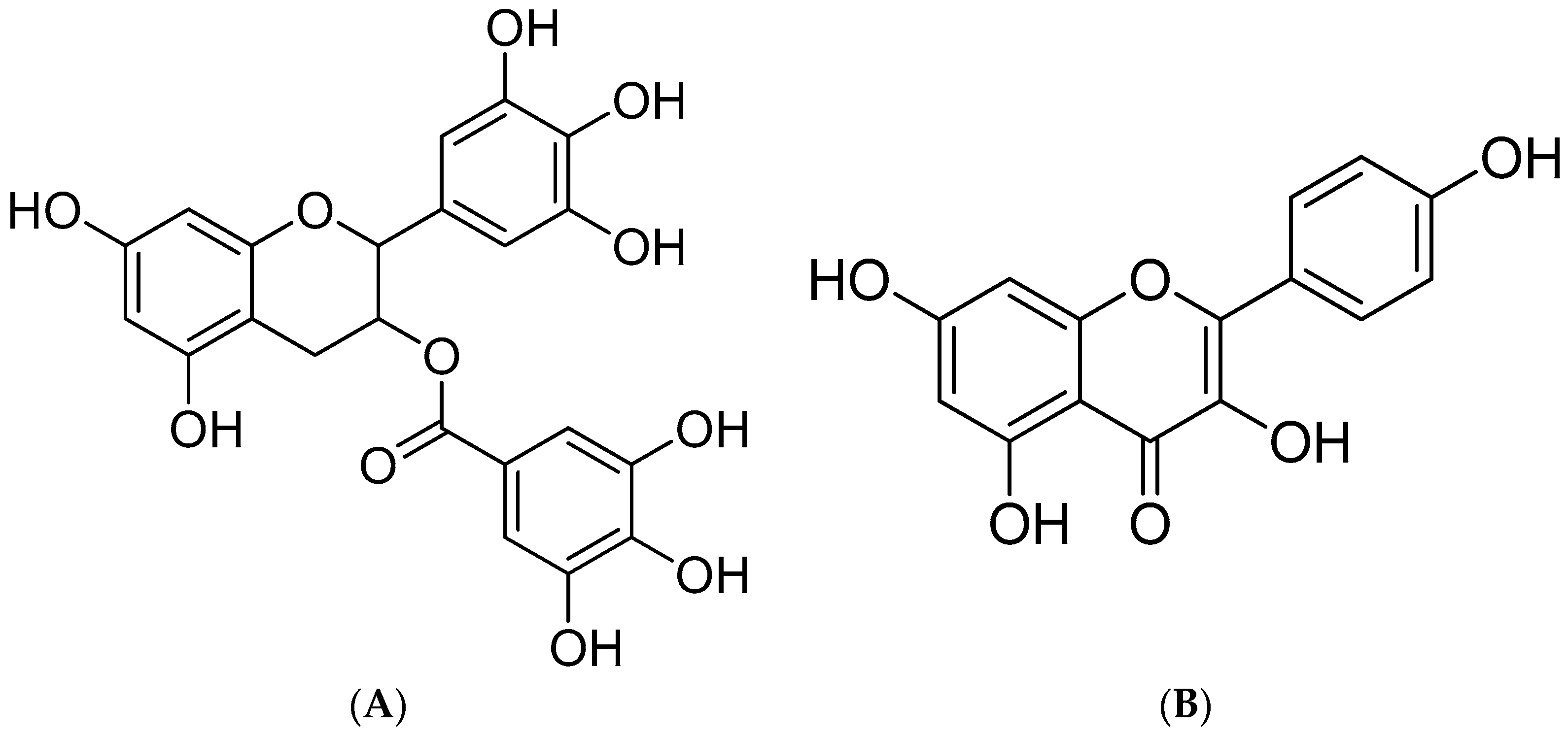
Characterization of the Synergistic Antioxidant Activity of Epigallocatechin Gallate (EGCG) and Kaempferol
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of EGCG and Kaempferol on Cytotoxicity Activity
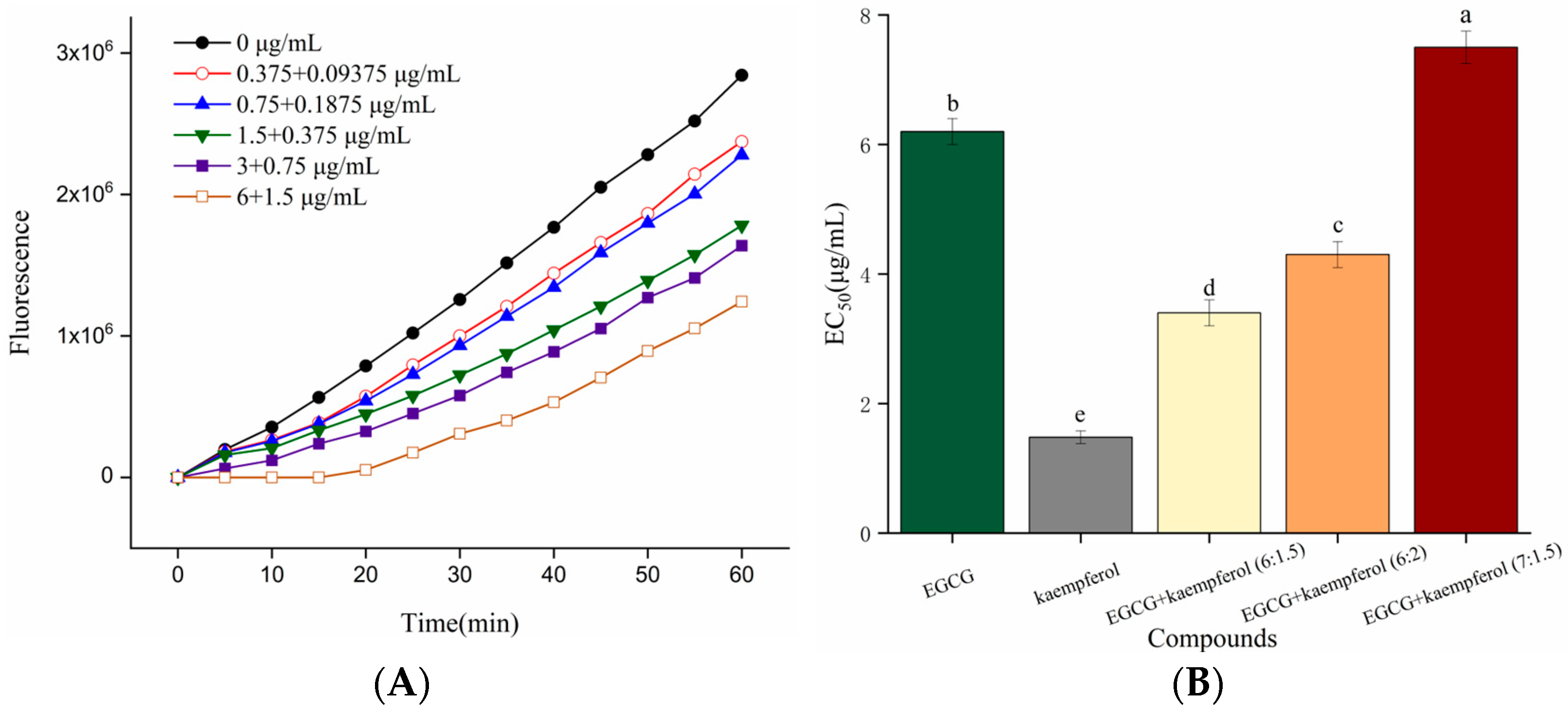
2.2. EGCG and Kaempferol Combination on Cellular Antioxidant Activity
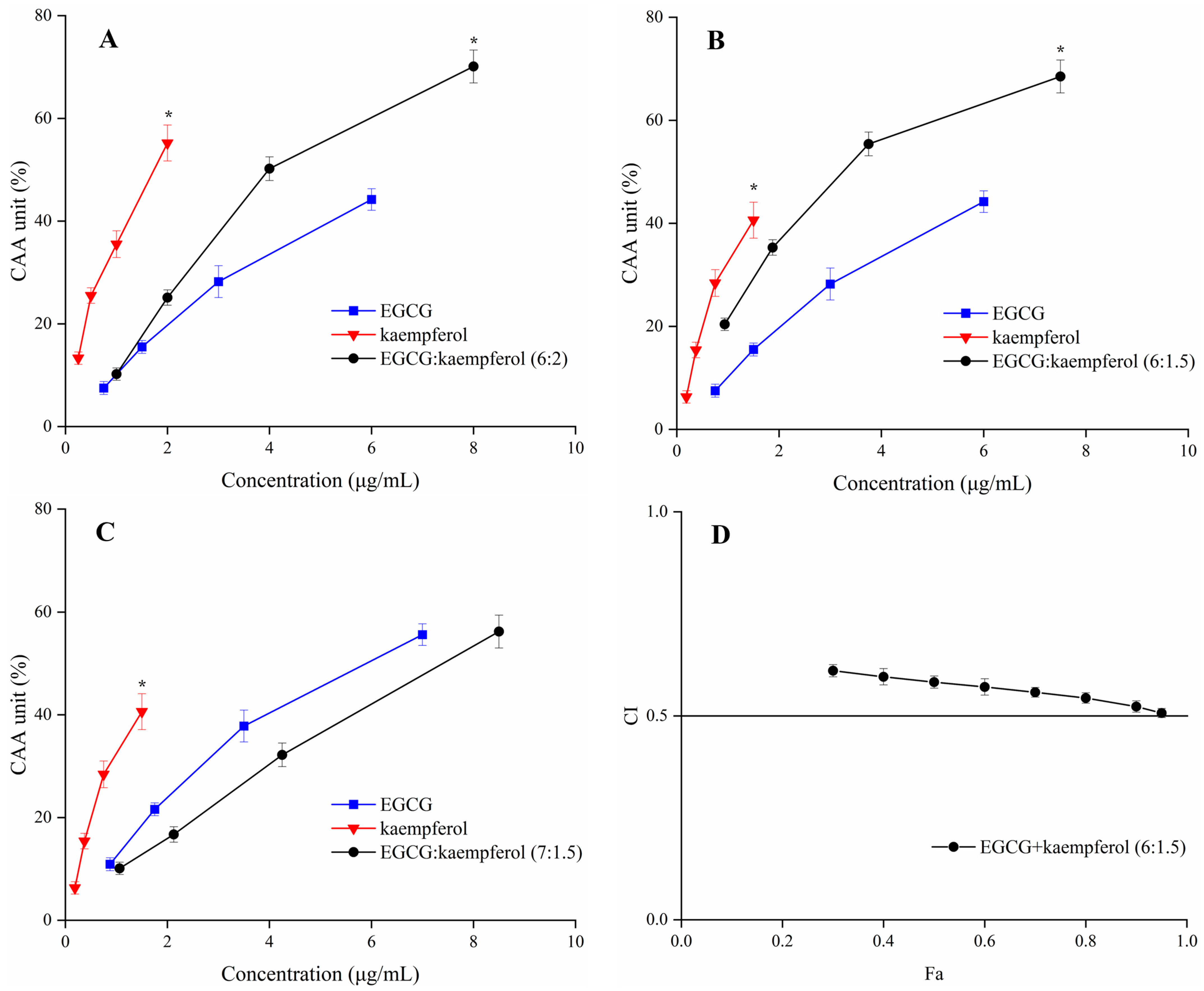
2.3. Synergistic Effect of EGCG and Kaempferol Combination
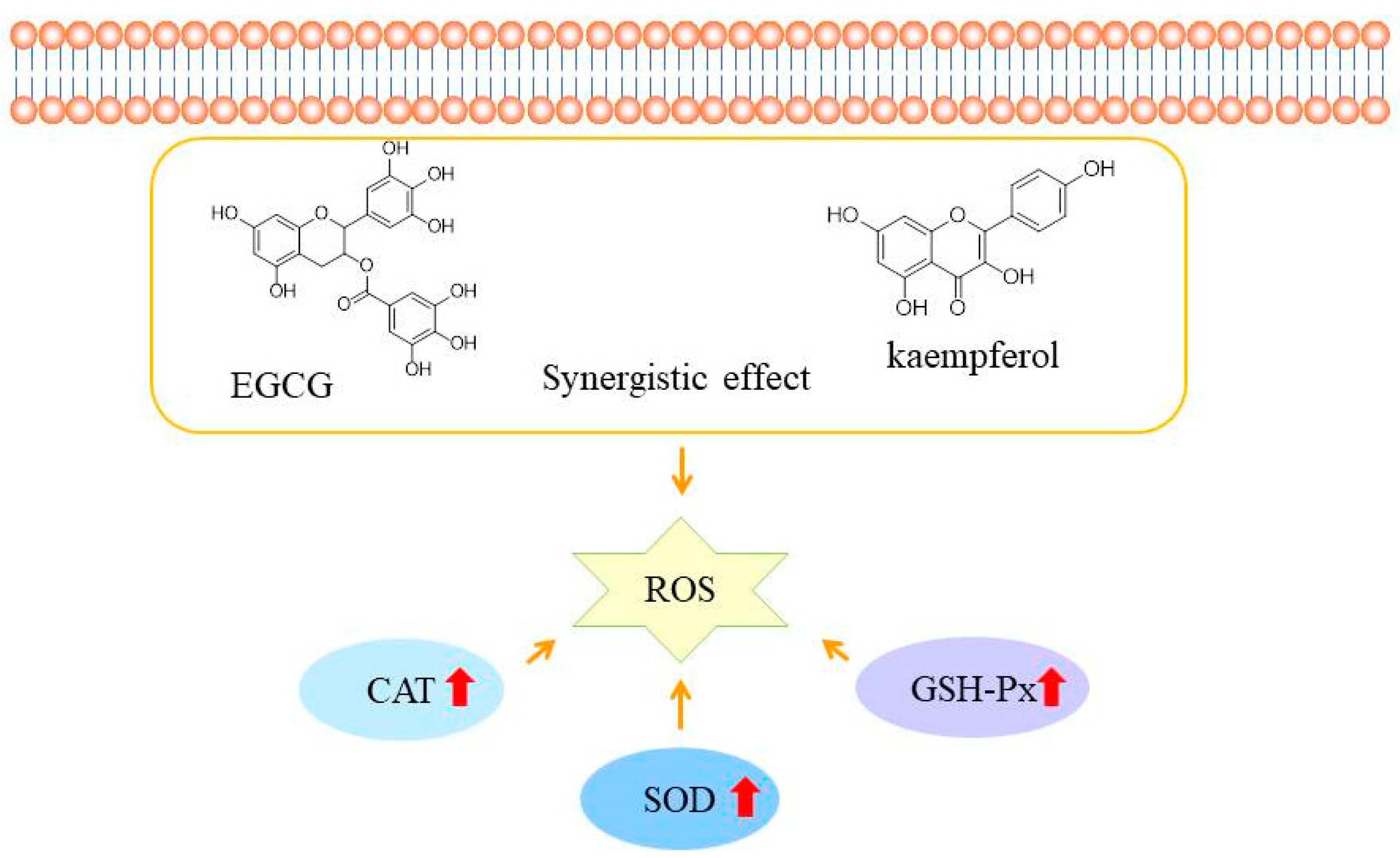
2.4. Effect on Antioxidant Enzymes
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cytotoxicity Assay
3.4. Cellular Antioxidant Activity (CAA) Assay
3.5. Determination of Combination Index (CI) Values
3.6. Cellular Antioxidant Enzymes Activities of SOD, GSH-Px, and CAT
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular reactive oxygen species (ROS) in cardiovascular pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Liu, Z.Q. What about the progress in the synthesis of flavonoid from 2020? Eur. J. Med. Chem. 2022, 243, 114671. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Y.; Sun-Waterhouse, D.X.; Zhai, H.; Guan, H.; Rong, X.; Li, F.; Yu, J.C.; Li, D.P. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H2O2-stimulated HepG2 cells: Roles of BACH1 in Nrf2-dependent pathways. Free Radic. Biol. Med. 2020, 153, 122–131. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Coombs, M.R.P. Editorial: Immune modulation by flavonoids. Front. Immunol. 2022, 13, 899577. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Lan, H.N.; Liu, R.Y.; Liu, Z.H.; Li, X.; Li, B.Z.; Yuan, Y.J. Biological valorization of lignin to flavonoids. Biotechnol. Adv. 2023, 64, 108107. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Z.; Zhao, L.; Chen, L.; Yu, Y.; Wang, D.; Mao, X.; Cao, G.; Zhao, Z.; Yang, H. Unique roles in health promotion of dietary flavonoids through gut microbiota regulation: Current understanding and future perspectives. Food Chem. 2023, 399, 133959. [Google Scholar] [CrossRef]
- Liu, H.; Guan, H.; Tan, X.; Jiang, Y.; Li, F.; Sun-Waterhouse, D.; Li, D. Enhanced alleviation of insulin resistance via the IRS-1/Akt/FOXO1 pathway by combining quercetin and EGCG and involving miR-27a-3p and miR-96-5p. Free Radic. Biol. Med. 2022, 181, 105–117. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liu, H.; Xie, J.; Yin, W.; Xu, Z.; Ma, H.; Wu, W.; Zheng, M.; Liu, M.; et al. Characterization of the synergistic inhibitory effect of cyanidin-3-O-glucoside and catechin on pancreatic lipase. Food Chem. 2023, 404, 134672. [Google Scholar] [CrossRef]
- Li, K.; Yu, X.H.; Maskey, A.R.; Musa, I.; Wang, Z.Z.; Garcia, V.; Guo, A.; Yang, N.; Srivastava, K.; Dunkin, D.; et al. Cytochrome P450 3A4 suppression by epimedium and active compound kaempferol leads to synergistic anti-inflammatory effect with corticosteroid. Front. Pharmacol. 2022, 13, 1042756. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Bergsten, T.M.; Li, K.; Lantvit, D.D.; Murphy, B.T.; Burdette, J.E. Kaempferol, a phytoprogestin, induces a subset of progesterone-regulated genes in the uterus. Nutrients 2023, 15, 1407. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, Y.; Zhang, H.; Mine, Y.; Tsao, R. Anti-inflammatory and anti-oxidative activities of daidzein and its sulfonic acid ester derivatives. J. Funct. Foods 2017, 35, 635–640. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Liu, X.Y.; Liu, J.H.; Li, D.P. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-kappa B and MAPK signaling pathways. Phytother. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef]
- Colon, M.; Nerin, C. Synergistic, antagonistic and additive interactions of green tea polyphenols. Eur. Food Res. Technol. 2016, 242, 211–220. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Long, C.; Xu, J.; Cen, J.; Yang, X. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, G.; Zhang, S.; Wang, H.; Ke, L.; Zhou, J.; Rao, P.; Wang, Q.; Li, J. Influences of calcium and magnesium ions on cellular antioxidant activity (CAA) determination. Food Chem. 2020, 320, 126625. [Google Scholar] [CrossRef]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes, X.; Ramirez, C.; Pung, D.; Kong, A.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Liu, Q.; Li, P.; Liu, E.H. Discovery of synergistic anti-inflammatory compound combination from herbal formula GuGe FengTong Tablet. Chin. J. Nat. Med. 2018, 16, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, D.; Sun, J.; Luo, X.; Li, H.; Sun, X.; Zheng, F. Analysis of antioxidant effect of two tripeptides isolated from fermented grains (Jiupei) and the antioxidative interaction with 4-methylguaiacol, 4-ethylguaiacol, and vanillin. Food Sci. Nutr. 2019, 7, 2391–2403. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, M.F.; You, L.J. Protective effect of peptides from Pinctada martensii meat on the H2O2-induced oxidative injured HepG2 cells. Antioxidants 2023, 12, 12020535. [Google Scholar] [CrossRef]
- Huo, J.; Ming, Y.; Li, H.; Li, A.; Zhao, J.; Huang, M.; Sun, W.; Wu, J.; Zhang, J. The protective effects of peptides from Chinese baijiu on AAPH-induced oxidative stress in HepG2 cells via Nrf2-mediated signaling pathway. Food Sci. Hum. Well. 2022, 11, 1527–1538. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.B.; Liu, J.C.; Liu, H.; Lv, Z.Z.; Zhang, C.L.; Chen, D.L.; Jiao, Z.G. Identification of sis flavonoids as novel cellular antioxidants and their structure-activity relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.M.; He, X.U.; Dong, M.; Zhang, Q.Y.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, Y.; Huang, R.; Zhao, Z. Identification and structure-activity relationship of recovered phenolics with antioxidant and antihyperglycemic potential from sugarcane molasses vinasse. Foods 2022, 11, 3131. [Google Scholar] [CrossRef]
- Banerjee, V.; Sharda, N.; Huse, J.; Singh, D.; Sokolov, D.; Czinn, S.J.; Blanchard, T.G.; Banerjee, A. Synergistic potential of dual andrographolide and melatonin targeting of metastatic colon cancer cells: Using the Chou-Talalay combination index method. Eur. J. Pharmacol. 2021, 897, 173919. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Shi, D.; Yang, J.; Jiang, Y.; Wen, L.; Wang, Z.; Yang, B. The antioxidant activity and neuroprotective mechanism of isoliquiritigenin. Free Radic. Biol. Med. 2020, 152, 207–215. [Google Scholar] [CrossRef]

| Compound | Proportion (c/c) | CI | CIavg | ||
|---|---|---|---|---|---|
| GI50 | GI75 | GI90 | |||
| EGCG + kaempferol | 6:2 | 1.07 ± 0.02 | 0.89 ± 0.01 | 0.74 ± 0.01 | 0.85 a |
| 6:1.5 | 0.58 ± 0.01 | 0.55 ± 0.02 | 0.52 ± 0.01 | 0.54 b | |
| 7:1.5 | >1 | >1 | >1 | >1 | |
| Compound (μg/mL) | SOD (U/mg Protein) | GSH-Px (mU/mg Protein) | CAT (U/mg Protein) |
|---|---|---|---|
| control | 29.3 ± 2.2 a | 310.5 ± 5.4 a | 61.3 ± 3.1 a |
| ABAP | 14.3 ± 1.1 f | 133.3 ± 2.3 e | 28.5 ± 2.2 e |
| 1.5 + 0.375 | 15.2 ± 0.8 e | 143.4 ± 4.1 d | 30.2 ± 1.2 d |
| 3 + 0.75 | 18.2 ± 1.3 c | 170.3 ± 3.7 c | 35.4 ± 2.4 c |
| 6 + 1.5 | 22.6 ± 1.5 b | 210.1 ± 4.8 b | 40.5 ± 2.5 b |
| 6 | 16.5 ± 1.2 d | 150.2 ± 2.5 d | 31.3 ± 1.3 d |
| 1.5 | 15.4 ± 0.6 e | 148.4 ± 2.5 d | 30.5 ± 0.5 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Pan, J.; Liu, H.; Jiao, Z. Characterization of the Synergistic Antioxidant Activity of Epigallocatechin Gallate (EGCG) and Kaempferol. Molecules 2023, 28, 5265. https://doi.org/10.3390/molecules28135265
Zhang Q, Pan J, Liu H, Jiao Z. Characterization of the Synergistic Antioxidant Activity of Epigallocatechin Gallate (EGCG) and Kaempferol. Molecules. 2023; 28(13):5265. https://doi.org/10.3390/molecules28135265
Chicago/Turabian StyleZhang, Qiang, Junkun Pan, Hui Liu, and Zhonggao Jiao. 2023. "Characterization of the Synergistic Antioxidant Activity of Epigallocatechin Gallate (EGCG) and Kaempferol" Molecules 28, no. 13: 5265. https://doi.org/10.3390/molecules28135265
APA StyleZhang, Q., Pan, J., Liu, H., & Jiao, Z. (2023). Characterization of the Synergistic Antioxidant Activity of Epigallocatechin Gallate (EGCG) and Kaempferol. Molecules, 28(13), 5265. https://doi.org/10.3390/molecules28135265








