Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment
Abstract
:1. Introduction
2. Noncovalent Interactions-Driven Carrier-Free Nanodrugs
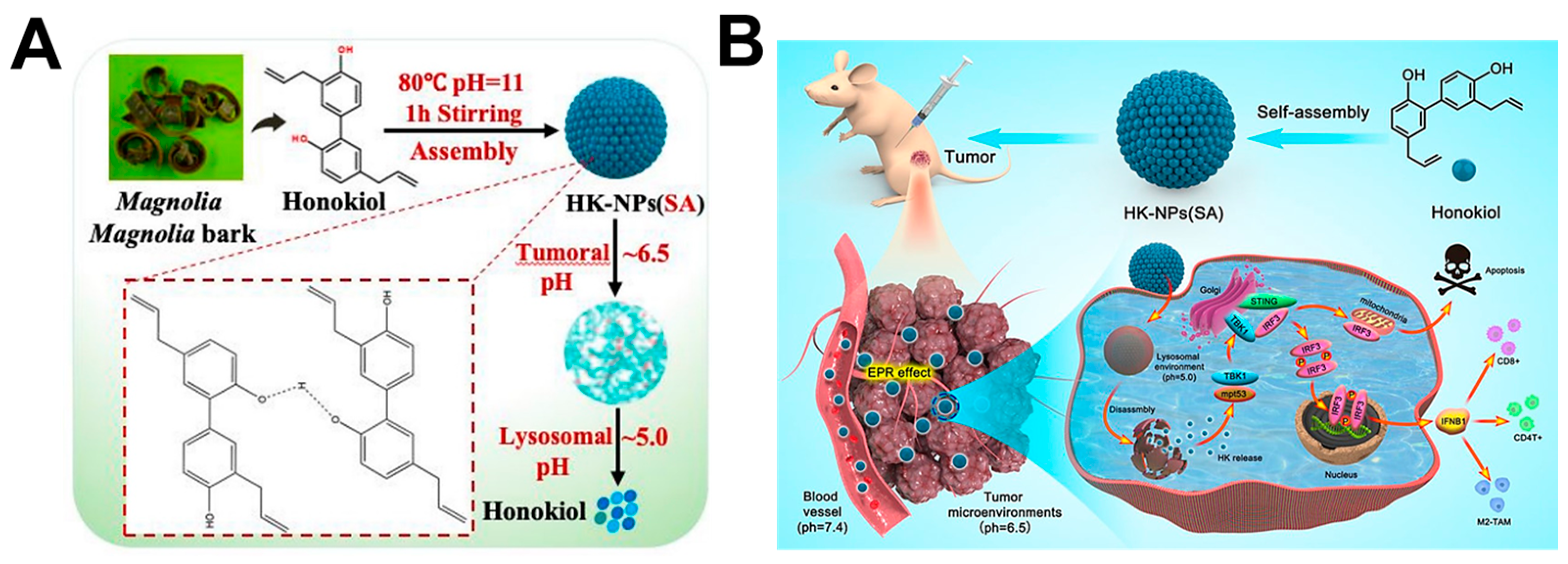
2.1. Hydrogen Bonding Interaction
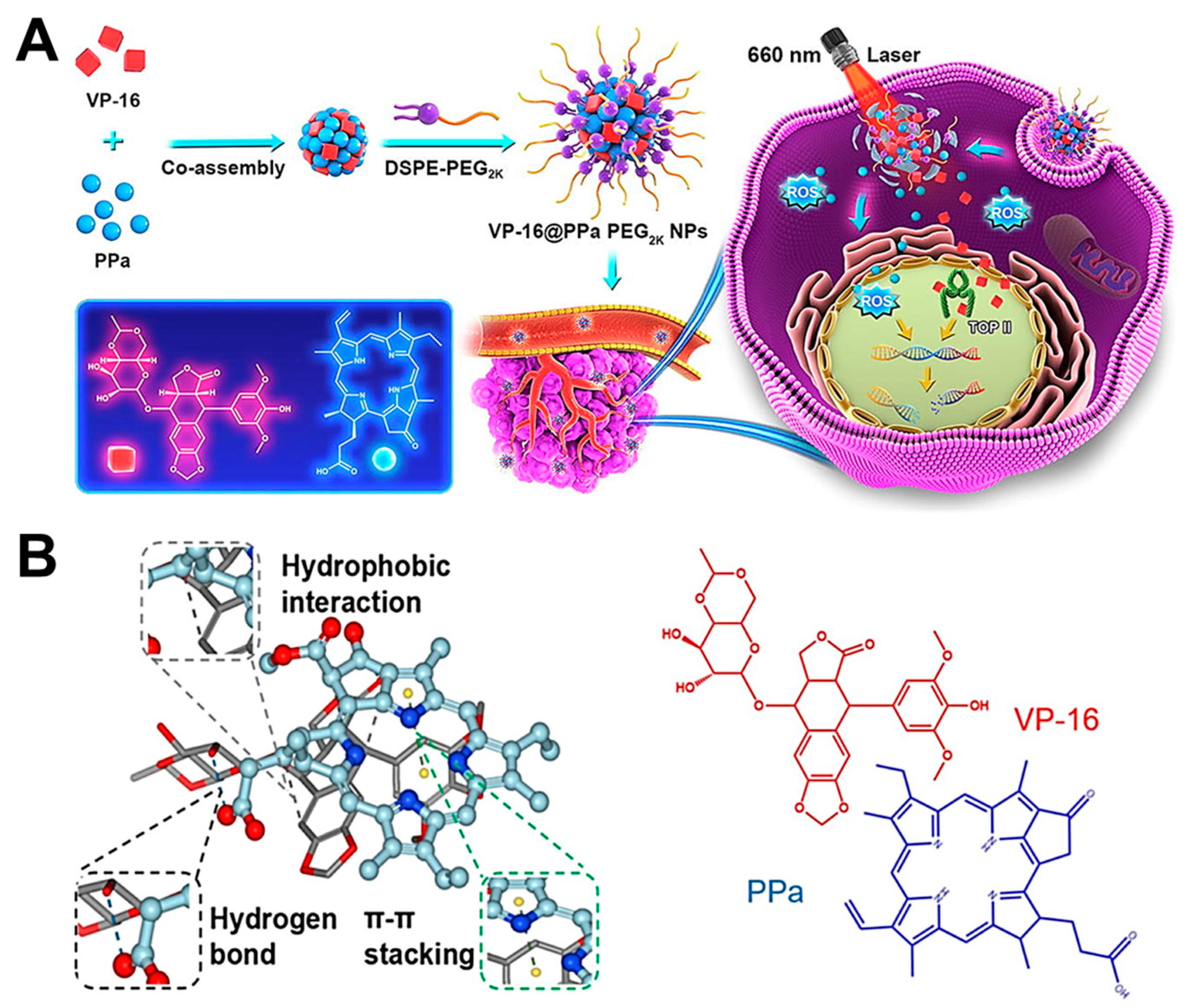
2.2. π-π Stacking Interaction
2.3. Electrostatic Interaction
2.4. Multiple Noncovalent Interactions
3. Covalent Bonds-Driven Carrier-Free Nanodrugs
3.1. Imine Bond
3.2. Disulfide Bond
3.3. Ester Bond
3.4. Specifically Peptide Linker
4. Metal Ions-Driven Carrier-Free Nanodrugs
4.1. Fe2+/3+ Ions Coordination
4.2. Cu2+ Ions Coordination
4.3. Mn2+ Ions Coordination
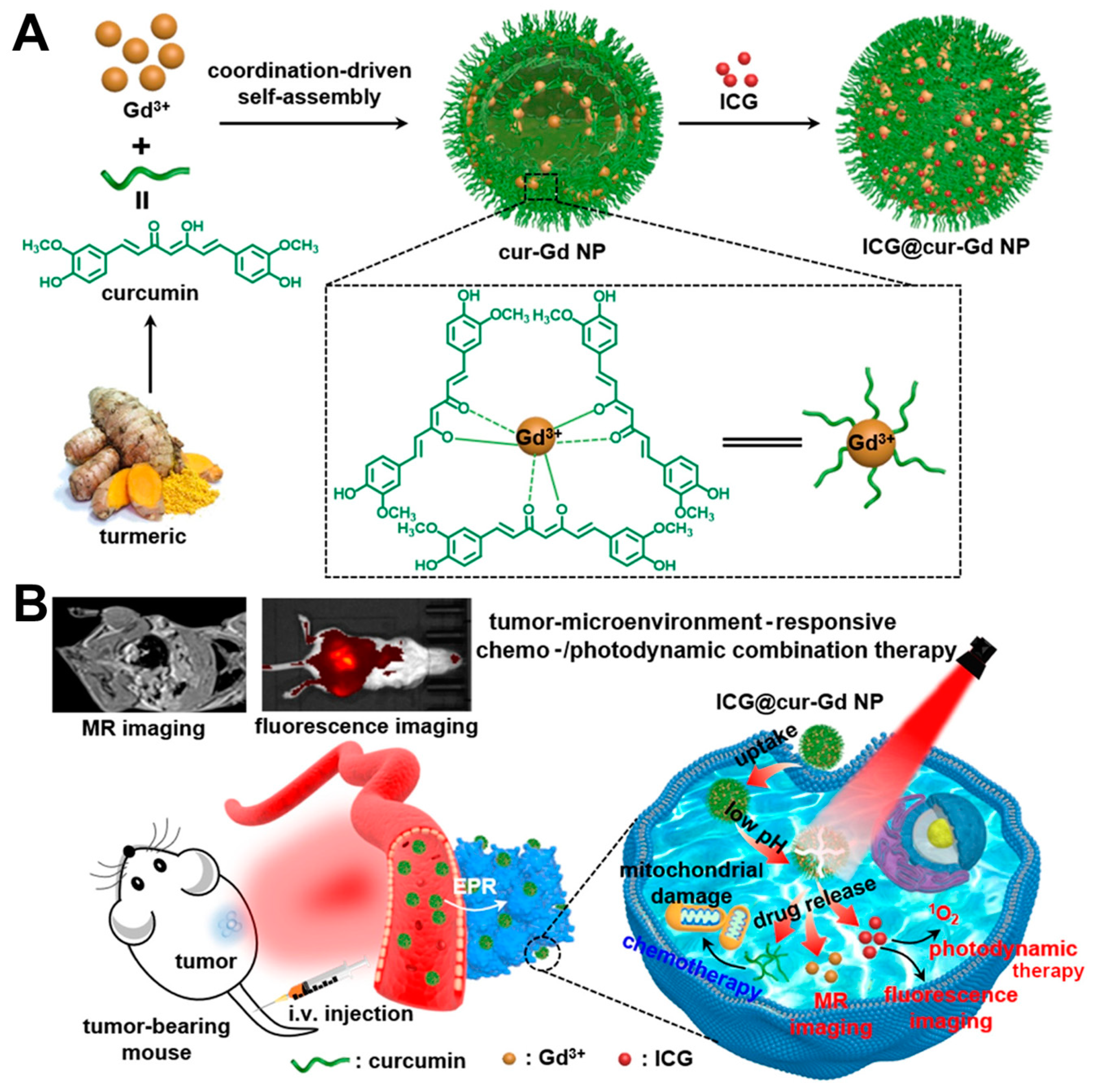
4.4. Gd3+ Ions Coordination
4.5. Zn2+ Ions Coordination
4.6. Other Metal Ions Coordination
5. Drug Nanocrystals
6. Summary and Perspectives
- (1)
- It is difficult to control active drug ratios precisely. Since drugs or functional biomolecules with various structures have an impact on their interactions during assembly, the actual resulting carrier-free nanodrugs are distinct from the original feed ratio. In particular, multiple drugs/functional biomolecules are co-assembled by strong and specific interaction forces, leading to difficulties in changing the proportion of drugs and accurately controlling the morphology, particle size, and surface charge of carrier-free nanodrugs [13]. Generally, precise screening of dose ratios will maximize the antitumor effect. Therefore, developing a hopeful strategy for the designation of carrier-free nanodrugs with accurate control of drug proportion is crucial.
- (2)
- Insufficient targeting efficiency. Most carrier-free nanodrugs enter the tumor mainly through the EPR effect, which is not enough to achieve high tumor selectivity. Recently, multifunctional nanodrugs with surface-modified targeting ligands (e.g., antibody, aptamer, peptide) have shown outstanding specific targeting ability toward cancer cells. Nevertheless, the targeting capacity is achieved through incorporating additional active targeted agents. Accordingly, exploring carrier-free nanodrugs with exceptional tumor-specific self-targeting deserves further studies.
- (3)
- The lack of a good understanding of pharmacokinetics in vivo. It is essential to have in-depth knowledge of the pharmacokinetic behaviors and destiny of carrier-free nanodrugs in the human body before clinical application. Although numerous reports have shown that carrier-free nanodrugs perform better in prolonging circulation time, enhancing cellar uptake and tumor accumulation, and avoiding rapid clearance, the mechanism of drug metabolism and pharmacological action in vivo should be deeply investigated to promote their clinical application.
- (4)
- The rationality of drug compatibility has not been well illustrated. Generally, there is antagonism, no interaction, additivity, or synergism between drug molecules. How to select the best optimal drug combination according to the synergistic effect and intermolecular interactions between drug molecules still lacks reliable research [187,188]. Thus, it is urgent to design carrier-free nanodrugs through reasonable drug compatibility for exerting the prime synergistic therapeutic efficiency, reducing potential metabolic risks and toxic-side effects.
- (5)
- The limited therapeutic applications. To date, although carrier-free nanodrugs can act as excellent nano-candidates, their applications mainly involve cancer therapy. There are few carrier-free nanodrugs designed for the treatment of bacterial infections or other diseases. We look forward to these advanced performances of carrier-free nanodrugs in cancer treatment, including PDT, PTT, CDT, RT, fluorescence imaging, and MRI, can be extended to antibacterial fields, which will undoubtedly bring innovative prospects for antibacterial [189,190] anti-inflammatory [191,192], antiviral [193], wound healing [194] and other fields.
- (6)
- The lack of deeper research. The exploration and study of carrier-free nanodrugs still stand in their infancy. The stability of long-term preservation needs to be fully considered. The potential toxicity to organs as well as the body’s immune response triggered by carrier-free nanodrugs have received little attention. Furthermore, large-scale industrial production and application in clinical practice are still big challenges to all the carrier-free nanodrugs. It needs to make more great efforts to conduct intensive research on carrier-free nanodrugs for clinical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef]
- Rivers, D. Lifestyle interventions for cancer survivors. Nat. Rev. Mater. 2022, 22, 130. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Mahabadi, V.P.; Ardekani, A.A.; Ghader, A.; Kamrava, S.K.; Dezfuli, A.S.; Ghaznavi, H.; Shakeri-Zadeh, A. Ultrastructural and optical characteristics of cancer cells treated by a nanotechnology based chemo-photothermal therapy method. J. Photochem. Photobiol. B 2019, 192, 19–25. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Liu, Y.; Hui, Y.; Ran, R.; Yang, G.Z.; Wibowo, D.; Wang, H.F.; Middelberg, A.P.J.; Zhao, C.X. Synergetic Combinations of Dual-Targeting Ligands for Enhanced In Vitro and In Vivo Tumor Targeting. Adv. Healthc. Mater. 2018, 7, e1800106. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Lyophilized mucoadhesive-dendrimer enclosed matrix tablet for extended oral delivery of albendazole. Eur. J. Pharm. Biopharm. 2016, 102, 202–213. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Gao, R.; Zhang, X.; Sun, Y.; Wu, J.; Liu, J.; Chen, C. Matrix Metalloproteinase-2-Responsive Surface-Changeable Liposomes Decorated by Multifunctional Peptides to Overcome the Drug Resistance of Triple-Negative Breast Cancer through Enhanced Targeting and Penetrability. ACS Biomater. Sci. Eng. 2022, 8, 2979–2994. [Google Scholar] [CrossRef]
- Kundu, P.K.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Borner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-controlled self-assembly of non-photoresponsive nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Zhang, A.-Q.; Cheng, S.-X.; Rong, L.; Zhang, X.-Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Zhang, X.; Lu, W.; Yu, J.; Liu, S. Carrier-free Janus nano-prodrug based on camptothecin and gemcitabine: Reduction-triggered drug release and synergistic in vitro antiproliferative effect in multiple cancer cells. Int. J. Pharm. 2018, 550, 45–56. [Google Scholar] [CrossRef]
- Huang, P.; Wang, D.; Su, Y.; Huang, W.; Zhou, Y.; Cui, D.; Zhu, X.; Yan, D. Combination of Small Molecule Prodrug and Nanodrug Delivery: Amphiphilic Drug-Drug Conjugate for Cancer Therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Lee, D.; Kim, G.; Lee, B.; Cho, Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D.; Nam, K.T.; et al. Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics that Overcome Drawbacks of Photodynamic Therapy. ACS Nano 2017, 12, 681–688. [Google Scholar] [CrossRef]
- Dong, C.; Jiang, Q.; Qian, X.; Wu, W.; Wang, W.; Yu, L.; Chen, Y. A self-assembled carrier-free nanosonosensitizer for photoacoustic imaging-guided synergistic chemo-sonodynamic cancer therapy. Nanoscale 2020, 12, 5587–5600. [Google Scholar] [CrossRef]
- Kung Sutherland, M.S.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455–1463. [Google Scholar] [CrossRef]
- Tan, X.; Li, B.B.; Lu, X.; Jia, F.; Santori, C.; Menon, P.; Li, H.; Zhang, B.; Zhao, J.J.; Zhang, K. Light-Triggered, Self-Immolative Nucleic Acid-Drug Nanostructures. J. Am. Chem. Soc. 2015, 137, 6112–6115. [Google Scholar] [CrossRef]
- Huang, L.; Hu, S.; Fu, Y.-N.; Wan, Y.; Li, G.; Wang, X. Multicomponent carrier-free nanodrugs for cancer treatment. J. Mater. Chem. B 2022, 10, 9735–9754. [Google Scholar] [CrossRef]
- Zhong, Y.T.; Cen, Y.; Xu, L.; Li, S.Y.; Cheng, H. Recent Progress in Carrier-Free Nanomedicine for Tumor Phototherapy. Adv. Healthc. Mater. 2023, 12, 2202307. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-H.; Zhang, X.-Z. Carrier-free nanomedicines for cancer treatment. Prog. Mater. Sci. 2022, 125, 100919. [Google Scholar] [CrossRef]
- Chen, M.; Hou, Y.; Chen, N.; Yang, E.; Sun, Z.; Wu, H.; Xu, X.; Yang, J.; Ma, G.; Huo, X. Co-assemblies based on natural Hemslecin A and β-sitosterol as a new sight for synergistic anti-gastric cancer efficacy in TCM. Colloids Interface Sci. Commun. 2022, 49, 100629. [Google Scholar] [CrossRef]
- Liu, J.; Peng, F.; Kang, Y.; Gong, D.; Fan, J.; Zhang, W.; Qiu, F. High-Loading Self-Assembling Peptide Nanoparticles as a Lipid-Free Carrier for Hydrophobic General Anesthetics. Int. J. Nanomed. 2021, 16, 5317–5331. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zheng, R.R.; Huang, J.Q.; Chen, X.Y.; Deng, F.A.; Liu, Y.B.; Huang, C.Y.; Yu, X.Y.; Cheng, H.; Li, S.Y. Self-Delivery Photo-Immune Stimulators for Photodynamic Sensitized Tumor Immunotherapy. ACS Nano 2020, 14, 17100–17113. [Google Scholar] [CrossRef]
- Li, H.; Zang, W.; Mi, Z.; Li, J.; Wang, L.; Xie, D.; Zhao, L.; Wang, D. Tailoring carrier-free nanocombo of small-molecule prodrug for combinational cancer therapy. J. Control. Release 2022, 352, 256–275. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, C.; Cheng, R.; Liu, Z.; Li, X.; Lu, A.; Tian, Z.; Yang, Z. Cargo-Free Nanomedicine with pH Sensitivity for Codelivery of DOX Conjugated Prodrug with SN38 To Synergistically Eradicate Breast Cancer Stem Cells. Mol. Pharm. 2018, 15, 3343–3355. [Google Scholar] [CrossRef]
- Hou, M.; Xue, P.; Gao, Y.-E.; Ma, X.; Bai, S.; Kang, Y.; Xu, Z. Gemcitabine-camptothecin conjugates: A hybrid prodrug for controlled drug release and synergistic therapeutics. Biomater. Sci. 2017, 5, 1889–1897. [Google Scholar] [CrossRef]
- Peng, M.; Qin, S.; Jia, H.; Zheng, D.; Rong, L.; Zhang, X. Self-delivery of a peptide-based prodrug for tumor-targeting therapy. Nano Res. 2015, 9, 663–673. [Google Scholar] [CrossRef]
- Xu, P.; Wang, X.; Li, T.; Li, L.; Wu, H.; Tu, J.; Zhang, R.; Zhang, L.; Guo, Z.; Chen, Q. Bioinspired Microenvironment Responsive Nanoprodrug as an Efficient Hydrophobic Drug Self-Delivery System for Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 33926–33936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Z.; Kong, Z.; Wang, Y.; Zhang, X.; Sun, B.; Zhang, H.; Kan, Q.; He, Z.; Luo, C.; et al. Photosensitizer-driven nanoassemblies of homodimeric prodrug for self-enhancing activation and synergistic chemo-photodynamic therapy. Theranostics 2021, 11, 6019–6032. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Ye, J.; Jiao, J.; Liu, Z.; Zhao, C.; Li, B.; Fu, Y. Metal-coordinated supramolecular self-assemblies for cancer theranostics. Adv. Sci. 2021, 8, e2101101. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Zhang, S.; Sun, B.; Chen, Q.; He, Z.; Luo, C.; Sun, J. Emerging carrier-free nanosystems based on molecular self-assembly of pure drugs for cancer therapy. Med. Res. Rev. 2020, 40, 1754–1775. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Zhao, R.-R.; Fang, Y.-F.; Jiang, J.-L.; Yuan, X.-T.; Shao, J.-W. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019, 570, 118663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, P.; Zhao, X.; Gao, D.; Sun, N.; Tian, Z.; Ma, T.; Yang, Z. Multifunctional cargo-free nanomedicine for cancer therapy. Int. J. Mol. Sci. 2018, 19, 2963. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Meijer, E.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Fu, S.; Li, G.; Zang, W.; Zhou, X.; Shi, K.; Zhai, Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm. Sin. B 2022, 12, 92–106. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Y.; Lortie, F.; Bernard, J.; Binder, W.H.; Zhu, J. Hydrogen-Bonds-Mediated Nanomedicine: Design, Synthesis, and Applications. Macromol. Rapid Commun. 2022, 43, e2200168. [Google Scholar] [CrossRef]
- Niu, D.; Jiang, Y.; Ji, L.; Ouyang, G.; Liu, M. Self-assembly through coordination and π-stacking: Controlled switching of circularly polarized luminescence. Angew. Chem. 2019, 58, 5946–5950. [Google Scholar] [CrossRef]
- Jiang, Z.; Bhaskaran, A.; Aitken, H.M.; Shackleford, I.C.; Connal, L.A. Using synergistic multiple dynamic bonds to construct polymers with engineered properties. Macromol. Rapid Commun. 2019, 40, e1900038. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, R.; Jiao, T.; Ma, K.; Chen, C.; Ma, G.; Yan, X. Carrier-Free, Chemophotodynamic Dual Nanodrugs via Self-Assembly for Synergistic Antitumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Wang, J.; Wu, J.; Ma, X.; Li, L.; Wei, X.; Ling, Q.; Song, P.; Zhou, L.; et al. Self-Assembling Prodrugs by Precise Programming of Molecular Structures that Contribute Distinct Stability, Pharmacokinetics, and Antitumor Efficacy. Adv. Funct. Mater. 2015, 25, 4956–4965. [Google Scholar] [CrossRef]
- Liu, L.; Bao, Y.; Wang, J.; Xiao, C.; Chen, L. Construction of carrier-free porphyrin-based drug self-framed delivery system to reverse multidrug resistance through photodynamic-chemotherapy. Dyes Pigments 2020, 177, 107922. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Fang, T.; Li, M.; Chen, G.; Chen, Q. Supramolecular biomaterials for enhanced cancer immunotherapy. J. Mater. Chem. B 2022, 10, 7183–7193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, Y.-H.; Zhao, Q.-Y.; Zheng, W.; Yang, J.-H.; Pei, H.-Y.; Liu, L.; Liu, K.-J.; Xue, L.-L.; Deng, D.-X. Synthesis and evaluation of new compounds bearing 3-(4-aminopiperidin-1-yl) methyl magnolol scaffold as anticancer agents for the treatment of non-small cell lung cancer via targeting autophagy. Eur. J. Med. Chem. 2021, 209, 112922. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, W.; Li, X.; Han, X.; Zhang, X.; Wang, J.; Liu, C.; Huang, L.; Gao, W. Natural Small Molecules Enabled Efficient Immunotherapy through Supramolecular Self-Assembly in P53-Mutated Colorectal Cancer. ACS Appl. Mater. Interfaces 2022, 14, 2464–2477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, W.; Li, X.; Zhao, H.; Zhang, H.; Dong, A.; Yang, X. A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy. J. Mater. Chem. B 2021, 9, 1040–1048. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Shi, N.; Li, W.; Bi, J.; Feng, X.; Shi, N.; Zhu, W.; Xie, Z. Self-assembly and self-delivery of the pure nanodrug dihydroartemisinin for tumor therapy and mechanism analysis. Biomater. Sci. 2023, 11, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Do special noncovalent π–π stacking interactions really exist? Angew. Chem. Int. Ed. Engl. 2008, 47, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Xu, C.; Shi, L.; Tayier, M.; Zhou, J.; Zhang, J.; Wu, J.; Ye, Z.; Fang, T. Cancer nanomedicines stabilized by π-π stacking between heterodimeric prodrugs enable exceptionally high drug loading capacity and safer delivery of drug combinations. Theranostics 2017, 7, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-R.; Wang, Y.; Cui, P.-F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.-L.; Oh, Y.-K. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Control. Release 2019, 294, 311–326. [Google Scholar] [CrossRef]
- Fu, S.; Yang, X. Recent advances in natural small molecules as drug delivery systems. J. Mater. Chem. B 2023, 11, 4584–4599. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Ren, C.; Wang, H.; Mao, D.; Zhang, X.; Fengzhao, Q.; Shi, Y.; Ding, D.; Kong, D.; Wang, L.; Yang, Z. When Molecular Probes Meet Self-Assembly: An Enhanced Quenching Effect. Angew. Chem. Int. Ed. Engl. 2015, 54, 4823–4827. [Google Scholar] [CrossRef]
- Wang, M.; Li, F.; Lu, T.; Wu, R.; Yang, S.; Chen, W. Photodynamic and ferroptotic Ce6@ ZIF-8@ ssPDA for head and neck cancer treatment. Mater. Des. 2022, 224, 111403. [Google Scholar] [CrossRef]
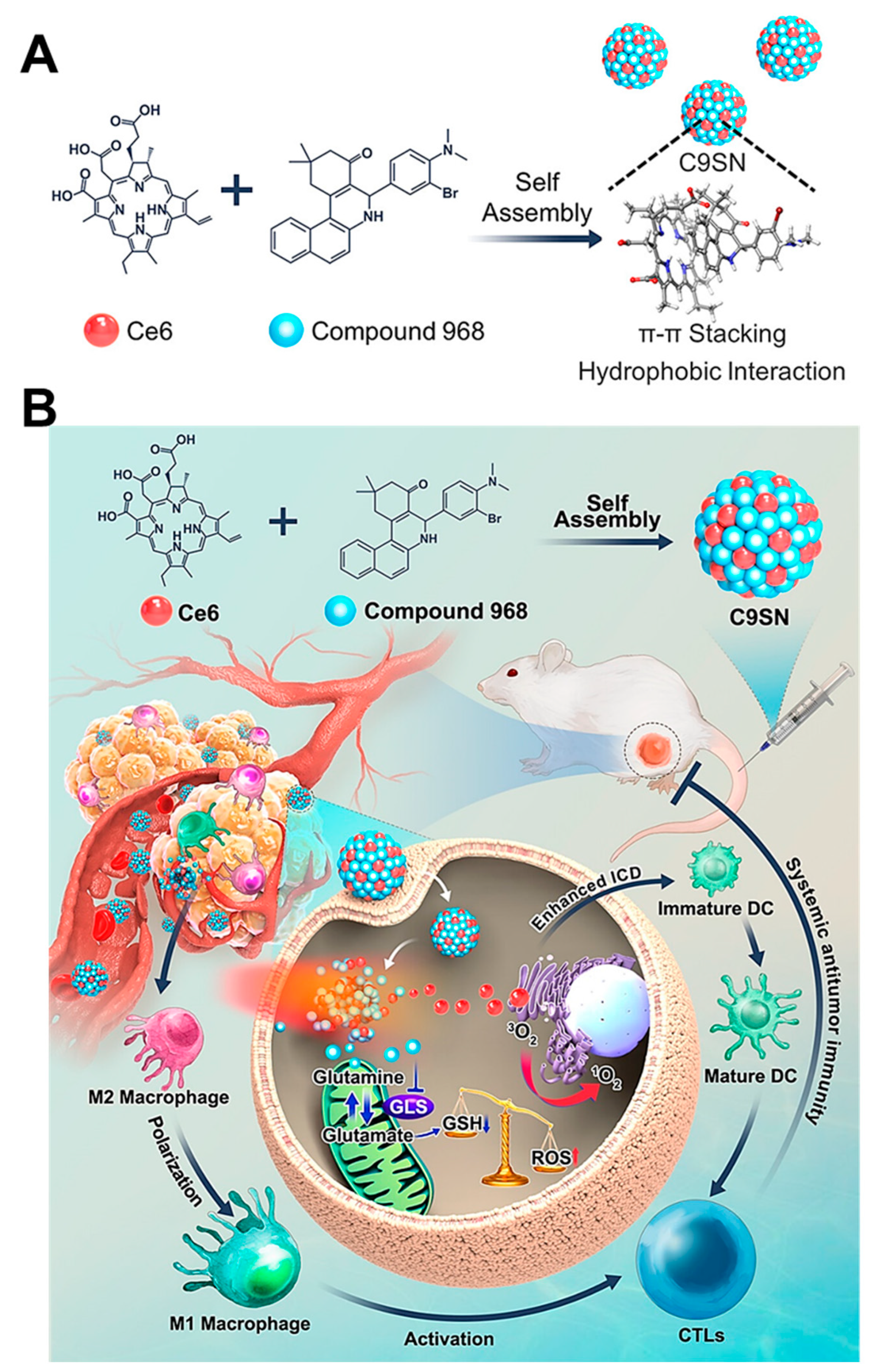
- Mai, Z.; Zhong, J.; Zhang, J.; Chen, G.; Tang, Y.; Ma, W.; Li, G.; Feng, Z.; Li, F.; Liang, X.-J. Carrier-free immunotherapeutic nano-booster with dual synergistic effects based on glutaminase inhibition combined with photodynamic therapy. ACS Nano 2023, 17, 1583–1596. [Google Scholar] [CrossRef]
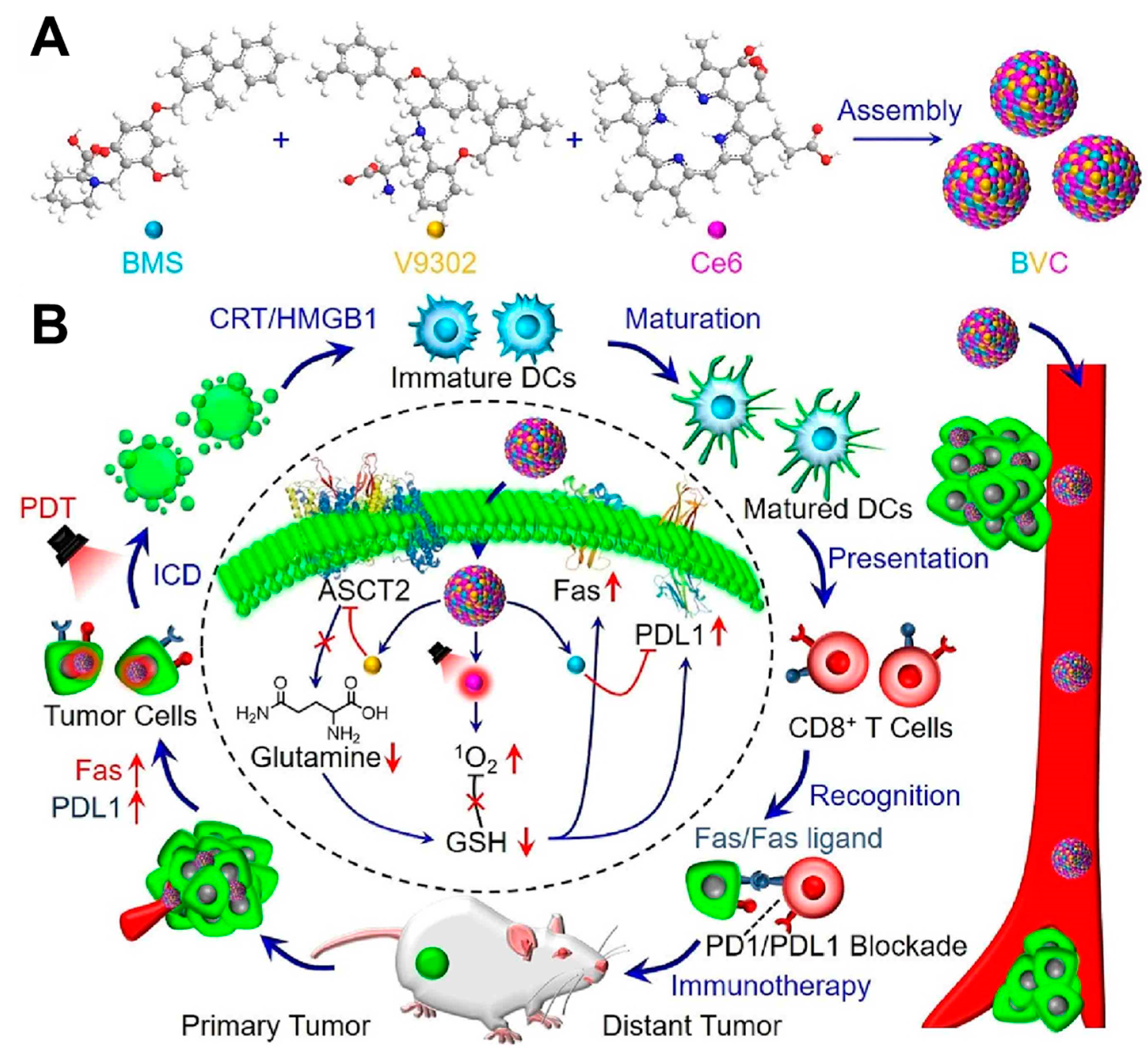
- Wang, C.; Yu, H.; Yang, X.; Zhang, X.; Wang, Y.; Gu, T.; Zhang, S.; Luo, C. Elaborately engineering of a dual-drug co-assembled nanomedicine for boosting immunogenic cell death and enhancing triple negative breast cancer treatment. Asian J. Pharm. Sci. 2022, 17, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, M.; Zhao, Z.; Du, Q.; Li, Q.; Jiang, Y.; Xue, F.; Luan, Y. A Carrier-Free Photodynamic Nanodrug to Enable Regulation of Dendritic Cells for Boosting Cancer Immunotherapy. Acta Biomater. 2022, 47, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Y.; Chen, K.; Huang, Y.; Liu, Y.; Xu, S.; Wang, W. CDK4/6 nano-PROTAC enhances mitochondria-dependent photodynamic therapy and anti-tumor immunity. Nano Today 2023, 50, 101890. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Zhao, L.; Rao, X.; Zheng, R.; Huang, C.; Kong, R.; Yu, X.; Cheng, H.; Li, S. Targeting glutamine metabolism with photodynamic immunotherapy for metastatic tumor eradication. J. Control. Release 2023, 357, 460–471. [Google Scholar] [CrossRef]
- Le, J.-Q.; Yang, F.; Yin, M.-D.; Zhao, R.-R.; Zhang, B.-C.; Li, C.; Lin, J.-F.; Fang, Y.-F.; Lin, Y.-T.; Shao, J.-W. Biomimetic polyphenol-coated nanoparticles by Co-assembly of mTOR inhibitor and photosensitizer for synergistic chemo-photothermal therapy. Colloids Surf. B 2022, 209, 112177. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Chen, S.-Y.; Zheng, R.-R.; Rao, X.-N.; Kong, R.-J.; Huang, C.-Y.; Liu, Y.-B.; Tang, Y.; Cheng, H.; Li, S.-Y. Photodynamic Therapy Initiated Ferrotherapy of Self-Delivery Nanomedicine to Amplify Lipid Peroxidation via GPX4 Inactivation. ACS Appl. Mater. Interfaces 2022, 14, 53501–53510. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Sun, X.; Wang, Y.; Zhang, X.; Zhang, S.; Zhang, H.; Kan, Q.; Sun, J.; He, Z. Precisely engineering a carrier-free hybrid nanoassembly for multimodal DNA damage-augmented photodynamic therapy. Chem. Eng. J. 2021, 426, 130838. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liu, J.; Zhu, Q.; Ma, J.; Zhu, X. Erythrocyte membrane bioengineered nanoprobes via indocyanine green-directed assembly for single NIR laser-induced efficient photodynamic/photothermal theranostics. J. Control. Release 2021, 335, 345–358. [Google Scholar] [CrossRef]
- Lan, J.-S.; Liu, L.; Zeng, R.-F.; Qin, Y.-H.; Hou, J.-W.; Xie, S.-S.; Yue, S.; Yang, J.; Ho, R.J.; Ding, Y. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021, 407, 127212. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; Mcneil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 2009, 5, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Rahman, M.S.U.; Mei, J.; Wang, X.; Jiang, J.; Chen, Y.; Xu, S.; Liu, Y. Pre-Induced ICD Membrane-Coated Carrier-Free Nanoparticles for the Personalized Lung Cancer Immunotherapy. Small Methods 2023, 7, e2201569. [Google Scholar] [CrossRef]
- Souza, C.; Pellosi, D.S.; Tedesco, A.C. Prodrugs for targeted cancer therapy. Expert Rev. Anticancer Ther. 2019, 19, 483–502. [Google Scholar] [CrossRef]
- Walther, R.; Rautio, J.; Zelikin, A.N. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv. Drug Deliv. Rev. 2017, 118, 65–77. [Google Scholar] [CrossRef]
- Harrisson, S.; Nicolas, J.; Maksimenko, A.; Bui, D.T.; Mougin, J.; Couvreur, P. Nanoparticles with in vivo anticancer activity from polymer prodrug amphiphiles prepared by living radical polymerization. Angew. Chem. Int. Ed. Engl. 2013, 52, 1678–1682. [Google Scholar] [CrossRef]
- Li, G.; Sun, B.; Li, Y.; Luo, C.; He, Z.; Sun, J. Small-molecule prodrug nanoassemblies: An emerging nanoplatform for anticancer drug delivery. Small 2021, 17, e2101460. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef]
- Low, L.E.; Wu, J.; Lee, J.; Tey, B.T.; Goh, B.H.; Gao, J.; Li, F.; Ling, D. Tumor-responsive dynamic nanoassemblies for targeted imaging, therapy and microenvironment manipulation. J. Control. Release 2020, 324, 69–103. [Google Scholar] [CrossRef]
- Nguyen, A.; Böttger, R.; Li, S.-D. Recent trends in bioresponsive linker technologies of prodrug-based self-assembling nanomaterials. Biomaterials 2021, 275, 120955. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shan, X.; Wang, Y.; Chen, Q.; Sun, J.; He, Z.; Sun, B.; Luo, C. Dimeric prodrug-based nanomedicines for cancer therapy. J. Control. Release 2020, 326, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Wang, J.; Han, X.; Wang, Y. Hydrophobic drug self-delivery systems as a versatile nanoplatform for cancer therapy: A review. Colloids Surf. B 2019, 180, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Ye, M.; Liu, L.; Xu, M.; Liu, H.; Zhang, H.; Li, Y.; Xu, Z.; Li, B. Azide-locked prodrug co-assembly into nanoparticles with indocyanine green for chemophotothermal therapy. Mol. Pharm. 2022, 19, 3279–3287. [Google Scholar] [CrossRef]
- Kyu Shim, M.; Yang, S.; Sun, I.C.; Kim, K. Tumor-activated carrier-free prodrug nanoparticles for targeted cancer Immunotherapy: Preclinical evidence for safe and effective drug delivery. Adv. Drug Deliv. Rev. 2022, 183, 114177. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Dong, X.; Brahma, R.K.; Fang, C.; Yao, S.Q. Stimulus-responsive self-assembled prodrugs in cancer therapy. Chem. Sci. 2022, 13, 4239–4269. [Google Scholar] [CrossRef]
- Mellman, I.; Fuchs, R.; Helenius, A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986, 55, 663–700. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Chen, L.; Yu, S.; Cao, Y.; He, C.; Chen, X. Intracellular pH-Sensitive PEG-block-Acetalated-Dextrans as Efficient Drug Delivery Platforms. ACS Appl. Mater. Interfaces 2013, 5, 10760–10766. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jazani, A.M.; Oh, J.K. Recent advances in development of imine-based acid-degradable polymeric nanoassemblies for intracellular drug delivery. Polymer 2021, 230, 124024. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, J.; Guan, Z.; Wu, H.; Cheng, H.; Yan, G.; Tang, R. pH-triggered small molecule nano-prodrugs emulsified from tryptamine-cinnamaldehyde twin drug for targeted synergistic glioma therapy. Colloids Surf. B 2021, 207, 112052. [Google Scholar] [CrossRef]
- Raguz, S.; Yague, E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Li, Q.; Jiang, Y.; Shen, W.; Xing, Y.; Liang, G.; Wu, Q.; Ban, S.; Zhang, R. Development of carrier-free nanodrugs based on low molecular weight heparin-doxorubicin conjugate assembly with smart pH-triggered drug release characteristics for combinatorial antitumor therapy. New J. Chem. 2022, 46, 820–831. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, H.; Sun, M.; Mao, S.; He, Q.; Shi, Y.; Deng, Y.; Dong, Z.; Xu, Q.; Zhao, C. Manipulating Offense and Defense Signaling to Fight Cold Tumors with Carrier-Free Nanoassembly of Fluorinated Prodrug and siRNA. Adv. Mater. 2022, 34, e2203019. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Seki, T.; Maeda, H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliv. Rev. 2009, 61, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, J.; Wan, J.; Li, Z. Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10, 24397–24409. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv. Sci. 2018, 5, 1700821. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y. Disulfide bond-driven oxidation-and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018, 18, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Liu, S.; Li, Y.; Xie, Z.; Jing, X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J. Control. Release 2017, 254, 23–33. [Google Scholar] [CrossRef] [PubMed]
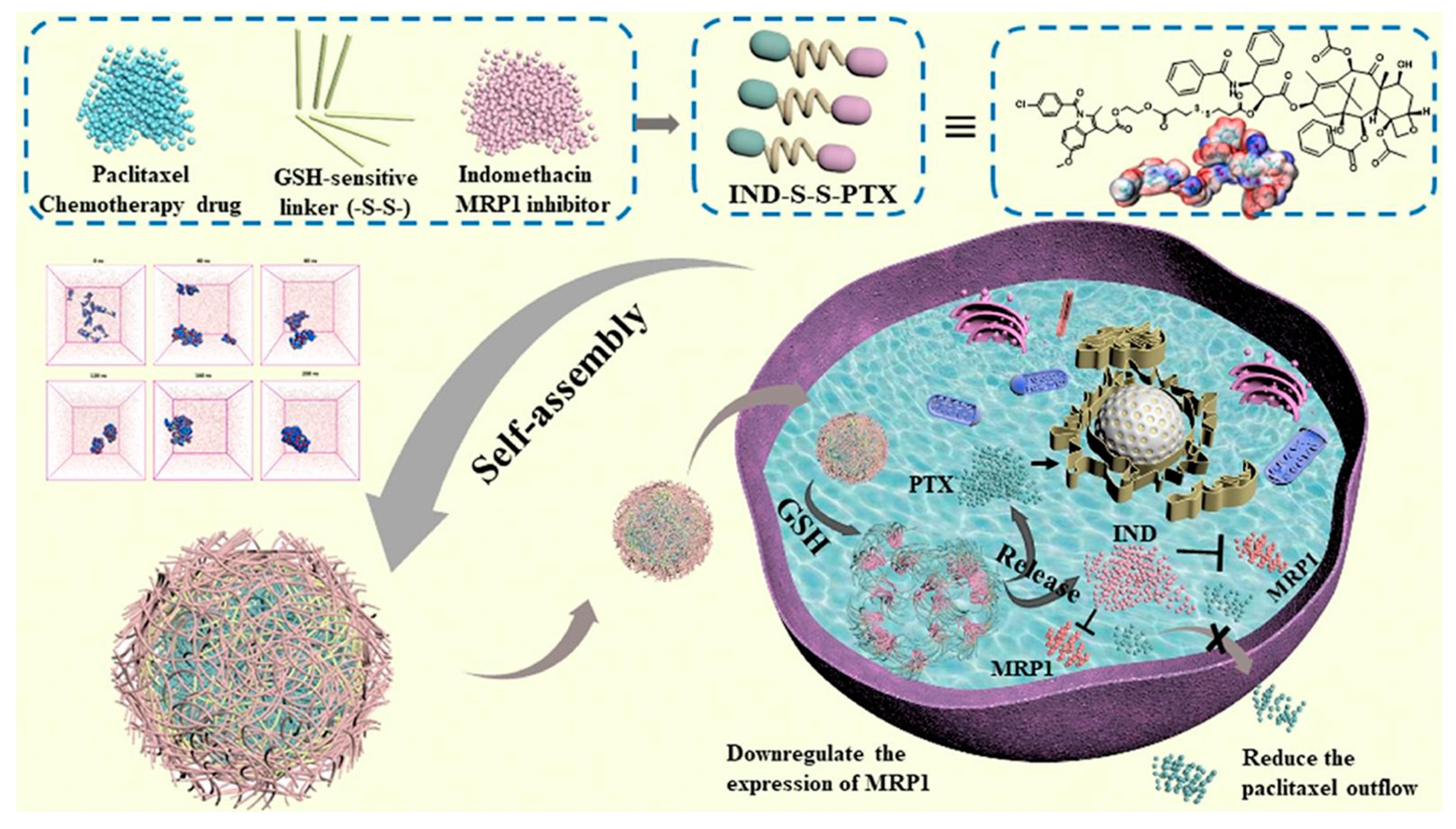
- Kang, W.; Ji, Y.; Cheng, Y. Van der Waals force-driven indomethacin-ss-paclitaxel nanodrugs for reversing multidrug resistance and enhancing NSCLC therapy. Int. J. Pharm. 2021, 603, 120691. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, X.; Wang, Y.; Han, F.; Xu, F.; Chen, Y. Mediating physicochemical properties and paclitaxel release of pH-responsive H-type multiblock copolymer self-assembly nanomicelles through epoxidation. J. Mater. Chem. B 2017, 5, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lv, Y.; Chen, Z.; Wang, F.; Wang, Y.; Pei, Y.; Jin, W.; Shi, C.; Wang, Y.; Qu, Y. A carrier-free multifunctional nano photosensitizer based on self-assembly of lactose-conjugated BODIPY for enhanced anti-tumor efficacy of dual phototherapy. Chem. Eng. J. 2021, 417, 129178. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, C.; Alfranca, G.; Yang, Y.; Jiang, X.; Yang, Y.; Pan, F.; de la Fuente, J.M.; Cui, D. Near-infrared light triggered ROS-activated theranostic platform based on Ce6-CPT-UCNPs for simultaneous fluorescence imaging and chemo-photodynamic combined therapy. Theranostics 2016, 6, 456–469. [Google Scholar] [CrossRef]
- Yang, B.; Wei, L.; Wang, Y.; Li, N.; Sun, J. Oxidation-strengthened disulfide-bridged prodrug nanoplatforms with cascade facilitated drug release for synergetic photochemotherapy. Asian J. Pharm. Sci. 2020, 15, 637–645. [Google Scholar] [CrossRef]
- Lavis, L.D. Ester bonds in prodrugs. ACS Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef]
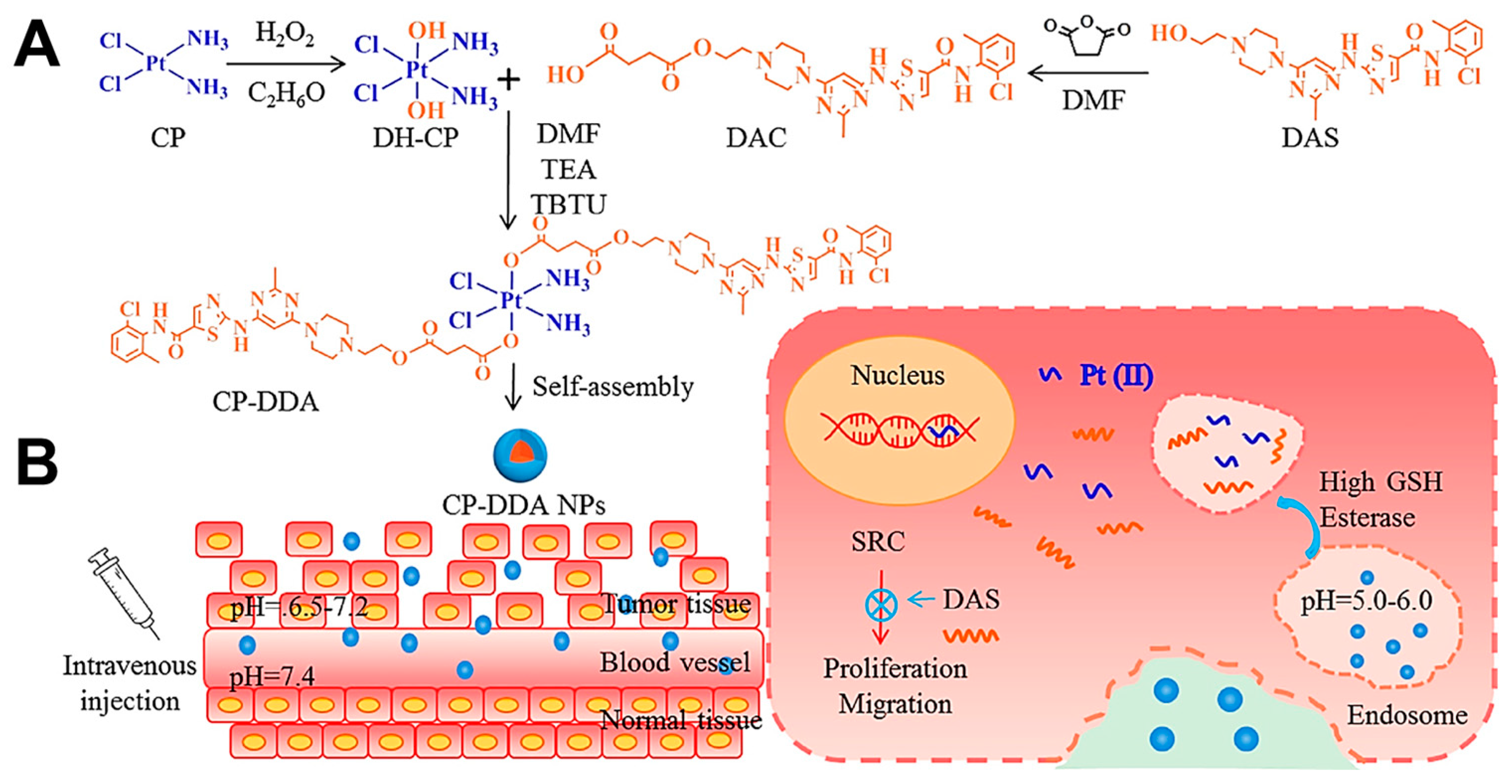
- Yang, L.; Xu, J.; Xie, Z.; Song, F.; Wang, X.; Tang, R. Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo. Asian J. Pharm. Sci. 2021, 16, 762–771. [Google Scholar] [CrossRef]
- Li, X.; Yu, N.; Li, J.; Bai, J.; Ding, D.; Tang, Q.; Xu, H. Novel “Carrier-Free” nanofiber codelivery systems with the synergistic antitumor effect of paclitaxel and tetrandrine through the enhancement of mitochondrial apoptosis. ACS Appl. Mater. Interfaces 2020, 12, 10096–10106. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, Y.; Xiang, S.; Zuo, W.; Huang, D.; Jiang, B.; Sun, H.; Yin, W.; Xie, L.; Hou, Z. Dual-self-recognizing, stimulus-responsive and carrier-free methotrexate–mannose conjugate nanoparticles with highly synergistic chemotherapeutic effects. J. Mater. Chem. B 2020, 8, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Olson, O.C.; Joyce, J.A. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 2015, 15, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110, 112–126. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Li, R.Q.; Qiu, W.X.; Zhuang, Z.N.; Cheng, S.X.; Zhang, X.Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018, 28, 1804492. [Google Scholar] [CrossRef]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Akinyemi, A.O.; Pereira, G.; Rocha, F.V. Role of Cathepsin B in Cancer Progression: A Potential Target for Coordination Compounds. Mini-Rev. Med. Chem. 2021, 21, 1612–1624. [Google Scholar] [CrossRef]
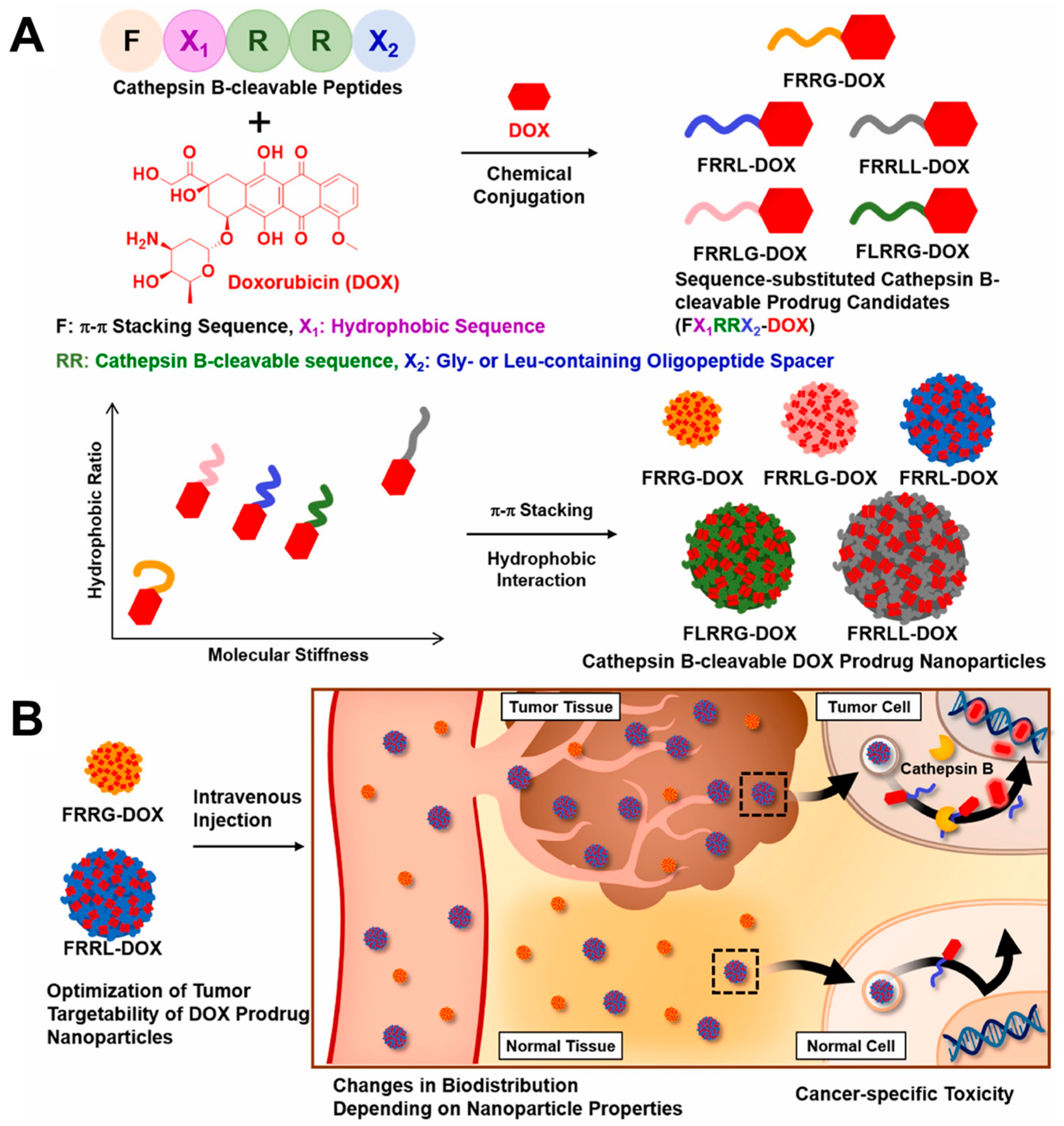
- Shim, M.K.; Moon, Y.; Yang, S.; Kim, J.; Cho, H.; Lim, S.; Yoon, H.Y.; Seong, J.-K.; Kim, K. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 2020, 261, 120347. [Google Scholar] [CrossRef] [PubMed]
- Shim, N.; Jeon, S.I.; Yang, S.; Park, J.Y.; Jo, M.; Kim, J.; Choi, J.; Yun, W.S.; Kim, J.; Lee, Y. Comparative study of cathepsin B-cleavable linkers for the optimal design of cathepsin B-specific doxorubicin prodrug nanoparticles for targeted cancer therapy. Biomaterials 2022, 289, 121806. [Google Scholar] [CrossRef]
- Hu, C.; He, X.; Chen, Y.; Yang, X.; Qin, L.; Lei, T.; Zhou, Y.; Gong, T.; Huang, Y.; Gao, H. Metformin mediated PD-L1 downregulation in combination with photodynamic-immunotherapy for treatment of breast cancer. Adv. Funct. Mater. 2021, 31, 2007149. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Fan, Z.; Zhu, F.; Zhang, Q.; Zeng, S.; Chen, Z.; Ren, L.; Hou, Z.; Ye, S.; Li, Y. Self-recognizing and stimulus-responsive carrier-free metal-coordinated nanotheranostics for magnetic resonance/photoacoustic/fluorescence imaging-guided synergistic photo-chemotherapy. J. Mater. Chem. B. 2020, 8, 5667–5681. [Google Scholar] [CrossRef]
- Liu, B.; Hu, F.; Zhang, J.; Wang, C.; Li, L. A biomimetic coordination nanoplatform for controlled encapsulation and delivery of drug-gene combinations. Angew. Chem. Int. Ed. Engl. 2019, 58, 8804–8808. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Ye, J.; Wang, J.; Wang, Q.; Liu, Z.; Xu, J.; Fu, Y. Metal-coordinated nanodrugs based on natural products for cancer theranostics. Chem. Eng. J. 2023, 456, 140892. [Google Scholar] [CrossRef]
- Huang, S.; Le, H.; Hong, G.; Chen, G.; Zhang, F.; Lu, L.; Zhang, X.; Qiu, Y.; Wang, Z.; Zhang, Q. An all-in-one biomimetic iron-small interfering RNA nanoplatform induces ferroptosis for cancer therapy. Acta Biomater. 2022, 148, 244–257. [Google Scholar] [CrossRef]
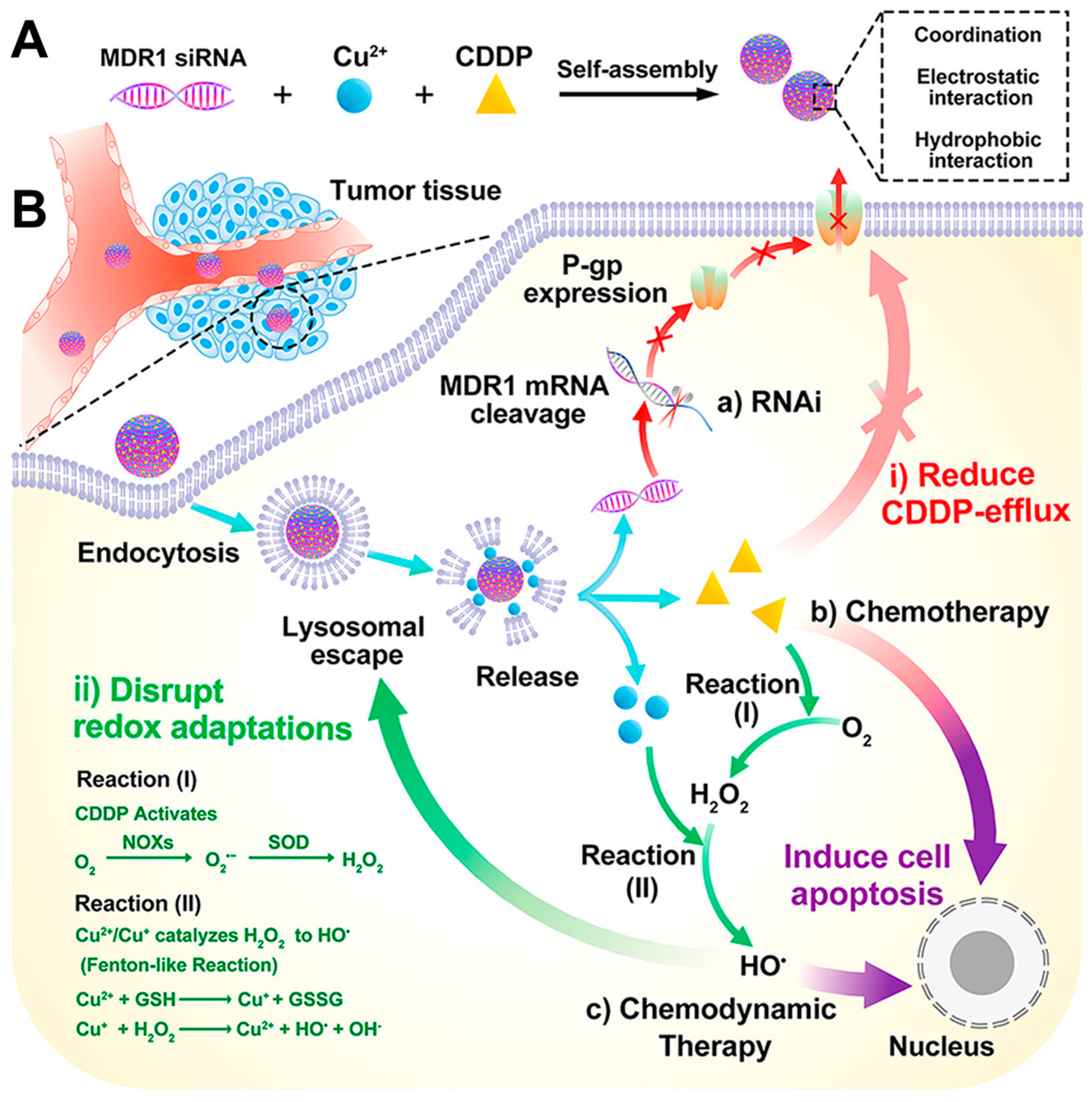
- Jiang, Y.; Liu, Y.; Wang, M.; Li, Z.; Su, L.; Xu, X.; Xing, C.; Li, J.; Lin, L.; Lu, C. siRNA-Based Carrier-Free System for Synergistic Chemo/Chemodynamic/RNAi Therapy of Drug-Resistant Tumors. ACS Appl. Mater. Interfaces 2021, 14, 361–372. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, W.; Jin, Q.; Liu, C.; Liu, N.; Tian, H.; Zhu, X. Mn (II)-directed dual-photosensitizers co-assemblies for multimodal imaging-guided self-enhanced phototherapy. Mater. Sci. Eng. C. 2021, 129, 112351. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Hu, J.; Liu, J.; Li, M. Degradable Carrier-Free Metal-Phenolic Network Theranostic Agent with Targeted Mitochondrial Damage for Efficient Cancer Theranostics. Chem. Mater. 2021, 33, 7089–7099. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, Y.; Zeng, J.; Li, Y.; Wang, Z.; Zhu, R.; Ma, T.; Gao, M. Coordinatively unsaturated Fe3+ based activatable probes for enhanced MRI and therapy of tumors. Angew. Chem. Int. Ed. 2019, 58, 11088–11096. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Han, S.; Fang, Y.; Huang, H.; Wu, J. Multidimensional transitional metal-actuated nanoplatforms for cancer chemodynamic modulation. Coord. Chem. Rev. 2022, 455, 214360. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Gong, S.; Zhang, C.; Qian, C.; Qiao, H.; Sun, M. Dual-mode avocado-like all-iron nanoplatform for enhanced T1/T2 MRI-guided cancer theranostic therapy. Nano Lett. 2020, 20, 4842–4849. [Google Scholar] [CrossRef]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Shang, L.; Yang, T.; Yang, C.; Li, Z.; Kong, L.; Zhang, Z. Metal ions-mediated self-assembly of nanomedicine for combinational therapy against triple-negative breast cancer. Chem. Eng. J. 2021, 425, 131420. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, D.; Zuo, W.; Feng, J.; Ge, D.; Su, G.; Yang, L.; Hou, Z. Trojan-Horse Diameter-Reducible Nanotheranostics for Macroscopic/Microscopic Imaging-Monitored Chemo-Antiangiogenic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 5033–5052. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, Y.; Zhang, S.; Liu, H.; Zhang, J.; Feng, H.; Li, B.; Wu, X.; Gao, Y. A redox-triggered C-centered free radicals nanogenerator for self-enhanced magnetic resonance imaging and chemodynamic therapy. Biomaterials 2021, 266, 120457. [Google Scholar] [CrossRef]
- Hao, Y.-N.; Zhang, W.-X.; Gao, Y.-R.; Wei, Y.-N.; Shu, Y.; Wang, J.-H. State-of-the-art advances of copper-based nanostructures in the enhancement of chemodynamic therapy. J. Mater. Chem. B 2021, 9, 250–266. [Google Scholar] [CrossRef]
- Pi, W.; Wu, L.; Lu, J.; Lin, X.; Huang, X.; Wang, Z.; Yuan, Z.; Qiu, H.; Zhang, J.; Lei, H. A metal ions-mediated natural small molecules carrier-free injectable hydrogel achieving laser-mediated photo-Fenton-like anticancer therapy by synergy apoptosis/cuproptosis/anti-inflammation. Bioact. Mater. 2023, 29, 98–115. [Google Scholar] [CrossRef]
- Lu, X.; Gao, S.; Lin, H.; Yu, L.; Han, Y.; Zhu, P.; Bao, W.; Yao, H.; Chen, Y.; Shi, J. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv. Mater. 2020, 32, 2002246. [Google Scholar] [CrossRef]
- Koo, S.; Park, O.K.; Kim, J.; Han, S.I.; Yoo, T.Y.; Lee, N.; Kim, Y.G.; Kim, H.; Lim, C.; Bae, J.-S. Enhanced chemodynamic therapy by Cu-Fe peroxide nanoparticles: Tumor microenvironment-mediated synergistic Fenton reaction. ACS Nano 2022, 16, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.N.; Huang, X.T.; Jiang, X.L.; Deng, T.W.; Li, Q.X.; Li, J.X.; Wu, Q.N.; Li, S.P.; Sun, X.Q.; Huang, Y.G.; et al. The Antibacterial Effects of Supermolecular Nano-Carriers by Combination of Silver and Photodynamic Therapy. Front. Chem. 2021, 9, 666408. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zuo, W.; Xiao, Z.; Jin, Q.; Liu, J.; Wu, L.; Liu, N.; Zhu, X. A carrier-free metal-coordinated dual-photosensitizers nanotheranostic with glutathione-depletion for fluorescence/photoacoustic imaging-guided tumor phototherapy. J. Colloid Interface Sci. 2021, 600, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, C.; Liu, H.; Wang, Y.; Dong, Z.; Lu, C.; Nan, B.; Yue, R.; Yin, X.; Zhang, X.B. Ternary Alloy PtWMn as a Mn Nanoreservoir for High-Field MRI Monitoring and Highly Selective Ferroptosis Therapy. Angew. Chem. Int. Ed. Engl. 2022, 134, e202117229. [Google Scholar] [CrossRef]
- Xu, K.F.; Jia, H.R.; Zhu, Y.X.; Liu, X.; Gao, G.; Li, Y.H.; Wu, F.G. Cholesterol-Modified Dendrimers for Constructing a Tumor Microenvironment-Responsive Drug Delivery System. ACS Biomater. Sci. Eng. 2019, 5, 6072–6081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, M.; Mu, Y.; Li, J.; Foda, M.F.; Zhang, W.; Han, K.; Han, H. Reasonably retard O2 consumption through a photoactivity conversion nanocomposite for oxygenated photodynamic therapy. Biomaterials 2019, 218, 119312. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Li, S.; Xin, X.; Yuan, S.; Ma, G.; Yan, X. Self-Assembled Minimalist Multifunctional Theranostic Nanoplatform for Magnetic Resonance Imaging-Guided Tumor Photodynamic Therapy. ACS Nano 2018, 12, 8266–8276. [Google Scholar] [CrossRef]
- Xing, R.; Zou, Q.; Yuan, C.; Zhao, L.; Chang, R.; Yan, X. Self-Assembling Endogenous Biliverdin as a Versatile Near-Infrared Photothermal Nanoagent for Cancer Theranostics. Adv. Mater. 2019, 31, e1900822. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Chen, F.; Wang, X.; Wang, L.; Pang, Y.; Liu, J. Combining anti-PD-1 antibodies with Mn2+-drug coordinated multifunctional nanoparticles for enhanced cancer therapy. Biomaterials. 2021, 275, 120897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO-DOX@ZIF-8 Core-Shell Nanoparticles for pH-Responsive Drug Delivery. ACS. Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Chu, C.; Lin, H.; Liu, H.; Wang, X.; Wang, J.; Zhang, P.; Gao, H.; Huang, C.; Zeng, Y.; Tan, Y. Tumor microenvironment-triggered supramolecular system as an in situ nanotheranostic generator for cancer phototherapy. Adv. Mater. 2017, 29, 1605928. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, H.; Li, Z.; Luo, Q.; Zhu, H.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Engineered gadolinium-based nanomaterials as cancer imaging agents. Appl. Mater. Today 2020, 20, 100686. [Google Scholar] [CrossRef]
- Fan, J.X.; Zheng, D.W.; Mei, W.W.; Chen, S.; Chen, S.Y.; Cheng, S.X.; Zhang, X.Z. A metal-polyphenol network coated nanotheranostic system for metastatic tumor treatments. Small 2017, 13, 1702714. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, C.; Key, J.; Ramirez, M.; Aryal, S.; Decuzzi, P. Radiolabeled Polymeric Nanoconstructs Loaded with Docetaxel and Curcumin for Cancer Combinatorial Therapy and Nuclear Imaging. Adv. Funct. Mater. 2015, 25, 3371–3379. [Google Scholar] [CrossRef]
- Fan, Z.; Jiang, B.; Zhu, Q.; Xiang, S.; Tu, L.; Yang, Y.; Zhao, Q.; Huang, D.; Han, J.; Su, G. Tumor-specific endogenous FeII-activated, MRI-guided self-targeting gadolinium-coordinated theranostic nanoplatforms for amplification of ROS and enhanced chemodynamic chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 14884–14904. [Google Scholar] [CrossRef]
- Detappe, A.; Kunjachan, S.; Rottmann, J.; Robar, J.; Tsiamas, P.; Korideck, H.; Tillement, O.; Berbeco, R. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, D.; Ye, Q.; Jiang, H.; Gu, R.; Ji, C.; Wu, J.; Hu, Y.; Yuan, A. Zoledronic Acid-Gadolinium Coordination Polymer Nanorods for Improved Tumor Radioimmunotherapy by Synergetically Inducing Immunogenic Cell Death and Reprogramming the Immunosuppressive Microenvironment. ACS Nano 2021, 15, 8450–8465. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, P.Y.; Yan, P.; Chen, P.L.; Shi, Q.Y.; Zhao, Z.A.; Chen, J.X.; Shu, X.; Wang, P.; Yang, B.; et al. Versatile Nanodrugs Containing Glutathione and Heme Oxygenase 1 Inhibitors Enable Suppression of Antioxidant Defense System in a Two-Pronged Manner for Enhanced Photodynamic Therapy. Adv. Healthc. Mater. 2021, 10, e2100770. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, L.; Huang, Y.; Wang, Y.; Zhang, M. Bioinspired fluorescent dipeptide nanoparticles for targeted cancer cell imaging and real-time monitoring of drug release. Nat. Nanotechnol. 2016, 11, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, W.; Duan, Y.; Xu, L.; Li, S.; Gao, X.; Liu, B. Carrier-Free Hybrid DNA Nanoparticles for Light-Induced Self-Delivery of Functional Nucleic Acid Enzymes. ACS Nano 2021, 15, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Chen, X. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Shi, B.; Ge, F.; Liu, Y.; Mao, Z.; Zhu, H.; Wang, S.; Yu, G.; Huang, F.; et al. Dual-Emissive Platinum(II) Metallacage with a Sensitive Oxygen Response for Imaging of Hypoxia and Imaging-Guided Chemotherapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 20208–20214. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yu, G.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef]
- Fu, X.; Yin, W.; Shi, D.; Yang, Y.; Zhang, D. Shuttle-Shape Carrier-Free Platinum-Coordinated Nanoreactors with O2 Self-Supply and ROS Augment for Enhanced Phototherapy of Hypoxic Tumor. ACS Appl. Mater. Interfaces 2021, 13, 32690–32702. [Google Scholar] [CrossRef]
- Xing, L.; Yang, C.X.; Zhao, D.; Shen, L.J.; Zhou, T.J.; Bi, Y.Y.; Huang, Z.J.; Wei, Q.; Li, L.; Li, F. A carrier-free anti-inflammatory platinum (II) self-delivered nanoprodrug for enhanced breast cancer therapy. J. Control. Release 2021, 331, 460–471. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Yoon, M.J.; Kim, E.H.; Kwon, T.K.; Park, S.A.; Choi, K.S. Simultaneous mitochondrial Ca2+ overload and proteasomal inhibition are responsible for the induction of paraptosis in malignant breast cancer cells. Cancer Lett. 2012, 324, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Palanikumar, L.; Choi, H.; Jeena, M.T.; Kim, C.; Ryu, J.-H. Intra-mitochondrial biomineralization for inducing apoptosis of cancer cells. Chem. Sci. 2018, 9, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Chenguang, L.; Lingxiao, G.; Yong, W.; Jianting, Z.; Caiyun, F. Delivering metal ions by nanomaterials: Turning metal ions into drug-like cancer theranostic agents. Coord. Chem. Rev. 2023, 494, 215332. [Google Scholar]
- Qin, M.; Li, M.; Song, G.; Yang, C.; Wu, P.; Dai, W.; Zhang, H.; Wang, X.; Wang, Y.; Zhou, D. Boosting innate and adaptive antitumor immunity via a biocompatible and carrier-free nanovaccine engineered by the bisphosphonates-metal coordination. Nano Today 2021, 37, 101097. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, M.; Wang, T.; Li, B.; Zhang, S.; Zhang, T.; Liu, P.; Wang, Q.; Qian, Z.R.; Zhu, C. Carrier-free multifunctional nanomedicine for intraperitoneal disseminated ovarian cancer therapy. J. Nanobiotechnol. 2022, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Hollis, C.; Weiss, H.; Leggas, M.; Evers, B.; Gemeinhart, R.A.; Li, T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: Lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release 2013, 172, 12–21. [Google Scholar] [CrossRef]
- Shete, G.; Pawar, Y.; Thanki, K.; Jain, S.; Bansal, A. Oral bioavailability and pharmacodynamic activity of hesperetin nanocrystals generated using a novel bottom-up technology. Mol. Pharm. 2015, 12, 1158–1170. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zhao, G.; Zhuang, J.; Wu, W. Improving systemic circulation of paclitaxel nanocrystals by surface hybridization of DSPE-PEG2000. Colloids Surf. B Biointerfaces 2019, 182, 110337. [Google Scholar] [CrossRef]
- Parmar, P.; Wadhawan, J.; Bansal, A. Pharmaceutical nanocrystals: A promising approach for improved topical drug delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yang, W.; Feng, T.; Lin, J.; Huang, P. Drug nanocrystals for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1499. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Xu, S.; Li, J.; Li, Y.; Xue, X.; Liu, Y.; Li, J.; Miao, X. Functional drug nanocrystals for cancer-target delivery. J. Drug Deliv. Sci. Technol. 2022, 76, 103807. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Zhao, Y.; Han, M.; Guo, Y.; Kuang, H.; Wang, X. A stabilizer-free and organic solvent-free method to prepare 10-hydroxycamptothecin nanocrystals: In vitro and in vivo evaluation. Int. J. Nanomed. 2016, 11, 2979–2994. [Google Scholar] [CrossRef]
- Zhang, C.; Long, L.; Xiong, Y.; Wang, C.; Peng, C.; Yuan, Y.; Liu, Z.; Lin, Y.; Jia, Y.; Zhou, X. Facile engineering of indomethacin-induced paclitaxel nanocrystal aggregates as carrier-free nanomedicine with improved synergetic antitumor activity. ACS Appl. Mater. Interfaces 2019, 11, 9872–9883. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Gao, J.; Dong, X.; Wheeler, K.; Wang, Z. Neutrophil-Mediated Delivery of Nanocrystal Drugs via Photoinduced Inflammation Enhances Cancer Therapy. ACS Nano 2023, 17, 15542–15555. [Google Scholar] [CrossRef]
- Lázár, V.; Snitser, O.; Barkan, D.; Kishony, R. Antibiotic combinations reduce Staphylococcus aureus clearance. Nature 2022, 610, 540–546. [Google Scholar] [CrossRef]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Zamarreño Beas, J.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.-J.; et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Feng, W.; Chittò, M.; Moriarty, T.F.; Li, G.; Wang, X. Targeted Drug Delivery Systems for Eliminating Intracellular Bacteria. Macromol. Biosci. 2022, 23, e2200311. [Google Scholar] [CrossRef]
- Wang, H.; Lin, F.; Wu, Y.; Guo, W.; Chen, X.; Xiao, C.; Chen, M. Carrier-Free Nanodrug Based on Co-Assembly of Methylprednisolone Dimer and Rutin for Combined Treatment of Spinal Cord Injury. ACS Nano 2023, 17, 12176–12187. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Di, Z.; Zhang, J.; Yin, F.; Li, L.; Zheng, L. Coordination-driven self-assembly of metallo-nanodrugs for local inflammation alleviation. Nano Res. 2023. [Google Scholar] [CrossRef]
- Kim, H.; Zhang, W.; Hwang, J.; An, E.-K.; Choi, Y.K.; Moon, E.; Loznik, M.; Huh, Y.H.; Herrmann, A.; Kwak, M.; et al. Carrier-free micellar CpG interacting with cell membrane for enhanced immunological treatment of HIV-1. Biomaterials 2021, 277, 121081. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, X.; Sun, Z.; Huo, X.; Hou, Y.; Xu, X.; Wu, H.; Shi, L.; Ma, G. Natural carrier-free self-assembled diterpene nanoparticles with its efficient anti-inflammation through the inhibition of NF-κB pathway for accelerated wound healing. Biomed. Pharmacother. 2023, 165, 115041. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hu, S.; Huang, L.; Chen, X.; Wang, X.; Fu, Y.-n.; Sun, H.; Li, G.; Wang, X. Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment. Molecules 2023, 28, 7065. https://doi.org/10.3390/molecules28207065
Zhang X, Hu S, Huang L, Chen X, Wang X, Fu Y-n, Sun H, Li G, Wang X. Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment. Molecules. 2023; 28(20):7065. https://doi.org/10.3390/molecules28207065
Chicago/Turabian StyleZhang, Xiaoyu, Shuyang Hu, Lifei Huang, Xiyue Chen, Xin Wang, Ya-nan Fu, Hui Sun, Guofeng Li, and Xing Wang. 2023. "Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment" Molecules 28, no. 20: 7065. https://doi.org/10.3390/molecules28207065
APA StyleZhang, X., Hu, S., Huang, L., Chen, X., Wang, X., Fu, Y.-n., Sun, H., Li, G., & Wang, X. (2023). Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment. Molecules, 28(20), 7065. https://doi.org/10.3390/molecules28207065








