The Anti-Arthritic Activity of Diclofenac Lipid-Core Nanocapsules: Stereological Analysis Showing More Protection of Deep Joint Components
Abstract
1. Introduction
2. Results
2.1. Preparation of DIC-LNC Dispersed in Water
2.2. DIC-LNC Reduces Edema Formation at the Hind Paws
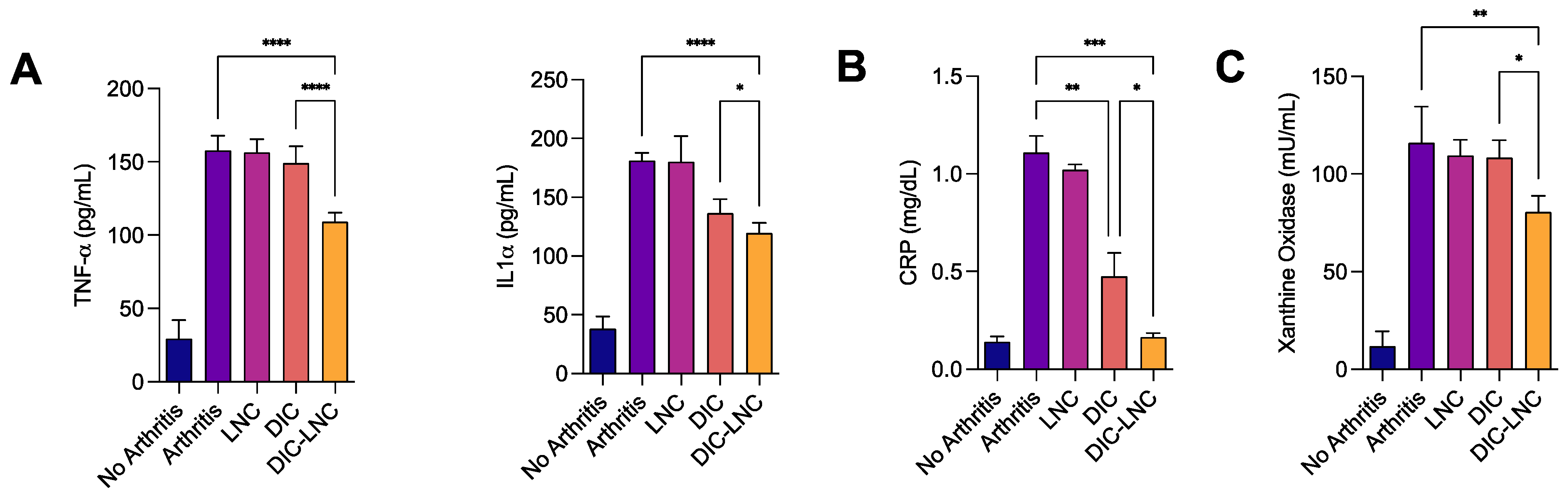
2.3. DIC-LNC Reduces the Serum Levels of Proinflammatory Cytokines and CRP
2.4. Absence of Liver and Renal Toxicity with DIC-LNC
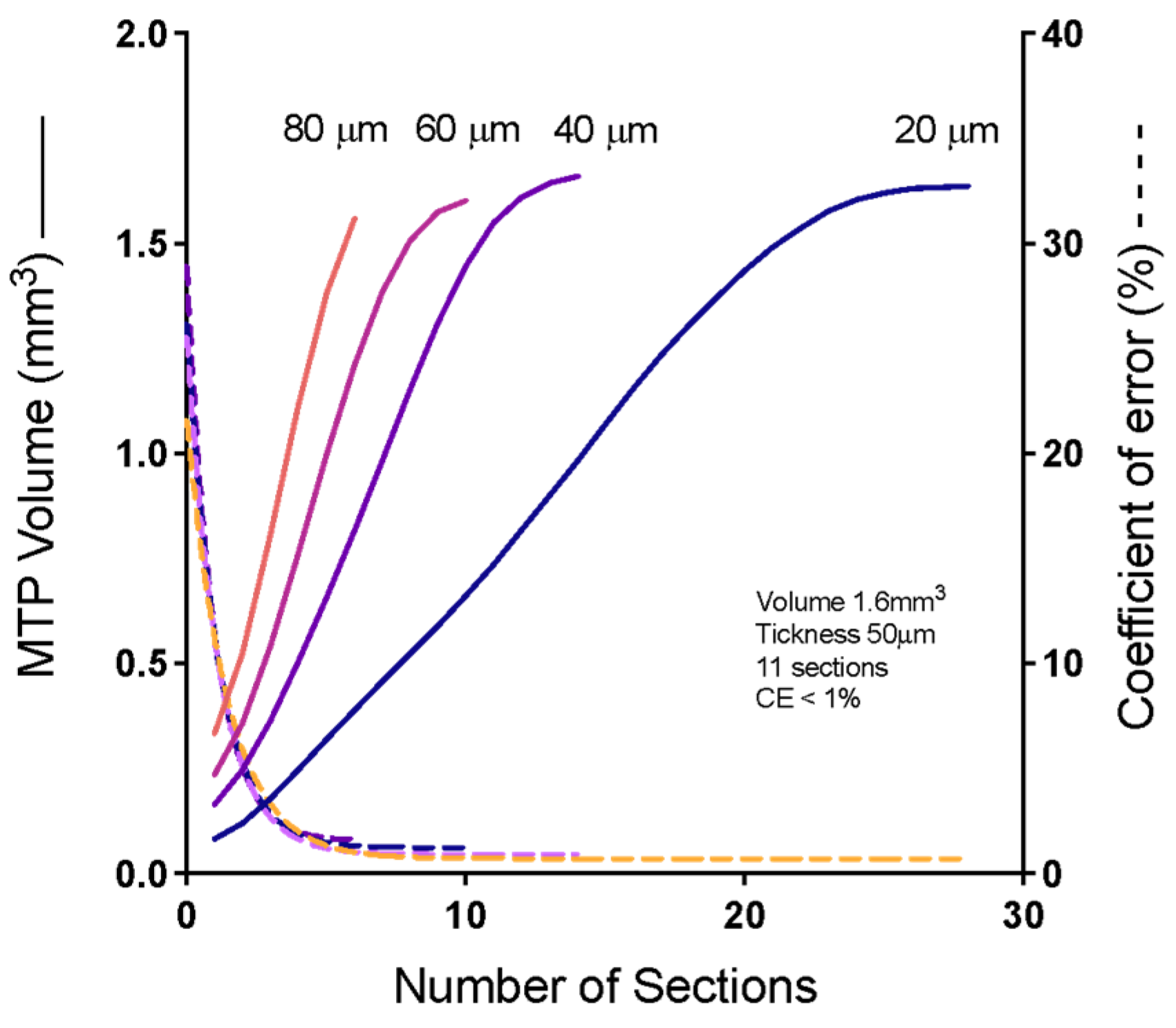
2.5. Cavalieri’s Volume of Metatarsophalangeal (MTP) Joints
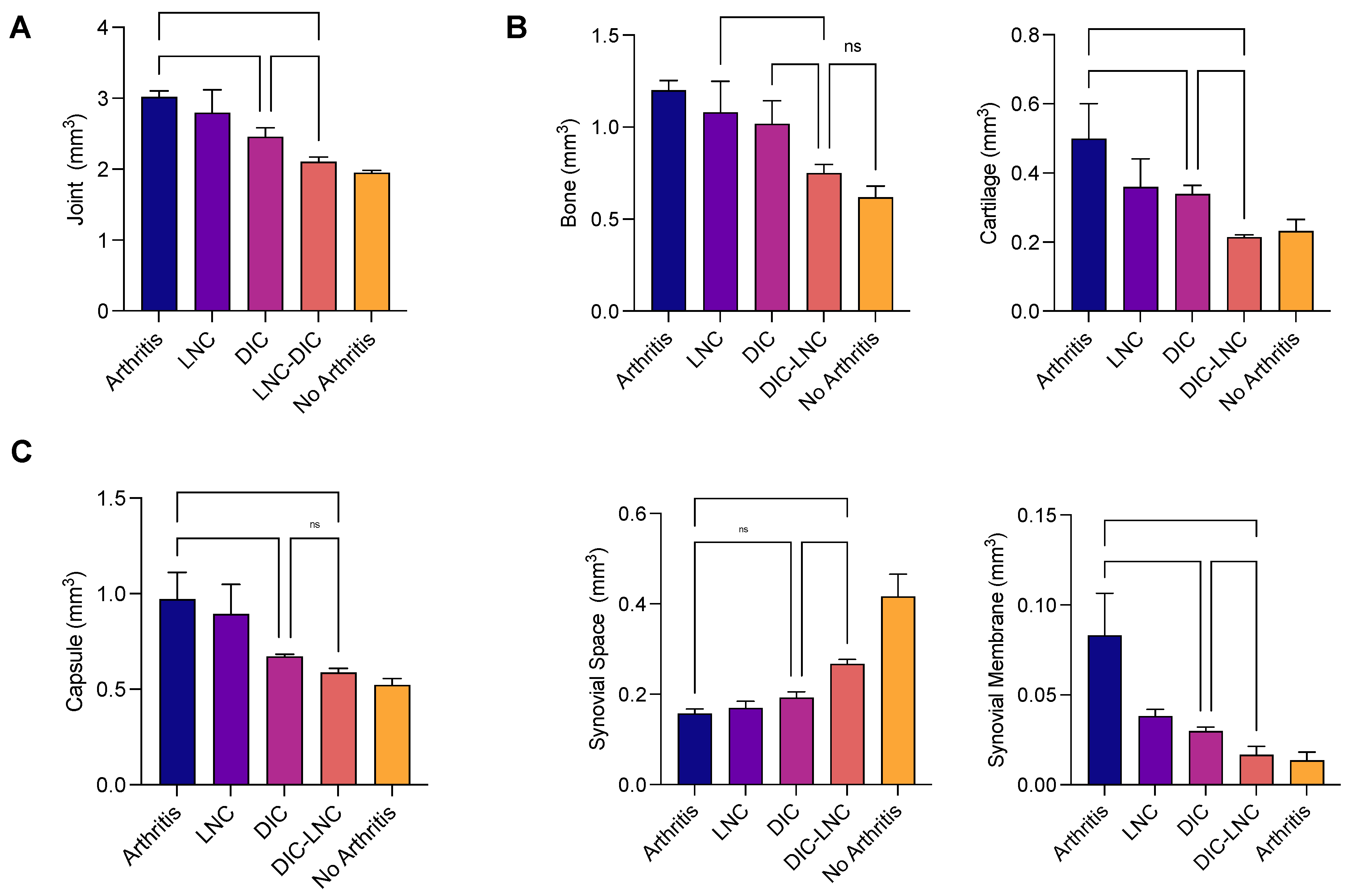
2.6. Density of the Joint Components and Absolute Volume
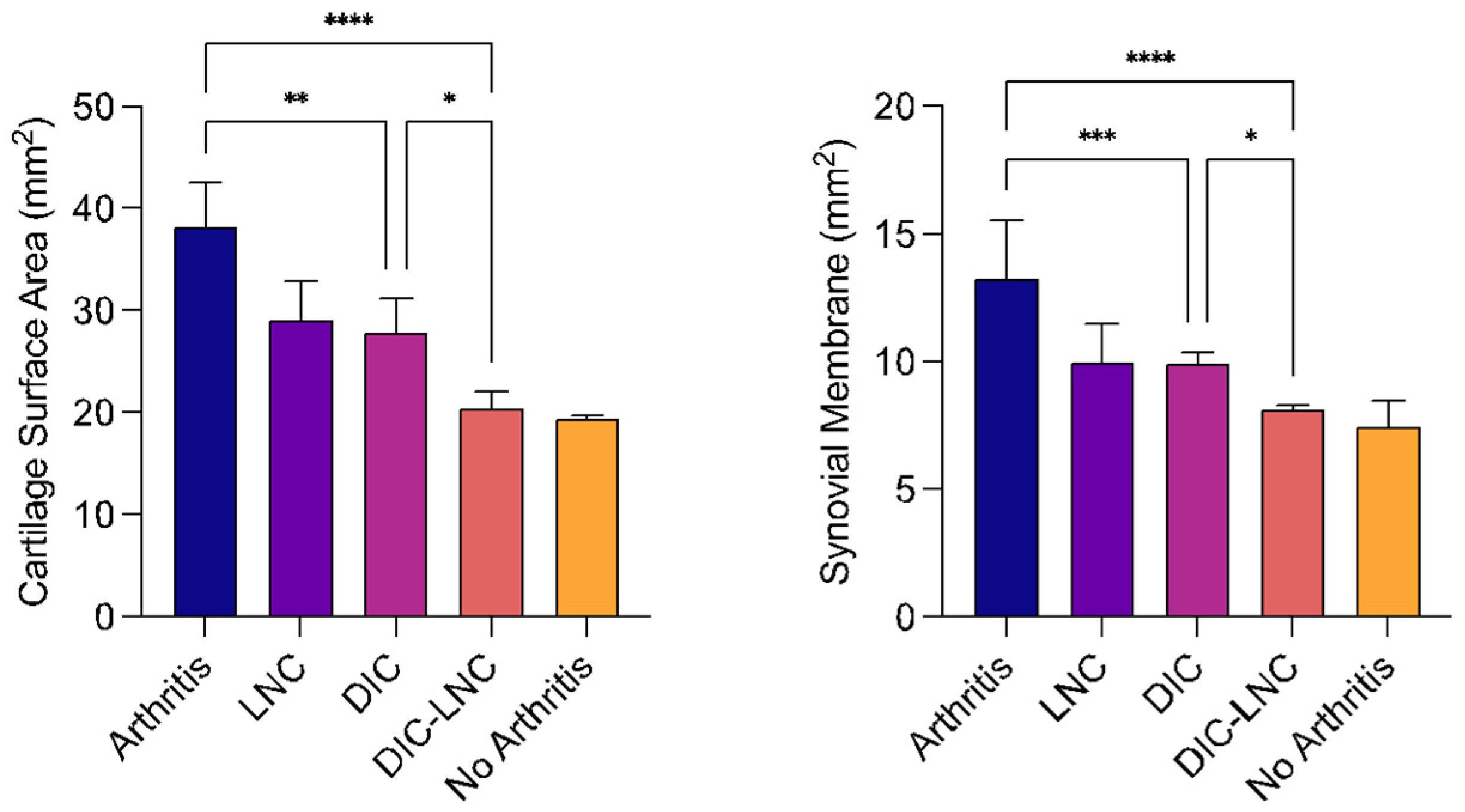
2.7. Surface Area of MTP Joints
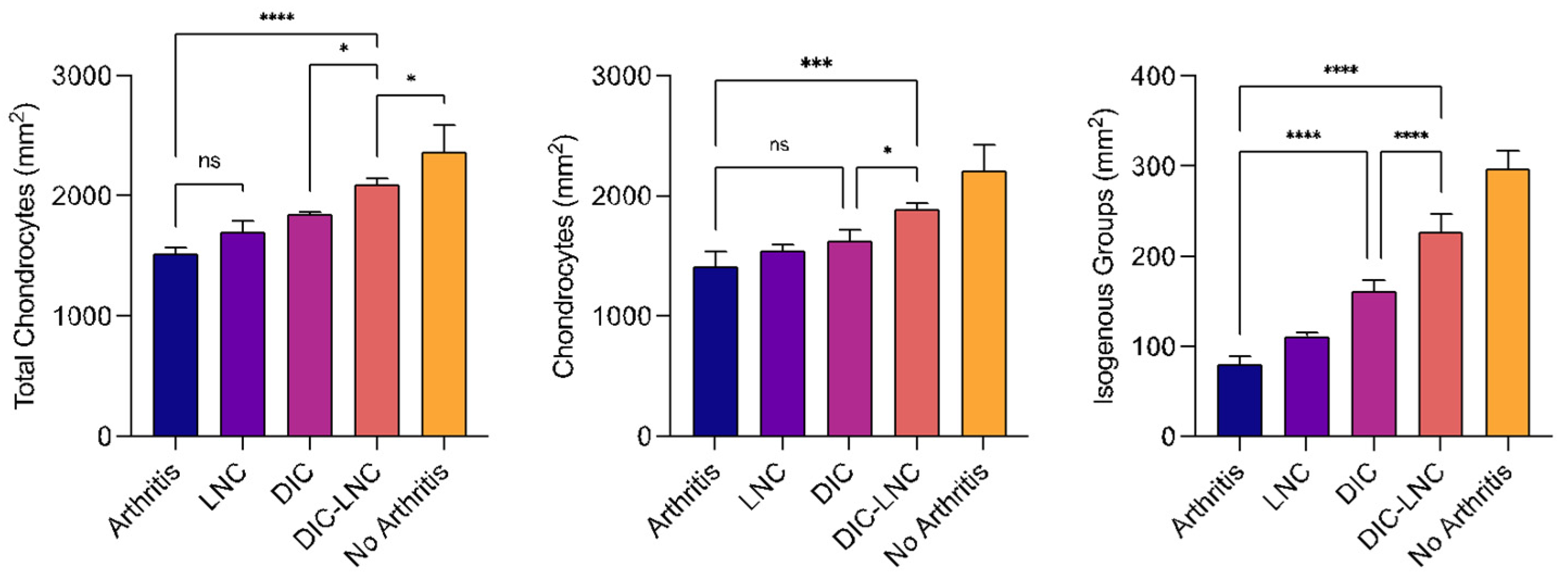
2.8. Quantification of Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Lipid-Core Nanocapsules Formulations
4.3. Physicochemical Characterization of the Formulations
4.3.1. pH Measurements
4.3.2. Electrophoretic Mobility and Zeta Potential
4.3.3. Laser Diffraction
4.3.4. Dynamic Light Scattering
4.3.5. Nanoparticle Tracking Analysis
4.3.6. Drug Content and Encapsulation Efficiency
4.4. Animals
4.5. Induction of Arthritis by Freund’s Complete Adjuvant and Experimental Design
4.6. Randomization, Blinding, and Allocation Concealment
4.7. Edema Volume and Arthritis Score of the Hind Paw
4.8. Quantification of Cytokines and Biochemical Markers
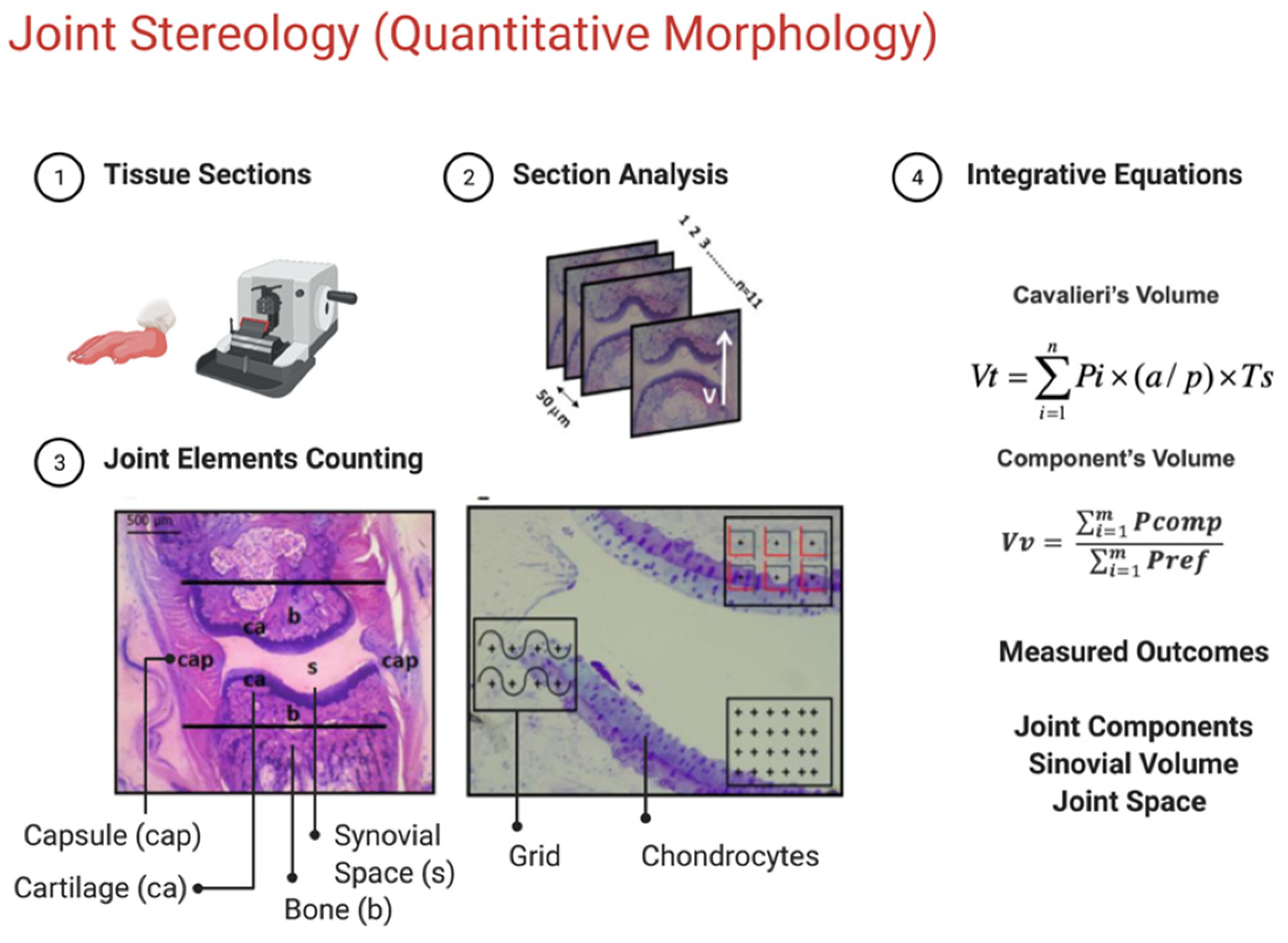
4.9. Stereological Analysis
4.10. Determination of Cavalieri’s Volume
4.11. Determination of Relative Volume
4.12. Determination of Surface Area
4.13. Counting the Number of Cellular Profiles
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [PubMed]
- Ku, E.C.; Lee, W.; Kothari, H.V.; Scholer, D.W. Effect of diclofenac sodium on the arachidonic acid cascade. Am. J. Med. 1986, 80, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [PubMed]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S2. [Google Scholar]
- Lanas, A. Nonsteroidal antiinflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: A trip from peptic ulcer to colon cancer. Am. J. Med. Sci. 2009, 338, 96–106. [Google Scholar]
- Prasad, L.K.; O’Mary, H.; Cui, Z. Nanomedicine delivers promising treatments for rheumatoid arthritis. Nanomedicine 2015, 10, 2063–2074. [Google Scholar]
- Nazrul-Hakim, M.; Yong, Y.K.; Chiong, H.S.; Sulaiman, M.R.; Zuraini; Zakaria, Z.A.; Yuen, K.H. Cytoprotective and enhanced anti-inflammatory activities of liposomal piroxicam formulation in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Nanomed. 2013, 8, 1245–1255. [Google Scholar] [CrossRef]
- Turk, C.T.S.; Oz, U.C.; Serim, T.M.; Hascicek, C. Formulation and optimization of nonionic surfactants emulsified nimesulide-loaded PLGA-based nanoparticles by design of experiments. AAPS PharmSciTech 2014, 15, 161–176. [Google Scholar]
- Venturini, C.G.; Jäger, E.; Oliveira, C.P.; Bernardi, A.; Battastini, A.M.O.; Guterres, S.S.; Pohlmann, A.R. Formulation of lipid core nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 200–208. [Google Scholar]
- Bernardi, A.; Zilberstein, A.; Jäger, E.; Campos, M.; Morrone, F.; Calixto, J.; Pohlmann, A.; Guterres, S.; Battastini, A. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br. J. Pharmacol. 2009, 158, 1104–1111. [Google Scholar]
- Keller, K.K.; Thomsen, J.S.; Stengaard-Pedersen, K.; Dagnæs-Hansen, F.; Nyengaard, J.R.; Hauge, E.-M. Bone formation and resorption are both increased in experimental autoimmune arthritis. PLoS ONE 2012, 7, e53034. [Google Scholar]
- Exner, H.E. Stereology and 3D microscopy: Useful alternatives or competitors in the quantitative analysis of microstructures? Image Anal. Stereol. 2004, 23, 73–82. [Google Scholar]
- Boyce, R.W.; Dorph-Petersen, K.A.; Lyck, L.; Gundersen, H.J.G. Design-based stereology: Introduction to basic concepts and practical approaches for estimation of cell number. Toxicol. Pathol. 2010, 38, 1011–1025. [Google Scholar] [CrossRef]
- Silva, M.D.; Savinainen, A.; Kapadia, R.; Ruan, J.; Siebert, E.; Avitahl, N.; Mosher, R.; Anderson, K.; Jaffee, B.; Schopf, L. Quantitative analysis of micro-CT imaging and histopathological signatures of experimental arthritis in rats. Mol. Imaging. 2004, 3, 312–318. [Google Scholar] [CrossRef]
- Thote, T.; Lin, A.; Raji, Y.; Moran, S.; Stevens, H.; Hart, M.; Kamath, R.; Guldberg, R.; Willett, N. Localized 3D analysis of cartilage composition and morphology in small animal models of joint degeneration. Osteoarthr. Cartil. 2013, 21, 1132–1141. [Google Scholar]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; Silva, T.d.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Frozza, R.L.; Horn, A.P.; Campos, M.M.; Calixto, J.B.; Salbego, C.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M.O. Protective effects of indomethacin-loaded nanocapsules against oxygen-glucose deprivation in organotypic hippocampal slice cultures: Involvement of neuroinflammation. Neurochem. Int. 2010, 57, 629–636. [Google Scholar] [PubMed]
- Cruz, L.; Schaffazick, S.R.; Costa, T.D.; Soares, L.U.; Mezzalira, G.; da Silveira, N.P.; Schapoval, E.E.; Pohlmann, A.R.; Guterres, S.S. Physico-chemical characterization and in vivo evaluation of indomethacin ethyl ester-loaded nanocapsules by PCS, TEM, SAXS, interfacial alkaline hydrolysis and antiedematogenic activity. J. Nanosci. Nanotechnol. 2006, 6, 3154–3162. [Google Scholar] [CrossRef]
- Bulcão, R.P.; de Freitas, F.A.; Dallegrave, E.; Venturini, C.G.; Baierle, M.; Durgante, J.; Sauer, E.; Cassini, C.; Cerski, C.T.; Zielinsky, P.; et al. In vivo toxicological evaluation of polymeric nanocapsules after intradermal administration. Eur. J. Pharm. Biopharm. 2014, 86, 167–177. [Google Scholar]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar]
- Schaible, H.-G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 2014, 16, 470. [Google Scholar] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar]
- Martel-Pelletier, J.; Pelletier, J.-P.; Fahmi, H. New insights into prostaglandin biology. J. Rheumatol. 2004, 31, 14–16. [Google Scholar] [PubMed]
- Gitter, B.D.; Labus, J.M.; Lees, S.L.; E Scheetz, M. Characteristics of human synovial fibroblast activation by IL-1 beta and TNF alpha. Immunology 1989, 66, 196–200. [Google Scholar]
- Gilroy, D.W.; Tomlinson, A.; Greenslade, K.; Seed, M.; Willoughby, D.A. The effects of cyclooxygenase 2 inhibitors on cartilage erosion and bone loss in a model of Mycobacterium tuberculosis-induced monoarticular arthritis in the rat. Inflammation 1998, 22, 509–519. [Google Scholar] [CrossRef]
- Anderson, G.D.; Keys, K.L.; De Ciechi, P.A.; Masferrer, J.L. Combination therapies that inhibit cyclooxygenase-2 and leukotriene synthesis prevent disease in murine collagen induced arthritis. Inflamm. Res. 2009, 58, 109–117. [Google Scholar]
- Korotkova, M.; Jakobsson, P.-J. Persisting eicosanoid pathways in rheumatic diseases. Nat. Rev. Rheumatol. 2014, 10, 229–241. [Google Scholar] [PubMed]
- Leng, P.; Li, D.; Sun, Y.; Wang, Y.; Zhang, H. Effects of human cyclooxygenase-2 gene silencing on synovial cells of rheumatoid arthritis mediated by lentivirus. Artif Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S274–S280. [Google Scholar]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar]
- Cai, X.; Wong, Y.F.; Zhou, H.; Xie, Y.; Liu, Z.Q.; Jiang, Z.H.; Bian, Z.X.; Xu, H.X.; Liu, L. The comparative study of Sprague-Dawley and Lewis rats in adjuvant-induced arthritis. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 373, 140–147. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New therapies for treatment of rheumatoid arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. S5), v3–v11. [Google Scholar] [CrossRef]
- Artacho-Pérula, E.; Roldéan-Villalobos, R.; Collantes-Estévez, E.; López-Beltréan, A. Stereological analysis of the synovial membrane in rheumatic disorders: Diagnostic value of volume-weighted mean nuclear volume estimation. Histopathology 1994, 25, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.D.; Stoustrup, P.; Küseler, A.; Pedersen, T.K.; Nyengaard, J.R.; Hauge, E.-M.; Herlin, T. Quantitative histological changes of repeated antigen-induced arthritis in the temporomandibular joints of rabbits treated with intra-articular corticosteroid. J. Oral Pathol. Med. 2008, 37, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Contri, R.V.; Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2015, 7, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, E.F.; Chassot, J.M.; Teixeira, F.C.; Azambuja, J.H.; Debom, G.; Beira, F.T.; Del Pino, F.A.B.; Lourenço, A.; Horn, A.P.; Cruz, L.; et al. Ketoprofen-loaded polymeric nanocapsules selectively inhibit cancer cell growth in vitro and in preclinical model of glioblastoma multiforme. Investig. New Drugs 2013, 31, 1424–1435. [Google Scholar] [CrossRef]
- Cattani, V.B.; Fiel, L.A.; Jäger, A.; Jäger, E.; Colomé, L.M.; Uchoa, F.; Stefani, V.; Costa, T.D.; Guterres, S.S.; Pohlmann, A.R. Lipid-core nanocapsules restrained the indomethacin ethyl ester hydrolysis in the gastrointestinal lumen and wall acting as mucoadhesive reservoirs. Eur. J. Pharm. Sci. 2010, 39, 116–124. [Google Scholar] [CrossRef]
- Van Walsem, A.; Pandhi, S.; Nixon, R.M.; Guyot, P.; Karabis, A.; Moore, R.A. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: A network meta-analysis. Arthritis Res. Ther. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.D.; Shadforth, M.F.; Crook, P.R.; John, V.A. Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur. J. Clin. Pharmacol. 1983, 25, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, H.; Cen, N.; Zhou, A.; Tao, H. A pharmacokinetic study of diclofenac sodium in rats. Biomed. Rep. 2017, 7, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kasperek, R.; Zimmer, Ł.; Jawień, W.; Poleszak, E. Pharmacokinetics of diclofenac sodium and papaverine hydrochloride after oral administration of tablets to rabbits. Acta Pol. Pharm. 2015, 72, 527–538. [Google Scholar]
- Hellms, S.; Gueler, F.; Gutberlet, M.; Schebb, N.H.; Rund, K.; Kielstein, J.T.; VoChieu, V.; Rauhut, S.; Greite, R.; Martirosian, P.; et al. Single-dose diclofenac in healthy volunteers can cause decrease in renal perfusion measured by functional magnetic resonance imaging. J. Pharm. Pharmacol. 2019, 71, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.S.; Müller, C.R.; Michalowski, C.B.; Pohlmann, A.R.; Dalla Costa, T. Gastro-intestinal tolerance following oral administration of spray-dried diclofenac-loaded nanocapsules and nanospheres. Pharma Sci. 2001, 11, 229–233. [Google Scholar]
- Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Nanoparticle-coated microparticles: Preparation and characterization. J. Microencapsul. 2004, 21, 499–512. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Venturini, C.G.; Donida, B.; Poletto, F.S.; Guterres, S.S.; Pohlmann, A.R. An algorithm to determine the mechanism of drug distribution in lipid-core nanocapsule formulations. Soft Matter 2013, 9, 1141–1150. [Google Scholar] [CrossRef]
- Boechat, A.L.; de Oliveira, C.P.; Tarragô, A.M.; da Costa, A.G.; Malheiro, A.; Guterres, S.S.; Pohlmann, A.R. Methotrexate-loaded lipid-core nanocapsules are highly effective in the control of inflammation in synovial cells and a chronic arthritis model. Int. J. Nanomed. 2015, 10, 6603–6614. [Google Scholar]
- Baddeley, A.J.; Gundersen, H.J.G.; Cruz-Orive, L.M. Estimation of surface area from vertical sections. J. Microsc. 1986, 142 Pt 3, 259–276. [Google Scholar] [CrossRef]
- Gundersen, H.J.G.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147 Pt 3, 229–263. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Howard, V.; Reed, M. Unbiased Stereology: Three-Dimensional Measurement in Microscopy, 2nd ed.; Garland Science: London, UK, 2004. [Google Scholar]
- Cruz-Orive, L.M. Precision of Cavalieri sections and slices with local errors. J. Microsc. 1999, 193 Pt 3, 182–198. [Google Scholar] [CrossRef] [PubMed]

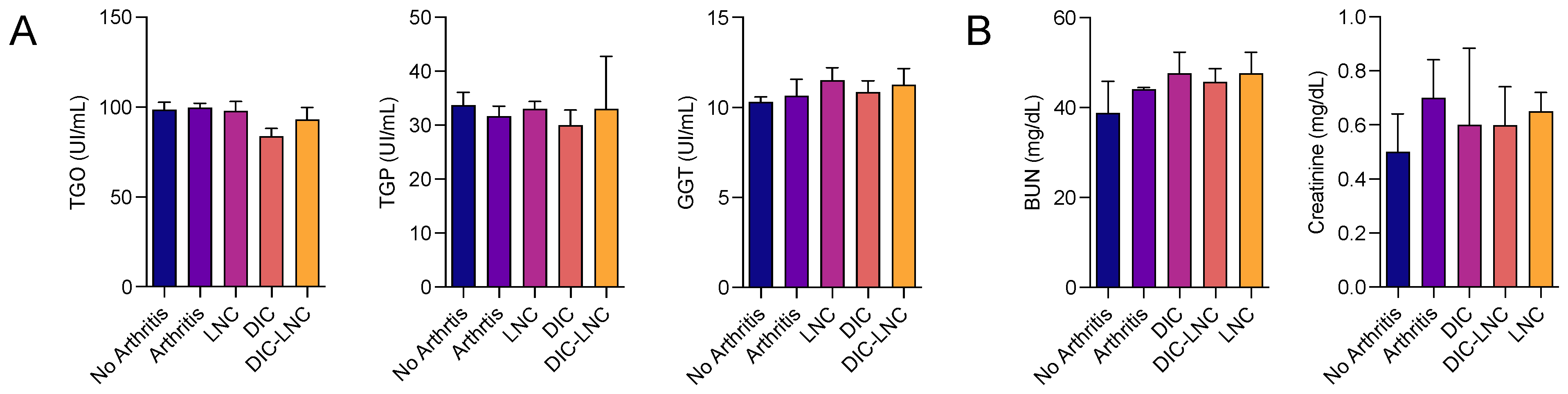
| LNC | DIC-LNC | |
|---|---|---|
| Potentiometry | ||
| pH | 5.43 ± 0.24 | 5.39 ± 0.16 |
| Laser diffraction | ||
| D[4,3] (nm) | 153 ± 10 | 204 ± 46 |
| Span | 1.4 ± 0.2 | 1.7 ± 0.1 |
| Dynamic Light Scattering | ||
| Dh (nm) | 170 ± 13 | 166 ± 13 |
| PDI | 0.06 ± 0.02 | 0.08 ± 0.02 |
| Electrophoretic mobility | ||
| Zeta Potential (mV) | −13 ± 6 | −11 ± 2 |
| Nanoparticle tracking analysis | ||
| Dh (nm) | 182 ± 9 | 196 ± 14 |
| D50 (nm) | 173 ± 13 | 186 ± 2 |
| D90 (nm) | 257 ± 10 | 309 ± 9 |
| PND (× 1012 particles mL−1) | 4.98 ± 0.25 | 4.76 ± 0.78 |
| Volume (mm3) | ||||||
|---|---|---|---|---|---|---|
| Groups | Joint | Cartilage | Bone | Capsule | Synovial Space | Synovial Membrane |
| Arthritis | 3.02 ± 0.08 | 0.50 ± 0.10 | 1.20 ± 0.05 | 0.97 ± 0.14 | 0.16 ± 0.01 | 0.08 ± 0.02 |
| LNC | 2.80 ± 0.32 | 0.36 ± 0.08 | 1.08 ± 0.16 | 0.89 ± 0.15 | 0.17 ± 0.01 | 0.04 ± 0.01 |
| Diclofenac | 2.46 ± 0.12 | 0.34 ± 0.03 | 1.02 ± 0.12 | 0.67 ± 0.02 | 0.19 ± 0.02 | 0.03 ± 0.00 |
| Diclofenac-LNC | 2.10 ± 0.10 | 0.21 ± 0.01 | 0.75 ± 0.05 | 0.58 ± 0.02 | 0.26 ± 0.01 | 0.02 ± 0.00 |
| No Arthritis | 1.95 ± 0.05 | 0.23 ± 0.03 | 0.61 ± 0.06 | 0.52 ± 0.03 | 0.41 ± 0.03 | 0.01 ± 0.05 |
| Surface Area (mm2) | Cellular Count (Cell/mm2) | ||||
|---|---|---|---|---|---|
| Groups | Cartilage | Synovial Membrane | Chondrocytes | Isogenous Groups | Total Chondrocytes |
| Arthritis | 38.25 ± 4.32 | 13.26 ± 2.78 | 1416 ± 126.3 | 80.55 ± 8.77 | 1517 ± 54.30 |
| LNC | 29.09 ± 3.75 | 9.98 ± 1.49 | 1543 ± 49.92 | 111.5 ± 4.51 | 1701 ± 91.96 |
| Diclofenac | 27.87 ± 3.31 | 9.92 ± 0.46 | 1627 ± 95.61 | 161.1 ± 11.84 | 1849 ± 16.73 |
| Diclofenac-LNC | 20.45 ± 1.58 | 8.01 ± 0.19 | 1894 ± 47.00 | 226.8 ± 20.5 | 2095 ± 52.33 |
| No Arthritis | 19.45 ± 0.28 | 7.43 ± 1.04 | 2208 ± 217.4 | 297.9 ± 19.5 | 2367 ± 225.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña, N.M.; de Oliveira, C.P.; Guterres, S.S.; Pohlmann, A.R.; da Costa, O.T.F.; Boechat, A.L. The Anti-Arthritic Activity of Diclofenac Lipid-Core Nanocapsules: Stereological Analysis Showing More Protection of Deep Joint Components. Molecules 2023, 28, 5219. https://doi.org/10.3390/molecules28135219
Ureña NM, de Oliveira CP, Guterres SS, Pohlmann AR, da Costa OTF, Boechat AL. The Anti-Arthritic Activity of Diclofenac Lipid-Core Nanocapsules: Stereological Analysis Showing More Protection of Deep Joint Components. Molecules. 2023; 28(13):5219. https://doi.org/10.3390/molecules28135219
Chicago/Turabian StyleUreña, Nathalie Marte, Catiúscia Padilha de Oliveira, Silvia Stanisçuaski Guterres, Adriana Raffin Pohlmann, Oscar Tadeu Ferreira da Costa, and Antonio Luiz Boechat. 2023. "The Anti-Arthritic Activity of Diclofenac Lipid-Core Nanocapsules: Stereological Analysis Showing More Protection of Deep Joint Components" Molecules 28, no. 13: 5219. https://doi.org/10.3390/molecules28135219
APA StyleUreña, N. M., de Oliveira, C. P., Guterres, S. S., Pohlmann, A. R., da Costa, O. T. F., & Boechat, A. L. (2023). The Anti-Arthritic Activity of Diclofenac Lipid-Core Nanocapsules: Stereological Analysis Showing More Protection of Deep Joint Components. Molecules, 28(13), 5219. https://doi.org/10.3390/molecules28135219







