Synthesis, Reactivity and Coordination Chemistry of Group 9 PBP Boryl Pincer Complexes: [(PBP)M(PMe3)n] (M = Co, Rh, Ir; n = 1, 2)
Abstract
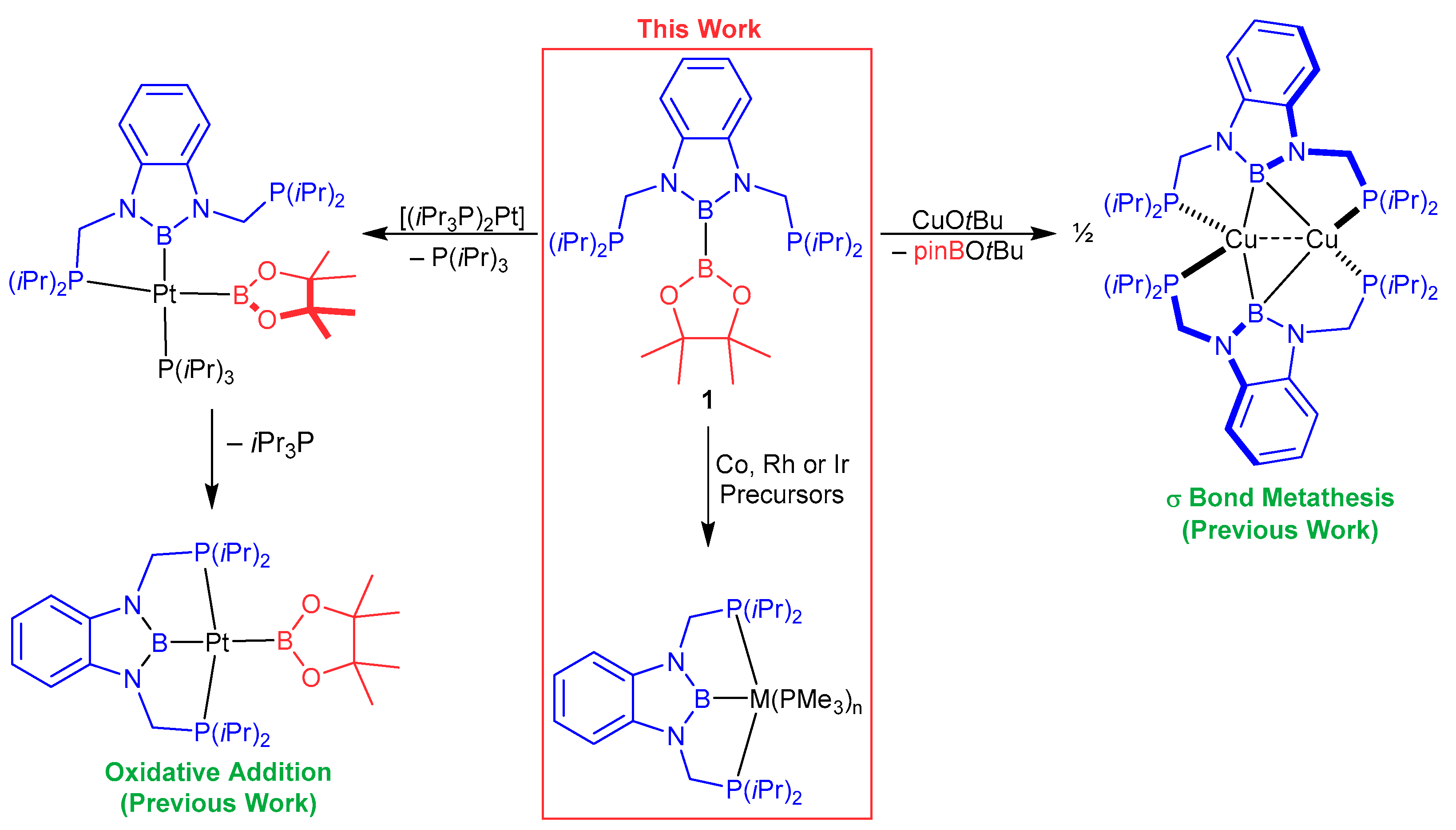
1. Introduction
2. Results
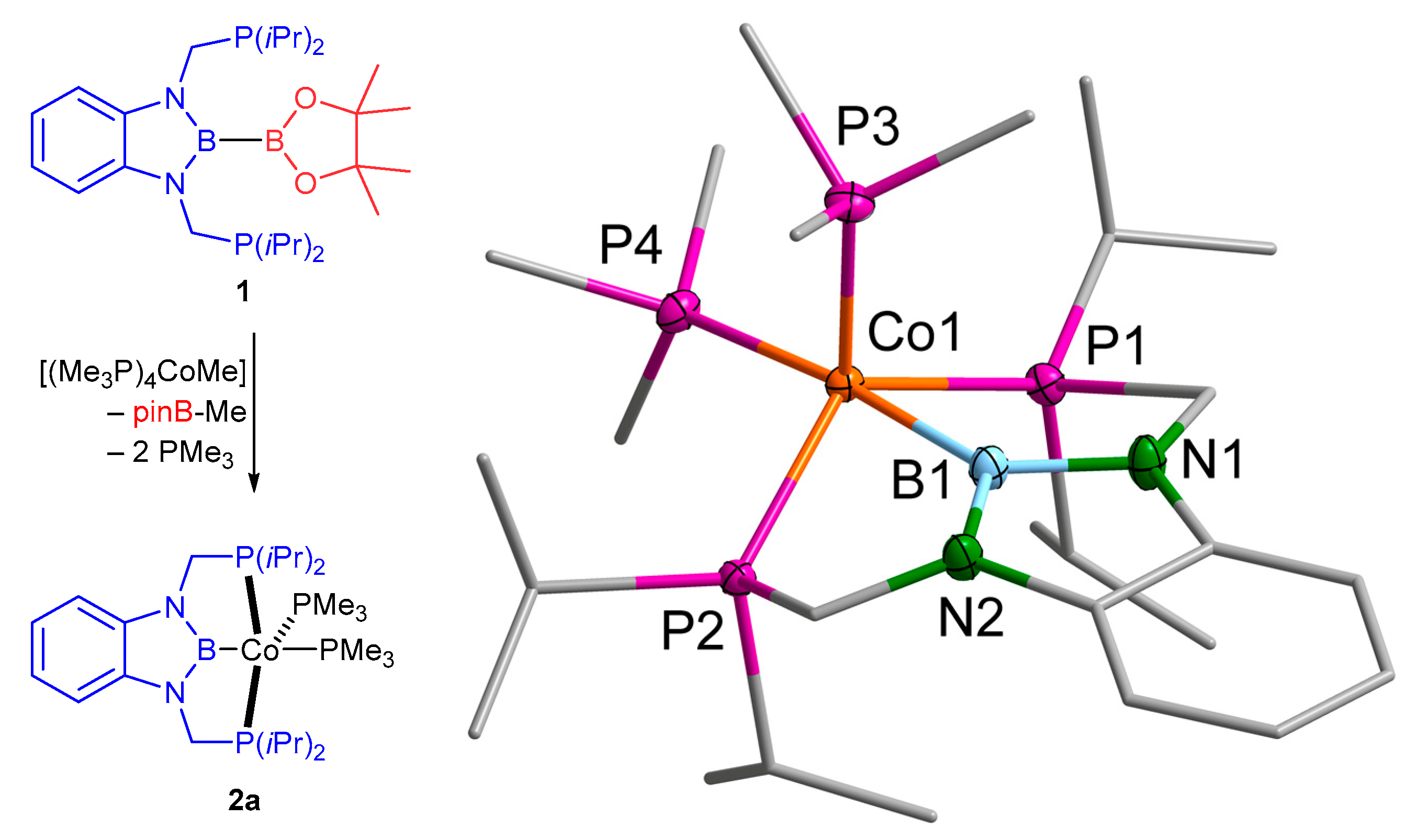
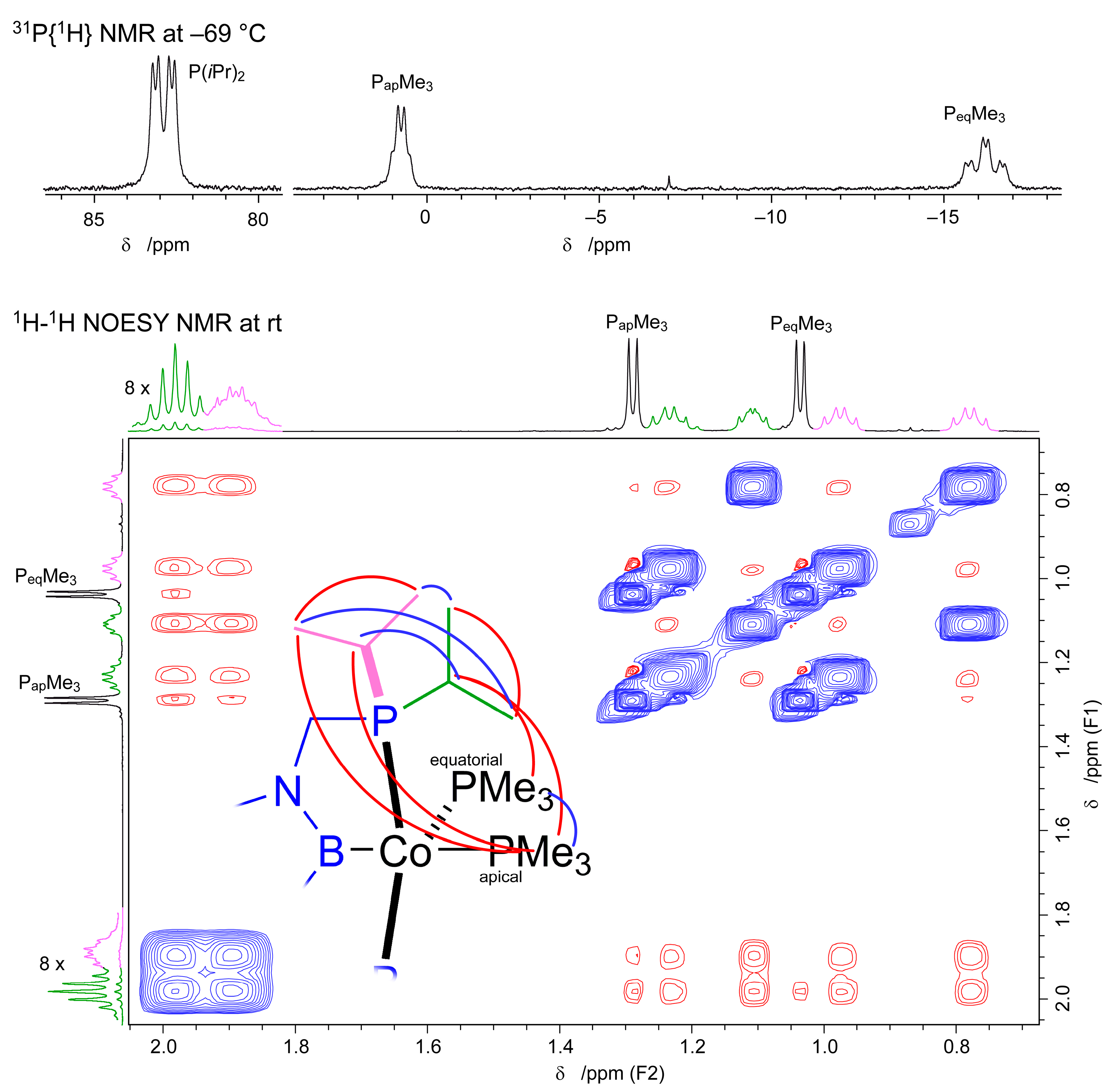
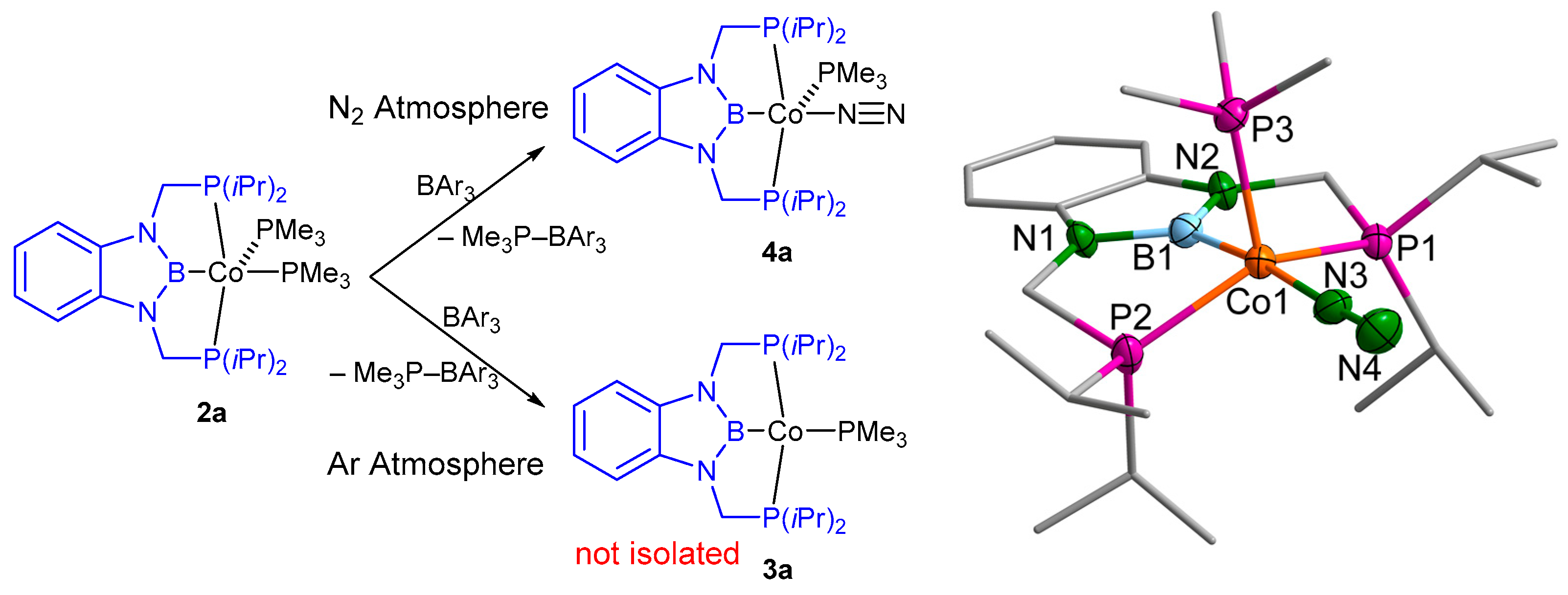
2.1. Cobalt Complexes
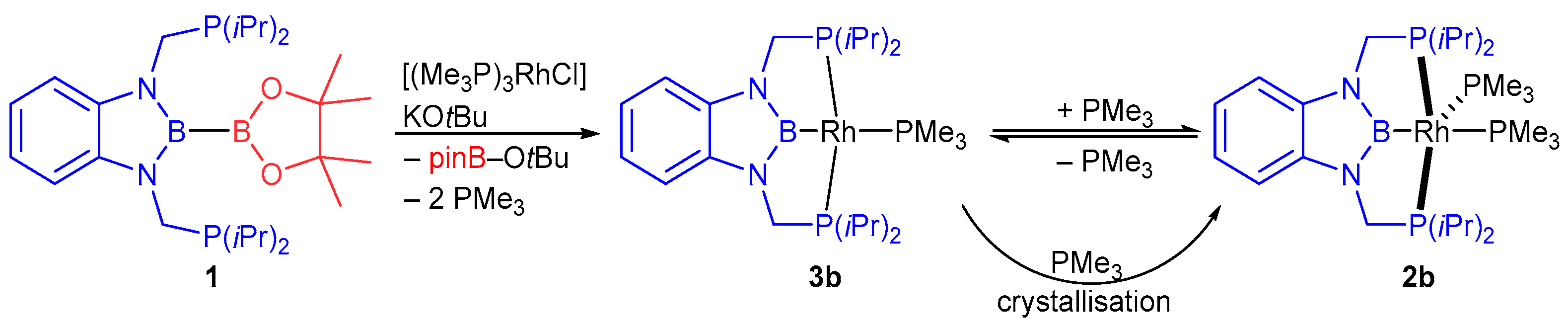
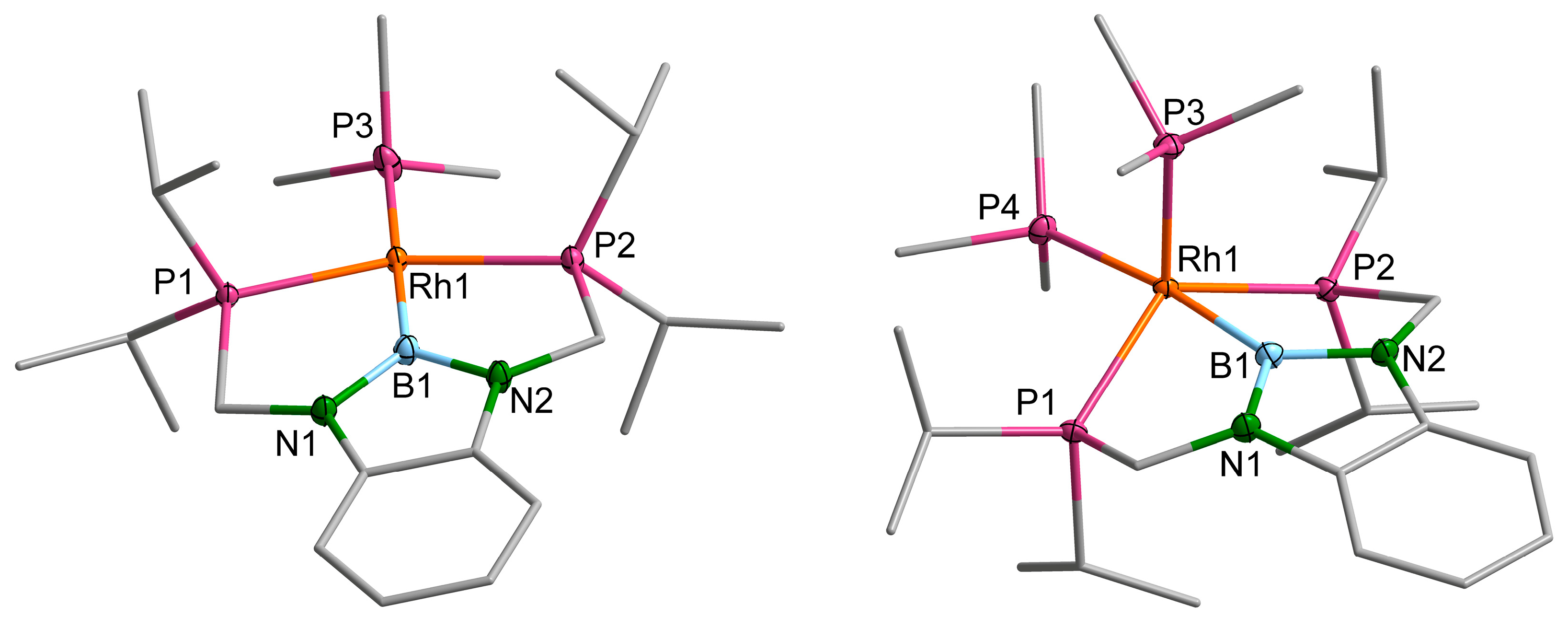
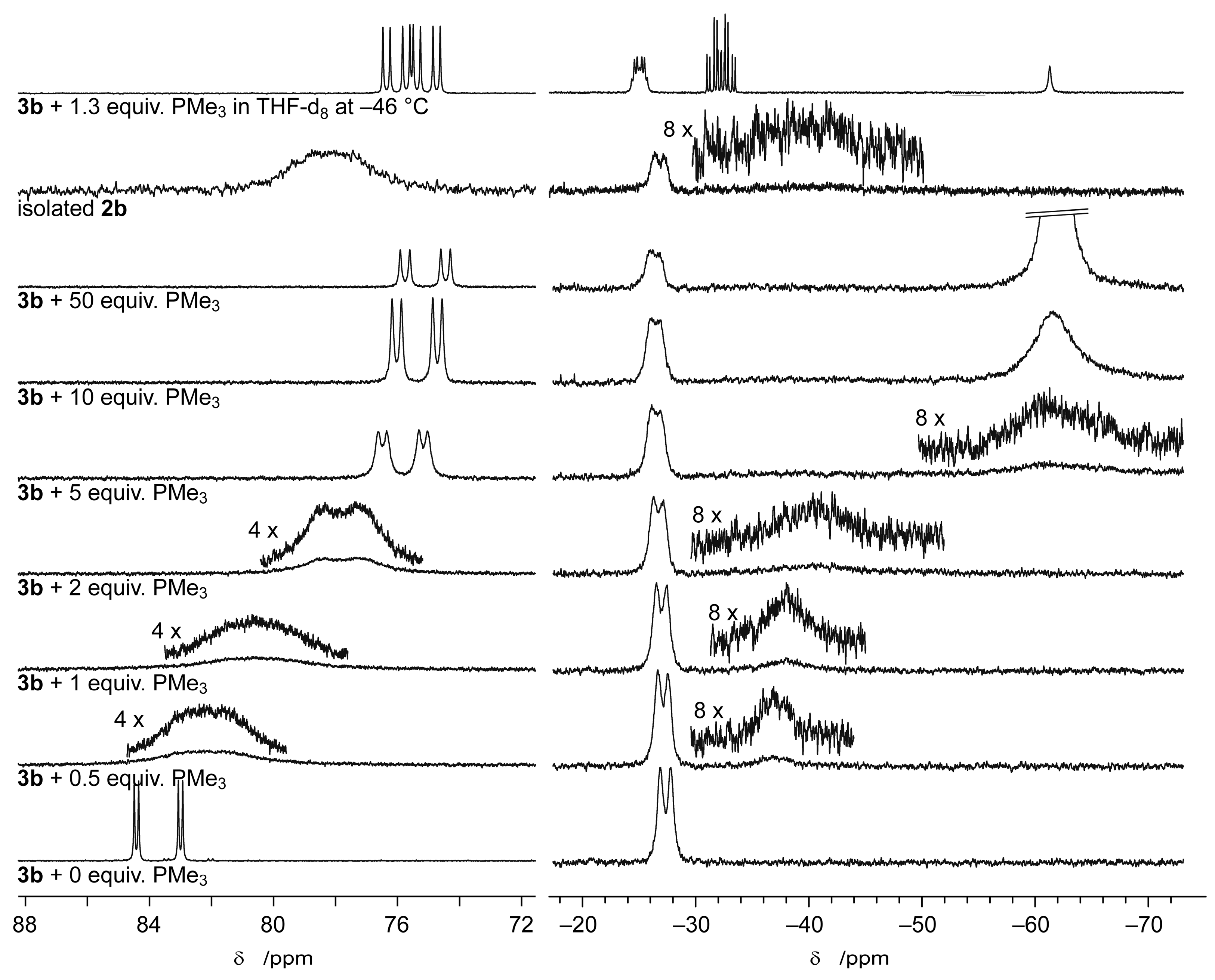
2.2. Rhodium Complexes
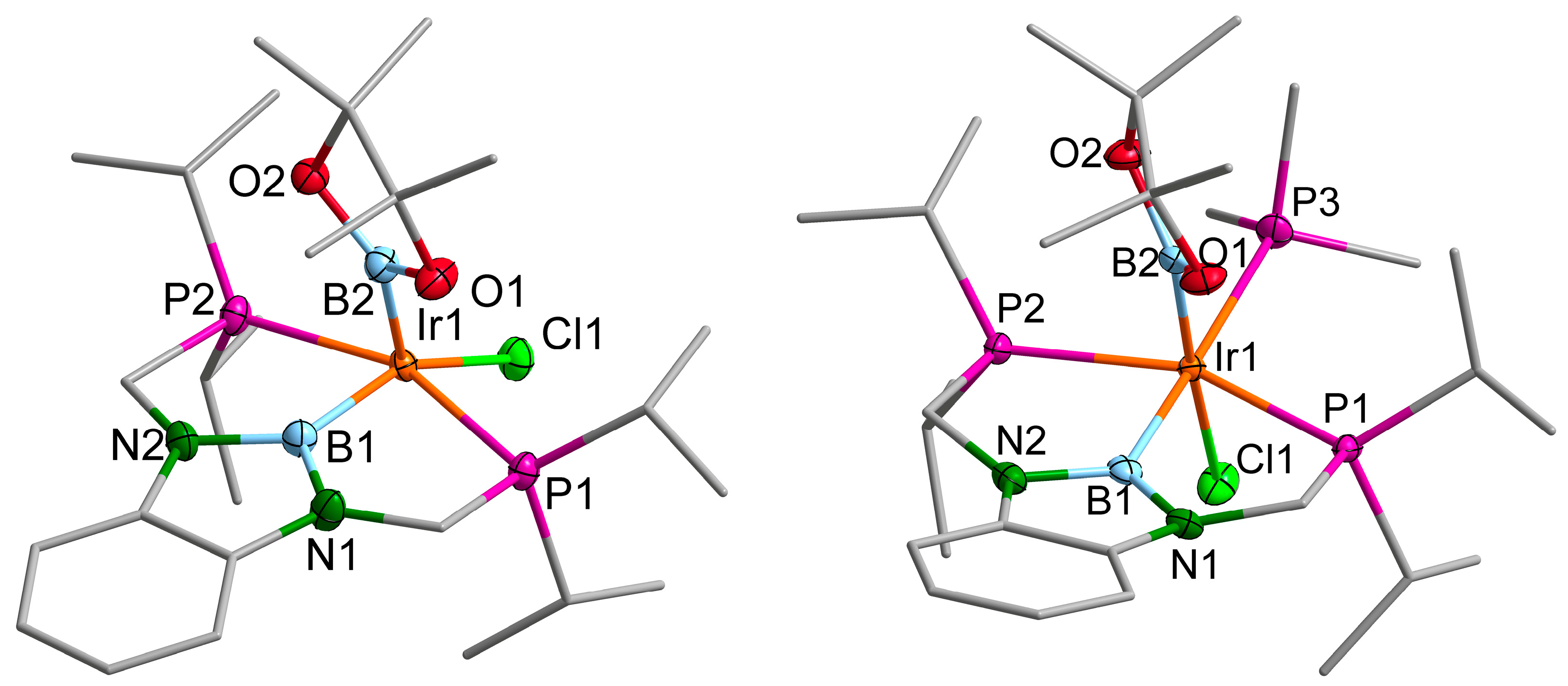
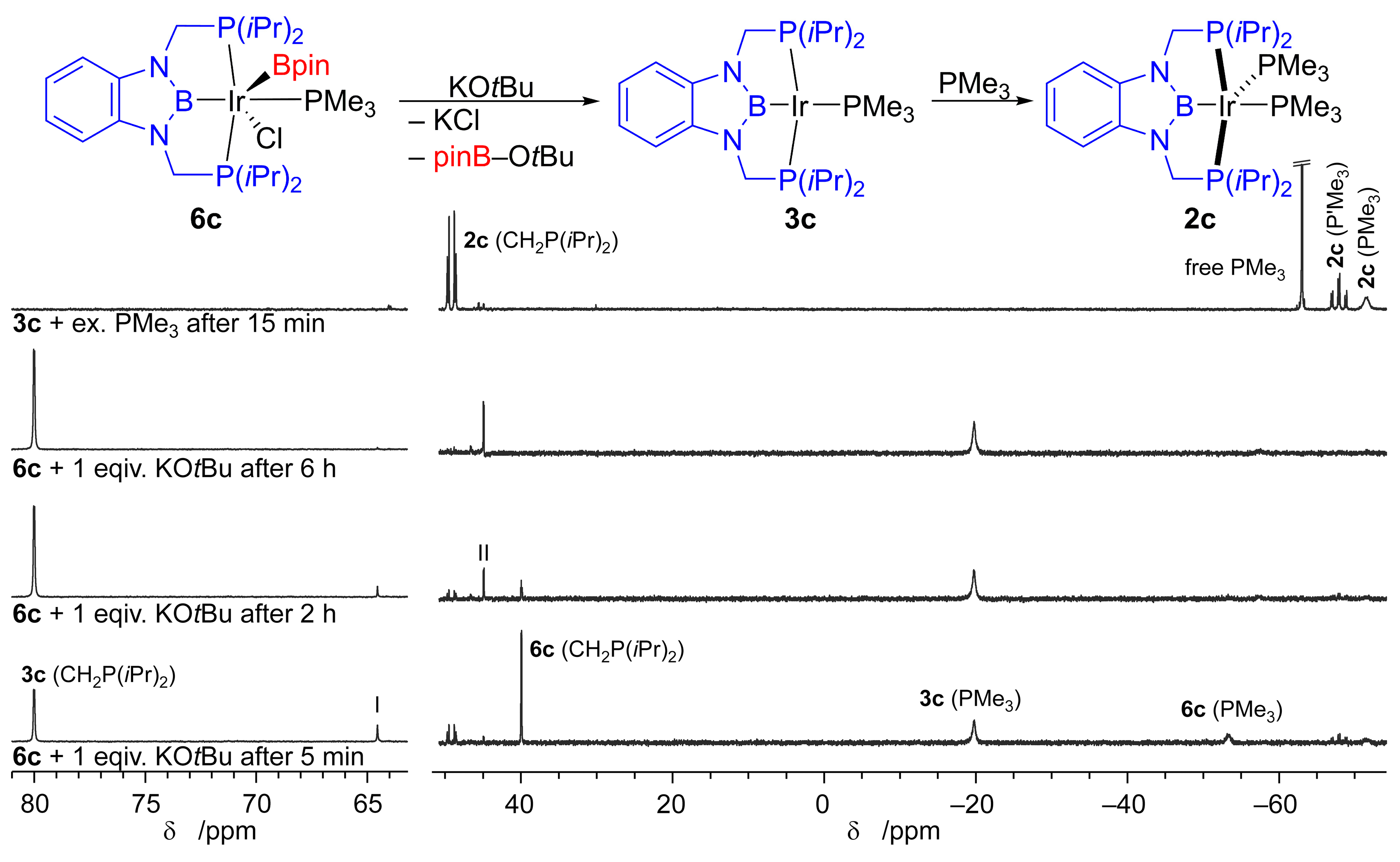
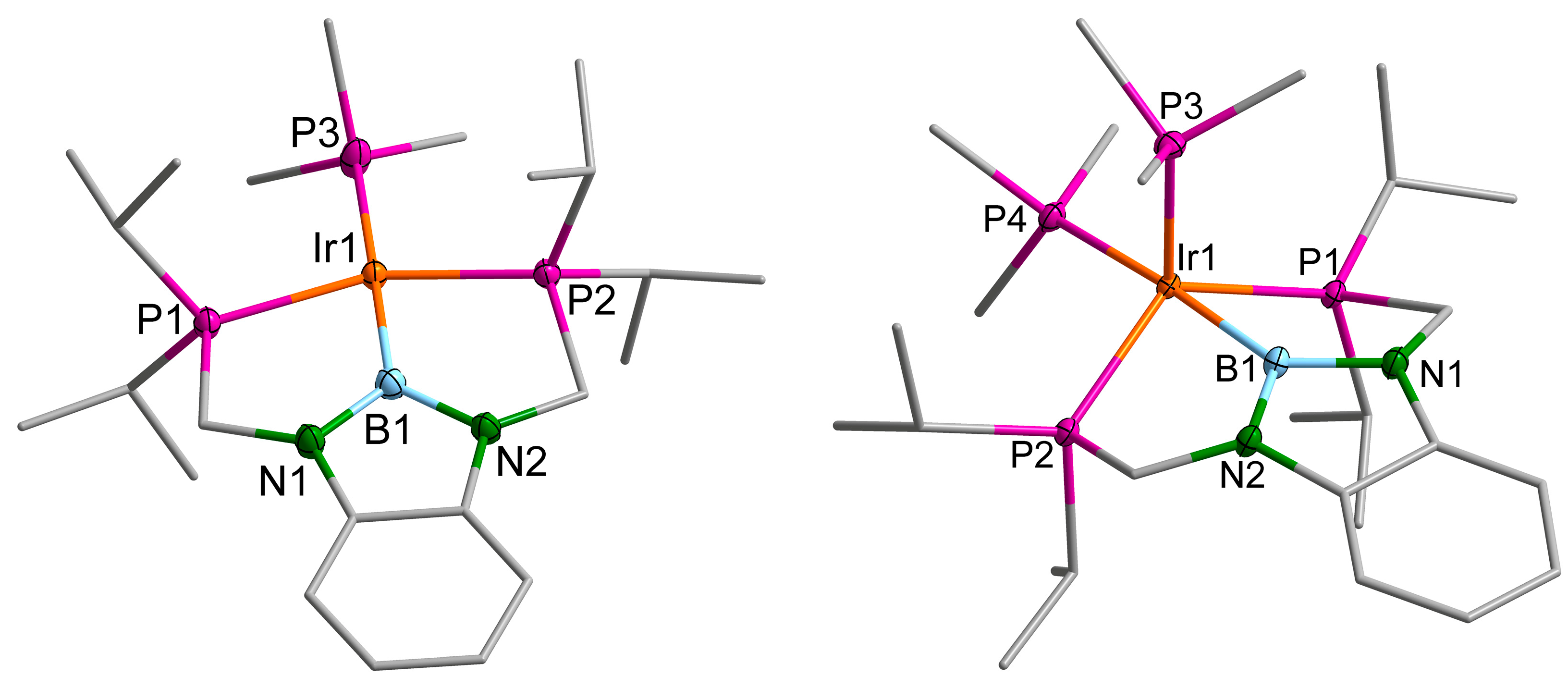
2.3. Iridium Complexes
3. Discussion
4. Materials and Methods
4.1. General Considerations
4.2. Experimental Procedures and Analysis Data
4.2.1. [(d(CH2P(iPr)2)abB)Co(PMe3)2] (2a)
4.2.2. [(d(CH2P(iPr)2)abB)Co(N2)(PMe3)] (4a)
4.2.3. [(d(CH2P(iPr)2)abB)Rh(PMe3)2] (2b)
4.2.4. [(d(CH2P(iPr)2)abB)Rh(PMe3)] (3b)
4.2.5. [(d(CH2P(iPr)2)abB)Ir(PMe3)2] (2c))
4.2.6. [(d(CH2P(iPr)2)abB)Ir(PMe3)] (3c)
4.2.7. [(d(CH2P(iPr)2)abB)IrCl(Bpin)] (5c)
4.2.8. [(d(CH2P(iPr)2)abB)IrCl(Bpin)(PMe3)] (6c)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Segawa, Y.; Yamashita, M.; Nozaki, K. Syntheses of PBP Pincer Iridium Complexes: A Supporting Boryl Ligand. J. Am. Chem. Soc. 2009, 131, 9201–9203. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Yamashita, M.; Nozaki, K. Diphenylphosphino- or Dicyclohexylphosphino-Tethered Boryl Pincer Ligands: Syntheses of PBP Iridium(III) Complexes and Their Conversion to Iridium-Ethylene Complexes. Organometallics 2009, 28, 6234–6242. [Google Scholar] [CrossRef]
- Lin, T.-P.; Peters, J.C. Boryl-Mediated Reversible H2 Activation at Cobalt: Catalytic Hydrogenation, Dehydrogenation, and Transfer Hydrogenation. J. Am. Chem. Soc. 2013, 135, 15310–15313. [Google Scholar] [CrossRef]
- van der Vlugt, J.I. Boryl-Based Pincer Systems: New Avenues in Boron Chemistry. Angew. Chem. Int. Ed. 2010, 49, 252–255. [Google Scholar] [CrossRef]
- Hasegawa, M.; Segawa, Y.; Yamashita, M.; Nozaki, K. Isolation of a PBP-Pincer Rhodium Complex Stabilized by an Intermolecular C–H σ Coordination as the Fourth Ligand. Angew. Chem. Int. Ed. 2012, 51, 6956–6960. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-P.; Peters, J.C. Boryl−Metal Bonds Facilitate Cobalt/Nickel-Catalyzed Olefin Hydrogenation. J. Am. Chem. Soc. 2014, 136, 13672–13683. [Google Scholar] [CrossRef]
- Tanoue, K.; Yamashita, M. Synthesis of Pincer Iridium Complexes Bearing a Boron Atom and iPr-Substituted Phosphorus Atoms: Application to Catalytic Transfer Dehydrogenation of Alkanes. Organometallics 2015, 34, 4011–4017. [Google Scholar] [CrossRef]
- Vondung, L.; Frank, N.; Fritz, M.; Alig, L.; Langer, R. Phosphine-Stabilized Borylenes and Boryl Anions as Ligands? Redox Reactivity in Boron-Based Pincer Complexes. Angew. Chem. Int. Ed. 2016, 55, 14450–14454. [Google Scholar] [CrossRef]
- Lai, Q.; Bhuvanesh, N.; Ozerov, O.V. Unexpected B/Al Transelementation within a Rh Pincer Complex. J. Am. Chem. Soc. 2020, 142, 20920–20923. [Google Scholar] [CrossRef]
- Shih, W.-C.; Gu, W.; MacInnis, M.C.; Timpa, S.D.; Bhuvanesh, N.; Zhou, J.; Ozerov, O.V. Facile Insertion of Rh and Ir into a Boron−Phenyl Bond, Leading to Boryl/Bis(phosphine) PBP Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 2086–2089. [Google Scholar] [CrossRef]
- Shih, W.-C.; Ozerov, O.V. Synthesis and Characterization of PBP Pincer Iridium Complexes and Their Application in Alkane Transfer Dehydrogenation. Organometallics 2017, 36, 228–233. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Reuter, M.G.; Stern, C.L.; Ratner, M.A.; Seideman, T.; Mirkin, C.A. Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS Pd(II) Complexes. J. Am. Chem. Soc. 2009, 131, 9482–9483. [Google Scholar] [CrossRef] [PubMed]
- El-Zaria, M.E.; Arii, H.; Nakamura, H. m-Carborane-Based Chiral NBN Pincer-Metal Complexes: Synthesis, Structure, and Application in Asymmetric Catalysis. Inorg. Chem. 2011, 50, 4149–4161. [Google Scholar] [CrossRef]
- Eleazer, B.J.; Smith, M.D.; Popov, A.A.; Peryshkov, D.V. (BB)-Carboryne Complex of Ruthenium: Synthesis by Double B−H Activation at a Single Metal Center. J. Am. Chem. Soc. 2016, 138, 10531–10538. [Google Scholar] [CrossRef]
- Rutz, P.M.; Grunenberg, J.; Kleeberg, C. Unsymmetrical Diborane(4) as a Precursor to PBP Boryl Pincer Complexes: Synthesis and Cu(I) and Pt(II) PBP Complexes with Unusual Structural Features. Organometallics 2022, 41, 3044–3054. [Google Scholar] [CrossRef]
- Klein, H.-F.; Karsch, H.H. Methylkobaltverbindungen mit nicht chelatisierenden Liganden, I. Methyltetrakis(trimethylphosphin)kobalt und seine Derivate. Chem. Berichte 1975, 108, 944–955. [Google Scholar] [CrossRef]
- Jones, R.A.; Real, F.M.; Wilkinson, G.; Galas, A.M.R.; Hursthouse, M.B.; Malik, K.M.A. Synthesis of trimethylphosphine complexes of rhodium and ruthenium. X-Ray crystal structures of tetrakis(trimethylphosphine)rhodium(I) chloride and chlorotris(trimethylphosphine)rhodium(I). J. Chem. Soc. Dalton Trans. 1980, 511–518. [Google Scholar] [CrossRef]
- Choudhury, J.; Podder, S.; Roy, S. Cooperative Friedel−Crafts Catalysis in Heterobimetallic Regime: Alkylation of Aromatics by π-Activated Alcohols. J. Am. Chem. Soc. 2005, 127, 6162–6163. [Google Scholar] [CrossRef]
- Adams, C.J.; Baber, R.A.; Batsanov, A.S.; Bramham, G.; Charmant, J.P.H.; Haddow, M.F.; Howard, J.A.K.; Lam, W.H.; Lin, Z.; Marder, T.B.; et al. Synthesis and reactivity of cobalt boryl complexes. Dalton Trans. 2006, volume, 1370–1373. [Google Scholar] [CrossRef]
- Borner, C.; Brandhorst, K.; Kleeberg, C. Selective B–B Bond Activation in an Unsymmetrical Diborane(4) by [(Me3P)4Rh–X] (X = Me, OtBu): A Switch of Mechanism? Dalton Trans. 2015, 44, 8600–8604. [Google Scholar] [CrossRef]
- Drescher, W.; Schmitt-Monreal, D.; Jacob, C.R.; Kleeberg, C. [(Me3P)3Co(Bcat)3]: Equilibrium Oxidative Addition of a B–B Bond and Interconversion between the fac-Tris-Boryl and the mer-Tris-Boryl Complex. Organometallics 2020, 39, 538–543. [Google Scholar] [CrossRef]
- Assefa, M.K.; Devera, J.L.; Brathwaite, A.D.; Mosley, J.D.; Duncan, M.A. Vibrational scaling factors for transition metal carbonyls. Chem. Phys. Lett. 2015, 640, 175–179. [Google Scholar] [CrossRef]
- Grunenberg, J. Ill-defined concepts in chemistry: Rigid force constants vs. compliance constants as bond strength descriptors for the triple bond in diboryne. Chem. Sci. 2015, 6, 4086–4088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Castillo, T.J.; Thompson, N.B.; Suess, D.L.M.; Ung, G.; Peters, J.C. Evaluating Molecular Cobalt Complexes for the Conversion of N2 to NH3. Inorg. Chem. 2015, 54, 9256–9262. [Google Scholar] [CrossRef]
- Schubert, H.; Leis, W.; Mayer, H.A.; Wesemann, L. A bidentate boryl ligand: Syntheses of platinum and iridium complexes. Chem. Commun. 2014, 50, 2738–2740. [Google Scholar] [CrossRef]
- Clegg, W.; Lawlor, F.J.; Marder, T.B.; Nguyen, P.; Norman, N.C.; Orpen, A.G.; Quayle, M.J.; Rice, C.R.; Robins, E.G.; Scott, A.J.; et al. Boron–boron bond oxidative addition to rhodium(I) and iridium(I) centres. J. Chem. Soc. Dalton Trans. 1998, 301–309. [Google Scholar] [CrossRef]
- Press, L.P.; Kosanovich, A.J.; McCulloch, B.J.; Ozerov, O.V. High-Turnover Aromatic C–H Borylation Catalyzed by POCOP-Type Pincer Complexes of Iridium. J. Am. Chem. Soc. 2016, 138, 9487–9497. [Google Scholar] [CrossRef]
- Lee, C.I.; DeMott, J.C.; Pell, C.J.; Christopher, A.; Zhou, J.; Bhuvanesh, N.; Ozerov, O.V. Ligand survey results in identification of PNP pincer complexes of iridium as long-lived and chemoselective catalysts for dehydrogenative borylation of terminal alkynes. Chem. Sci. 2015, 6, 6572–6582. [Google Scholar] [CrossRef]
- Foley, B.J.; Bhuvanesh, N.; Zhou, J.; Ozerov, O.V. Combined Experimental and Computational Studies of the Mechanism of Dehydrogenative Borylation of Terminal Alkynes Catalyzed by PNP Complexes of Iridium. ACS Catal. 2020, 10, 9824–9836. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, Z.; Marder, T.B. Trans Influence of Boryl Ligands and Comparison with C, Si, and Sn Ligands. Inorg. Chem. 2005, 44, 9384–9390. [Google Scholar] [CrossRef]
- Chotana, G.A.; Vanchura, B.A., II; Tse, M.K.; Staples, R.J.; Maleczka, R.E., Jr.; Smith, M.R., III. Getting the sterics just right: A five-coordinate iridium trisboryl complex that reacts with C–H bonds at room temperature. Chem. Commun. 2009, 5731–5733. [Google Scholar] [CrossRef] [PubMed]
- Putz, H.; Brandenburg, K. Cole Research Group. 11B NMR Chemical Shifts; SDSU Department of Chemistry & Biochemistr: San Diego, CA, USA, 2015. [Google Scholar]
- Price, R.T.; Andersen, R.A.; Muetterties, E.L. Arene C-H bond activation: Reaction of (Me3P)3Rh(Me) with toluene to give (Me3P)3Rh(Ar) where Ar is o-, m- and p-tolyl. J. Organomet. Chem. 1989, 376, 407–417. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Stalke, D. Cryo crystal structure determination and application to intermediates. Chem. Soc. Rev. 1998, 27, 171–178. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysalisPro, Version 1.171.40.84–1.171.41.122; Agilent Technologies: Santa Clara, CA, USA, 2020–2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization, Crystal Impact; GbR: Bonn, Germany, 2018. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta-Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401-1–146401-4. [Google Scholar] [CrossRef] [PubMed]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Brandhorst, K.; Grunenberg, J. Efficient computation of compliance matrices in redundant internal coordinates from Cartesian Hessians for nonstationary points. J. Chem. Phys. 2010, 132, 184101-1–184101-7. [Google Scholar] [CrossRef]
- Brandhorst, K.; Grunenberg, J. How strong is it? The interpretation of force and compliance constants as bond strength descriptors. Chem. Soc. Rev. 2008, 37, 1558–1567. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutz, P.M.; Grunenberg, J.; Kleeberg, C. Synthesis, Reactivity and Coordination Chemistry of Group 9 PBP Boryl Pincer Complexes: [(PBP)M(PMe3)n] (M = Co, Rh, Ir; n = 1, 2). Molecules 2023, 28, 6191. https://doi.org/10.3390/molecules28176191
Rutz PM, Grunenberg J, Kleeberg C. Synthesis, Reactivity and Coordination Chemistry of Group 9 PBP Boryl Pincer Complexes: [(PBP)M(PMe3)n] (M = Co, Rh, Ir; n = 1, 2). Molecules. 2023; 28(17):6191. https://doi.org/10.3390/molecules28176191
Chicago/Turabian StyleRutz, Philipp M., Jörg Grunenberg, and Christian Kleeberg. 2023. "Synthesis, Reactivity and Coordination Chemistry of Group 9 PBP Boryl Pincer Complexes: [(PBP)M(PMe3)n] (M = Co, Rh, Ir; n = 1, 2)" Molecules 28, no. 17: 6191. https://doi.org/10.3390/molecules28176191
APA StyleRutz, P. M., Grunenberg, J., & Kleeberg, C. (2023). Synthesis, Reactivity and Coordination Chemistry of Group 9 PBP Boryl Pincer Complexes: [(PBP)M(PMe3)n] (M = Co, Rh, Ir; n = 1, 2). Molecules, 28(17), 6191. https://doi.org/10.3390/molecules28176191





