Anti-Inflammatory Effects of β-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes
Abstract
1. Introduction
2. Results
2.1. Cell Growth and Morphology of 5-FU and β-Cry Treated Human Normal Oral Mucosal Keratinocytes (hOMK)
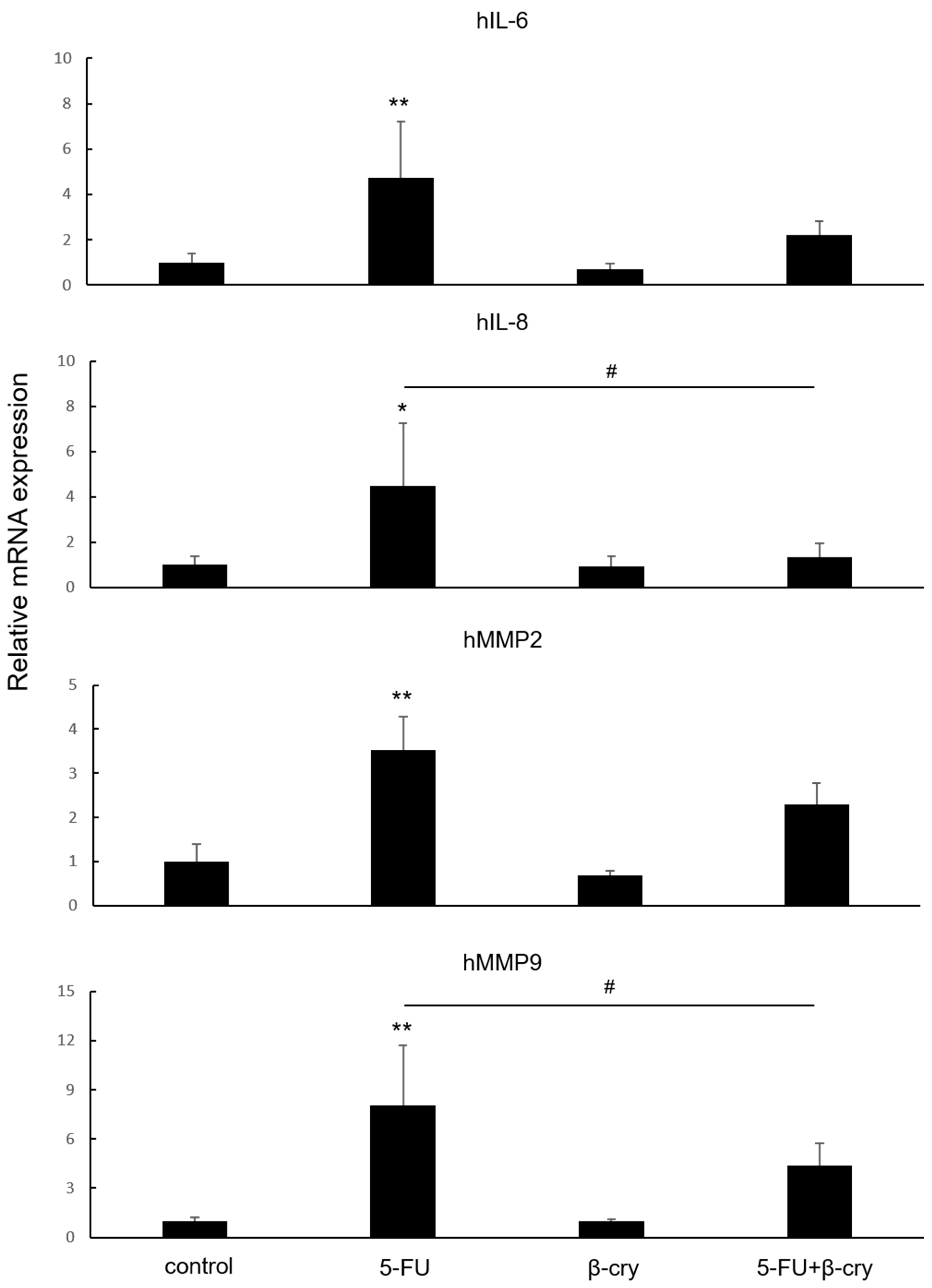
2.2. Effects of β-Cry on 5-FU-Induced mRNA Expression of Inflammatory Cytokines and MMPs in hOMK
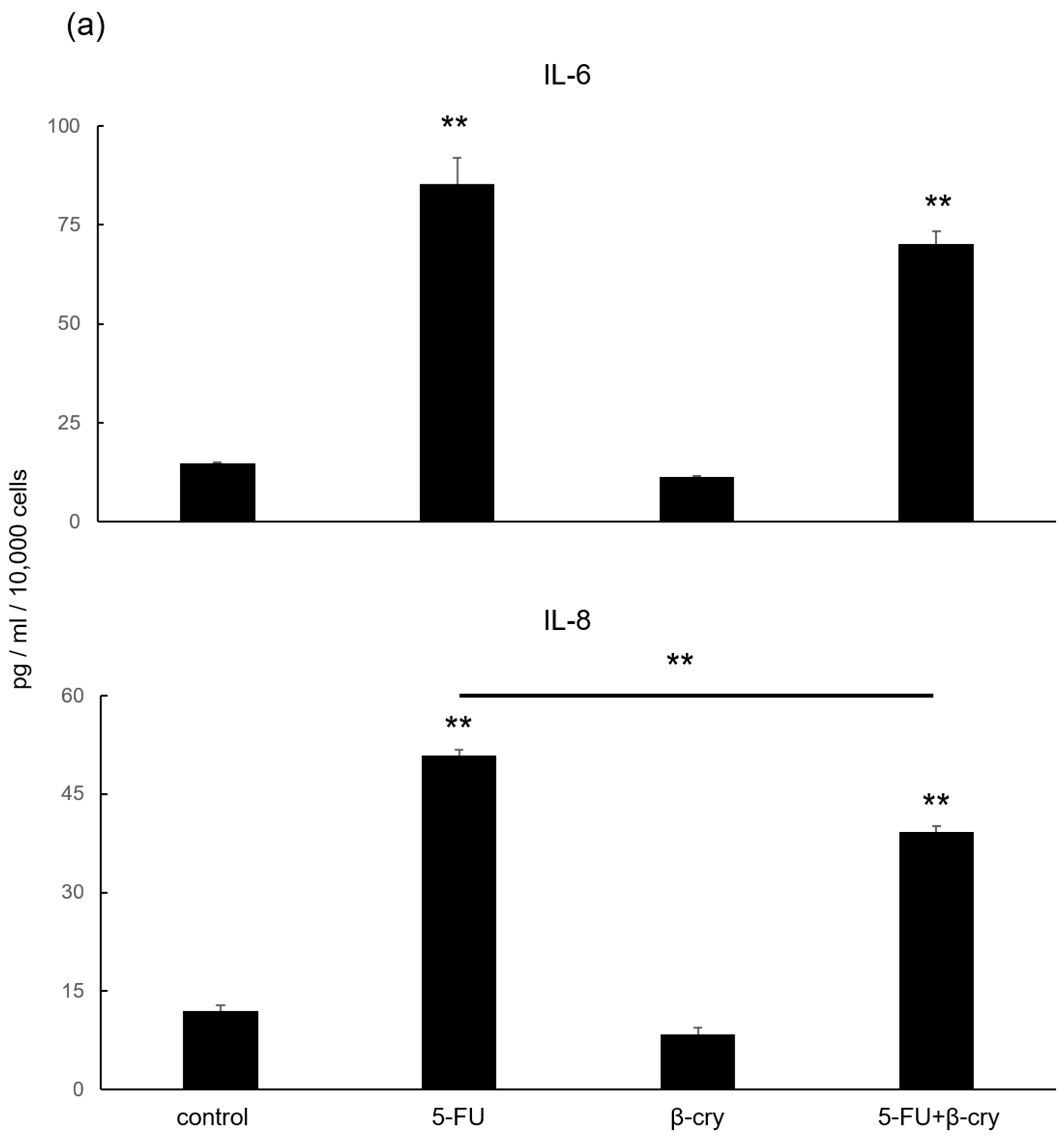
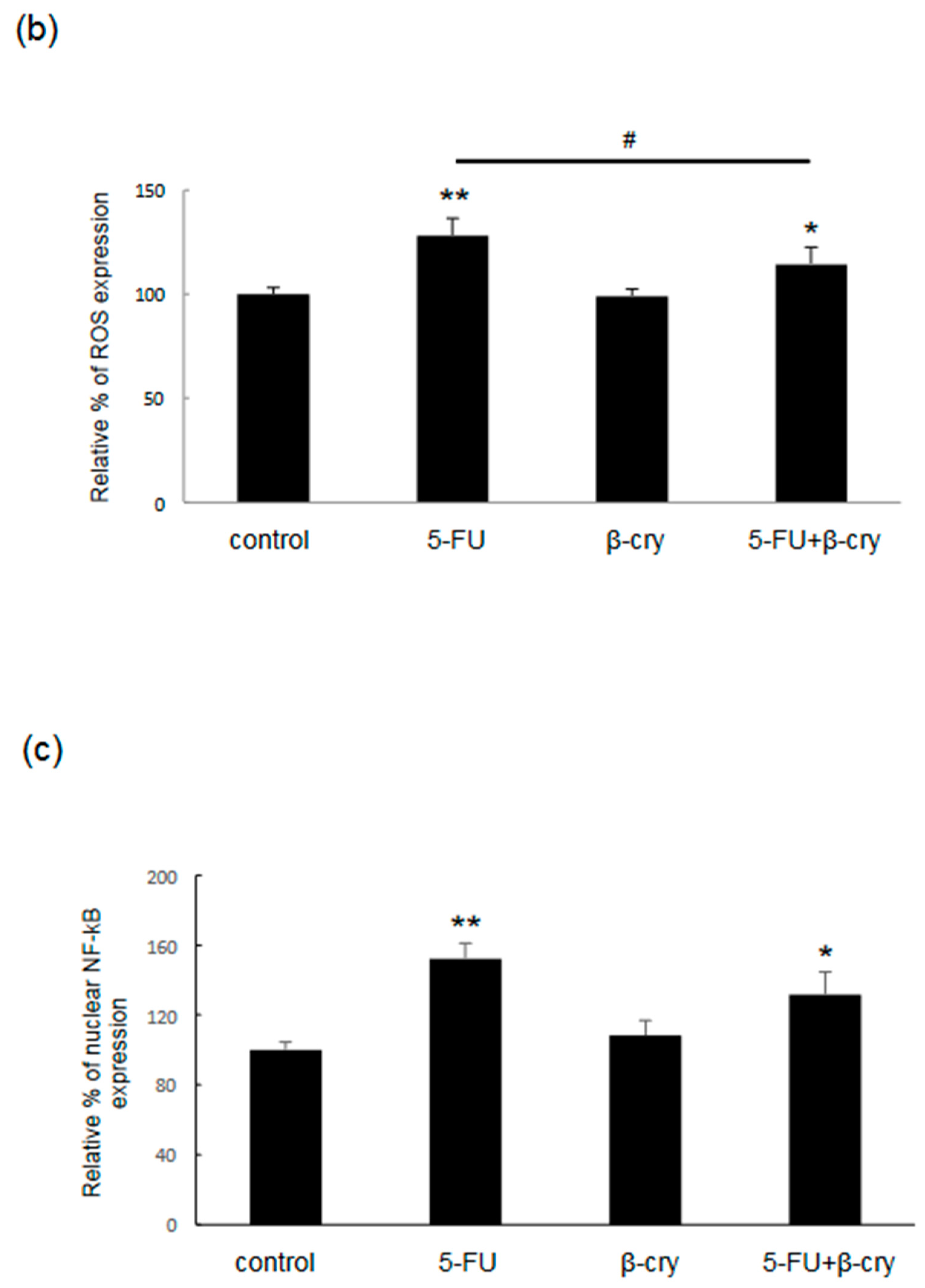
2.3. Effects of β-Cry on 5-FU-Induced IL-6, IL-8, and ROS Production in hOMK
2.4. Effects of β-Cry on 5-FU-Induced NF-κB Activation in hOMK
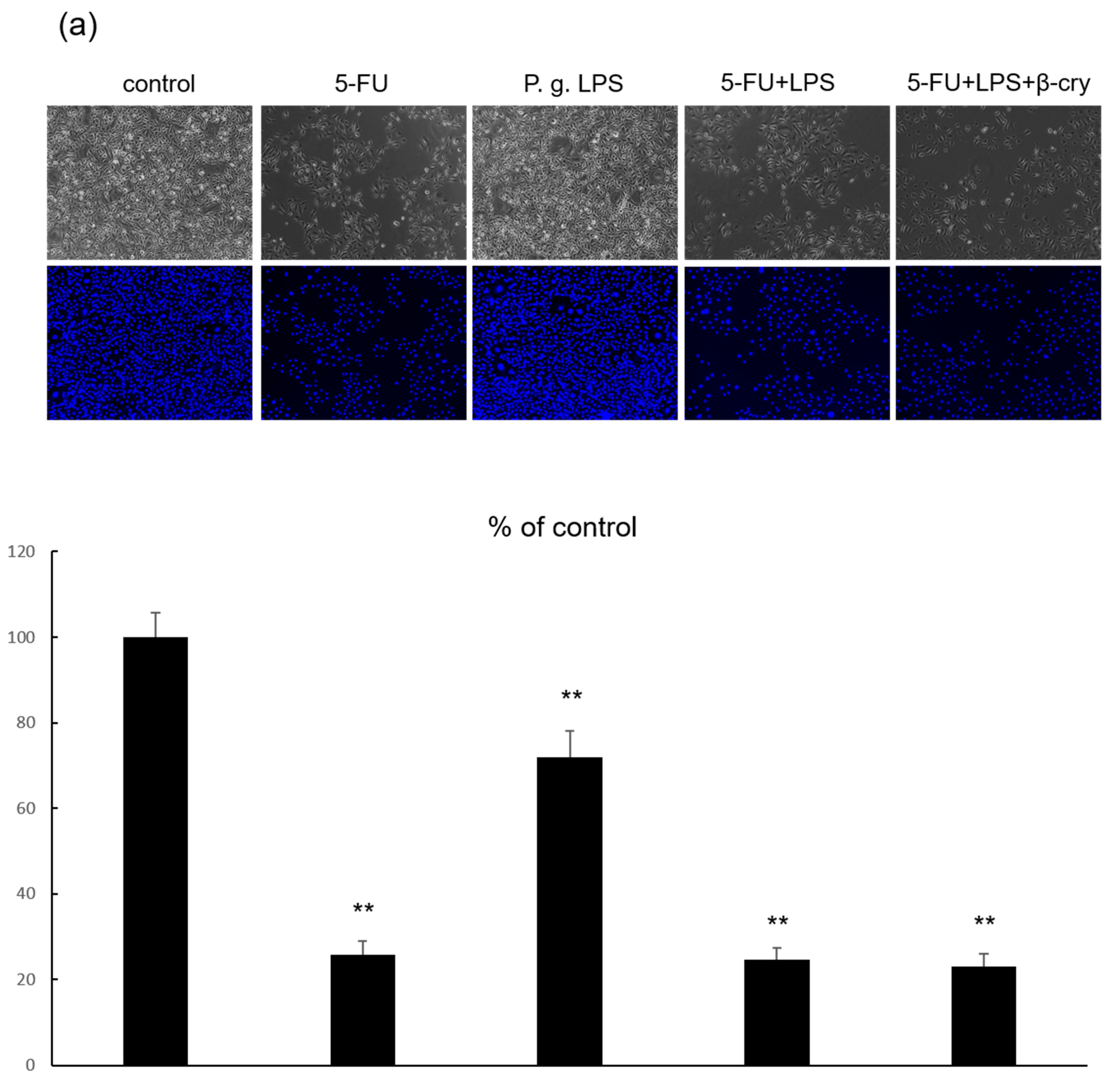
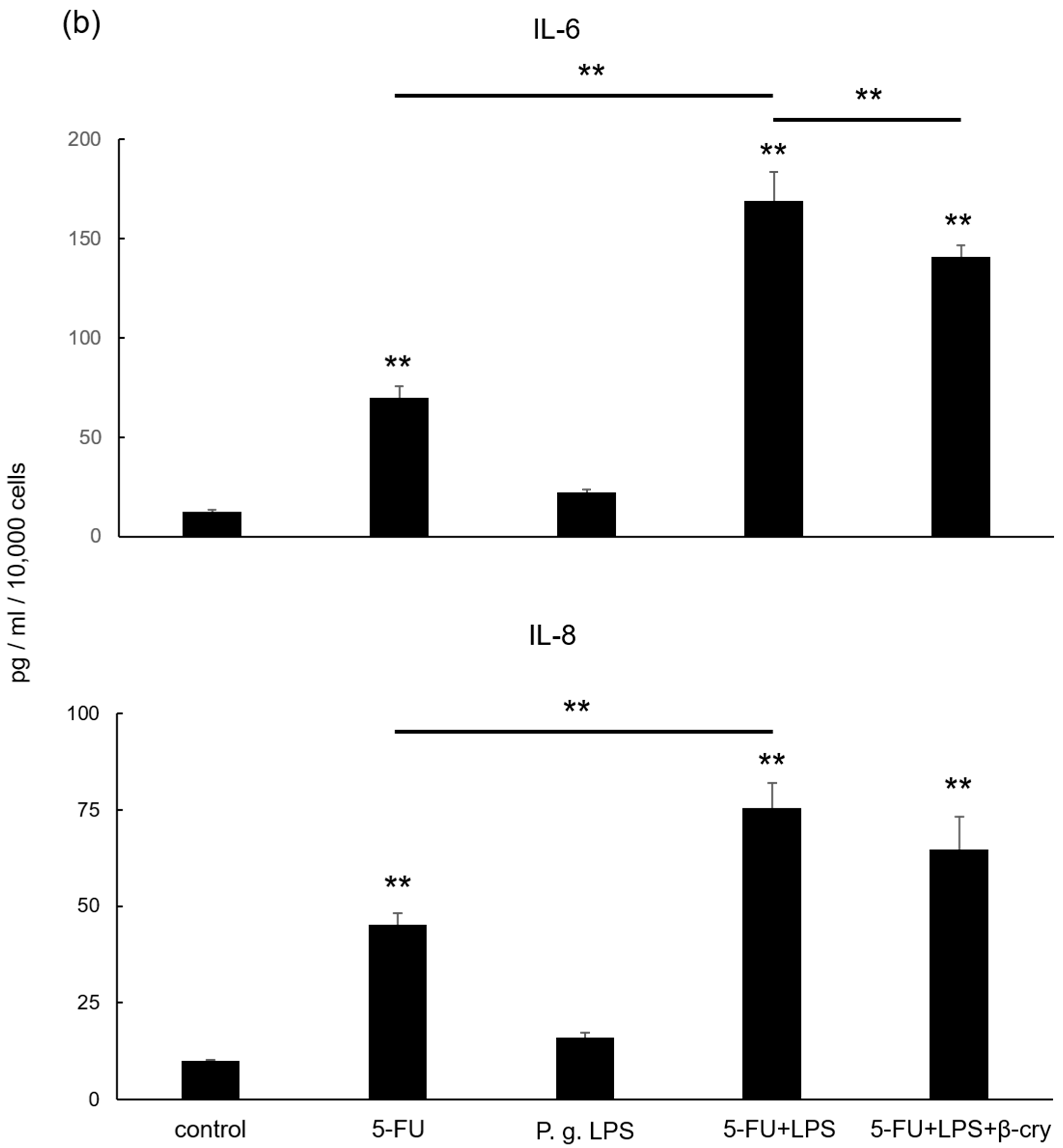
2.5. Effects of P. gingivalis Lipopolysaccharide (LPS) on IL-6 and IL-8 Production in 5-FU-Treated hOMK
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Cellular Growth and Morphology
4.4. Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. ROS Assay
4.7. NF-κB Activity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scully, C.; Epstein, J.B. Oral health care for the cancer patient. Eur. J. Cancer B Oral Oncol. 1996, 32B, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef]
- Oba, M.K.; Innocentini, L.M.A.R.; Viani, G.; Ricz, H.M.A.; de Carvalho Reis, T.; Ferrari, T.C.; de Macedo, L.D. Evaluation of the correlation between side effects to oral mucosa, salivary glands, and general health status with quality of life during intensity-modulated radiotherapy for head and neck cancer. Support Care Cancer 2021, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Alsheyyab, F.; Al-Momani, D.; Kasht, R.; Kamal, A.; Abusalem, D.; Al-Qasem, W. Impact of severe oral mucositis in pediatric cancer patients on resource utilization and cancer treatment plans. Int. J. Clin. Pharm. 2021, 43, 1322–1326. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer, & International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Schneider, J.J.; Galettis, P.; Martin, J.H. Overcoming barriers to implementing precision dosing with 5-fluorouracil and capecitabine. Br. J. Clin. Pharmacol. 2021, 87, 317–325. [Google Scholar] [CrossRef]
- Cardona-G, W.; Herrera-R, A.; Castrillón-L, W.; Ramírez-Malule, H. Chemistry and anticancer activity of hybrid molecules and derivatives based on 5-Fluorouracil. Curr. Med. Chem. 2021, 28, 5551–5601. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Nakajima, A.; Wada-Takahashi, S.; Takahashi, S.; Lee, M.C. Alteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamsters. PLoS ONE 2013, 8, e82834. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Mangoni, M.; Gerini, C.; Salvatore, G.; Castiglione, F.; Desideri, I.; Bonomo, P.; Meattini, I.; Greto, D.; Loi, M.; et al. Peroxisome proliferator activated receptor-gamma stimulation for prevention of 5-fluorouracil-induced oral mucositis in mice. Head Neck 2018, 40, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of oral mucositis due to chemotherapy. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, C.E. Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment. Pharmacotherapy 2005, 25, 540–554. [Google Scholar] [CrossRef]
- Hayashi, H.; Kobayashi, R.; Suzuki, A.; Yamada, Y.; Ishida, M.; Shakui, T.; Kitagawa, J.; Hayashi, H.; Sugiyama, T.; Takeuchi, H.; et al. Preparation and clinical evaluation of a novel lozenge containing polaprezinc, a zinc-L-carnosine, for prevention of oral mucositis in patients with hematological cancer who received high-dose chemotherapy. Med. Oncol. 2016, 33, 91. [Google Scholar] [CrossRef]
- Manzi, N.d.M.; Silveira, R.C.; dos Reis, P.E. Prophylaxis for mucositis induced by ambulatory chemotherapy: Systematic review. J. Adv. Nurs. 2016, 72, 735–746. [Google Scholar] [CrossRef]
- Sugiura, M. Beta-Cryptoxanthin and the risk for lifestyle-related disease: Findings from recent nutritional epidemiologic studies. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2015, 135, 67–76. [Google Scholar] [CrossRef]
- Lorenzo, Y.; Azqueta, A.; Luna, L.; Bonilla, F.; Domínguez, D.; Collins, A.R. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009, 30, 308–314. [Google Scholar] [CrossRef]
- Liu, X.R.; Wang, Y.Y.; Dan, X.G.; Kumar, A.; Ye, T.Z.; Yu, Y.Y.; Yang, L.G. Anti-inflammatory potential of β-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod. Toxicol. 2016, 60, 148–155. [Google Scholar] [CrossRef]
- Quesada-Gómez, J.M.; Santiago-Mora, R.; Durán-Prado, M.; Dorado, G.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Casado-Díaz, A. β-cryptoxanthin inhibits angiogenesis in human umbilical vein endothelial cells through retinoic acid receptor. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Okamoto, M.; Fukasawa, K.; Iezaki, T.; Onishi, Y.; Yoneda, Y.; Sugiura, M.; Hinoi, E. Daily intake of β-cryptoxanthin prevents bone loss by preferential disturbance of osteoclastic activation in ovariectomized mice. J. Pharmacol. Sci. 2015, 129, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Horie, T.; Fukasawa, K.; Ozaki, K.; Onishi, Y.; Kanayama, T.; Iezaki, T.; Kaneda, K.; Sugiura, M.; Hinoi, E. Amelioration of the development of osteoarthritis by daily intake of β-cryptoxanthin. Biol. Pharm. Bull. 2017, 40, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Swendseid, M.E.; Jacob, R.A.; McKee, R.W. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J. Nutr. 1992, 122, 96–100. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. β-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef]
- Jianhua, L.; Liqing, Z.; Yingxin, L.; Zhihua, Z.; Li, Z.; Lixia, S.; Xiulong, Z.; Haixia, Q. Effect of DTPP-mediated photodynamic therapy on cell morphology, viability, cell cycle, and cytotoxicity in a murine lung adenocarcinoma cell line. Lasers Med. Sci. 2015, 30, 181–191. [Google Scholar] [CrossRef]
- Chauvin, A.; Bergeron, D.; Vencic, J.; Lévesque, D.; Paquette, B.; Scott, M.S.; Boisvert, F.M. Downregulation of KRAB zinc finger proteins in 5-fluorouracil resistant colorectal cancer cells. BMC Cancer 2022, 22, 363. [Google Scholar] [CrossRef]
- Avallone, A.; Di Gennaro, E.; Silvestro, L.; Iaffaioli, V.R.; Budillon, A. Targeting thymidylate synthase in colorectal cancer: Critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin. Drug Saf. 2014, 13, 113–129. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Wanderley, C.W.S.; Wong, D.V.; Mota, J.M.; Leite, C.A.; Souza, M.H.; Cunha, F.Q.; Lima, R.C., Jr. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Gibson, R.J.; Bowen, J.M.; Keefe, D.M. Matrix metalloproteinases: Key regulators in the pathogenesis of chemotherapy-induced mucositis? Cancer Chemother. Pharmacol. 2009, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol. Ther. 2008, 7, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Anthony, L.; Bowen, J.; Garden, A.; Hewson, I.; Sonis, S. New thoughts on the pathobiology of regimen-related mucosal injury. Support Care Cancer 2006, 14, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshioka, M.; Okamura, H.; Moriyama, S.; Kawazoe, K.; Grenier, D.; Hinode, D. Preventive effect of Daiokanzoto (TJ-84) on 5-fluorouracil-induced human gingival cell death through the inhibition of reactive oxygen species production. PLoS ONE 2014, 9, e112689. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, T.; Azuma, M.; Ashida, Y.; Motegi, K.; Takashima, R.; Harada, K.; Kawaguchi, S.; Sato, M. Enhanced radiosensitization and chemosensitization in NF-kappaB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int. J. Cancer 2004, 108, 912–921. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel milk ameliorates 5-fluorouracil-induced renal injury in rats: Targeting MAPKs, NF-κB and PI3K/Akt/eNOS pathways. Cell Physiol. Biochem. 2018, 46, 1628–1642. [Google Scholar] [CrossRef]
- Stringer, A.M.; Logan, R.M. The role of oral flora in the development of chemotherapy-induced oral mucositis. J. Oral Pathol. Med. 2015, 44, 81–87. [Google Scholar] [CrossRef]
- Vanlancker, E.; Vanhoecke, B.; Sieprath, T.; Bourgeois, J.; Beterams, A.; De Moerloose, B.; De Vos, W.H.; Van de Wiele, T. Oral microbiota reduce wound healing capacity of epithelial monolayers, irrespective of the presence of 5-fluorouracil. Exp. Biol. Med. 2018, 243, 350–360. [Google Scholar] [CrossRef]
- Haverman, T.M.; Laheij, A.M.G.A.; de Soet, J.J.; Lange, J.; Rozema, F.R. Candida and Porphyromonas gingivalis: The effect on wound closure in vitro. J. Oral Microbiol. 2017, 9, 1328266. [Google Scholar] [CrossRef] [PubMed]
- Haverman, T.M.; Laheij, A.M.G.A.; Nie, M.; Deng, D.M.; Raber-Durlacher, J.E.; de Soet, J.J.; Rozema, F.R. Exploring the role of oral microorganisms in the pathogenesis of mucositis by assessing their impact on metabolic activity and reproductive capacity of epithelial cells in vitro. Support Care Cancer 2020, 28, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ferdous, T.; Mizukami, Y.; Mishima, K. Elemental diet inhibits pro-inflammatory cytokine production in keratinocytes through the suppression of NF-κB activation. Oncol. Rep. 2018, 40, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Momota, Y.; Kani, K.; Aota, K.; Yamamura, Y.; Yamanoi, T.; Azuma, M. γ-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2. Int. J. Oncol. 2015, 46, 1453–1460. [Google Scholar] [CrossRef]
- Kobori, M.; Ni, Y.; Takahashi, Y.; Watanabe, N.; Sugiura, M.; Ogawa, K.; Nagashimada, M.; Kaneko, S.; Naito, S.; Ota, T. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLoS ONE 2014, 9, e98294. [Google Scholar] [CrossRef]
- Haidari, F.; Hojhabrimanesh, A.; Helli, B.; Seyedian, S.S.; Ahmadi-Angali, K. An energy-restricted high-protein diet supplemented with β-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: A randomized controlled trial. Nutr. Res. 2020, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J.; Chang, J.S.; Neidlinger, T.R. β-Cryptoxanthin- and α-carotene-rich foods have greater apparent bioavailability than β-carotene-rich foods in Western diets. Br. J. Nutr. 2011, 105, 212–219. [Google Scholar] [CrossRef]
- De Pee, S.; West, C.E.; Permaesih, D.; Martuti, S.; Muhilal, H.J. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and β-carotene in schoolchildren in Indonesia. Am. J. Clin. Nutr. 1998, 68, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.M.W.; et al. Beta-cryptoxanthin inhibits lipopolysaccharide-induced osteoclast differentiation and bone resorption via the suppression of inhibitor of NF-κB Kinase activity. Nutrients 2019, 11, 368. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, D.; Wang, X.; Zhang, Y.; Duan, W.; Li, Y. β-cryptoxanthin alleviates myocardial ischaemia/reperfusion injury by inhibiting NF-κB-mediated inflammatory signalling in rats. Arch. Physiol. Biochem. 2022, 128, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Lee, S.E.; Yoon, J.W.; Park, H.J.; Kim, S.H.; Oh, S.H.; Lee, D.G.; Pyeon, D.B.; Kim, E.Y.; Park, S.P. Comparison of three antioxidants in chemical and biological assays on porcine oocytes during ageing in vitro. Zygote 2022, 30, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Hayaishi, O. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. USA 1965, 54, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.M.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary b-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Gao, M.; Dang, F.; Deng, C. β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sato, Y.; Honjo, K.; Ichioka, H.; Oseko, F.; Sowa, Y.; Yamamoto, T.; Kanamura, N.; Kishida, T.; Mazda, O. Generation of Directly Converted Human Osteoblasts That Are Free of Exogenous Gene and Xenogenic Protein. J. Cell. Biochem. 2016, 117, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kishida, T.; Nakai, K.; Sato, Y.; Kotani, S.I.; Nishizawa, Y.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal. Sci. Rep. 2018, 8, 8463. [Google Scholar] [CrossRef]
- Fekrazad, R.; Chiniforush, N. Oral mucositis prevention and management by therapeutic laser in head and neck cancers. J. Lasers Med. Sci. 2014, 5, 1–7. [Google Scholar]
- Alterio, D.; Jereczek-Fossa, B.; Fiore, M.R.; Piperno, G.; Mohssen, A.; Orecchia, R. Cancer treatment induced oral mucositis. Anticancer Res. 2007, 27, 1105–1125. [Google Scholar]
- Block, K.I.; Koch, A.C.; Mead, M.K.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemo therapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef]
- Camphausen, K.; Citrin, D.; Krishna, M.C.; Mitchell, J.B. Implications for tumour control during protection of normal tissues with anti oxidants. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5455–5457. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanobe, H.; Yamamoto, K.; Kishimoto, S.; Nakai, K.; Oseko, F.; Yamamoto, T.; Mazda, O.; Kanamura, N. Anti-Inflammatory Effects of β-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes. Molecules 2023, 28, 2935. https://doi.org/10.3390/molecules28072935
Yamanobe H, Yamamoto K, Kishimoto S, Nakai K, Oseko F, Yamamoto T, Mazda O, Kanamura N. Anti-Inflammatory Effects of β-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes. Molecules. 2023; 28(7):2935. https://doi.org/10.3390/molecules28072935
Chicago/Turabian StyleYamanobe, Hironaka, Kenta Yamamoto, Saki Kishimoto, Kei Nakai, Fumishige Oseko, Toshiro Yamamoto, Osam Mazda, and Narisato Kanamura. 2023. "Anti-Inflammatory Effects of β-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes" Molecules 28, no. 7: 2935. https://doi.org/10.3390/molecules28072935
APA StyleYamanobe, H., Yamamoto, K., Kishimoto, S., Nakai, K., Oseko, F., Yamamoto, T., Mazda, O., & Kanamura, N. (2023). Anti-Inflammatory Effects of β-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes. Molecules, 28(7), 2935. https://doi.org/10.3390/molecules28072935




