Functionally Active Microheterogeneous Systems for Elastomer Fire- and Heat-Protective Materials
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zaikov, G.E.; Kalugina, E.V.; Gumargalieva, K.Z. Fundamental Regularities of Thermal Oxidation of Heat-Resistant Heterochain Polymers—Thermal Stability of Engineering Heterochain Thermoresistant Polymers; Utrecht: Boston, MA, USA, 2004; pp. 15–57. [Google Scholar]
- Mikhailin, Y.A. Structural Polymeric Composite Materials; Nauka: St. Petersburg, Russia, 2010; pp. 25–67. [Google Scholar]
- Walter, M.D.; Wajer, M.T. Overveiw of Flame Retardants Including Magnesium Hydroxine; Martin Marietta Magnesia Specialties LLC: Nottingham, MD, USA, 2010. [Google Scholar]
- Bhuvaneswari, C.M.; Surehkumar, M.S.; Kakade, S.D. Manoj Gupta Ethylene-propylene Diene Rubber as a Futuristic Elastomer for Insulation of Solid Rocket Motors. Def. Sci. J. 2006, 56, 309–320. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hoa, S.V. Thermal insulation by heat resistant polymers for solid rocket motor insulation. J. Compos. Mater. 2012, 46, 1544–1599. [Google Scholar] [CrossRef]
- Mariappan, T. Fire Retardant Coatings. In New Technologies in Protective Coatings; BoD—Books on Demand: Paris, France, 2017. [Google Scholar] [CrossRef]
- Kablov, V.F.; Novopoltseva, O.M.; Kochetkov, V.G.; Lapina, A.G.; Pudovkin, V.V. Elastomer heat-shielding materials containing aluminosilicate microspheres. Russ. Eng. Res. 2017, 37, 1059–1061. [Google Scholar] [CrossRef]
- Markowski, J. Cenospheres. Anuniversal construction material. Przem. Chem. 2019, 98, 940–943. [Google Scholar] [CrossRef]
- Bezzaponnaya, O.V.; Golovina, E.V. Effect of mineral fillers on the heat resistance and combustibility of an intumescent fire proofing formulation on silicone base. Russ. J. Appl. Chem. 2018, 91, 96–100. [Google Scholar] [CrossRef]
- Kablov, V.F.; Keibal, N.A.; Kochetkov, V.G.; Motchenko, A.O.; Antonov, Y.M. Research of the influence of carbon microfiber on the properties of elastomer fire-protective materials. Russ. J. Appl. Chem. 2018, 91, 1160–1164. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The effects of triphenylphosphate and recorcinolhis on the thermal degradation of polycarbonate in air. Thermochim. Acta 2005, 433, 1–12. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Yang, L.; Cai, Y.; Song, L.; Chen, Z.; Fan, W. Fire retardant synergism between melamine and triphenyl phosphate in poly(butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 2093–2100. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B.; Fichera, M.A.; Jager, C. Flame Retardancy Mechanisms of Bisphenol a- Bis(diphenyl phosphate) in Combination with Zinc Borate in Bisphenol a-Polycarbonate/Acrylonitrile-Butadiene-Styrene Blends. Thermochim. Acta 2010, 49, 92–99. [Google Scholar] [CrossRef]
- Kashiwagi, T. Flame Retardant Mechanism of the Nanotubes-Based Nanocomposites; Final Report. NIST GCR 07-912; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2007; p. 65. [Google Scholar]
- Bourbigot, S.; Le Bras, M. Flame Retardant Plastics. In Plastics Flammability Handbook; Manser: Munich, Germany, 2004; Volume 5, pp. 133–172. [Google Scholar] [CrossRef]
- Beach, M.W.; Rondan, N.G.; Froese, R.D.; Gerhart, B.B.; Green, J.G.; Stobby, B.G.; Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P. Studies of degradation enhancement of polystyrene by flame retardant additives. Polym. Degrad. Stab. 2008, 9, 1664–1673. [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Professional: New York, NY, USA, 2006; pp. 159–176. [Google Scholar]
- Koo, J.H.; Miller, M.J.; Weispfenning, J.; Blackmon, C. Silicone polymer composites for thermal protection system: Fiber reinforcements and microstructures. J. Compos. Mater. 2011, 45, 1363–1380. [Google Scholar] [CrossRef]
- Li, J.; Hu, B.; Hui, K.; Li, K.; Wang, L. Erosion resistance of ethylene propylene diene monomer insulations reinforced with precoated multi-walled carbon nanotubes. Acta Astronaut. 2022, 198, 251–257. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Marzec, A.; Szadkowski, B.; Kuśmierek, M.; Śliwka-Kaszyńska, M.; Mirkhodjaev, U.Z. Effects of Basalt and Carbon Fillers on Fire Hazard, Thermal, and Mechanical Properties of EPDM Rubber Composites. Materials 2021, 14, 5245. [Google Scholar] [CrossRef]
- Raask, E. Cenospheres in pulverized fuel ash. J. Inst. Fuel 1968, 43, 339–344. [Google Scholar]
- Rose, N.L. Inorganie flu-ash spheres as pollution. Trasers Environ. Pollut. 1996, 91, 245–252. [Google Scholar] [CrossRef]
- Pandey, G.S.; Gain, V.K. Cenosphere-load in coal ash diseharge ofthermal power plant. Res. Ind. 1993, 38, 99–100. [Google Scholar]
- Wang, S.; Wang, L.; Su, H.; Li, C.; Fan, W.; Jing, X. Enhanced thermal resistance and ablation properties of ethylene-propylene-diene monomer rubber with boron-containing phenolic resins. React. Funct. Polym. 2022, 170, 105136. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Wang, Y. Effects of carbon nanotubes on char structure and heat transfer in ethylene propylene diene monomer composites at high temperature. Compos. Sci. Technol. 2021, 211, 108852. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Ge, X. Effect of various fibers on the ablation resistance of poly(diaryloxyphosphazene) elastomer. J. Appl. Polym. Sci. 2020, 137, 48534. [Google Scholar] [CrossRef]
- Iqbal, N.; Sagar, S.; Khan, M.B.; Rafique, H.M. Elastomeric ablative nanocomposites used in hyperthermal environments. Polym. Eng. Sci. 2014, 54, 255–263. [Google Scholar] [CrossRef]
- Tate, J.S.; Gaikwad, S.; Theodoropoulou, N.; Trevino, E.; Koo, J.H. Carbon/phenolic nanocomposites as advanced thermal protection material in aerospace applications. J. Compos. 2013, 2013, 403656. [Google Scholar] [CrossRef]
- Kablov, V.F.; Novopol’tseva, O.M.; Kochetkov, V.G.; Pudovkin, V.V. Physicomechanical, thermal, and flame-retardant properties of elastomer compounds based on ethylene–propylene–diene rubber and filled with hollow aluminosilicate microspheres. Russ. J. Appl. Chem. 2017, 90, 257–261. [Google Scholar] [CrossRef]
- Ravishankar, P.S. Treatise on EPDM. Rubber Chem. Technol. 2012, 85, 327–349. [Google Scholar] [CrossRef]
- Kablov, V.F.; Bondarenko, S.N.; Vasilkova, L.A. Properties of fire-retardant coating. Nov. Mater. 2013, 5, 61–66. [Google Scholar]
- ISO Standard 37-2013; Rubber, Vulcanized or Thermoplastic; Determination of Tensile Stress-Strain Properties. Standartinform Publ.: Moscow, Russia, 2014; p. 32.
- Kablov, V.F.; Kochetkov, V.G.; Keibal, N.A.; Novopol’tseva, O.M.; Kryukova, D.A. Modifier Based on Dicyandiamide and Dimethyl Phosphite for Fire and Heat Resistant Elastomer Materials. Russ. J. Appl. Chem. 2022, 95, 661–668. [Google Scholar] [CrossRef]

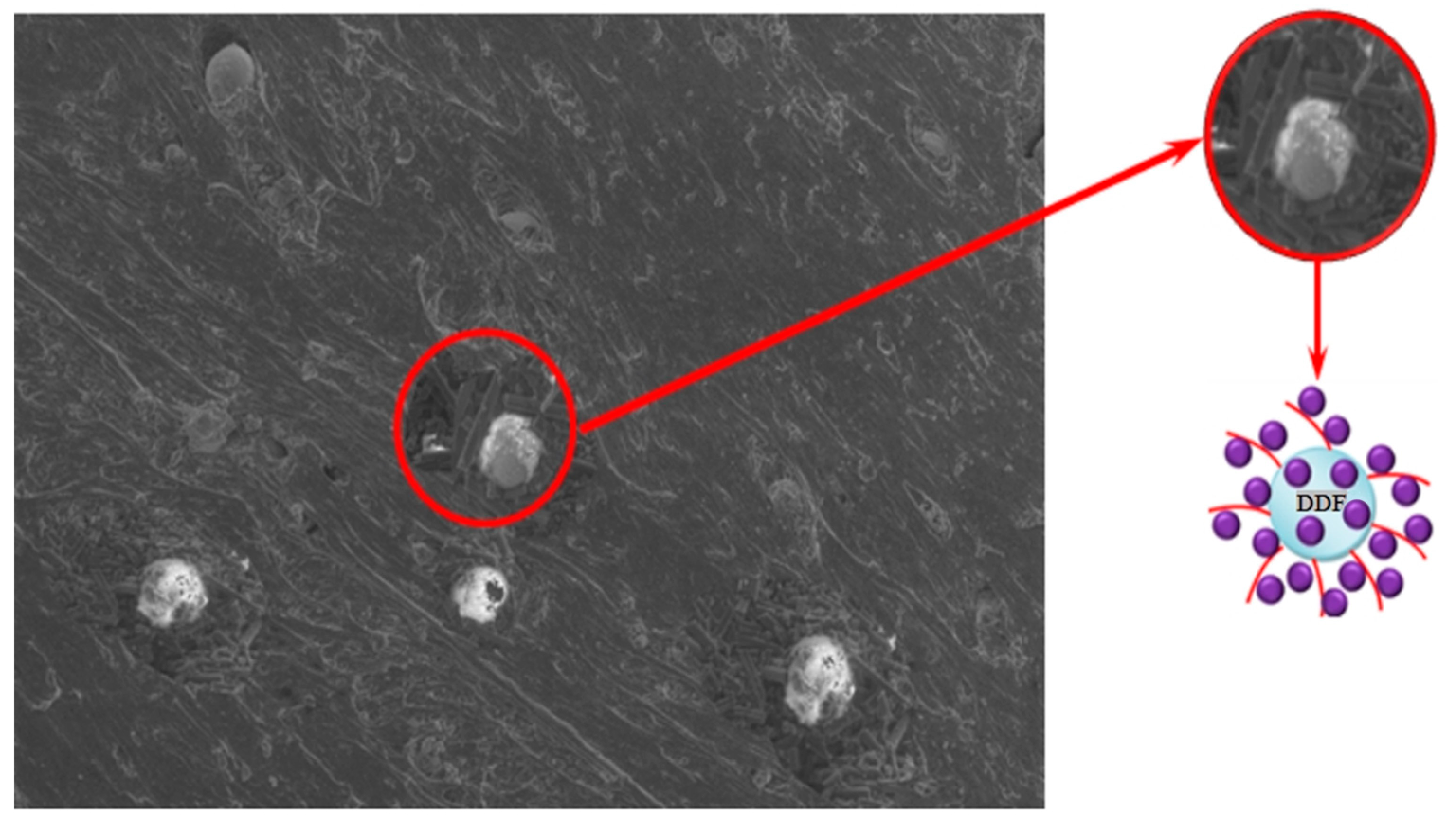
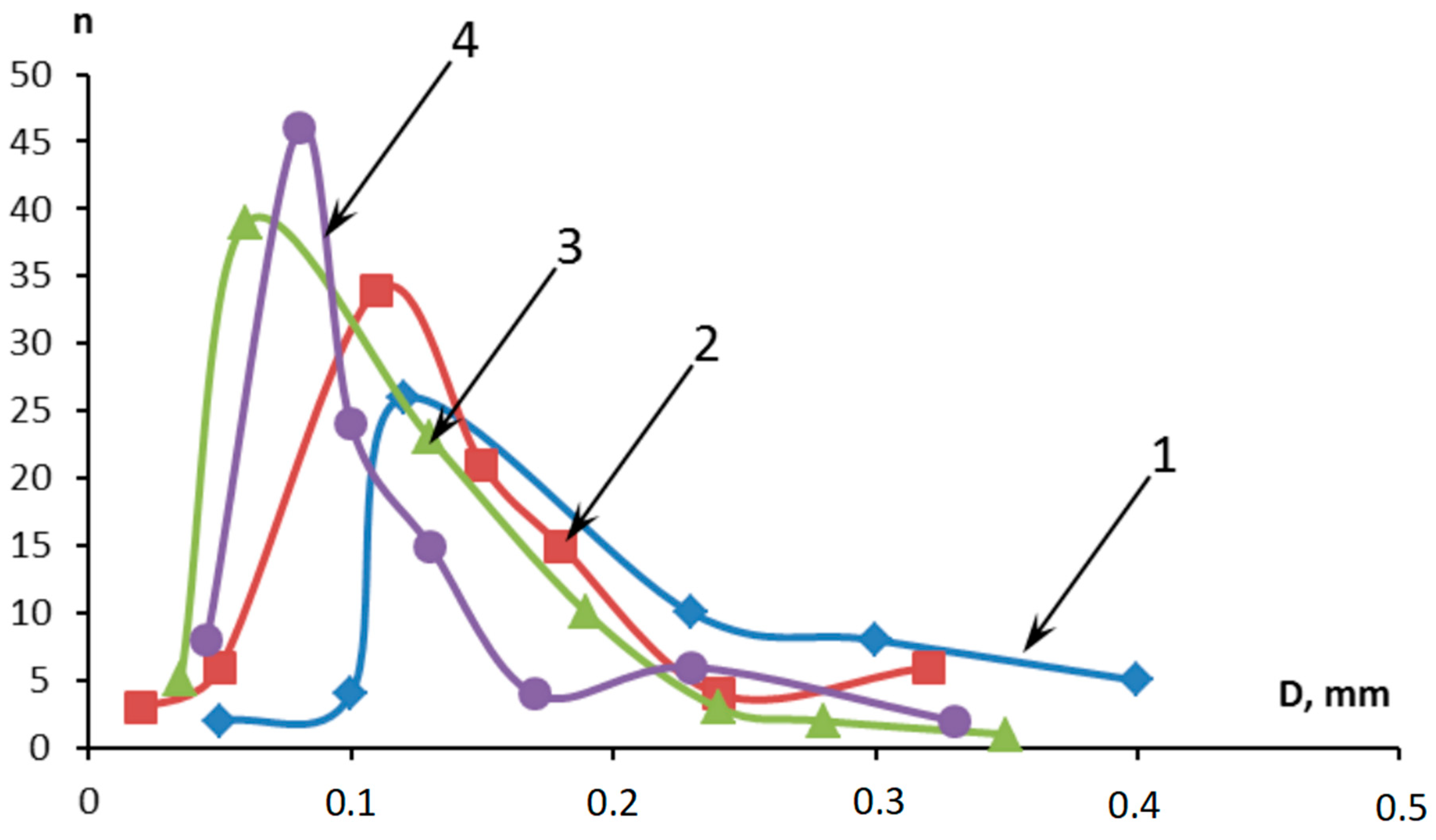
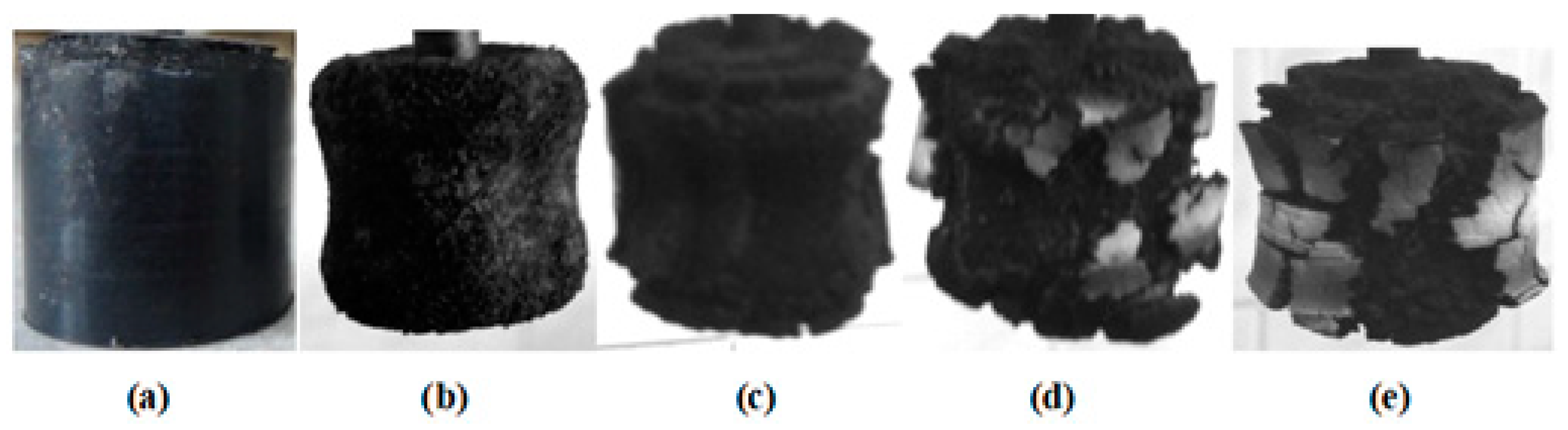
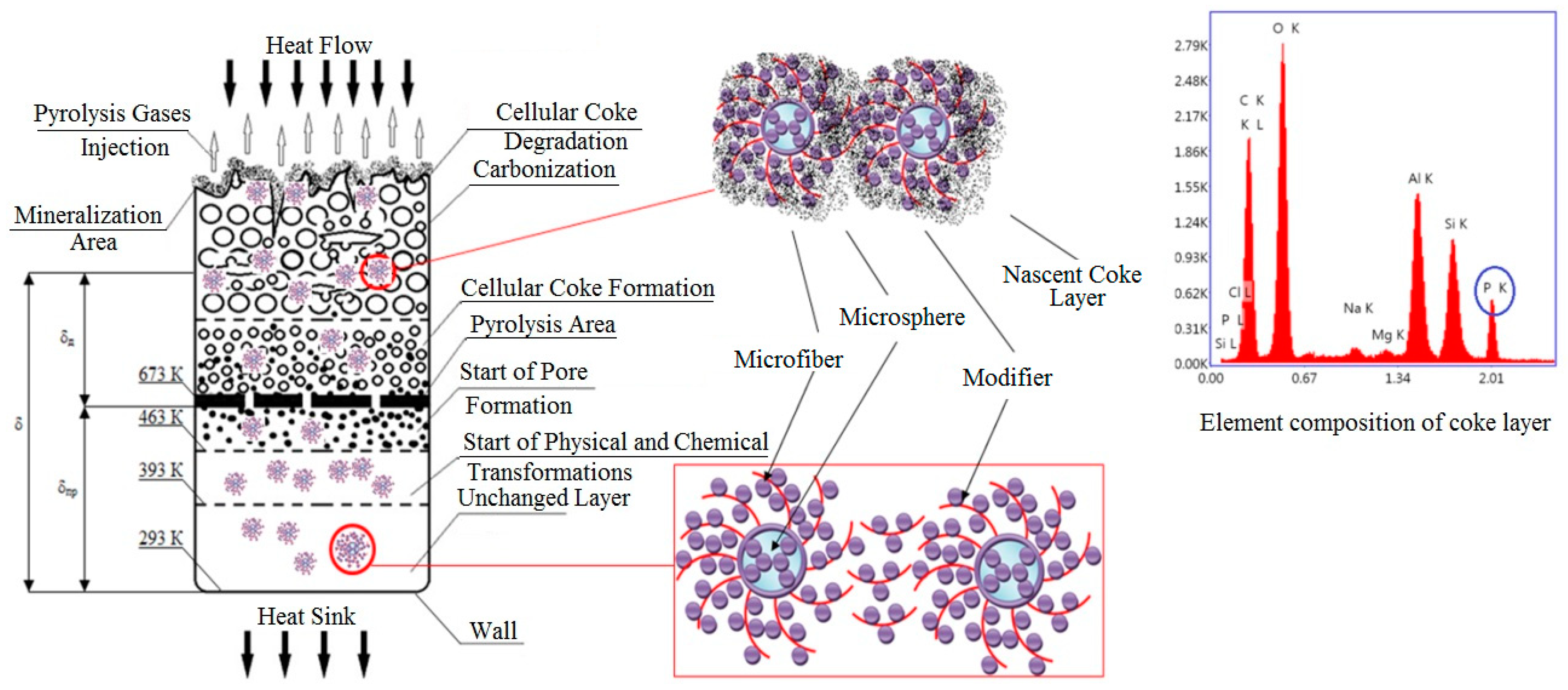


| Ingredient | Sample Number | ||
|---|---|---|---|
| 5MUV:10MSF | 10MUV:5MSF | 15MUV:5MSF | |
| Content, wt. pts. per 100 wt. pts. Rubber | |||
| Aluminosilicate microspheres | 10 | 5 | 5 |
| Carbon microfibers | 5 | 10 | 15 |
| DDF | 1 | 1 | 1 |
| Parameter | Sample Number | ||||
|---|---|---|---|---|---|
| Ref. | Control Sample | 5MUV:10MSF | 10MUV:5MSF | 15MUV:5MSF | |
| Tensile strength ft, MPa | Not less than 6.0 | 16.5 | 8.5 | 11.8 | 12.4 |
| Breaking elongation εrel, % | Not less than 300 | 450 | 400 | 350 | 380 |
| Permanent elongation θperm, % | Not more than 30 | 20 | 25 | 18 | 18 |
| Density ρ, kg m−3 | Not more than 1100 | 1080 | 1065 | 1082 | 1105 |
| Heating time of unheated surface of a sample up to 100 °C, s | – | 62 | 70 | 87 | 83 |
| Coke number CCV, % | – | 2.4 | 14.8 | 15.9 | 16.7 |
| Linear burning speed Vl.b., mm min−1 | – | 32.1 | 30.4 | 26.7 | 25.4 |
| Coke layer tear propagation strength σ, mPa | – | 37.3 | 40.1 | 41.4 | 41.6 |
| Ingredient | Sample Number | |||
|---|---|---|---|---|
| Control Sample | 5MUV:10MSF | 10MUV:5MSF | 15MUV:5MSF | |
| Content, wt. pts. per 100 wt. pts. Rubber | ||||
| EPDM-40 | 100 | 100 | 100 | 100 |
| BS-120 | 30 | 30 | 30 | 30 |
| Zinc oxide | 5 | 5 | 5 | 5 |
| Stearine | 1 | 1 | 1 | 1 |
| Captax | 2 | 2 | 2 | 2 |
| Sulphur | 2 | 2 | 2 | 2 |
| FAS | 0 | 16 | 16 | 16 |
| Total | 140 | 156 | 156 | 156 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kablov, V.F.; Novopoltseva, O.M.; Kryukova, D.A.; Keibal, N.A.; Burmistrov, V.; Kochetkov, V.G. Functionally Active Microheterogeneous Systems for Elastomer Fire- and Heat-Protective Materials. Molecules 2023, 28, 5267. https://doi.org/10.3390/molecules28135267
Kablov VF, Novopoltseva OM, Kryukova DA, Keibal NA, Burmistrov V, Kochetkov VG. Functionally Active Microheterogeneous Systems for Elastomer Fire- and Heat-Protective Materials. Molecules. 2023; 28(13):5267. https://doi.org/10.3390/molecules28135267
Chicago/Turabian StyleKablov, Victor F., Oksana M. Novopoltseva, Daria A. Kryukova, Natalia A. Keibal, Vladimir Burmistrov, and Vladimir G. Kochetkov. 2023. "Functionally Active Microheterogeneous Systems for Elastomer Fire- and Heat-Protective Materials" Molecules 28, no. 13: 5267. https://doi.org/10.3390/molecules28135267
APA StyleKablov, V. F., Novopoltseva, O. M., Kryukova, D. A., Keibal, N. A., Burmistrov, V., & Kochetkov, V. G. (2023). Functionally Active Microheterogeneous Systems for Elastomer Fire- and Heat-Protective Materials. Molecules, 28(13), 5267. https://doi.org/10.3390/molecules28135267









