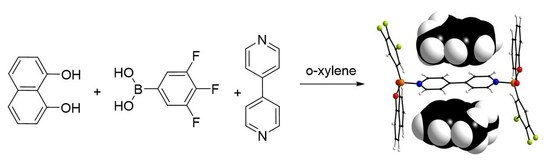
1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons
Abstract
1. Introduction
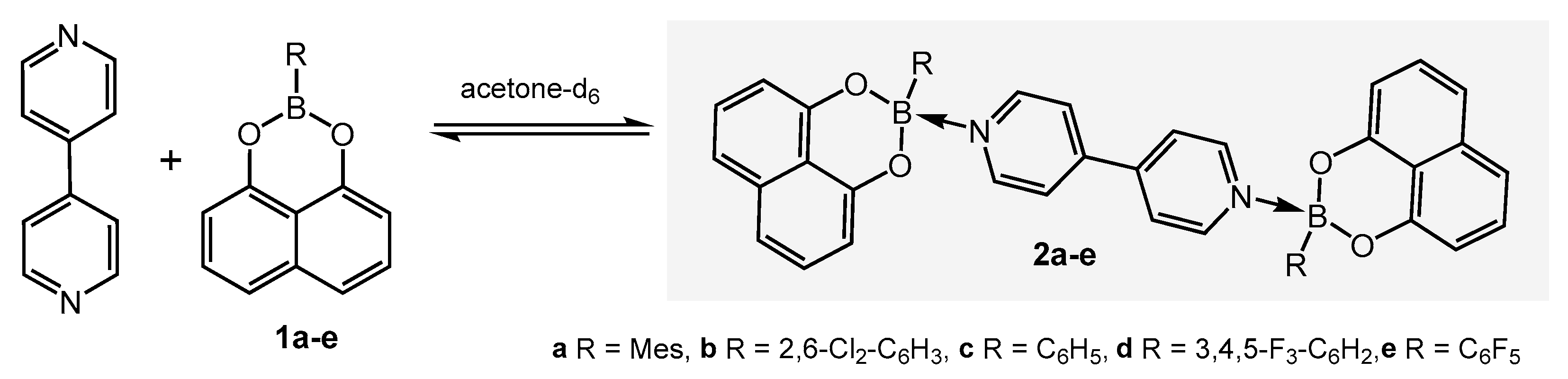
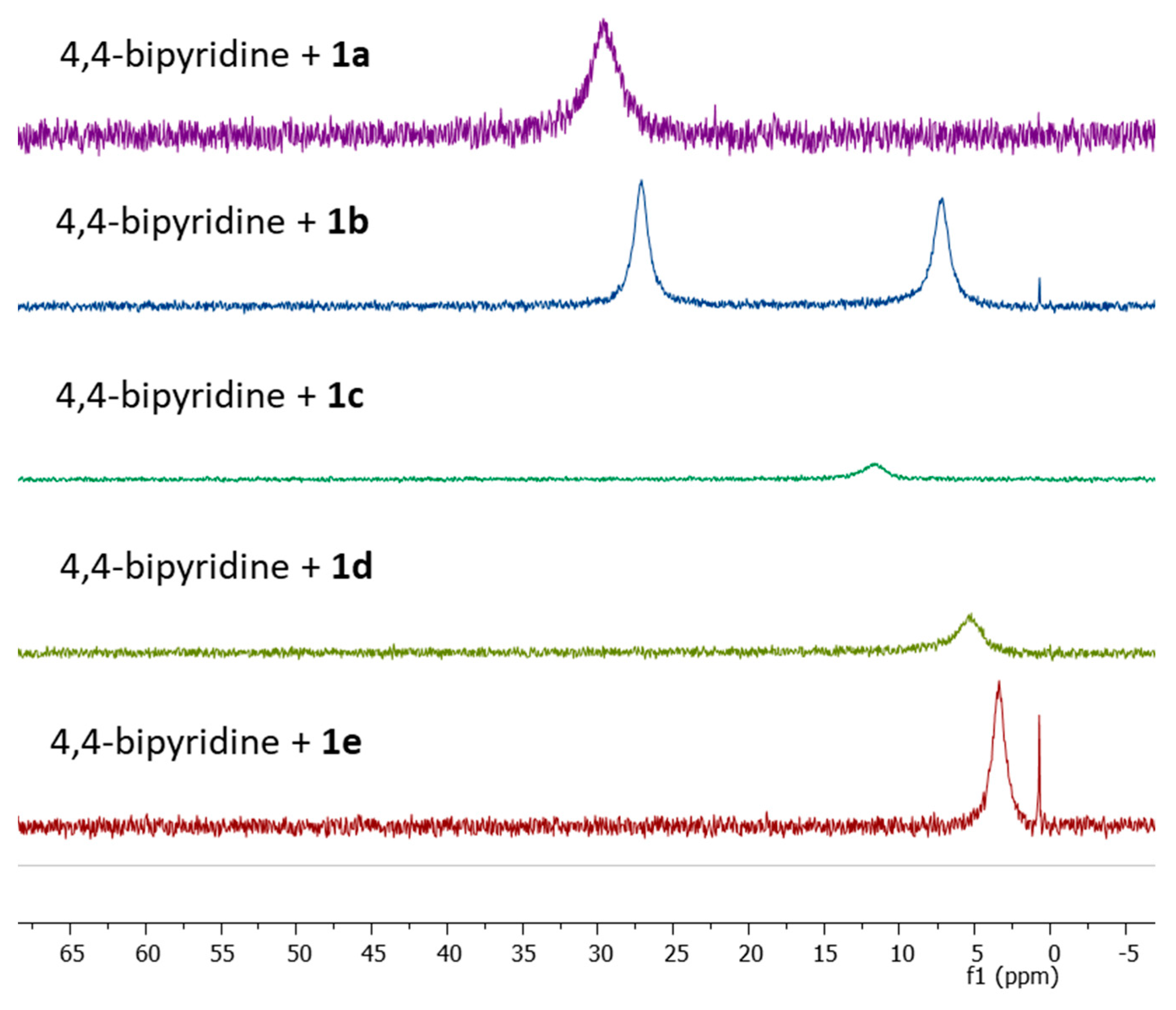
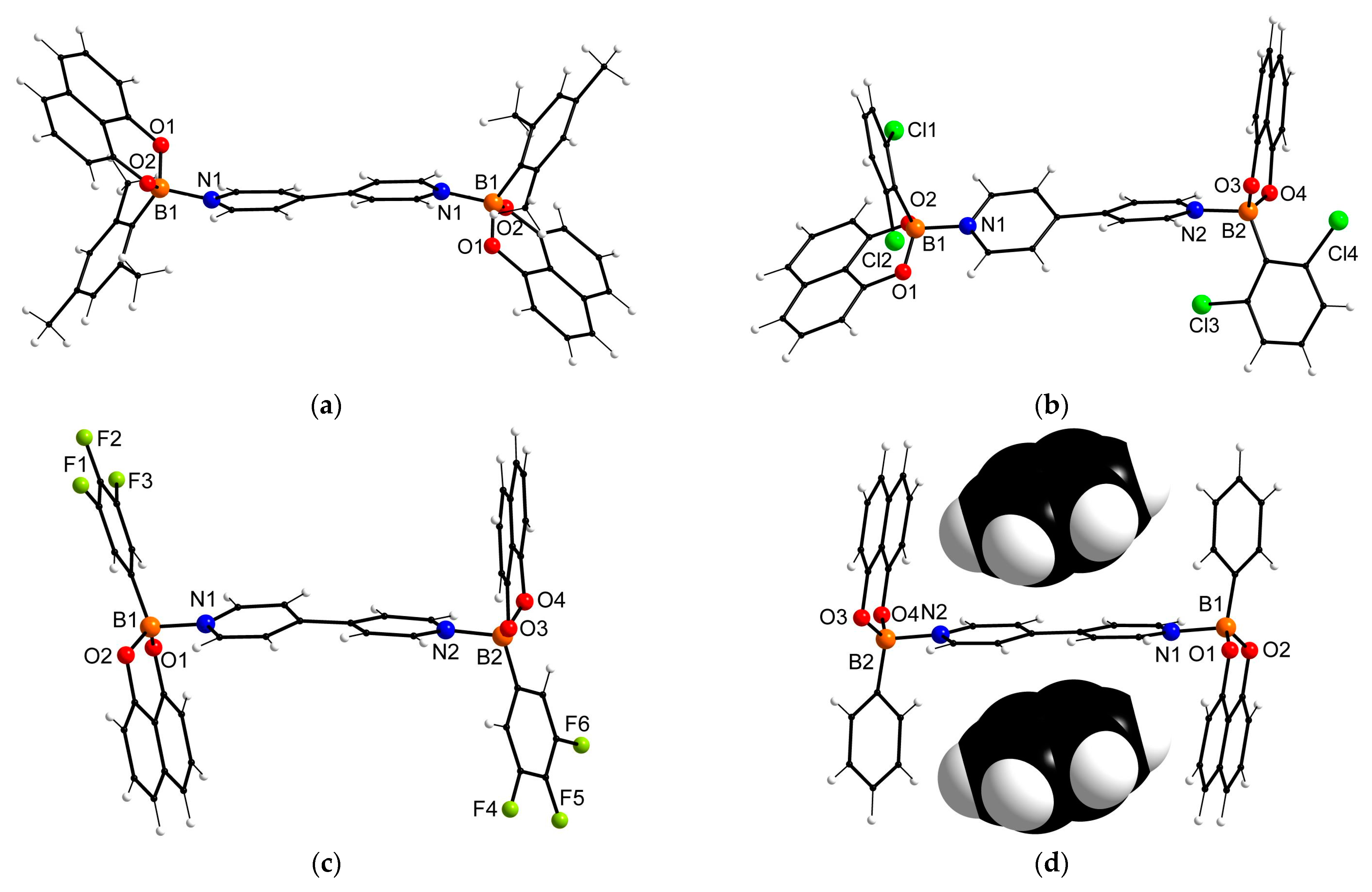
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Brunet, P.; Simard, M.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 1997, 119, 2737–2738. [Google Scholar] [CrossRef]
- Hosseini, M.W. Molecular tectonics: From simple tectons to complex molecular networks. Acc. Chem. Res. 2005, 38, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M. Shape-Persistent Organic Cage Compounds by Dynamic Covalent Bond Formation. Angew. Chem. Int. Ed. 2010, 49, 5042–5053. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef]
- Goesten, M.G.; Kapteijn, F.; Gascon, J. Fascinating chemistry or frustrating unpredictability: Observations in crystal engineering of metal−organic frameworks. CrystEngComm 2013, 15, 9249–9257. [Google Scholar] [CrossRef]
- Harris, K.; Fujita, D.; Fujita, M. Giant hollow MnL2n spherical complexes: Structure, functionalisation and applications. Chem. Commun. 2013, 49, 6703–6712. [Google Scholar] [CrossRef]
- Song, M.; Sun, Z.; Han, C.; Tian, D.; Li, H.; Kim, J.S. Calixarene based chemosensors by means of click chemistry. Chem. Asian J. 2014, 9, 2344–2357. [Google Scholar] [CrossRef]
- Durola, F.; Heitz, V.; Reviriego, F.; Roche, C.; Sauvage, J.-P.; Sour, A.; Trolez, Y. Cyclic [4]rotaxanes containing two parallel porphyrinic plates: Toward switchable molecular receptors and Compressors. Acc. Chem. Res. 2014, 47, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mastalerz, M. Organic cage compounds—From shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.-J.; Liu, D.; Jin, G.-X. Multi-component coordination-driven self-assembly toward heterometallic macrocycles and cages. Coord. Chem. Rev. 2015, 293–294, 139–157. [Google Scholar]
- Torres-Huerta, A.; Velásquez-Hernández, M.; Martínez-Otero, D.; Höpfl, H.; Jancik, V. Structural induction via solvent variation in assemblies of triphenylboroxine and piperazine—Potential application as self-assembly molecular sponge. Cryst. Growth Des. 2017, 17, 2438–2452. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Du, F.; Wang, G.-D.; Hou, L.; Zhu, Z.; Liu, B.; Wang, Y.-Y. Dative B←N bonds based crystalline organic framework with permanent porosity for acetylene storage and separation. Chem. Sci. 2023, 14, 533–539. [Google Scholar]
- Stephens, A.J.; Scopelliti, R.; Tirani, F.F.; Solari, E.; Severin, K. Crystalline Polymers Based on Dative Boron-Nitrogen Bonds and the Quest for Porosity. ACS Mater. Lett. 2019, 1, 3–7. [Google Scholar] [CrossRef]
- Bhandary, S.; Shukla, R.; Van Hecke, K. Effect of chemical substitution on the construction of boroxine-based supramolecular crystalline polymers featuring B←N dative bonds. CrystEngComm 2022, 24, 1695–1699. [Google Scholar] [CrossRef]
- Madura, I.D.; Czerwinska, K.; Jakubczyk, M.; Pawelko, A.; Adamczyk-Wozniak, A.; Sporzynski, A. Weak C–H···O and Dipole–Dipole Interactions as Driving Forces in Crystals of Fluorosubstituted Phenylboronic Catechol Esters. Cryst. Growth Des. 2013, 13, 5344–5352. [Google Scholar] [CrossRef]
- Luisier, N.; Scopelliti, R.; Severin, K. Supramolecular gels based on boronate esters and imidazolyl donors. Soft Matter 2016, 12, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Mendoza, D.; Cruz-Huerta, J.; Höpfl, H.; Hernández-Ahuactzi, I.F.; Sanchez, M. Macrocycles and coordination polymers derived from self-complementary tectons based on N-containing boronic acids. Cryst. Growth Des. 2013, 13, 2441–2454. [Google Scholar] [CrossRef]
- Ray, K.K.; Campillo-Alvarado, G.; Morales-Rojas, H.; Hopfl, H.; MacGillivray, L.R.; Tivanski, A.V. Semiconductor Cocrystals Based on Boron: Generated Electrical Response with π-Rich Aromatic Molecules. Cryst. Growth Des. 2020, 20, 3–8. [Google Scholar] [CrossRef]
- Icli, B.; Solari, E.; Kilbas, B.; Scopelliti, R.; Severin, K. Multicomponent Assembly of Macrocycles and Polymers by Coordination of Pyridyl Ligands to 1,4-Bis(benzodioxaborole)benzene. Chem. Eur. J. 2012, 18, 14867–14874. [Google Scholar] [CrossRef]
- Christinat, N.; Scopelliti, R.; Severin, K. Boron-based rotaxanes by multicomponent self-assembly. Chem. Commun. 2008, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Hartwick, C.J.; Yelgaonkar, S.P.; Reinheimer, E.W.; Campillo-Alvarado, G.; MacGillivray, L.R. Self-Assembly of Diboronic Esters with U-Shaped Bipyridines: “Plugin-Socket” Assemblies. Cryst. Growth Des. 2021, 21, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Christinat, N.; Croisier, E.; Scopelliti, R.; Cascella, M.; Rothlisberger, U.; Severin, K. Formation of boronate ester polymers with efficient intrastrand charge-transfer transitions by three-component reactions. Eur. J. Inorg. Chem. 2007, 2007, 5177–5181. [Google Scholar] [CrossRef]
- Herrera-España, A.D.; Campillo-Alvarado, G.; Román-Bravo, P.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H. Selective Isolation of Polycyclic Aromatic Hydrocarbons by Self-Assembly of a Tunable N→B Clathrate. Cryst. Growth Des. 2015, 15, 1572–1576. [Google Scholar] [CrossRef]
- Dhara, A.; Beuerle, F. Reversible Assembly of a Supramolecular Cage Linked by Boron–Nitrogen Dative Bonds. Chem. Eur. J. 2015, 21, 17391–17396. [Google Scholar] [CrossRef]
- Icli, B.; Sheepwash, E.; Riis-Johannessen, T.; Schenk, K.; Filinchuk, Y.; Scopelliti, R.; Severin, K. Dative boron–nitrogen bonds in structural supramolecular chemistry: Multicomponent assembly of prismatic organic cages. Chem. Sci. 2011, 2, 1719–1721. [Google Scholar]
- Campillo-Alvarado, G.; Vargas-Olvera, E.C.; Hopfl, H.; Herrera-Espana, A.D.; Sanchez-Guadarrama, O.; Morales-Rojas, H.; MacGillivray, L.R.; Rodriguez-Molina, B.; Farfan, N. Self-Assembly of Fluorinated Boronic Esters and 4,4′-Bipyridine into 2:1 N→B Adducts and Inclusion of Aromatic Guest Molecules in the Solid State: Application for the Separation of o,m,p-Xylene. Cryst. Growth Des. 2018, 18, 2726–2743. [Google Scholar] [CrossRef]
- Herrera-Espana, A.D.; Hoepfl, H.; Morales-Rojas, H. Boron-Nitrogen Double Tweezers Comprising Arylboronic Esters and Diamines: Self-Assembly in Solution and Adaptability as Hosts for Aromatic Guests in the Solid State. ChemPlusChem 2020, 85, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Manankandayalage, C.P.; Unruh, D.K.; Krempner, C. Boronic, diboronic and boric acid esters of 1,8-naphthalenediol–synthesis, structure and formation of boronium salts. Dalton Trans. 2020, 49, 4834–4842. [Google Scholar] [CrossRef]
- Bruker (2021) SADABS v2016/2, Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- CrysAlisPRO. Oxford Diffraction; Agilent Technologies UK Ltd.: Yarnton, UK, 2018. [Google Scholar]
- SCALE3 ABSPACK, Oxford Diffraction Ltd.: Abingdon, UK, 2005.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


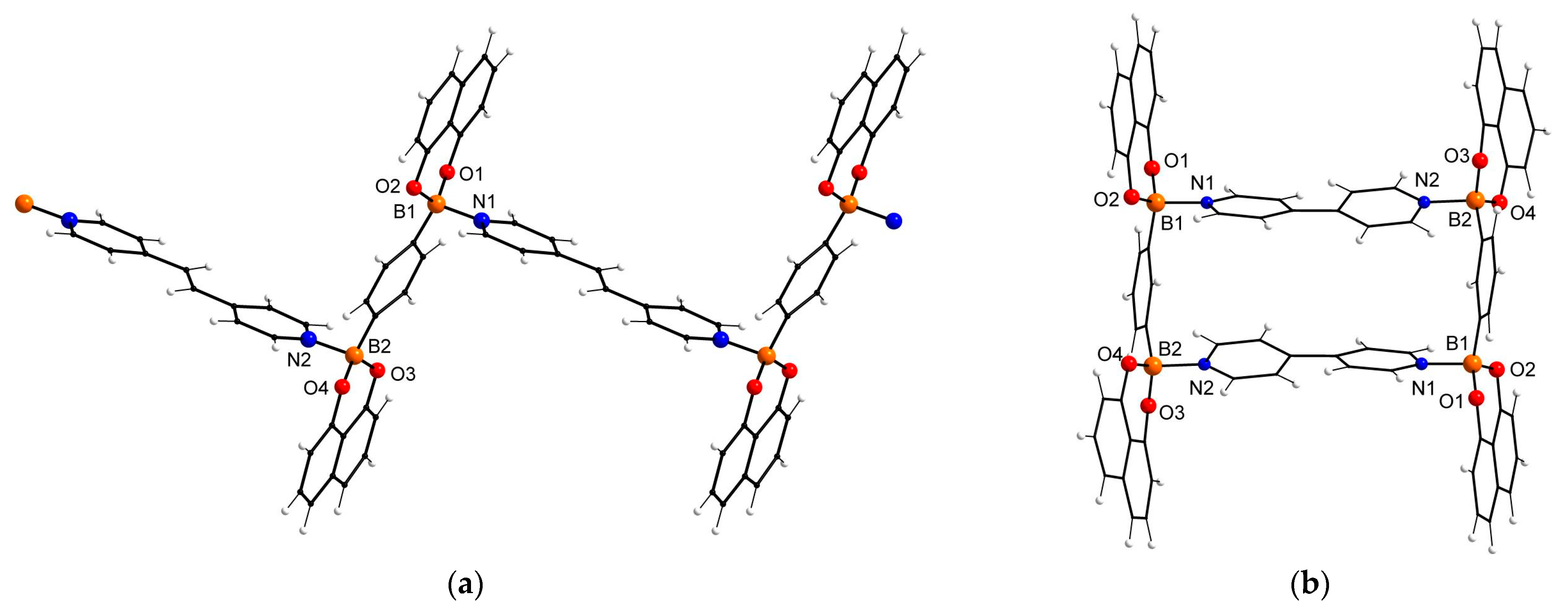


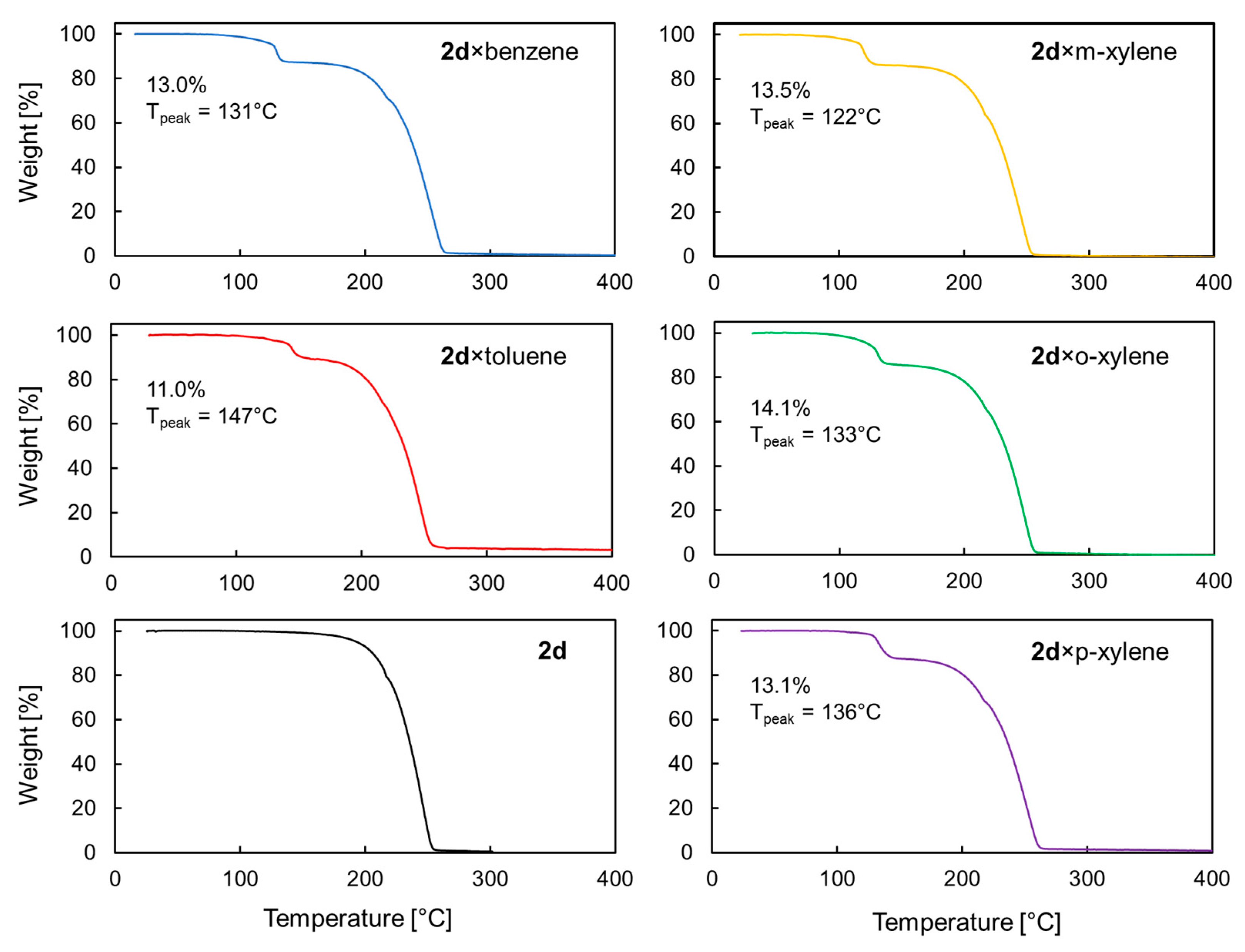
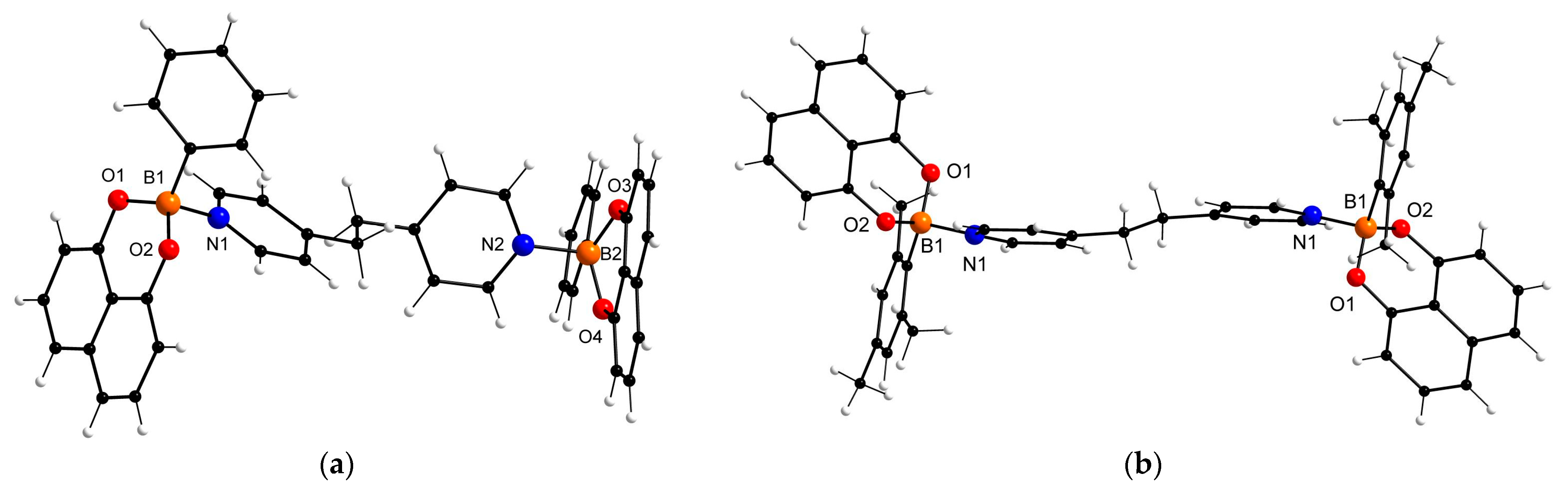
| 2d × Solvent | 2d/Solvent Ratio (from X-ray Data) | 2d/Solvent Ratio (from 1H NMR Data) 1 |
|---|---|---|
| 2d × benzene | 2:3 | 1:1.5 |
| 2d × toluene | 1:1 | 1:1 |
| 2d × o-xylene | 1:2 | 1:1 |
| 2d × m-xylene 2 | 3:6 3 and 1:1 | 1:1 |
| 2d × p-xylene | 1:1 | 1:1 |
| 2d × mesitylene | 1:2 4 | 1:2.66 |
| 2d × aniline | 2:3 | 1:1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manankandayalage, C.P.; Unruh, D.K.; Perry, R.; Krempner, C. 1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons. Molecules 2023, 28, 5394. https://doi.org/10.3390/molecules28145394
Manankandayalage CP, Unruh DK, Perry R, Krempner C. 1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons. Molecules. 2023; 28(14):5394. https://doi.org/10.3390/molecules28145394
Chicago/Turabian StyleManankandayalage, Chamila P., Daniel K. Unruh, Ryan Perry, and Clemens Krempner. 2023. "1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons" Molecules 28, no. 14: 5394. https://doi.org/10.3390/molecules28145394
APA StyleManankandayalage, C. P., Unruh, D. K., Perry, R., & Krempner, C. (2023). 1,8-Dihydroxy Naphthalene—A New Building Block for the Self-Assembly with Boronic Acids and 4,4′-Bipyridine to Stable Host–Guest Complexes with Aromatic Hydrocarbons. Molecules, 28(14), 5394. https://doi.org/10.3390/molecules28145394