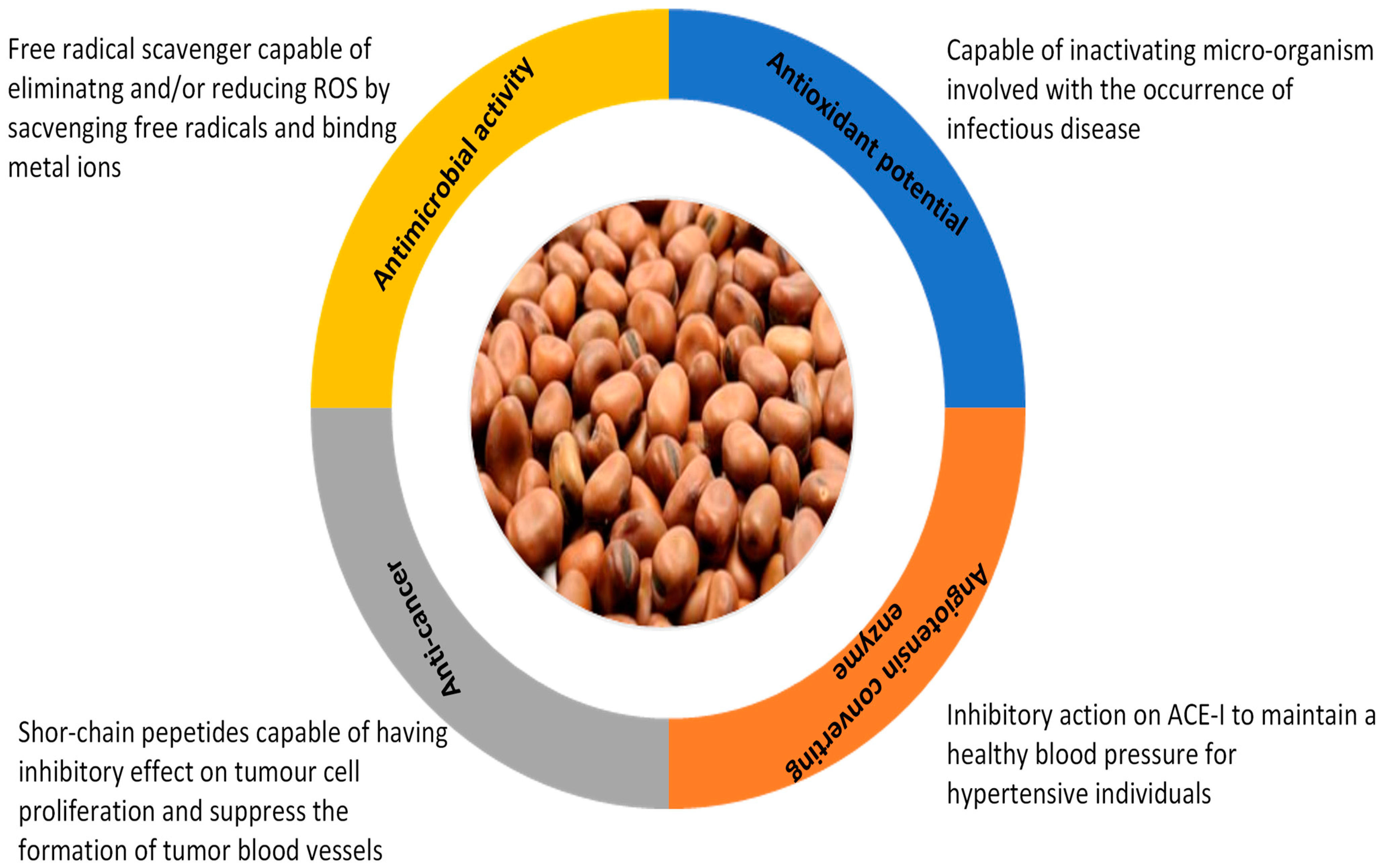
4. Bioactivity
5. Major Antinutritional Factors
5.1. Vicine and Convicine
5.2. Phytic Acid
5.3. Condensed Tannins
5.4. Oligosaccharides
5.5. Other Antinutritional Factors
6. Processing Effect on Toxicants and Pharmacological Activity
6.1. Soaking
6.2. Dehulling
6.3. Germination
| Non-Thermal Technology | Processing Conditions | % Major Toxin Reduction | Reference |
|---|---|---|---|
| Extraction | Protein concentrate Protein isolate | ↑Trypsin inhibitors increased by 64.79% ↓Vicine reduced by 8.5% ↓Convicine reduced by 12% ↓Trypsin inhibitors reduced by 80% ↓Vicine 100% reduction ↓Convicine 100% reduction | [34] |
| Alkaline extraction (pH 10.5); protein isolate | ↓Vicine and convicine reduced by 99% | [33] | |
| Germination | Time: 0–9 days; temperature: 20 °C | ↓Vicine reduced by 0.7–9.8% | [88] |
| Germination | Germination at 24–72 h at 25 °C | ↓Phytic acid content reduced by 53.46–61% ↓Condensed tannins reduced by 56–60% | [43] |
| Germination | Time: 0–144 h | ↓Trypsin inhibitors (TIU/mg) reduced by 26–38% ↓Phytic acid (mg/g) reduced by 55–58% ↓Condensed tannins reduced by 29–39% | [54] |
| Fermentation | Fermentation with Lactobacillus plantarum (DPPMAB24W); time: 0–48 h; temperature: 30 °C | ↓Convicine content reduced by 14–93% ↓Vicine content reduced by 35–96% | [29] |
| Fermentation using L. plantarum | Fermentation with L. plantarum for 24 h at 25 °C | ↓Phytic acid (mg/g) reduced by 8% | [123] |
| Fermentation | Fermentation with starter culture; time: 1–3 days at 30 °C | ↓Phytic acid (mg/g) reduced 48–84% | [22] |
| L. plantarum (sourdough) 48 h at 30 °C | ↓Vicine and convicine reduced by 100% | [56] | |
| Enzyme treatment | Flour treated with phytase (0, 2, 10, and 20 U) for 1–24 h using L. plantarum | ↓Phytic acid (mg/g) reduced by 15.46–90.72% | [123] |
| Dry fractionation | Micronized flour, protein-rich fraction, coarse-starch-rich fraction, wet-extracted protein fraction, deproteinated starch-rich flour | Phytic acid (mg/g) content was 4.3, 10.5, 7, 10.2, and 1, respectively Trypsin inhibitor (TIU/g) content was 10.5, 14.5, 5.8, 14.8, and 3.2, respectively | [124] |
| High-pressure processing | Concentrate (600 MPa for 4 min) | ↓Relative trypsin activity was reduced by 90.8% | [125] |
6.4. Thermal Processing
7. Impact of Processing Technologies on Faba Bean Seeds’ Pharmacological Activity
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chávez-Murillo, C.E.; Veyna-Torres, J.I.; Cavazos-Tamez, L.M.; de la Rosa-Millán, J.; Serna-Saldívar, S.O. Physicochemical Characteristics, ATR-FTIR Molecular Interactions and in Vitro Starch and Protein Digestion of Thermally-Treated Whole Pulse Flours. Food Res. Int. 2018, 105, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lazarte, C.E.; Carlsson, N.-G.; Almgren, A.; Sandberg, A.-S.; Granfeldt, Y. Phytate, Zinc, Iron and Calcium Content of Common Bolivian Food, and Implications for Mineral Bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted Extraction of Phenolics from Pomegranate Peels: Optimization, Kinetics, and Comparison with Ultrasounds Extraction. Chem. Eng. Process. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Johnson, C.R.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens culinaris L.): A Prebiotic-Rich Whole Food Legume. Food Res. Int. 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Moreno-Vilet, L.; Villanueva-Rodríguez, S.J. Current Status of Emerging Food Processing Technologies in Latin America: Novel Non-Thermal Processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of Short-Term Germination on the Chemical Compositions, Technological Characteristics and Nutritional Quality of Yellow Pea and Faba Bean Flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Ben Mlouka, M.A.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, Antityrosinase and Antibiofilm Activities of Synthesized Peptides Derived from Vicia Faba Protein Hydrolysate: A Powerful Agents in Cosmetic Application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C.G.; Flander, L.; Poutanen, K. Lactic Acid Fermentation as a Pre-Treatment Process for Faba Bean Flour and Its Effect on Textural, Structural and Nutritional Properties of Protein-Enriched Gluten-Free Faba Bean Breads. Foods 2019, 8, 431. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of Tea Tannins and Condensed Tannins with Proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Re, M.; Karsma, A.; Laitila, A.; Nordlund, E. Phytic Acid Reduction by Bioprocessing as a Tool To Improve the In Vitro Digestibility of Faba Bean Protein. J. Agric. Food Chem. 2018, 66, 10394–10399. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Mahoonak, A.S.; Aalami, M. Antioxidant Activity of Faba Bean (Vicia faba) Proteins Hydrolysates Produced by Alcalase and Trypsin. Res. Innov. Food Sci. Technol. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Turco, I.; Bacchetti, T.; Bender, C.; Zimmermann, B.; Oboh, G.; Ferretti, G. Polyphenol Content and Glycemic Load of Pasta Enriched with Faba Bean Flour. Funct. Foods Health Dis. 2016, 6, 291. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant Foods Hum. Nut. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hassanien, A.A.E.; Wong, J.H.; Bah, C.S.F.; Soliman, S.S.; Ng, T.B. Isolation of a New Trypsin Inhibitor from the Faba Bean (Vicia faba Cv. Giza 843) with Potential Medicinal Applications. Protein Pept. Lett. 2011, 18, 64–72. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, R.; Meena, M.K.; Khan, Y.J. Assessment of Soil Fertility Augmenting Potential of Faba Bean (Vicia faba L.) Germplasm and Their Performance. J. Agric. Search 2016, 3, 136–141. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Structure and Function of Pea, Lentil and Faba Bean Proteins Treated by High Pressure Processing and Heat Treatment. J. Food Sci. Technol. 2021, 152, 112349. [Google Scholar] [CrossRef]
- Luo, Y.; Gu, Z.; Han, Y.; Chen, Z. The Impact of Processing on Phytic Acid, in Vitro Soluble Iron and Phy/Fe Molar Ratio of Faba Bean (Vicia faba L.). J. Sci. Food Agric. 2009, 89, 861–866. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the In Vitro and In Vivo Protein Quality of Beans (Phaseolus vulgaris and Vicia faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Orúe, E.; Marzo, F.F. Effects of Extrusion and Conventional Processing Methods on Protein and Antinutritional Factor Contents in Pea Seeds. Food Chem. 1998, 63, 505. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Mishra, A. In Vitro and in Silico Interaction of Faba Bean (Vicia faba L.) Seed Extract with Xanthine Oxidase and Evaluation of Antioxidant Activity as a Therapeutic Potential. Nat. Prod. Res. 2019, 33, 2689–2693. [Google Scholar] [CrossRef]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in Levels of Enzyme Inhibitors during Soaking and Cooking for Pulses Available in Canada. Food Res. Int. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Osae, R.; Essilfie, G.; Alolga, R.N.; Akaba, S.; Song, X.; Owusu-ansah, P.; Zhou, C. Application of Non-thermal Pretreatment Techniques on Agricultural Products Prior to Drying: A Review. J. Sci. Food Agric. 2020, 100, 2585. [Google Scholar] [CrossRef] [PubMed]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Flavor Effects from Processing to Consumer Acceptability. Foods 2023, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic Cooking Methods Affect the Stability and Bioaccessibility of Dark Purple Eggplant (Solanum melongena) Phenolic Compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Sadiq, M.B.; Anal, A.K. Comparative Study of Physicochemical and Functional Properties of Soaked, Germinated Pressure-Cookedooked Faba Bean. J. Food Sci. Technol. 2021, 59, 257–267. [Google Scholar] [CrossRef]
- Vadivel, V.; Pugalenthi, M. Effect of various processing methods on the levels of antinutritional constituents and protein digestibility Ofmucuna pruriens (L.) dc. var.utilis (wall. ex wight) baker ex burck (velvet bean) seeds. J. Food Biochem. 2008, 32, 795–812. [Google Scholar] [CrossRef]
- Neacsu, M.; McBey, D.; Johnstone, A. Chapter 22—Meat Reduction and Plant-Based Food: Replacement of Meat: Nutritional, Health, and Social Aspects. In Sustainable Protein Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 359–375. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; FitzGerald, R.J. Structure and in Vitro Bioactive Properties of O/W Emulsions Generated with Fava Bean Protein Hydrolysates. Food Res. Int. 2021, 150, 110780. [Google Scholar] [CrossRef]
- Ashraf, J.; Awais, M.; Liu, L.; Khan, M.I.; Tong, L.-T.; Ma, Y.; Wang, L.; Zhou, X.; Zhou, S. Effect of Thermal Processing on Cholesterol Synthesis, Solubilisation into Micelles and Antioxidant Activities Using Peptides of Vigna angularis and Vicia faba. J. Food Sci. Technol. 2020, 129, 109504. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, W.; Luo, F. Effect of Several Germination Treatments on Phosphatases Activities and Degradation of Phytate in Faba Bean (Vicia faba L.) and Azuki Bean (Vigna angularis L.). J. Food Sci. 2012, 77, C1023–C1029. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Yang, X.-Q.; Gao, W.-R. Characterization, Amino Acid Composition and in Vitro Digestibility of Hemp (Cannabis sativa L.) Proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Maya-Ocaña, K.; Ortiz-Moreno, A.; Herrera-Cabrera, B.E.; Dávila-Ortiz, G.; Múzquiz, M.; Martín-Pedrosa, M.; Burbano, C.; Cuadrado, C.; Jiménez-Martínez, C. Effect of Roasting and Boiling on the Content of Vicine, Convicine and L-3,4-Dihydroxyphenylalanine in Vicia faba L. J. Food Qual. 2012, 35, 419–428. [Google Scholar] [CrossRef]
- Duc, G. Faba Bean (Vicia faba L.). J. Food Qual. 1997, 53, 99. [Google Scholar] [CrossRef]
- Vogelsang-O’dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.M.; Maia, M.R.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.; Cabrita, A.R. Profiling of Phenolic Compounds and Antioxidant Properties of European Varieties and Cultivars of Vicia faba L. Pods. Phytochem. 2018, 152, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.A.; Hassan, A.B.; Osman, G.A.M.; Mohammed, N.; Rushdi, M.A.H.; Diab, E.E.; Babiker, E.E. Effects of Gamma Irradiation and/or Cooking on Nutritional Quality of Faba Bean (Vicia faba L.) Cultivars Seeds. J. Food Sci. Technol. 2012, 51, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Dueik, V.; Chen, B.K.; Diosady, L.L. Iron-Polyphenol Interaction Reduces Iron Bioavailability in Fortified Tea: Competing Complexation to Ensure Iron Bioavailability. J. Food Qual. 2017, 2017, 1805047. [Google Scholar] [CrossRef]
- Duan, S.-C.; Kwon, S.-J.; Eom, S.-H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants 2021, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.D. 10—Adventitious Toxins. In Fish Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 601–649. [Google Scholar] [CrossRef]
- Lattanzio, V.; Bianco, V.V.; Miccolis, V.; Linsalata, V. Mono- and Oligosaccharides in Fifteen Vicia faba L. Cultivars. Food Chem. 1986, 22, 17–25. [Google Scholar] [CrossRef]
- Liang, J.; Han, B.-Z.; Nout, M.J.R.; Hamer, R.J. Effects of Soaking, Germination and Fermentation on Phytic Acid, Total and in Vitro Soluble Zinc in Brown Rice. Food Chem. 2008, 110, 821–828. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Effect of High-Pressure Processing and Heat Treatment on in Vitro Digestibility and Trypsin Inhibitor Activity in Lentil and Faba Bean Protein Concentrates. J. Food Sci. Technol. 2021, 152, 112342. [Google Scholar] [CrossRef]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for Carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Lestienne, I.; Icard-Vernière, C.; Mouquet, C.; Picq, C.; Trèche, S. Effects of Soaking Whole Cereal and Legume Seeds on Iron, Zinc and Phytate Contents. Food Chem. 2005, 89, 421–425. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, W.; Hao, Z.; Jin, X.; Wang, Q. The impact of processing on in vitro bioactive compounds bioavailability and antioxidant activities in faba bean (Vicia faba L.) and azuki bean (Vigna angularis L.). Int. Food Res. 2014, 21, 1031. [Google Scholar]
- Loizzo, M.R.; Bonesi, M.; Leporini, M.; Falco, T.; Sicari, V.; Tundis, R. Chemical Profile and In Vitro Bioactivity of Vicia faba Beans and Pods. Proceedings 2020, 70, 45. [Google Scholar] [CrossRef]
- Ali, M. Functional Properties of Faba Bean Protein and Effect of Enzymatic Hydrolysis on Its Antioxidant Activity. J. Agric. Res. 2019, 46, 99–114. [Google Scholar] [CrossRef]
- Jamalian, J. Removal of Favism-Inducing Factors Vicine and Convicine and the Associated Effects on the Protein Content and Digestibility of Fababeans (Vicia faba L). J. Sci. Food Agric. 1999, 79, 1909. [Google Scholar] [CrossRef]
- Hassan, A.B.; Babiker, E.E. Nutritional Evaluation of Cooked Faba Bean Vicia faba L. and white bean Phaseolus vulgaris L. Cultivars. Aust. J. Basic Appl. Sci. 2009, 3, 2484–2490. [Google Scholar]
- Dumoulin, L.; Jacquet, N.; Malumba, P.; Richel, A.; Blecker, C. Dry and Wet Fractionation of Plant Proteins: How a Hybrid Process Increases Yield and Impacts Nutritional Value of Faba Beans Proteins. Innov. Food Sci. Emerg. Technol. 2021, 72, 102747. [Google Scholar] [CrossRef]
- Sánchez-velázquez, O.A.; Ribéreau, S.; Mondor, M.M.; Cuevas-rodríguez, E.O.; Arcand, Y.; Hernández-álvarez, A.J. Impact of Processing on the in Vitro Protein Quality, Bioactive Compounds, and Antioxidant Potential of 10 Selected Pulses. Legume Sci. 2021, 3, e88. [Google Scholar] [CrossRef]
- Hegazy, M.I.; Marquardt, R.R. Development of a Simple Procedure for the Complete Extraction of Vicine and Convicine from Fababeans (Vicia faba L.). J. Sci. Food Agric. 1983, 34, 100–108. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P. Flavonoids and Saponins: What Have We Got or Missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S.; Kumar, M. Effect of Processing Method on Quality Characteristics of Harit Soybean (Glycine Max): In Vitro Protein Digestibility, Hplc, Ftir Analysis. Nutr. Food Sci. 2022, 52, 684–697. [Google Scholar] [CrossRef]
- Zhu, F.; Cui, R. Comparison of Physicochemical Properties of Oca (Oxalis tuberosa), Potato, and Maize Starches. Int. J. Biol. Macromol. 2020, 148, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic Profiles of Ten Australian Faba Bean Varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and Functional Properties of Vicia faba Protein Isolates and Related Fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Roux, L.L.; Chacon, R.; Dupont, D.; Jeantet, R.; Deglaire, A.; Nau, F. In Vitro Static Digestion Reveals How Plant Proteins Modulate Model Infant Formula Digestibility. Food Res. Int. 2020, 130, 108917. [Google Scholar] [CrossRef]
- Mínguez, M.I.; Rubiales, D. Chapter 15—Faba Bean. In Crop Physiology Case Histories for Major Crops; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 452–481. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive Compounds and Bioactivities of Germinated Edible Seeds and Sprouts: An Updated Review. Trends Food Sci. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Mayer Labba, I.-C.; Frøkiær, H.; Sandberg, A.-S. Nutritional and Antinutritional Composition of Fava Bean (Vicia faba L., Var. Minor) Cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J. Food 2014, 79, R273–R283. [Google Scholar] [CrossRef] [PubMed]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive Peptides and Proteins from Foods: Indication for Health Effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Paximada, P.; Howarth, M.; Dubey, B.N. Double Emulsions Fortified with Plant and Milk Proteins as Fat Replacers in Cheese. J. Food Eng. 2021, 288, 110229. [Google Scholar] [CrossRef]
- Khazaei, H.; Hawkins, G.; Vandenberg, A. Historical Review of Faba Bean Improvement in Western Canada. Legume Sci. 2021, 3, e92. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, Nutritional, Antioxidant, Sensory Properties and Comparative Peptidomic Profile of Faba Bean (Vicia faba L.) Seed Protein Hydrolysates and Fortified Apple Juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Sandberg, A.-S.; Andersson, H.; Carlsson, N.-G.; Sandstrom, B. Degradation Products of Bran Phytate Formed during Digestion in the Human Small Intestine: Effect of Extrusion Cooking on Digestibility. J. Nutr. Sci. 1987, 117, 2061–2065. [Google Scholar] [CrossRef]
- Di Stefano, E.; Tsopmo, A.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. Bioprocessing of Common Pulses Changed Seed Microstructures, and Improved Dipeptidyl Peptidase-IV and α-Glucosidase Inhibitory Activities. Sci. Rep. 2019, 9, 15308. [Google Scholar] [CrossRef]
- Deaville, E.R.; Green, R.J.; Mueller-Harvey, I.; Willoughby, I.; Frazier, R.A. Hydrolyzable Tannin Structures Influence Relative Globular and Random Coil Protein Binding Strengths. J. Agric. Food Chem. 2007, 55, 4554–4561. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Sathe, S.K.; Salunkhe, D.K.; Cornforth, D.P. Effects of Dehulling on Phytic Acid, Polyphenols, and Enzyme Inhibitors of Dry Beans (Phaseolus vulgaris L.). J. Food Sci. 1982, 47, 1846–1850. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Costantini, M.; Ricciardi, L.; Lotti, C.; Pavan, S.; Summo, C. Data on the Proximate Composition, Bioactive Compounds, Physicochemical and Functional Properties of a Collection of Faba Beans (Vicia faba L.) and Lentils (Lens culinaris Medik.). Data Brief 2021, 34, 106660. [Google Scholar] [CrossRef]
- Bi, S.; Xu, X.; Luo, D.; Lao, F.; Pang, X.; Shen, Q.; Hu, X.; Wu, J. Characterization of Key Aroma Compounds in Raw and Roasted Peas (Pisum sativum L.) by Application of Instrumental and Sensory Techniques. J. Agric. Food Chem. 2020, 68, 2718. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of Pea, Lentil and Faba Bean Starches Isolated from Air-Classified Flours in Comparison with Commercial Starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.M.; Schwingshackl, L.; Hoffmann, G.; Ekmekcioglu, C. The Effect of Vegetarian Diets on Iron Status in Adults: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of Vicine, Convicine and Their Aglycones during Fermentation of Faba Bean Flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.; Waelchli, K.; Çabuk, B.; McIntosh, T.; Wanasundara, J.; Arntfield, S.; Nickerson, M. The Levels of Bioactive Compounds Found in Raw and Cooked Canadian Pulses. Food Sci. Technol. Int. 2021, 27, 528–538. [Google Scholar] [CrossRef]
- Sandberg, A.-S. Bioavailability of Minerals in Legumes. Br. J. Nutr. 2002, 88, 281–285. [Google Scholar] [CrossRef]
- Siah, S.D.; Konczak, I.; Agboola, S.; Wood, J.A.; Blanchard, C.L. In Vitro Investigations of the Potential Health Benefits of Australian-Grown Faba Beans (Vicia faba L.): Chemopreventative Capacity and Inhibitory Effects on the Angiotensin-Converting Enzyme, α-Glucosidase and Lipase. Br. J. Nutr. 2012, 108, S123–S134. [Google Scholar] [CrossRef]
- Bautista-Expósito, S.; Vandenberg, A.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Lentil and Fava Bean With Contrasting Germination Kinetics: A Focus on Digestion of Proteins and Bioactivity of Resistant Peptides. Front. Plant Sci. 2021, 12, 754287. [Google Scholar] [CrossRef]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Varela, A.; Guillamón, E.; Pedrosa, M.M.; Muzquiz, M. Content and Distribution of Vicine, Convicine and l-DOPA during Germination and Seedling Growth of Two Vicia faba L. Varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary Triggers of Abdominal Symptoms in Patients With Irritable Bowel Syndrome: Randomized Placebo-Controlled Evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U.; Baraniak, B.; Bochnak, J. Peptides Obtained from Fermented Faba Bean Seeds (Vicia faba) as Potential Inhibitors of an Enzyme Involved in the Pathogenesis of Metabolic Syndrome. Food Sci. Technol. 2019, 105, 306–313. [Google Scholar] [CrossRef]
- Trikusuma, M.; Paravisini, L.; Peterson, D.G. Identification of Aroma Compounds in Pea Protein UHT Beverages. Food Chem. 2020, 312, 126082. [Google Scholar] [CrossRef]
- León-Espinosa, E.B.; Sánchez-Chino, X.; Garduño-Siciliano, L.; Álvarez-González, R.I.; Dávila-Ortiz, G.; Madrigal-Bujaidar, E.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Hypocholesterolemic and Anticarcinogenic Effect of Vicia faba Protein Hydrolyzates. Nutr. Cancer. 2016, 68, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Saldanha do Carmo, C.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is Dehulling of Peas and Faba Beans Necessary Prior to Dry Fractionation for the Production of Protein- and Starch-Rich Fractions? Impact on Physical Properties, Chemical Composition and Techno-Functional Properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- El-Sayed, S.T.; Al- Azzouny, R.A.; Ali, O.S. Purification of a Novel Monophenolase Inhibitory Peptides Prepared from Vicia faba Pods Protein via Enzymatic Hydrolysis. Biocatal. Agric. Biotechnol. 2019, 19, 101123. [Google Scholar] [CrossRef]
- Jiang, Z.; Pulkkinen, M.; Wang, Y.; Lampi, A.-M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba Bean Flavour and Technological Property Improvement by Thermal Pre-Treatments. Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Spencer, C.M.; Cai, Y.A.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Magnolato, D.; Lilley, T.H.; Haslam, E. Polyphenol Complexation—Some Thoughts and Observations. Phytochemistry 1988, 27, 2397–2409. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Oz, F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring Structure and Technological Properties of Plant Proteins Using High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Adamidou, S.; Nengas, I.; Grigorakis, K.; Nikolopoulou, D.; Jauncey, K. Chemical Composition and Antinutritional Factors of Field Peas (Pisum sativum), Chickpeas (Cicer arietinum), and Faba Beans (Vicia faba) as Affected by Extrusion Preconditioning and Drying Temperatures. Cereal Chem. 2011, 88, 80–86. [Google Scholar] [CrossRef]
- Savelkoul, F.H.M.G.; Van der Poel, A.F.B.; Tamminga, S. The Presence and Inactivation of Trypsin Inhibitors, Tannins, Lectins and Amylase Inhibitors in Legume Seeds during Germination. A Review. Plant Foods Hum. Nutr. 1992, 42, 71–85. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of Extrusion and Traditional Processing Methods on Antinutrients and in Vitro Digestibility of Protein and Starch in Faba and Kidney Beans. Food Chem. 2000, 68, 159. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S. Influence of Growing Environment on the Biochemical Composition and Physical Characteristics of Soybean Seed. J. Food Compos. Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Heiniö, R.-L.; Cassan, D.; Holopainen-Mantila, U.; Micard, V.; Lantto, R.; Sozer, N. Effect of Bioprocessing and Fractionation on the Structural, Textural and Sensory Properties of Gluten-Free Faba Bean Pasta. J. Food Sci. Technol. 2016, 67, 27–36. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating Vicine and Convicine, the Main Anti-Nutritional Factors Restricting Faba Bean Usage. Legume Sci. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Khalil, M.I.; Salih, M.A.; Mustafa, A.A. Study of Fatty Acid Composition, Physiochemical Properties and Thermal Stability of Broad Beans (Vicia faba) Seed Oil. Agric. Biol. J. N. Am. 2015, 8, 141–146. [Google Scholar] [CrossRef]
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Modulating Effect of Cotyledon Cell Microstructure on in Vitro Digestion of Starch in Legumes. Food Hydrocoll. 2019, 96, 112–122. [Google Scholar] [CrossRef]
- Abd El-Hady, E.A.; Habiba, R.A. Effect of Soaking and Extrusion Conditions on Antinutrients and Protein Digestibility of Legume Seeds. Food Sci. Technol. 2003, 36, 285. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in Foods Are More Complex than Often Thought 1–3 Véronique Cheynier. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Etzler, M.E. Plant Lectins: Molecular and Biological Aspects. Annu. Rev. Plant Physiol. 1985, 36, 209–234. [Google Scholar] [CrossRef]
- Roland, W.S.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Kader, Z.M.A. Study of Some Factors Affecting Water Absorption by Faba Beans during Soaking. Food Chem. 1995, 53, 235. [Google Scholar] [CrossRef]
- van Barneveld, R.J. Understanding the Nutritional Chemistry of Lupin (Lupinus Spp.) Seed to Improve Livestock Production Efficiency. Nutr. Res. Rev. 1999, 12, 203–230. [Google Scholar] [CrossRef]
- Chaieb, N.; González, J.L.; López-Mesas, M.; Bouslama, M.; Valiente, M. Polyphenols Content and Antioxidant Capacity of Thirteen Faba Bean (Vicia faba L.) Genotypes Cultivated in Tunisia. Food Res. Int. 2011, 44, 970–977. [Google Scholar] [CrossRef]
- Johns, P.W.; Hertzler, S.R. Substantial Depletion of Vicine, Levodopa, and Tyramine in a Fava Bean Protein-Based Nutritional Product. Int. J. Food Sci. 2021, 2021, 6669544. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between Total, Processed, Red and White Meat Consumption and All-Cause, CVD and IHD Mortality: A Meta-Analysis of Cohort Studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Reading, N.S.; Sirdah, M.M.; Shubair, M.E.; Nelson, B.E.; Al-Kahlout, M.S.; Al-Tayeb, J.M.; Aboud, L.N.; Shaban, M.A.; Luzzatto, L.; Prchal, J.T. Favism, the Commonest Form of Severe Hemolytic Anemia in Palestinian Children, Varies in Severity with Three Different Variants of G6PD Deficiency within the Same Community. Blood Cells Mol. Dis. 2016, 60, 58–64. [Google Scholar] [CrossRef]
- Luo, Y.-W.; Xie, W.-H. Effect of Different Processing Methods on Certain Antinutritional Factors and Protein Digestibility in Green and White Faba Bean (Vicia faba L.). J. Food Sci. 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Silventoinen-Veijalainen, P.; Zobel, H.; Holopainen-Mantila, U.; Sahlstrøm, S.; Knutsen, S.H. The Effect of Dehulling of Yellow Peas and Faba Beans on the Distribution of Carbohydrates upon Dry Fractionation. Food Sci. Technol. 2022, 163, 113509. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and Health Benefits of Pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus Beans: Impact on Glycaemic Response and Chronic Disease Risk in Human Subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the Protein Quality of Wheat Bread through Faba Bean Sourdough Addition. Food Sci. Technol. 2017, 82, 296–302. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M. Global Diets Link Environmental Sustainability and Human Health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Renin and Angiotensin Converting Enzyme Inhibition with Antioxidant Properties of African Yam Bean Protein Hydrolysate and Reverse-Phase HPLC-Separated Peptide Fractions. Food Res. Int. 2013, 52, 437–444. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.J.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive Constituents in Pulses and Their Health Benefits. J. Food Sci. Technol. 2016, 54, 858–870. [Google Scholar] [CrossRef]
- Ballard, C.R.; Maróstica, M.R. Health Benefits of Flavonoids. In Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 185–201. [Google Scholar] [CrossRef]
- Jamalian, J.; Ghorbani, M. Extraction of Favism-Inducing Agents from Whole Seeds of Faba Bean (Vicia faba L. Var Major). J. Sci. Food Agric. 2005, 85, 1055–1060. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochemistry 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøm, S. Meat Analogues from a Faba Bean Concentrate Can Be Generated by High Moisture Extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Hou, W.; Li, P.; Sha, W.; Tian, Y. Structure and Function of Seed Storage Proteins in Faba Bean (Vicia faba L.). 3 Biotech. 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Pang, X.; Tian, S.; Hu, Q.; Li, X.; Liu, J.; Wang, J.; Lu, Y. The Effect of Processing and Cooking on Glucoraphanin and Sulforaphane in Brassica Vegetables. Food Chem. 2021, 360, 130007. [Google Scholar] [CrossRef]
- Maleki, S.; Razavi, S.H. Pulses’ Germination and Fermentation: Two Bioprocessing against Hypertension by Releasing ACE Inhibitory Peptides. Crit. Rev. Food Sci. 2021, 61, 2876–2893. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Valverde, C.; Frias, J.; Sierra, I.; Blazquez, I.; Lambein, F.; Kuo, Y.-H. New Functional Legume Foods by Germination: Effect on the Nutritive Value of Beans, Lentils and Peas. Eur. Food Res. Technol. 2002, 215, 472–477. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, L.; Liu, Y.; Huang, L.; Shen, S.; Zhang, R.; Chen, J.; Zhao, Y.; Shen, H.; Chen, F. A Systematic Review and Meta-Analysis Reveals Long and Dispersive Incubation Period of COVID-19. MedRexiV 2020. [Google Scholar] [CrossRef]
- Jamalian, J.; Bassiri, A. Variation in Vicine Concentration during Pod Development in Broad Bean (Vicia faba L.). J. Agric. Food Chem. 1978, 26, 1454–1456. [Google Scholar] [CrossRef]
- Pakfetrat, S.; Amiri, S.; Radi, M.; Abedi, E.; Torri, L. Reduction of Phytic Acid, Aflatoxins and Other Mycotoxins in Wheat during Germination. J. Sci. Food Agric. 2019, 99, 4695–4701. [Google Scholar] [CrossRef]
- McMillan, D.C.; Schey, K.L.; Meier, G.P.; Jollow, D.J. Chemical Analysis and Hemolytic Activity of the Fava Bean Aglycon Divicine. Chem. Res. Toxicol. 1993, 6, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Marambe, P.; Wanasundara, J. Seed Storage Proteins as Sources of Bioactive Peptides. In Bioactive Molecules in Plant Foods; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 49–80. [Google Scholar]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, X.; Wang, P.; Jiang, H.; Li, W. Compositional, Morphological, and Physicochemical Properties of Starches from Red Adzuki Bean, Chickpea, Faba Bean, and Baiyue Bean Grown in China. Nutr. Food Sci. 2019, 7, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Verni, M.; Koivula, H.; Montemurro, M.; Seppa, L.; Kemell, M.; Katina, K.; Coda, R.; Gobbetti, M. Influence of Fermented Faba Bean Flour on the Nutritional, Technological and Sensory Quality of Fortified Pasta. Food Funct. 2017, 8, 860–871. [Google Scholar] [CrossRef]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in Levels of Phytic Acid, Lectins and Oxalates during Soaking and Cooking of Canadian Pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef]
- Valente, I.M.; Cabrita, A.R.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.; Maia, M.R. Unravelling the Phytonutrients and Antioxidant Properties of European Vicia faba L. Seeds. Food Res. Int. 2019, 116, 888–896. [Google Scholar] [CrossRef]
- Baginsky, C.; Peña-Neira, Á.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuzé, R. Phenolic Compound Composition in Immature Seeds of Fava Bean (Vicia faba L.) Varieties Cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Dugardin, C.; Cudennec, B.; Tourret, M.; Caron, J.; Guérin-Deremaux, L.; Behra-Miellet, J.; Lefranc-Millot, C.; Ravallec, R. Explorative Screening of Bioactivities Generated by Plant-Based Proteins after In Vitro Static Gastrointestinal Digestion. Nutrients 2020, 12, 3746. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Legumes as a Source of Natural Antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- Landry, E.J.; Fuchs, S.J.; Hu, J. Carbohydrate Composition of Mature and Immature Faba Bean Seeds. J. Food Compos. Anal. 2016, 50, 55–60. [Google Scholar] [CrossRef]
- Purves, R.W.; Zhang, H.; Khazaei, H.; Vandenberg, A. Rapid Analysis of Medically Relevant Compounds in Faba Bean Seeds Using FAIMS and Mass Spectrometry. Int. J. Ion Mobil. Spectrom. 2017, 20, 125–135. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Gawalko, E.J. Effect of Variety and Processing on Nutrients and Certain Anti-Nutrients in Field Peas (Pisum sativum). Food Chem. 2008, 111, 132–138. [Google Scholar] [CrossRef]
- Björnsdotter, E.; Nadzieja, M.; Chang, W.; Escobar-Herrera, L.; Mancinotti, D.; Angra, D.; Xia, X.; Tacke, R.; Khazaei, H.; Crocoll, C.; et al. VC1 Catalyses a Key Step in the Biosynthesis of Vicine in Faba Bean. Nature 2021, 7, 923. [Google Scholar] [CrossRef] [PubMed]
- Marcela, G.-M. Bioactive Peptides from Legumes as Anticancer Therapeutic Agents. Int. J. Cancer Clin. Res. 2017, 4, 81. [Google Scholar] [CrossRef]
- Hussein, L.; Motawei, H.; Nassib, A.; Khalil, S.; Marquadt. The Complete Elimination of Vicine and Convicine from the Faba Beans by Combinations of Genetic Selection and Processing Techniques. Plant Foods Hum. Nutr. 1986, 36, 231–242. [Google Scholar] [CrossRef]
- Amarakoon, D.; Thavarajah, D.; McPhee, K.; Thavarajah, P. Iron-, Zinc-, and Magnesium-Rich Field Peas (Pisum sativum L.) with Naturally Low Phytic Acid: A Potential Food-Based Solution to Global Micronutrient Malnutrition. Br. J. Nutr. 2012, 27, 8–13. [Google Scholar] [CrossRef]

| Sample | Protein % | Fat % | Ash % | Carbohydrate % |
|---|---|---|---|---|
| Whole a | 30.8–31.60 | 2–2.2 | 3.3–3.5 | 62.5–63.8 |
| Hulled a | 35.5 | 2.1 | 4.0 | 57.5–59.1 |
| Protein concentrate b | 64.1 | 2.43 | 4.8 | 28.7 |
| Protein isolate b | 90.1 | 4.36 | 5.2 | 0.34 |
| Amino Acids | Faba Bean | Protein Concentrate | Protein Isolate | Other Protein | Casein e | FAO/WHO Requirement | ||
|---|---|---|---|---|---|---|---|---|
| Whole Seeds a | Dehulled Seeds a | FBC b | FBI c | SPI d | 2–5-Year-Old f | Adult f | ||
| Histidine | 2.56 | 2.43 | 2.39 | 2.80 | 2.81 | 2.70 | 1.90 | 1.60 |
| Isoleucine | 4.1 | 3.97 | 3.73 | 3.80 | 4.35 | 4.90 | 2.80 | 1.3 |
| Leucine | 7.5 | 7.25 | 7.10 | 8.0 | 6.79 | 8.40 | 6.60 | 1.90 |
| Lysine | 6.43 | 6.16 | 6.34 | 7.0 | 5.23 | 7.10 | 5.80 | 1.60 |
| Methionine | 0.89 | 0.79 | 0.60 | 0.100 | 0.92 | 2.60 | - | - |
| Phenylalanine | 4.25 | 4.07 | 4.13 | 4.90 | 5.14 | 4.50 | - | - |
| Threonine | 3.65 | 3.45 | 3.54 | 3.70 | 3.98 | 3.70 | 3.40 | 0.90 |
| Valine | 4.75 | 4.56 | 4.14 | 4.10 | 4.28 | 6.0 | 3.50 | 1.30 |
| Alanine | 3.97 | 3.78 | 3.85 | 4.40 | 3.72 | 2.7 | - | - |
| Arginine | 9.73 | 10.21 | 10.48 | 10.00 | 7.35 | 3.3 | - | - |
| Aspartic acid | 11.2 | 10.71 | 10.30 | 13.30 | 11.47 | 6.3 | - | - |
| Cysteine | 1.18 | 1.13 | - | 5.00 | 0.05 | 0.04 | - | |
| Glutamic acid | 16.78 | 16.05 | 16.25 | 19.90 | 20.67 | 19.0 | - | - |
| Glycine | 4.38 | 4.06 | 3.81 | 4.90 | 3.74 | 1.60 | - | - |
| Serine | 4.98 | 4.74 | 4.87 | 6.30 | 5.32 | 4.60 | - | - |
| Tyrosine | 3.67 | 3.50 | 3.05 | 2.63 | 3.61 | 5.50 | - | |
| Proline | 4.22 | 4.09 | 4.24 | 3.40 | 5.13 | - | - | - |
| Technology | Conditions | Results | Reference |
|---|---|---|---|
| Baking | Bread with 30% faba bean flour; faba bean sourdough bread | ↑IVPD: 0.79–16.51 (%) | [56] |
| Extrusion | Temperature: (140–180 °C); moisture: 18–22%); unsoaked beans | ↑IVPD: 3.71–4.26 (%) | [57] |
| Temperature: (140–180 °C); moisture: 18–22%); soaked for 16 h at 30 °C | ↑IVPD: 2.71–5.80 (%). | ||
| Extraction | Cooked starch extract | ↑Rapidly digestible starch increased by 475% ↓Slowly digestible starch reduced by 93% ↓Resistant starch reduced by 87% | [37] |
| Protein concentrate Protein isolate | IVPD: 33.9 IVPD: 9.2 | [34] | |
| Protein isolate Optimized ultrasound-treated isolate | IVPD: 68.42 IVPD: 65.98 | [58] | |
| Thermal treatment | Extruded flour; cooking flour; baked flour | ↓IVPD 2–4(%) | [34] |
| Soaking | Soaked flour in acidic and acidic + hydrogen peroxide Soaked flour in water and neutral conditions | ↑IVPD 11.78–14.75% ↓IVPD 2.0–7.4% | [59] |
| Time: 12–48 h at room temperature | ↑IVPD 0.82–1.4% | [26] | |
| Thermal treatment | Extrusion (barrel temperature between 30 and 120 °C); cooking; extrusion followed by baking for 27–35 min | IVPD (%) was found to be 82.22, 81.41, and 76.79, respectively | [60] |
| Cooking | Soaked for 8 h followed by cooking | ↑Albumin digestibility 29% ↑Glutelin digestibility 23.61% | [7] |
| Cooking (100 °C; 30 min); soaking + cooking (100 °C; 30 min); hulling + soaking + cooking (100 °C; 30 min) | ↑IVPD 7.33–9.46 (%) | [26] | |
| Heat treatment | Annealing with incubation at 65 °C for 24 h Heat–moisture treatment; incubation at 120 °C for 24 h | ↑IVPD 8.13 (%) ↓IVPD 4% | [55] |
| Fermented product | Fermented and unfermented pasta formulation (10–50%). | ↑IVPD 16.86–81% | [61] |
| Bread with 50% faba flour Bread with 50% fermented four using L. plantarum | IVPD 53.9% IVPD 72.3% | [62] | |
| Dehulling | Hulled seeds | ↑IVPD 0.79% | [26] |
| Microwave Treatment | Microwave for 6 min; soaking + microwave (6 min); hulling + soaking + microwave (6 min) | ↑IVPD 1.75–3.5% | [26] |
| Autoclaving | Autoclaving (121 °C for 20 min) | ↑IVPD 7.2–11% | [26] |
| Irradiation | Irradiated at 0.5 and 1 kGy Irradiated at 0.5 and 1 KGy followed by cooking | ↑IVPD 10–20% ↓IVPD 10–16% | [63] |
| Method | Conditions | Toxin Results | Reference |
|---|---|---|---|
| Cooking | Dehulling followed by soaking (1:10 w/v); cooking at 100 °C for 30 min | ↓Phytic acid reduced by 13% ↓Trypsin inhibitors reduced by 15% ↓Tannins reduced from 63% | [26] |
| Soaked for 4 h, followed by cooking at 95 °C for 1 h | ↓Hemagglutinin activity reduced by 98% ↓Phytic acid reduced by 19% ↓Oxalate content reduced by 31% | [24] | |
| Extrusion | Feeder at 383 and 385 g/min with moisture content at constant 25%. Outlet temperatures were 152 and 156 °C | ↓Trypsin inhibitors reduced by 99% ↓Chymotrypsin inhibitor was reduced by 53% ↓a-Amylase inhibitor reduced by 100% reduction. | [107] |
| Extrusion at 30–120 °C Cooking (soaked for 16 h before cooking (25–35 min)) Baked (193 °C for 35 min) | ↓Saponins reduced by 13% ↓Phytic acid reduced by 50% | [34] | |
| Autoclaving | Pre-treatment: dehulling and soaking followed by autoclaving at 1.5 × 106 Pa (121 °C) for 20 min | ↓Phytic acid reduced by 23–39 ↓Trypsin inhibitors reduced by 22–50% ↓Tannins reduced by 65% | [26] |
| Microwave cooking | Pre-treatment: soaking and dehulling followed by microwave cooking for 6 min | ↓Trypsin inhibitors reduced by 8–52% ↓Tannins reduced by 0.5–58% | [26] |
| Heat treatment | Protein concentrate (95 °C for 15 min) | ↓Relative trypsin activity reduced by 98% | [125] |
| Boiling seeds for 0.33 h at 121 °C Roasting seeds for 0.17 h at 120 °C | ↓Vicine reduced by 30% and convicine by 61% ↓Vicine reduced by 12% and convicine by 40% | [131] | |
| Irradiation | Treatment: 0.5 and 0.1 KGy at a dose rate of 3.2 kGy/h at 25 °C, followed by cooking | ↓Tannins reduced by 53% Phytic acid increased from 7.2 to 7.47 at 0.5 kGy but reduced at 6.5 mg/g at 1.0 kGy | [97] |
| Soaking–cooking | Soaked for 8 h; cooked in boiling water | Phytic acid (mg/100 g) decreased from 183.65 to 153.44 Tannin content (mg/100 g) reduced from 1120 to 50 | [132] |
| Soaking | Solid: liquid (1:5 w/w) Time: 4 h at room temperature | ↓Hemagglutinin activity reduced by 0.5–5% ↓Phytic acid reduced by 1–3% ↓Oxalate reduced by 17.40–37% | [23] |
| 1% acid (flow 0.5 mL/min) whole beans; 72 h at 50 °C | ↓Vicine and convicine reduced by 100% | [108] | |
| Autoclaving | Autoclaving after soaking (48 h) for 0.5 h at 121 °C | ↓Vicine and convicine reduced by 50% | [108] |
| Technology | Processing Conditions | Compound Tested | Major Findings | Unit | Reference |
|---|---|---|---|---|---|
| Untreated | Raw faba bean | Total phenols | 0.45 | mg/g | [136] |
| Immature seed | Total phenols | 817–1337.82 | mg/GAE/Kg | [73] | |
| Seeds | Total phenols Total flavonoids Total carotenoids Antioxidants DPPH ABTS FRAP | 2.5 2 2.1 74.71 2.35 70.16 | mg/g μg/mL µg/mL µg Fe (III)/g | [148] | |
| Young leaves | Total phenols Total flavonoids DPPH | 53.59–55.03 62.41–64.93 73.23–74.17 | mg/GAE/g d.w mg/CE/g d.w mg VCE/g d.w | [149] | |
| Old leaves | Total phenols Total flavonoids DPPH | 42.68–44.3 43.46–43.90 53.83–55.27 | |||
| Soaked–cooked | Whole seeds (soaked for 4 h, followed by cooking for 1 h) | Total polyphenols | −54% reduction | [63] | |
| Split beans | −47% reduction | ||||
| Enzymatic treatment | Alcalase-treated protein concentrate for 8 min | Antioxidants FRAP ORAC | FRAP increased by 6–36% ORAC increased by 2% | [146] | |
| Protein hydrolysates with different enzymes | Antioxidant ABTS DPPH | ABTS increased by 14,800% DPPH increased by 460% | [147] | ||
| Solvent extraction | Acetone extract | Total phenols Flavonoids | 33.87 27.93 | [150] | |
| Germination | Time: 96 h; followed by digestion till the end of the small intestine | Antioxidants ORAC ABTS | ORAC increased by 112% ABTS increased by 95% | [27] | |
| Bioprocess | Pasta replaced with 35% faba bean flour | Total phenols Total flavonoids Antioxidant activity | Phenol content increased by 190% Flavonoids increased by 74% Antioxidant content increased by 18% | [151] | |
| Dry heating | 100 °C for 15–60 min | TPC TFC Antioxidant activity | ↓TPC after 15 min ↓TFC reduced ↓Antioxidant activity reduced | [149] | |
| Steaming | Time: 15–60 min | TPC TFC Antioxidant activity | ↓TPC after 15 min ↓TFC ↓Antioxidant activity reduced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules 2023, 28, 5431. https://doi.org/10.3390/molecules28145431
Badjona A, Bradshaw R, Millman C, Howarth M, Dubey B. Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules. 2023; 28(14):5431. https://doi.org/10.3390/molecules28145431
Chicago/Turabian StyleBadjona, Abraham, Robert Bradshaw, Caroline Millman, Martin Howarth, and Bipro Dubey. 2023. "Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties" Molecules 28, no. 14: 5431. https://doi.org/10.3390/molecules28145431
APA StyleBadjona, A., Bradshaw, R., Millman, C., Howarth, M., & Dubey, B. (2023). Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules, 28(14), 5431. https://doi.org/10.3390/molecules28145431







