Review of the Role of Nanotechnology in Overcoming the Challenges Faced in Oral Cancer Diagnosis and Treatment
Abstract
1. Introduction
2. Oral Carcinogenesis
3. Challenges in OC Diagnosis
4. Challenges in OC Treatment
5. Nanotechnology
6. Nanoparticles
6.1. Types of NPs
6.2. Properties of NPs
7. Cancer Nanotechnology
8. NPs for OC Therapy
Liposomes for OC Diagnosis and Therapy
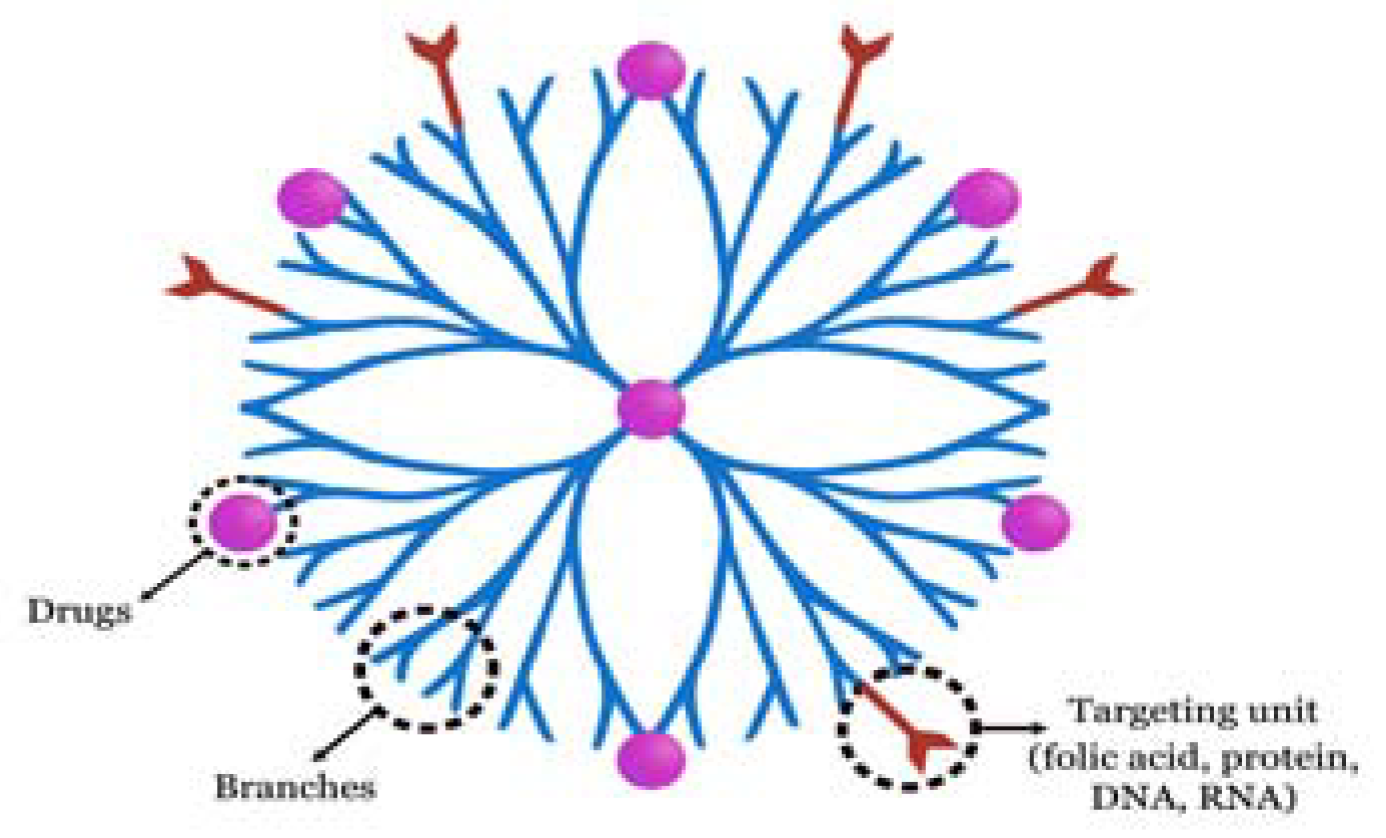
9. Dendrimers for OC Diagnosis and Therapy
10. Magnetic NPs for OC Diagnosis and Therapy
11. Quantum Dots for OC Diagnosis and Therapy
12. Nanotechnology-Based Carriers for OC Therapy
13. DNA-Based Molecular NPs
14. Cyclodextrins for OC Therapy
15. Nanolipids for OC Therapy
16. Peptides/Proteins for OC Therapy
17. Virus-like Particles for OC Therapy
18. Future Perspective
19. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Treatment of Oral Cancer: A Review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishigamori, R. Understanding Carcinogenesis for Fighting Oral Cancer. J. Oncol. 2011, 2011, 603740. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.A.; Brennan, M.T. Impact of Oral Cancer on Quality of Life. Dent. Clin. N. Am. 2018, 62, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global Epidemiology of Oral and Oropharyngeal Cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Iyer, N.G.; Tan, M.H.; Edgren, G. Changing Epidemiology of Oral Squamous Cell Carcinoma of the Tongue: A Global Study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Arakeri, G.; Patil, S.G.; Aljabab, A.S.; Lin, K.C.; Merkx, M.A.W.; Gao, S.; Brennan, P.A. Oral Submucous Fibrosis: An Update on Pathophysiology of Malignant Transformation. J. Oral Pathol. Med. 2017, 46, 413–417. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, S.; Christianson, L.; Rehman, S.; Ekwunife, O.; Samkange-Zeeb, F. Smokeless Tobacco and Oral Potentially Malignant Disorders in South Asia: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2017, 20, 12–21. [Google Scholar] [CrossRef]
- Balasubramaniam, A.M.; Sriraman, R.; Sindhuja, P.; Mohideen, K.; Parameswar, R.A.; Muhamed Haris, K.T. Autofluorescence Based Diagnostic Techniques for Oral Cancer. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 2), S374–S377. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Rahimi-Nedjat, R.K.; Ziebart, T.; Bemsch, A.; Walter, C.; Al-Nawas, B.; Koch, F.P. A Chemiluminescent Light System in Combination with Toluidine Blue to Assess Suspicious Oral Lesions-Clinical Evaluation and Review of the Literature. Clin. Oral Investig. 2015, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Benergossi, J.; Calixto, G.; Fonseca-Santos, B.; Aida, K.L.; de Cássia Negrini, T.; Duque, C.; Gremião, M.P.; Chorilli, M. Highlights in Peptide Nanoparticle Carriers Intended to Oral Diseases. Curr. Top. Med. Chem. 2015, 15, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, X.; Zeng, X.; Dan, H.; Chen, Q. Non-invasive Techniques for Detection and Diagnosis of Oral Potentially Malignant Disorders. Tohoku J. Exp. Med. 2016, 238, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, V.; Paderni, C.; Campisi, G. Novel Non-invasive Adjunctive Techniques for Early Oral Cancer Diagnosis and Oral Lesions Examination. Curr. Pharm. Des. 2012, 18, 5442–5451. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Zhang, L.; Rosin, M. Advances in the Diagnosis of Oral Premalignant and Malignant Lesions. J. Can. Dent. Assoc. 2002, 68, 617–621. [Google Scholar]
- Gharat, S.A.; Momin, M.; Bhavsar, C. Oral Squamous Cell Carcinoma: Current Treatment Strategies and Nanotechnology-Based Approaches for Prevention and Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef]
- Bao, C.; Conde, J.; Curtin, J.; Artzi, N.; Tian, F.; Cui, D. Bioresponsive Antisense DNA Gold Nanobeacons as a Hybrid In Vivo Theranostics Platform for the Inhibition of Cancer Cells and Metastasis. Sci. Rep. 2015, 5, 12297. [Google Scholar] [CrossRef]
- Han, Y.; An, Y.; Jia, G.; Wang, X.; He, C.; Ding, Y.; Tang, Q. Theranostic Micelles Based on Upconversion Nanoparticles for Dual-Modality Imaging and Photodynamic Therapy in Hepatocellular Carcinoma. Nanoscale 2018, 10, 6511–6523. [Google Scholar] [CrossRef]
- Halo, T.L.; McMahon, K.M.; Angeloni, N.L.; Xu, Y.; Wang, W.; Chinen, A.B.; Malin, D.; Strekalova, E.; Cryns, V.L.; Cheng, C.; et al. NanoFlares for the Detection, Isolation, and Culture of Live Tumor Cells from Human Blood. Proc. Natl. Acad. Sci. USA 2014, 111, 17104–17109. [Google Scholar] [CrossRef]
- Ho, D.; Wang, C.H.; Chow, E.K. Nanodiamonds: The Intersection of Nanotechnology, Drug Development, and Personalized Medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Liu, K.; Huo, Z.J.; Li, X.C.; Wang, M.; Liu, P.; Pang, B.; Wang, S.J. A Cell-Targeted Chemotherapeutic Nanomedicine Strategy for Oral Squamous Cell Carcinoma Therapy. J. Nanobiotechnol. 2015, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Zdobnova, T.A.; Lebedenko, E.N.; Deyev, S.M. Quantum Dots for Molecular Diagnostics of Tumors. Acta Nat. 2011, 3, 29–47. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, D.H.; Jung, S.W.; Lee, K.N.; Park, Y.S.; Seong, W.K. Measurements of Serum C-Reactive Protein Levels in Patients with Gastric Cancer and Quantification Using Silicon Nanowire Arrays. Nanomedicine 2010, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.; Ramya, A.N.; Maiti, K.K.; Ramaiah, D. Unveiling NIR Aza–Boron-dipyrromethene (BODIPY) Dyes as Raman Probes: Surface-Enhanced Raman Scattering (SERS)-Guided Selective Detection and Imaging of Human Cancer Cells. Chemistry 2017, 23, 14286–14291. [Google Scholar] [CrossRef]
- Gonda, K.; Watanabe, M.; Tada, H.; Miyashita, M.; Takahashi-Aoyama, Y.; Kamei, T.; Ishida, T.; Usami, S.; Hirakawa, H.; Kakugawa, Y.; et al. Quantitative Diagnostic Imaging of Cancer Tissues by Using Phosphor-Integrated Dots with Ultra-high Brightness. Sci. Rep. 2017, 7, 7509. [Google Scholar] [CrossRef]
- Pande, P.; Shrestha, S.; Park, J.; Gimenez-Conti, I.; Brandon, J.; Applegate, B.E.; Jo, J.A. Automated Analysis of Multimodal Fluorescence Lifetime Imaging and Optical Coherence Tomography Data for the Diagnosis of Oral Cancer in the Hamster Cheek Pouch Model. Biomed. Opt. Express 2016, 7, 2000–2015. [Google Scholar] [CrossRef]
- Jackson, A.W.; Chandrasekharan, P.; Ramasamy, B.; Goggi, J.; Chuang, K.H.; He, T.; Robins, E.G. Octreotide Functionalized Nano-contrast Agent for Targeted Magnetic Resonance Imaging. Biomacromolecules 2016, 17, 3902–3910. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Xiang, J.; He, Y.; Zhang, Z.; Cheng, Z.; Liu, W.; Tan, S. A Critical Review of Spray-Dried Amorphous Pharmaceuticals: Synthesis, Analysis and Application. Int. J. Pharm. 2021, 594, 120165. [Google Scholar] [CrossRef]
- Yu, L.; Hou, Y.; Xie, W.; Camacho, J.L.C.; Cheng, C.; Holle, A.; Young, J.; Trappmann, B.; Zhao, W.; Melzig, M.F.; et al. Ligand Diffusion Enables Force-Independent Cell Adhesion via Activating α5β1 Integrin and Initiating rac and RhoA Signalling. Adv. Mater. 2020, 32, 2002566. [Google Scholar] [CrossRef]
- Yu, L.; Hou, Y.; Xie, W.; Cuellar-Camacho, J.L.; Wei, Q.; Haag, R. Self-Strengthening Adhesive Force Promotes Cell Mechanotransduction. Adv. Mater. 2020, 32, 2006986. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, W.; Yu, L.; Camacho, L.C.; Nie, C.; Zhang, M.; Haag, R.; Wei, Q. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Small 2020, 16, 1905422. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Cao, G.; Li, K.; Zhang, R.; Li, X. Nanocomposites for the Delivery of Bioactive Molecules in Tissue Repair: Vital Structural Features, Application Mechanisms, Updated Progress and Future Perspectives. J. Mater. Chem. B 2020, 8, 10271–10289. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Feng, X.; Cao, G.; She, Z.; Tan, R.; Aifantis, K.E.; Zhang, R.; Li, X. The Effect of Carbon Nanotubes on Osteogenic Functions of Adipose-Derived Mesenchymal Stem Cells In Vitro and Bone Formation In Vivo Compared with That of Nano-Hydroxyapatite and the Possible Mechanism. Bioact. Mater. 2021, 6, 333–345. [Google Scholar] [CrossRef]
- Bi, X.; Liu, B.; Mao, Z.; Wang, C.; Dunne, N.; Fan, Y.; Li, X. Applications of Materials for Dural Reconstruction in Pre-clinical and Clinical Studies: Advantages and Drawbacks, Efficacy, and Selections. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111326. [Google Scholar] [CrossRef]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of Decellularized Materials in Tissue Engineering: Advantages, Drawbacks and Current Improvements, and Future Perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Liu, F.; Pan, Y.; Miao, L.; Zhu, Q.; Tan, S. The Feasibility of Antioxidants Avoiding Oxidative Damages from Reactive Oxygen Species in Cryopreservation. Front. Chem. 2021, 9, 648684. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Chen, X.; Zhou, Y.; He, B.; Tan, S. Analysis of the Biodegradation Performance and Biofouling in a Halophilic MBBR-MBR to Improve the Treatment of Disinfected Saline Wastewater. Chemosphere 2021, 269, 128716. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. [Google Scholar] [CrossRef]
- Yang, L.; Pijuan-Galito, S.; Rho, H.S.; Vasilevich, A.S.; Eren, A.D.; Ge, L.; Habibović, P.; Alexander, M.R.; de Boer, J.; Carlier, A.; et al. High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology. Chem. Rev. 2021, 121, 4561–4677. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Zhou, Y.; Yu, Z.; Yuan, H.; Feng, B.; van Rijn, P.; Zhang, Y. Alkali-Mediated Miscibility of Gelatin/Polycaprolactone for Electrospinning Homogeneous Composite Nanofibers for Tissue Scaffolding. Macromol. Biosci. 2017, 17, 1700268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, Z.; Zhou, Z.; Zhang, G.; Chiechi, R.C.; van Rijn, P. Directing Mesenchymal Stem Cells with Gold Nanowire Arrays. Adv. Mater. Interfaces 2018, 5, 1800334. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Q.; Jiang, Z.; Wang, Y.; Yang, K.; Qiu, X.; Ji, Q. The Effect of Doxycycline-Containing Chitosan/Carboxymethyl Chitosan Nanoparticles on NLRP3 Inflammasome in Periodontal Disease. Carbohydr. Polym. 2020, 237, 116163. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical Modifications and Bioconjugate Reactions of Nanomaterials for Sensing, Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef]
- Shim, M.S.; Kwon, Y.J. Stimuli-Responsive Polymers and Nanomaterials for Gene Delivery and Imaging Applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Luan, Y.; Vainikka, P.A.; Thallmair, S.; Marrink, S.J.; Feringa, B.L.; van Rijn, P. Unidirectional Rotating Molecular Motors Dynamically Interact with Adsorbed Proteins to Direct the Fate of Mesenchymal Stem Cells. Sci. Adv. 2020, 6, eaay2756. [Google Scholar] [CrossRef]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in Cancer Imaging and Therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef]
- Jones, D.L.; Rankin, K.V. Oral Cancer and Associated Risk Factors. In Prevention in Clinical Oral Health Care; Cappelli, D.P., Mobley, C.C., Eds.; Mosby Elsevier: Amsterdam, The Netherlands, 2008; pp. 68–77. [Google Scholar]
- Mithani, S.K.; Mydlarz, W.K.; Grumbine, F.L.; Smith, I.M.; Califano, J.A. Molecular Genetics of Premalignant Oral Lesions. Oral Dis. 2007, 13, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bisen, P.S. Oncoapoptotic Signaling and Deregulated Target Genes in Cancers: Special Reference to Oral Cancer. Biochim. Biophys. Acta 2013, 1836, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wood, R.E.; Tenenbaum, H.C. Delays in Diagnosis of Head and Neck Cancers. J. Can. Dent. Assoc. 2008, 74, 61. [Google Scholar] [PubMed]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef]
- Shah, J.P.; Lydiatt, W. Treatment of Cancer of the Head and Neck. CA Cancer J. Clin. 1995, 45, 352–368. [Google Scholar] [CrossRef]
- Scully, C. (Ed.) Oral and Maxillofacial Medicine. The Basis of Diagnosis and Treatment, 3rd ed.; Churchill Livingstone; Elsevier: Amsterdam, The Netherlands, 2013; pp. 2014–2017. [Google Scholar]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An Intergroup Phase III Comparison of Standard Radiation Therapy and Two Schedules of Concurrent Chemoradiotherapy in Patients with Unresectable Squamous Cell Head and Neck Cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef]
- Agüeros, M.; Ruiz-Gatón, L.; Vauthier, C.; Bouchemal, K.; Espuelas, S.; Ponchel, G.; Irache, J.M. Combined Hydroxypropyl-Beta-Cyclodextrin and Poly(Anhydride) Nanoparticles Improve the Oral Permeability of Paclitaxel. Eur. J. Pharm. Sci. 2009, 38, 405–413. [Google Scholar] [CrossRef]
- Baselga, J.; Trigo, J.M.; Bourhis, J.; Tortochaux, J.; Cortés-Funes, H.; Hitt, R.; Gascón, P.; Amellal, N.; Harstrick, A.; Eckardt, A. Phase II Multicenter Study of the Antiepidermal Growth Factor Receptor Monoclonal Antibody Cetuximab in Combination with Platinum-Based Chemotherapy in Patients with Platinum-Refractory Metastatic and/or Recurrent Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2005, 23, 5568–5577. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Haddad, R.; Sonis, S.; Posner, M.; Wirth, L.; Costello, R.; Braschayko, P.; Allen, A.; Mahadevan, A.; Flynn, J.; Burke, E.; et al. Randomized Phase 2 Study of Concomitant Chemoradiotherapy Using Weekly Carboplatin/Paclitaxel with or Without Daily Subcutaneous Amifostine in Patients with Locally Advanced Head and Neck Cancer. Cancer 2009, 115, 4514–4523. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab as a Single Agent in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Terwogt, J.M.M.; Schellens, J.H.M.; Huinink, W.W.; Beijnen, J.H. Clinical pharmacology of Anticancer Agents in Relation to Formulations and Administration Routes. Cancer Treat. Rev. 1999, 25, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of Nanotechnology in Pharmaceutical Product Development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Kruijtzer, C.M.F.; Beijnen, J.H.; Schellens, J.H.M. Improvement of Oral Drug Treatment by Temporary Inhibition of Drug Transporters and/or Cytochrome P450 in the Gastrointestinal Tract and Liver: An Overview. Oncologist 2002, 7, 516–530. [Google Scholar] [CrossRef]
- Grodzinski, P.; Silver, M.; Molnar, L.K. Nanotechnology for Cancer Diagnostics: Promises and Challenges. Expert Rev. Mol. Diagn. 2006, 6, 307–318. [Google Scholar] [CrossRef]
- Srinivasa Rao, B.; Bhushanam, K.; Das, U.N.; Prasad, T.N.V.K.V.; Subbarao, K. Recent Advances of Nanoparticles in Cancer Therapy and Diagnosis. J. Med. Sci. Res. 2013, 1, 95–102. [Google Scholar] [CrossRef]
- Pisani, P.; Parkin, D.M.; Bray, F.; Ferlay, J. Estimates of the Worldwide Mortality from 25 Cancers in 1990. Int. J. Cancer 1999, 83, 18–29. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic Surfactant Vesicular Systems for Effective Drug Delivery—An Overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Neha, B.; Ganesh, B.; Preeti, K. Drug Delivery to the Brain Using Polymeric Nanoparticles: A Review. Int. J. Pharma. Life Sci. 2013, 2, 107–132. [Google Scholar] [CrossRef]
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle Drug Loading as a Design Parameter to Improve Docetaxel Pharmacokinetics and Efficacy. Biomaterials 2013, 34, 8424–8429. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, D.S.; Tyagi, P.; Mirvish, S.S.; Kompella, U.B. Supercritical fluid Technology Based Large Porous Celecoxib–PLGA Microparticles Do Not Induce Pulmonary Fibrosis and Sustain Drug Delivery and Efficacy for Several Weeks Following a Single Dose. J. Control. Release 2013, 168, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation Mechanism of Monodisperse, Low Molecular Weight Chitosan Nanoparticles by Ionic Gelation Technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, P.; Liang, X.; Gong, X.; Song, T.; Niu, R.; Chang, J. Folate-PEG Coated Cationic Modified Chitosan—Cholesterol Liposomes for Tumor-Targeted Drug Delivery. Biomaterials 2010, 31, 4129–4138. [Google Scholar] [CrossRef]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging Technologies of Polymeric Nanoparticles in Cancer Drug Delivery. J. Nanomater. 2011, 2011, 408675. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and Targeted Systems for Cancer Therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable Nanoparticles Are Excellent Vehicle for Site Directed In-Vivo Delivery of Drugs and Vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Li, Z.; Chai, F.; Yang, L.; Luo, X.; Yang, C. Mechanical Properties and Nanoparticles Precipitation Behavior of Multi-component Ultra High Strength Steel. Mater. Des. 2020, 191, 108637. [Google Scholar] [CrossRef]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I. Green synthesis of Mg 0.99 Zn 0.01O nanoparticles for the fabrication of κ-Carrageenan/NaCMC hydrogel in order to deliver catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Khatir, N.; Abdul-Malek, Z.; Zak, A.K.; Akbari, A.; Sabbagh, F. Sol–gel grown Fe-doped ZnO nanoparticles: Antibacterial and structural behaviors. J. Sol.-Gel. Sci. Technol. 2016, 78, 91–98. [Google Scholar] [CrossRef]
- Sindura, C.S.; Babu, N.C. Vinod. Unbounding the Future: Nanobiotechnology in Detection and Treatment of Oral Cancer. J. Adv. Med. Dent. Scie 2013, 1, 66–77. [Google Scholar]
- Shetty, N.J.; Swati, P.; David, K. Nanorobots: Future in Dentistry. Saudi Dent. J. 2013, 25, 49–52. [Google Scholar] [CrossRef]
- Sujatha, R.; Robert, H.B. Daniel WvdW. Radio Frequency Rectification on Membrane Bound Pores. Nanotechnology 2010, 21, 075201. [Google Scholar]
- Craig, M.; Jenner, A.L.; Namgung, B.; Lee, L.P.; Goldman, A. Engineering in medicine to address the challenge of cancer drug resistance: From micro-and nanotechnologies to computational and mathematical modelling. Chem. Rev. 2020, 121, 3352–3389. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Del. Rev. 2016, 98, 3. [Google Scholar] [CrossRef]
- Tuthill, M.H.; Montinaro, A.; Zinngrebe, J.; Prieske, K.; Draber, P.; Prieske, S.; Newsom-Davis, T.; von Karstedt, S.; Graves, J.; Walczak, H. TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells. Oncogene 2015, 34, 2138. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, T.H.; Jiang, H.H.; Park, C.W.; Lee, S.; Chen, X.; Lee, K.C. Improved biological half-life and anti-tumor activity of TNF-related apoptosis-inducing ligand (TRAIL) using PEG-exposed nanoparticles. Biomaterials 2011, 32, 3538. [Google Scholar] [CrossRef]
- Zhao, P.; Tang, X.; Huang, Y. Teaching new tricks to old dogs: A review of drug repositioning of disulfiram for cancer nanomedicine. View 2021, 2, 20200127. [Google Scholar] [CrossRef]
- YashRoy, R.C. Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; p. 341. [Google Scholar]
- Gerritzen, M.J.; Martens, D.E.; Wijfels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T.J. Co-delivery of silybin and paclitaxel by dextran-based nanoparticles for effective anti-tumor treatment through chemotherapy sensitization and microenvironment modulation. Control. Release 2020, 321, 198. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhang, M.; Li, J.; Young, K.H. PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor immune response? Front. Immunol. 2017, 8, 1597. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Qiu, N.; Zhou, Q.; Wang, G.; Jiang, H.; Piao, Y.; Zhou, Z.; Tang, J.; Shen, Y. Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy. Nature communications. Nat. Commun. 2021, 12, 2425. [Google Scholar] [CrossRef]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1. [Google Scholar]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, S.K. Nanotechnology in Oral Cancer: A Comprehensive Review. J. Oral Maxillofac. Pathol. 2017, 21, 407–414. [Google Scholar] [CrossRef]
- Luiza Ribeiro de Souza, A.; Priscila Kiill, C.; Kolenyak dos Santos, F.; Marielli da Luz, G.; Rocha e Silva, H.; Chorilli, M.; Palmira Daflon Gremiao, M. Nanotechnology-Based Drug Delivery Systems for Dermatomycosis Treatment. Curr. Nanosci. 2012, 8, 512–519. [Google Scholar] [CrossRef]
- Mezei, M.; Gulasekharam, V. Liposomes–A Selective Drug Delivery System for the Topical Route of Administration. Lotion Dosage Form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J. Trends and Developments in Liposome Drug Delivery Systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Pan, X.Q.; Wang, H.; Lee, R.J. Antitumor Activity of Folate Receptor-Targeted Liposomal Doxorubicin in a KB Oral Carcinoma Murine Xenograft Model. Pharm. Res. 2003, 20, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gosangari, S.L.; Watkin, K.L. Effect of Preparation Techniques on the Properties of Curcumin Liposomes: Characterization of Size, Release and Cytotoxicity on a Squamous Oral Carcinoma Cell Line. Pharm. Dev. Technol. 2012, 17, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mahakian, L.M.; Farwell, D.G.; Zhang, H.; Seo, J.W.; Poirier, B.; Tinling, S.P.; Afify, A.M.; Haynam, E.M.; Shaye, D.; Ferrara, K.W. Comparison of PET Imaging with 64Cu-Liposomes and 18F-fdg in the 7,12-Dimethylbenz[a]Anthracene (DMBA)-Induced Hamster Buccal Pouch Model of Oral Dysplasia and Squamous Cell Carcinoma. Mol. Imaging Biol. 2014, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid, E.S.A.; Gamal-Eldeen, A.M.; Sharaf Eldeen, A.M. Liposome-Coated Nano Doxorubicin Induces Apoptosis on Oral Squamous Cell Carcinoma CAL-27 Cells. Arch. Oral Biol. 2019, 103, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.E.; Rotsides, J.M.; Balogun, F.O.; Persky, M.S.; Muggia, F.M.; Persky, M.J. Oral Squamous Cell Carcinoma as a Complication of Treatment for Recurrent High-Grade Serous Cancer. Laryngoscope 2020, 130, 2607–2610. [Google Scholar] [CrossRef]
- Nomura, H.; Sakamoto, K.; Sugihara, T.; Okamoto, S.; Aoki, Y.; Tanigawa, T.; Matoda, M.; Omatsu, K.; Kanao, H.; Kato, K.; et al. Oral Leukoplakia, a Precancerous Lesion of Squamous Cell Carcinoma, in Patients with Long-Term Pegylated Liposomal Doxorubicin Treatment. Medicine 2018, 97, e9932. [Google Scholar] [CrossRef]
- Zhang, N.N.; Zhang, L.G.; Liu, Z.N.; Huang, G.L.; Zhang, L.; Yi, J.; Yao, L.; Hu, X.H. Therapeutic Efficacy of Paclitaxel and Carboplatin via Arterial or Venous Perfusion in Rabbits with VX-2 Tongue Cancer. Int. J. Clin. Exp. Med. 2015, 8, 4979–4988. [Google Scholar]
- Mohan, A.; Narayanan, S.; Balasubramanian, G.; Sethuraman, S.; Krishnan, U.M. Dual Drug Loaded Nanoliposomal Chemotherapy: A Promising Strategy for Treatment of Head and Neck Squamous Cell Carcinoma. Eur. J. Pharm. Biopharm. 2016, 99, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Overlid, N.; Konopka, K.; Düzgünes, N. Gene Therapy for Oral Cancer: Efficient Delivery of a ‘Suicide Gene’ to Murine Oral Cancer Cells in Physiological Milieu. J. Calif. Dent. Assoc. 2005, 33, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.; Faneca, H.; Bertin, S.; Konopka, K.; Düzgüneş, N.; Pierrefite-Carle, V.; Simões, S.; Pedroso de Lima, M.C. Transferrin Lipoplex-Mediated Suicide Gene Therapy of Oral Squamous Cell Carcinoma in an Immunocompetent Murine Model and Mechanisms Involved in the Antitumoral Response. Cancer Gene Ther. 2009, 16, 91–101. [Google Scholar] [CrossRef] [PubMed]
- French, J.T.; Goins, B.; Saenz, M.; Li, S.; Garcia-Rojas, X.; Phillips, W.T.; Otto, R.A.; Bao, A. Interventional Therapy of Head and Neck Cancer with Lipid Nanoparticle-Carried Rhenium 186 Radionuclide. J. Vasc. Interv. Radiol. 2010, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.; Rao, T.; He, X.; Xu, T. Pharmaceutical Applications of Dendrimers: Promising Nanocarriers for Drug Delivery. Front. Biosci. 2008, 13, 1447–1471. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liao, W.; Xu, Z.; Yang, Y.; Wong, D.T.; Ho, C.M. A Bio-abiotic Interface Constructed by Nanoscale DNA-Dendrimer and Conducting Polymer for Ultrasensitive Bio-molecular Diagnosis. Small 2010, 5, 1784–1790. [Google Scholar] [CrossRef]
- Ward, B.B.; Dunham, T.; Majoros, I.J.; Baker, J.R. Targeted Dendrimer Chemotherapy in an Animal Model for Head and Neck Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2011, 69, 2452–2459. [Google Scholar] [CrossRef]
- Liu, X.; Huang, H.; Wang, J.; Wang, C.; Wang, M.; Zhang, B.; Pan, C. Dendrimers-Delivered Short Hairpin RNA Targeting HTERT Inhibits Oral Cancer Cell Growth In Vitro and In Vivo. Biochem. Pharmacol. 2011, 82, 17–23. [Google Scholar] [CrossRef]
- Eskiizmir, G.; Ermertcan, A.T.; Nanomaterials, Y.K. Promising Structures for the Management of Oral Cancer; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–544. [Google Scholar]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Shabestari Khiabani, S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic Nanoparticles: Preparation Methods, Applications in Cancer Diagnosis and Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S.K. Magnetic Nanoparticles: A Novel Platform for Cancer Theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhuang, L.; Lin, Y.; Yan, M.; Lv, J.; Li, X.; Lin, H.; Zhu, P.; Lin, Q.; Xu, Y. Novel Drug Delivery System Based on Hollow Mesoporous Magnetic Nanoparticles for Head and Neck Cancers—Targeted Therapy In Vitro and In Vivo. Am. J. Cancer Res. 2020, 10, 350–364. [Google Scholar] [PubMed]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient Delivery of Therapeutic SiRNA by Fe3O4 Magnetic Nanoparticles into Oral Cancer Cells. Pharmaceutics 2019, 11, 615. [Google Scholar] [CrossRef]
- Miao, L.; Liu, C.; Ge, J.; Yang, W.; Liu, J.; Sun, W.; Yang, B.; Zheng, C.; Sun, H.; Hu, Q. Antitumor Effect of TRAIL on Oral Squamous Cell Carcinoma Using Magnetic Nanoparticle-Mediated Gene Expression. Cell Biochem. Biophys. 2014, 69, 663–672. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted Magnetic Nanoparticle Hyperthermia for the Treatment of Oral Cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Yue, K.; Yang, C.; You, Y.; Wang, X.; Zhang, X. Experimental Investigation of Temperature Influence on Nanoparticle Adhesion in an Artificial Blood Vessel. Int. J. Nanomed. 2023, 18, 425–436. [Google Scholar] [CrossRef]
- Wu, F.G.; Zhang, X.; Chen, X.; Sun, W.; Bao, Y.W.; Hua, X.W.; Gao, G.; Jia, H.R. Quantum Dots for Cancer Therapy and Bioimaging; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Liu, L.; Miao, Q.; Liang, G. Quantum Dots as Multifunctional Materials for Tumor Imaging and Therapy. Materials 2013, 6, 483–499. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum Dots and Their Multi-Modal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. BioCompatible Quantum Dots for Biological Applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef]
- Bakalova, R.; Zhelev, Z.; Kokuryo, D.; Spasov, L.; Aoki, I.; Saga, T. Chemical Nature and Structure of Organic Coating of Quantum Dots Is Crucial for Their Application in Imaging Diagnostics. Int. J. Nanomed. 2011, 6, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, In Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, C.; Cao, Y.A.; Tang, H.; Bai, Y.L.; Huang, H.; Zhao, C.R.; Chen, R.; Zhao, D. In Vivo and In Situ Imaging of Head and Neck Squamous Cell Carcinoma Using Near-Infrared Fluorescent Quantum Dot Probes Conjugated with Epidermal Growth Factor Receptor Monoclonal Antibodies in Mice. Oncol. Rep. 2012, 27, 1925–1931. [Google Scholar] [CrossRef]
- Yang, K.; Cao, Y.A.; Shi, C.; Li, Z.G.; Zhang, F.J.; Yang, J.; Zhao, C. Quantum Dot-Based Visual In Vivo Imaging for Oral Squamous Cell Carcinoma in Mice. Oral Oncol. 2010, 46, 864–868. [Google Scholar] [CrossRef]
- Aswathy, R.G.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Near-Infrared Quantum Dots for Deep Tissue Imaging. Anal. Bioanal. Chem. 2010, 397, 1417–1435. [Google Scholar] [CrossRef]
- Zhu, C.N.; Chen, G.; Tian, Z.Q.; Wang, W.; Zhong, W.Q.; Li, Z.; Zhang, Z.; Pang, D. Near-Infrared Fluorescent Ag2Se-cetuximab Nanoprobes for Targeted Imaging and Therapy of Cancer. Small 2017, 13, 1602309. [Google Scholar] [CrossRef]
- Zhao, J.J.; Chen, J.; Wang, Z.P.; Pan, J.; Huang, Y.H. Double Labeling and Comparison of Fluorescence Intensity and Photostability Between Quantum Dots and FITC in Oral Tumors. Mol. Med. Rep. 2011, 4, 425–429. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, F.J.; Tang, H.; Zhao, C.; Cao, Y.A.; Lv, X.Q.; Chen, D.; Li, Y.D. In-Vivo Imaging of Oral Squamous Cell Carcinoma by EGFR Monoclonal Antibody Conjugated Near-Infrared Quantum Dots in Mice. Int. J. Nanomed. 2011, 6, 1739–1745. [Google Scholar] [CrossRef]
- Xue, J.; Chen, H.; Fan, M.; Zhu, F.; Diao, L.; Chen, X.; Fan, L.; Li, P.; Xia, D. Use of Quantum Dots to Detect Human Papillomavirus in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2009, 38, 668–671. [Google Scholar] [CrossRef]
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S.M. N-Doped Carbon Quantum Dot (NCQD)-Deposited Carbon Capsules for Synergistic Fluorescence Imaging and Photothermal Therapy of Oral Cancer. Langmuir 2019, 35, 15320–15329. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor Effect of a Pt-Loaded Nanocomposite Based on Graphene Quantum Dots Combats Hypoxia-Induced Chemoresistance of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic Nanoparticles for Cancer Imaging and Therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; Rodriguez-Torres, M.D.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.E.; Su, H.J.; Marras, A.E.; Zhou, L.; Johnson, J. Mechanical Design of DNA Nanostructures. Nanoscale 2015, 7, 5913–5921. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B.; et al. DNA Origami as an In Vivo Drug Delivery Vehicle for Cancer Therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, Y.; Li, J.; Li, Q.; Lv, M.; Zhu, B.; Tian, T.; Cheng, D.; Xia, J.; Zhang, L.; et al. Multiple-Armed Tetrahedral DNA Nanostructures for Tumor-Targeting, Dual-Modality In Vivo Imaging. ACS Appl. Mater. Interfaces 2016, 8, 4378–4384. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and Bone Regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Zhou, T.; Lin, S.; Shi, S.; Lin, Y. Synthesis of an Ethyleneimine/Tetrahedral DNA Nanostructure Complex and Its Potential Application as a Multi-functional Delivery Vehicle. Nanoscale 2017, 9, 18402–18412. [Google Scholar] [CrossRef]
- Cai, X.; Xie, J.; Yao, Y.; Cun, X.; Lin, S.; Tian, T.; Zhu, B.; Lin, Y. Angiogenesis in a 3D Model Containing Adipose Tissue Stem Cells and Endothelial Cells Is Mediated by Canonical Wnt Signaling. Bone Res. 2017, 5, 17048. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, H.; Liu, D.; Liu, J.; Ji, N.; Chen, F.; Luo, X.; Zhou, Y.; Dan, H.; Zeng, X.; et al. Self-Assembling Monomeric Nucleoside Molecular Nanoparticles Loaded with 5-FU Enhancing Therapeutic Efficacy against Oral Cancer. ACS Nano 2015, 9, 9638–9651. [Google Scholar] [CrossRef] [PubMed]
- Soldi, M.; Cuomo, A.; Bremang, M.; Bonaldi, T. Mass spectrometry-based proteomics for the analysis of chromatin structure and dynamics. Int. J. Mol. Sci. 2013, 14, 5402–5431. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kawai, T. DNA electronics. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 1–12. [Google Scholar] [CrossRef]
- Hamidinezhad, H.; Abdul-Malek, Z.; Wahab, Y. Effects of gas pressure on the synthesis and photoluminescence properties of Si nanowires in VHF-PECVD method. Appl. Phys. A 2012, 108, 739–744. [Google Scholar] [CrossRef]
- Viljas, J.; Pauly, F.J. Modeling elastic and photoassisted transport in organic molecular wires: Length dependence and current-voltage characteristics. Cuevas Phys. Rev. B 2008, 77, 155119. [Google Scholar] [CrossRef]
- Joachim, C.; Ratner, M. Molecular wires: Guiding the super-exchange interactions between two electrodes. Nanotechnology 2004, 15, 1065. [Google Scholar] [CrossRef]
- Endres, R.; Cox, D.; Singh, R. Colloquium: The quest for high-conductance DNA. Rev. Mod. Phys. 2004, 76, 195–214. [Google Scholar] [CrossRef]
- Mao, Y.; Luo, C.; Ouyang, Q. Studies of temperatured—Ependent electronic transduction on DNA hairpin loop sensor. Nucleic. Acids Res. 2003, 31, e108–e131. [Google Scholar] [CrossRef]
- Khatir, N.; Abdul-Malek, Z.; Banihashemian, S. Temperature and magnetic field driven modifications in the IV features of gold-DNA-Gold structure. Sensors 2014, 14, 19229–19241. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Persson, B.; Uhlen, M.; Nygren, P.-A. Real-time monitoring of DNA manipulations using biosensor technology. Anal. Biochem. 1995, 224, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. DNA biosensors based on peptide nucleic acid (PNA) recognition layers. A review. Biosens. Bioelectron. 1998, 13, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Palecek, E.; Nielsen, P.E.; Rivas, G.; Cai, X.; Shiraishi, H.; Dontha, N.; Luo, D.; Farias, P.A. Peptide nucleic acid probes for sequence-specific DNA biosensors. J. Am. Chem. Soc. 1996, 118, 7667–7670. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ito, K.; Ishimori, Y. Novel DNA sensor for electrochemical gene detection. DNA. Novel. Anal. Chim. Acta 1994, 286, 219–224. [Google Scholar] [CrossRef]
- Hartzell, B.; McCord, B.; Asare, D.; Chen, H.; Heremans, J.; Soghomonian, V. Graphene Science Handbook Applications and Industrialization. Appl. Phys. Lett. 2003, 82, 4800–4802. [Google Scholar] [CrossRef]
- Yoo, K.H.; Ha, D.; Lee, J.O.; Park, J.; Kim, J.; Kim, J.; Lee, H.Y.; Kawai, T.; Choi, H.Y. Electrical conduction through poly (dA)-poly (dT) and poly (dG)-poly (dC) DNA molecules. Phys. Rev. Lett. 2001, 87, 198102–198104. [Google Scholar] [CrossRef]
- DeVault, D. Quantum-Mechanical Tunnelling in Biological Systems; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Khatir, N.M.; Banihashemian, S.M.; Periasamy, V.; Ritikos, R.; Abd Majid, W.H.; Rahman, S.A. Electrical characterization of Gold-DNA-Gold structures in presence of an external magnetic field by means of IV curve analysis. Sensors 2012, 12, 3578–3586. [Google Scholar] [CrossRef]
- Khatir, N.M.; Abdul-Malek, Z.; Banihashemian, S.M. Investigation of The Electrical Resistivity Of 20 μm-Gap Gold-DNA-Gold Structure: Exploiting the Current-Voltage Characteristics Under a Variable External Magnetic Field. Appl. Mech. Mater. Trans. Tech. Publ. 2014, 554, 155–159. [Google Scholar] [CrossRef]
- Zadegan, R.M.; Norton, M.L. Structural DNA nanotechnology: From design to applications. Int. J. Mol. Sci. 2012, 13, 7149–7162. [Google Scholar] [CrossRef]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Hill MG Barton JK Nat. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.; Eichen, Y.; Sivan, U.; Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 1998, 391, 775–778. [Google Scholar] [CrossRef]
- Schildkraut, C.; Lifson, S. Dependence of the melting temperature of DNA on salt concentration. Biopolymers 1965, 3, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Poturnayová, A.; Šnejdárková, M.; Hianik, T.; KorriYoussoufi, H. Binding kinetics of human cellular prion detection by DNA aptamers immobilized on a conducting polypyrrole. Anal. Bioanal. Chem. 2013, 405, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.M. Unimolecular rectifiers and what lies ahead. Coll. Surf. A Physicochem. Eng. Asp. 2006, 284, 2–10. [Google Scholar] [CrossRef]
- Metzger, R.M. Monolayer rectifiers. J. Solid State Chem. 2002, 168, 696–711. [Google Scholar] [CrossRef]
- Aviram, A.; Ratner, M.A. Molecular rectifiers. Chem. Phys. Lett. 1974, 29, 277–283. [Google Scholar] [CrossRef]
- Cohen, H.; Nogues, C.; Naaman, R.; Porath, D. Direct measurement of electrical transport through single DNA molecules of complex sequence. Proc. Natl. Acad. Sci. USA 2005, 102, 11589–11593. [Google Scholar] [CrossRef]
- Porath, D.; Bezryadin, A.; DeVries, S.; Dekker, C. Direct measurement of electrical transport through DNA molecules. Nature 2000, 403, 635–637. [Google Scholar] [CrossRef]
- Kasumov, A.Y.; Kociak, M.; Gueron, S.; Reulet, B.; Volkov, V.; Klinov, D.; Bouchiat, H. Proximity-induced superconductivity in DNA. Science 2001, 291, 280–282. [Google Scholar] [CrossRef]
- De Pablo, P.; Moreno-Herrero, F.; Colchero, J.; Gómez Herrero, J.; Herrero, P.; Baro, A.; Ordejón, P.; Soler, J.; Artacho, E. Absence of dc-Conductivity in λ-DNA. Phys. Rev. Lett. 2000, 85, 4992–4995. [Google Scholar] [CrossRef] [PubMed]
- Porath, D.; Cuniberti, G.; Di Felice, R. Charge transport in DNA-based devices. Top. Curr. Chem. 2004, 237, 183–228. [Google Scholar]
- Rife, J.; Miller, M.; Sheehan, P.; Tamanaha, C.; Tondra, M.; Whitman, L. Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuators A Phys. 2003, 107, 209–218. [Google Scholar] [CrossRef]
- Freitas, P.; Ferreira, H.; Graham, D.; Clarke, L.; Amaral, M.; Martins, V.; Fonseca, L.; Cabral, J. Magnetoresistive DNA Chips; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Ejsing, L.; Hansen, M.F.; Menon, A.K.; Ferreira, H.; Graham, D.; Freitas, P. Planar hall effect sensor for magnetic micro- and nanobead detection. Appl. Phys. Lett. 2004, 84, 4729–4731. [Google Scholar] [CrossRef]
- Anguelouch, A.; Reich, D.; Chien, C.; Tondra, M. Detection of ferromagnetic nanowires using GMR sensors. IEEE Trans. Magn. 2004, 40, 2997–2999. [Google Scholar] [CrossRef]
- Shen, W.; Liu, X.; Mazumdar, D.; Xiao, G. Insitu detection of single micron-sized magnetic beads using magnetic tunnel junction sensors. Appl. Phys. Lett. 2005, 86, 253901–253903. [Google Scholar] [CrossRef]
- Ferreira, H.A.; Graham, D.L.; Feliciano, N.; Clarke, L.A.; Amaral, M.D.; Freitas, P.P. Detection of cystic fibrosis related DNA targets using AC field focusing of magnetic labels and spin-valve sensors. IEEE Trans. Magn. 2005, 41, 4140–4142. [Google Scholar] [CrossRef]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical Applications of Cyclodextrins. 2. In Vivo Drug Delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly Soluble Cyclodextrin Derivatives: Chemistry, Properties, and Trends in Development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin Based Novel Drug Delivery Systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.Y.; Shi, Y.; Jia, F. Editorial. Editorial: Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Front. Chem. 2020, 8, 245. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured Lipid Carriers as Novel Carrier for Parenteral Delivery of Docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, J.; Zhang, Y.; Shen, Q.; Pan, W. Characterization and Evaluation of Nanostructured Lipid Carrier as a Vehicle for Oral Delivery of Etoposide. Eur. J. Pharm. Sci. 2011, 43, 174–179. [Google Scholar] [CrossRef]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Nutraceuticals as New Treatment Approaches for Oral Cancer-I: Curcumin. Oral Oncol. 2013, 49, 187–191. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-Trimethyl Chitosan Nanoparticles and CSKSSDYQC Peptide: N-Trimethyl Chitosan Conjugates Enhance the Oral Bioavailability of Gemcitabine to Treat Breast Cancer. J. Control. Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Du, W.; Fan, Y.; Zheng, N.; He, B.; Yuan, L.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Transferrin Receptor Specific Nanocarriers Conjugated with Functional 7peptide for Oral Drug Delivery. Biomaterials 2013, 34, 794–806. [Google Scholar] [CrossRef]
- Yang, G.; Chen, S.; Zhang, J. Bioinspired and Biomimetic Nanotherapies for the Treatment of Infectious Diseases. Front. Pharmacol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Wu, S.Y.; Lin, C.H. Oral Immunization with Cell-Free Self-Assembly Virus-Like Particles against Orange-Spotted Grouper Nervous Necrosis Virus in Grouper Larvae, Epinephelus coioides. Vet. Immunol. Immunopathol. 2018, 197, 69–75. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, Y.; Liu, J.; Ji, X.; Meng, L.; Wang, T.; Sun, W.; Zhang, K.; Sang, X.; Yu, Z.; et al. Inclusion of Membrane-Anchored LTB or Flagellin Protein in H5N1 Virus-Like Particles Enhances Protective Responses Following Intramuscular and Oral Immunization of Mice. Vaccine 2018, 36, 5990–5998. [Google Scholar] [CrossRef] [PubMed]
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernández, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient Oral Vaccination by Bioengineering Virus-Like Particles with Protozoan Surface Proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical Implications of Chemotherapeutic Agent Organ Toxicity on Perioperative Care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 05, 105–129. [Google Scholar] [CrossRef]
- Guo, J.; Dong, F.; Ding, L.; Wang, K.; Zhang, J.; Fang, J. A Novel Drug-Free Strategy of Nano-pulse Stimulation Sequence (NPSS) in Oral Cancer Therapy: In Vitro and In Vivo Study. Bioelectrochemistry 2018, 123, 26–33. [Google Scholar] [CrossRef]
- Roberts, S.A.; Parikh, N.; Blower, R.J.; Agrawal, N. SPIN: Rapid Synthesis, Purification, and Concentration of Small Drug-Loaded Liposomes. J. Liposome Res. 2018, 28, 331–340. [Google Scholar] [CrossRef]
- Shi, S.; Peng, Q.; Shao, X.; Xie, J.; Lin, S.; Zhang, T.; Li, Q.; Li, X.; Lin, Y. Self-Assembled Tetrahedral DNA Nanostructures Promote Adipose-Derived Stem Cell Migration via lncRNA XLOC 010623 and RHOA/ROCK2 Signal Pathway. ACS Appl. Mater. Interfaces 2016, 8, 19353–19363. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Gupta, B.; Choi, H.G.; Yong, C.S.; Kim, J.O. Multiple Polysaccharide-Drug Complex-Loaded Liposomes: A Unique Strategy in Drug Loading and Cancer Targeting. Carbohydr. Polym. 2017, 173, 57–66. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Review of the Role of Nanotechnology in Overcoming the Challenges Faced in Oral Cancer Diagnosis and Treatment. Molecules 2023, 28, 5395. https://doi.org/10.3390/molecules28145395
Umapathy VR, Natarajan PM, Swamikannu B. Review of the Role of Nanotechnology in Overcoming the Challenges Faced in Oral Cancer Diagnosis and Treatment. Molecules. 2023; 28(14):5395. https://doi.org/10.3390/molecules28145395
Chicago/Turabian StyleUmapathy, Vidhya Rekha, Prabhu Manickam Natarajan, and Bhuminathan Swamikannu. 2023. "Review of the Role of Nanotechnology in Overcoming the Challenges Faced in Oral Cancer Diagnosis and Treatment" Molecules 28, no. 14: 5395. https://doi.org/10.3390/molecules28145395
APA StyleUmapathy, V. R., Natarajan, P. M., & Swamikannu, B. (2023). Review of the Role of Nanotechnology in Overcoming the Challenges Faced in Oral Cancer Diagnosis and Treatment. Molecules, 28(14), 5395. https://doi.org/10.3390/molecules28145395





