Naringin from Coffee Inhibits Foodborne Aspergillus fumigatus via the NDK Pathway: Evidence from an In Silico Study
Abstract
1. Introduction
2. Results
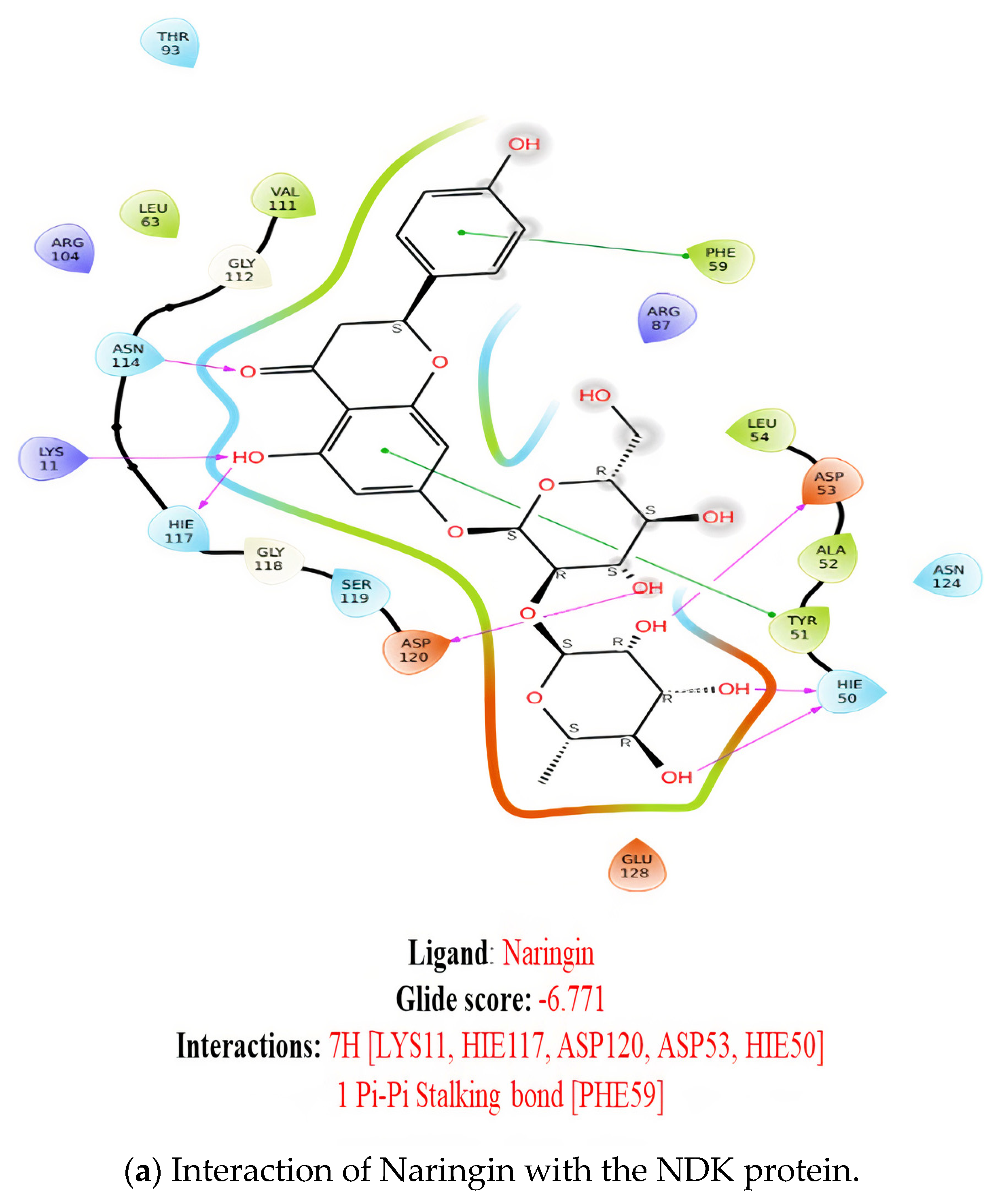
2.1. Molecular Docking
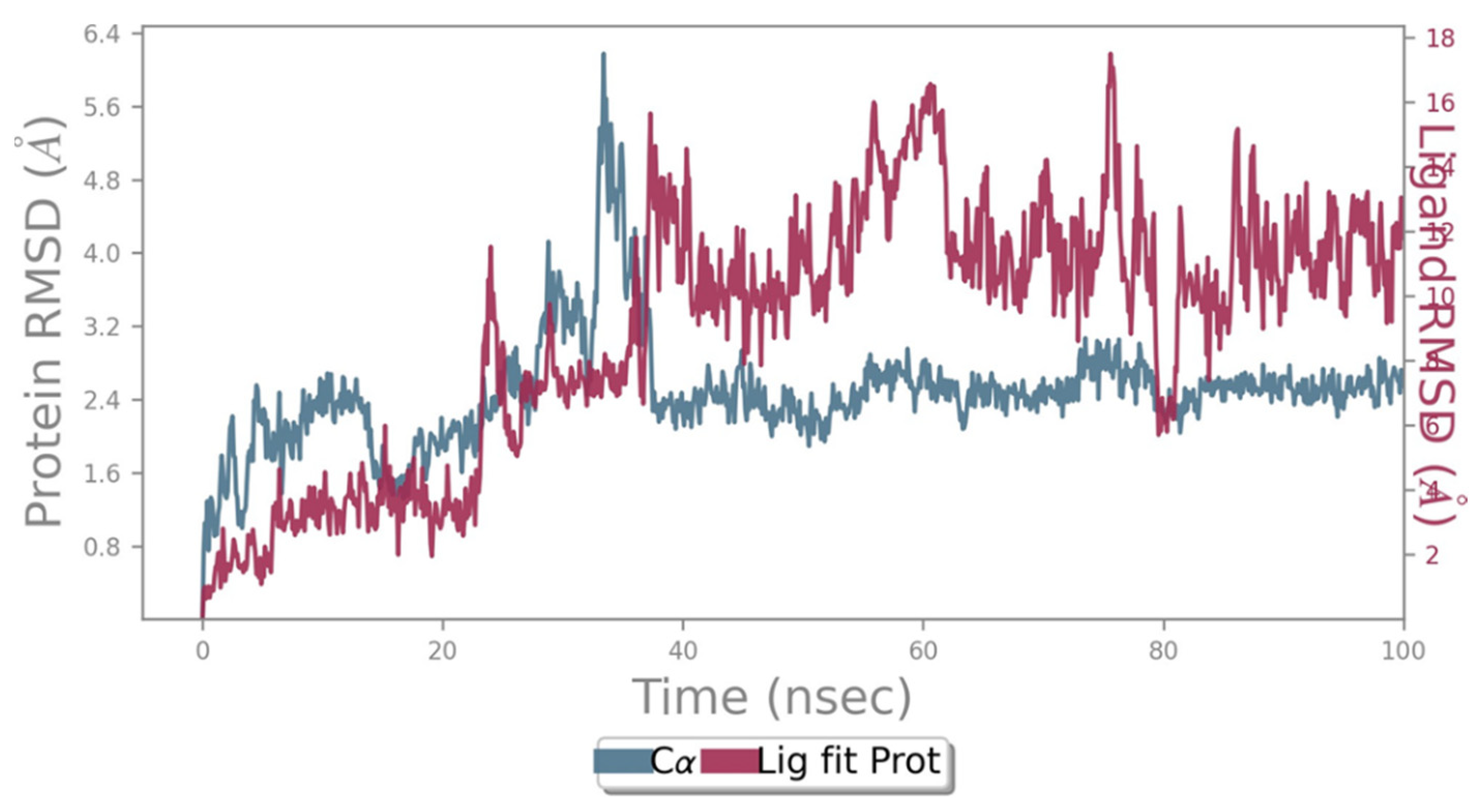
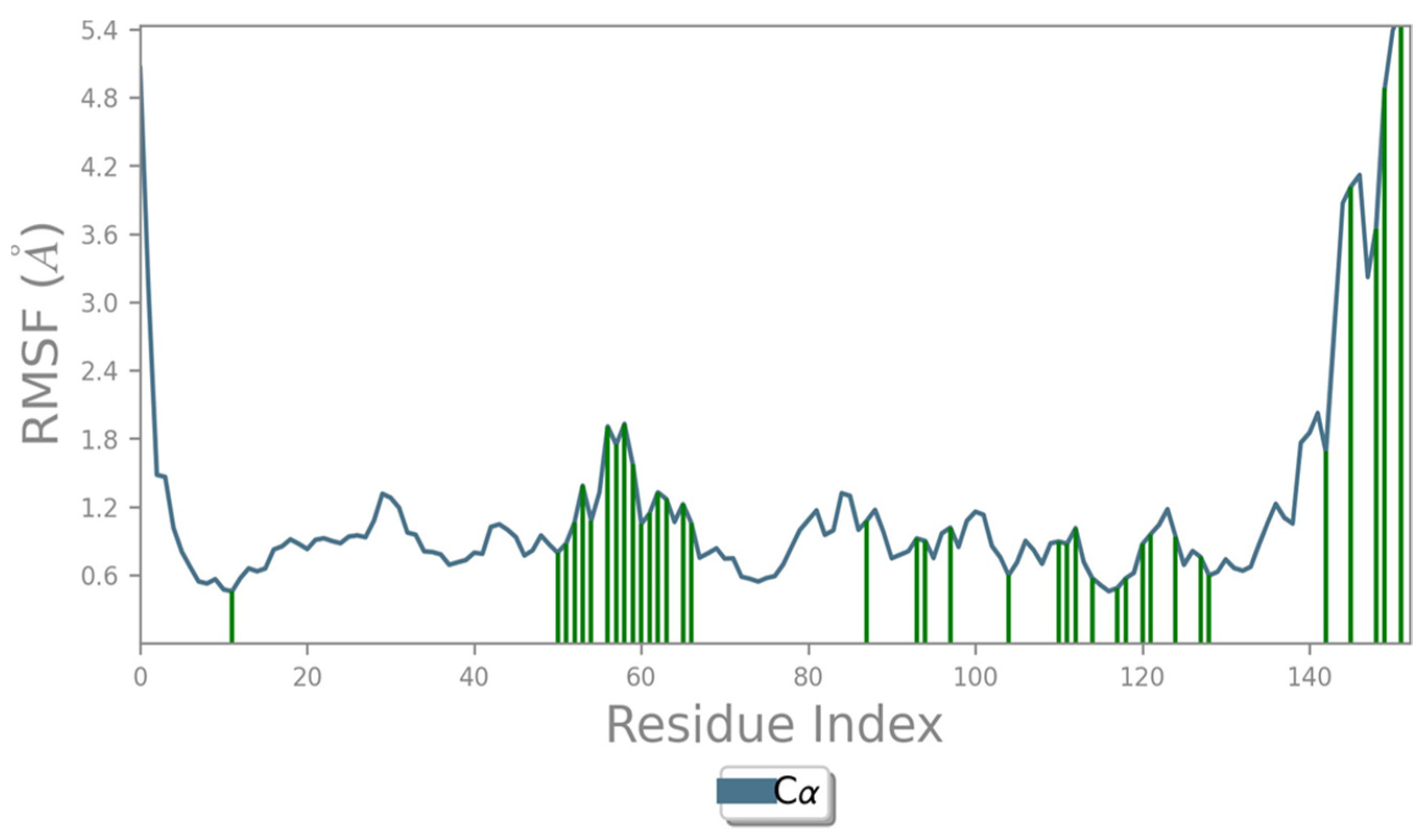
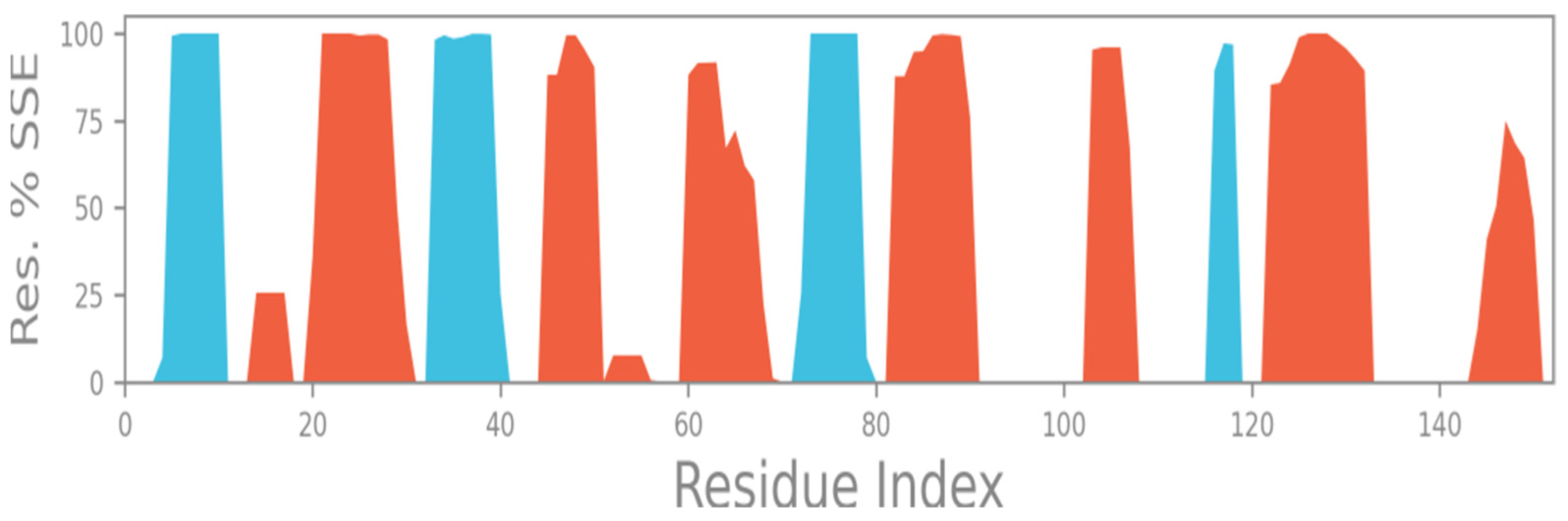
2.2. Molecular Dynamics Simulation
2.3. RMSD
2.4. Protein RMSF
2.5. Total Secondary Structures (SSE)
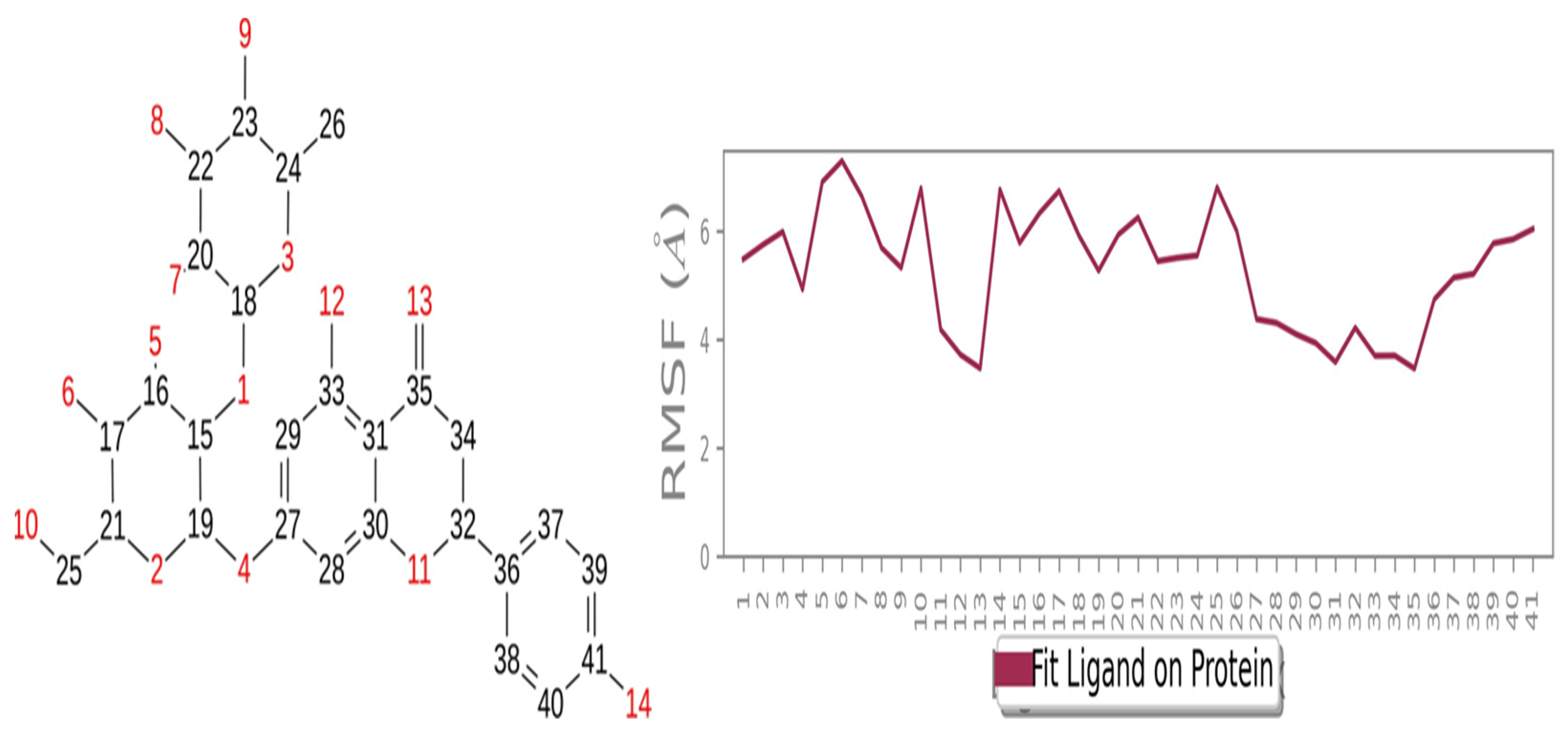
2.6. Ligand RMSF
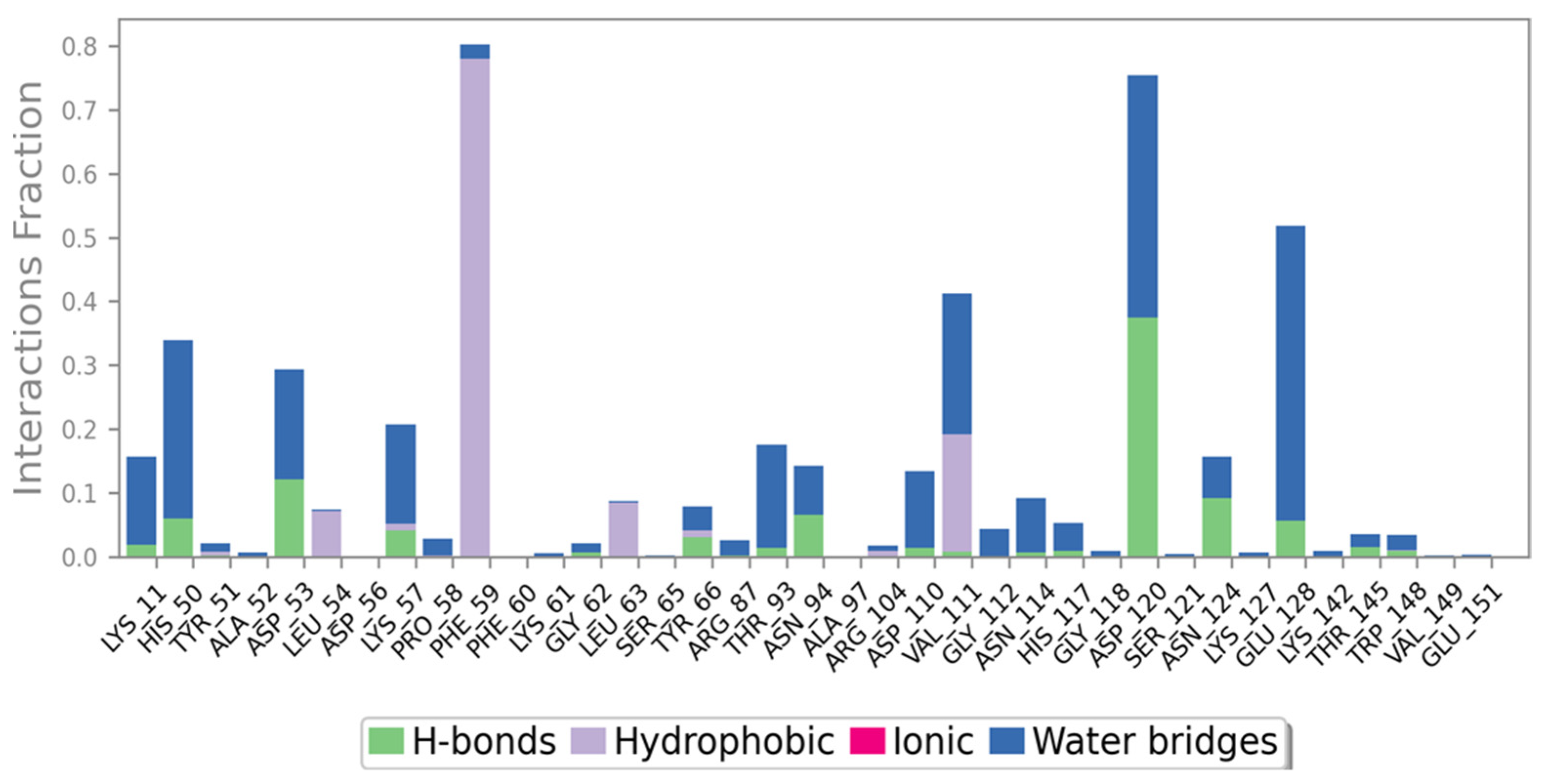
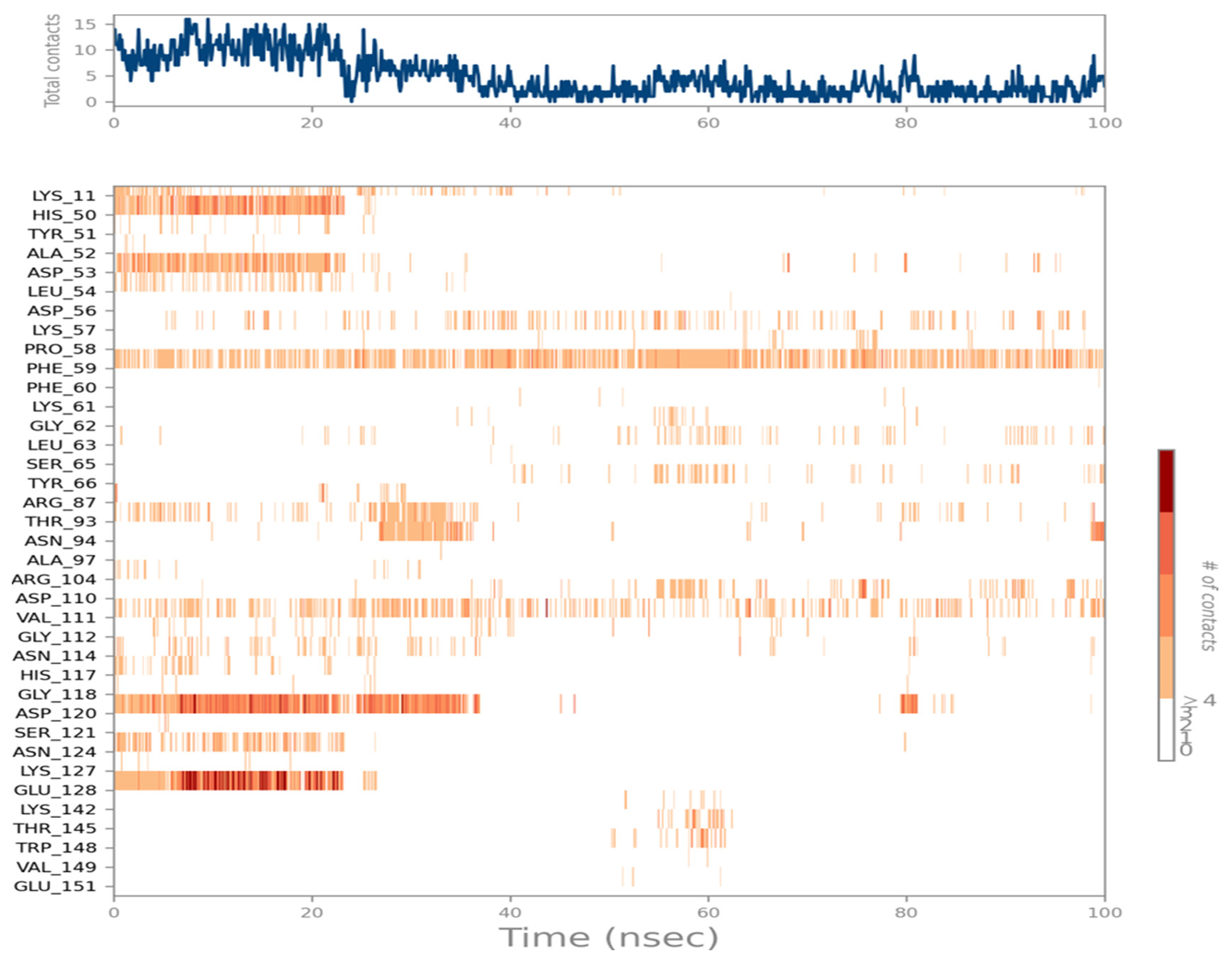
2.7. Ligand Interactions
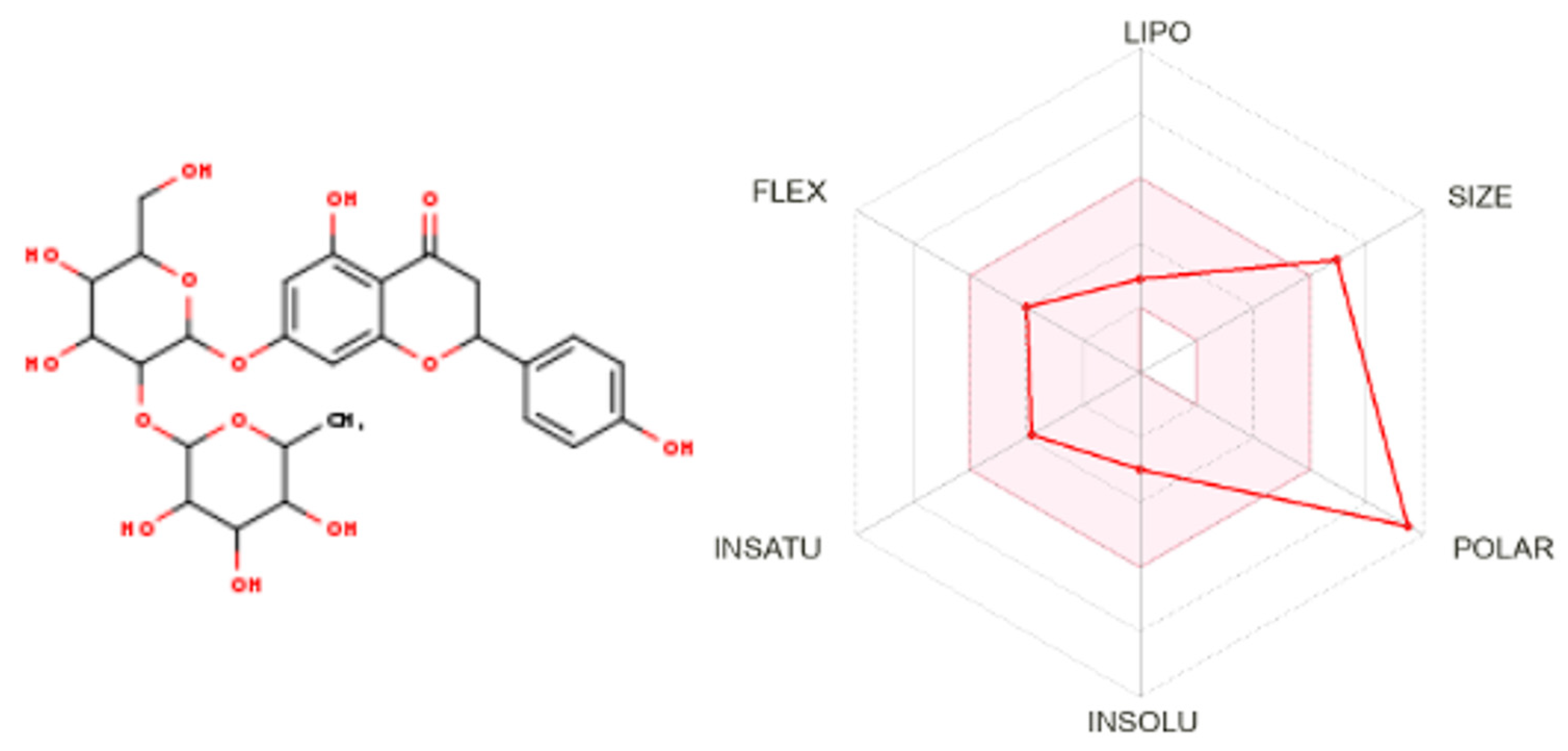
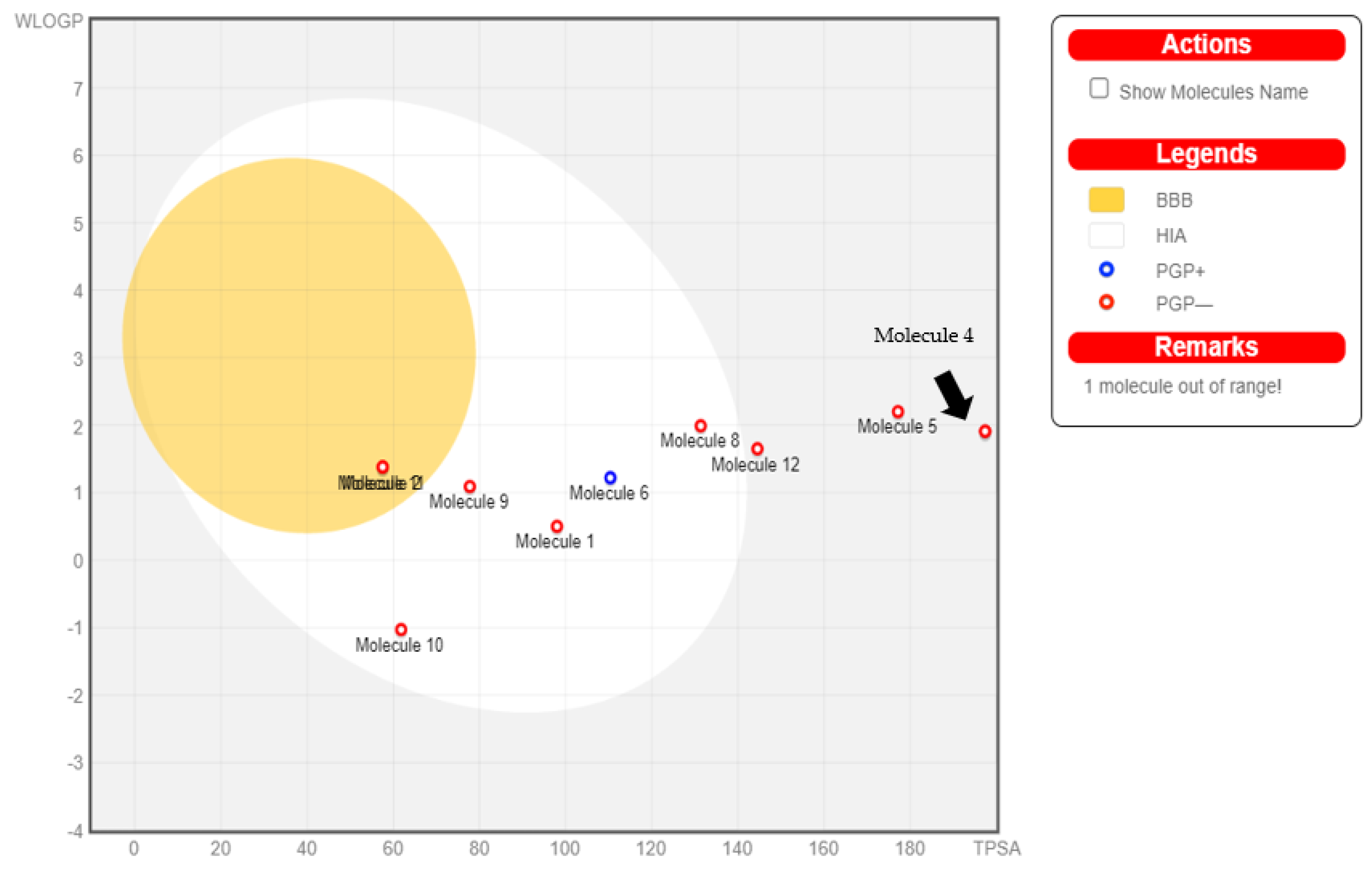
2.8. ADME and Gastrointestinal Absorbability
2.9. Boiled Egg Analysis
2.10. Toxicity Prediction
3. Discussion
4. Materials and Methods
4.1. Molecular Docking
4.1.1. Protein Preparation
4.1.2. Receptor Grid Generation
4.1.3. Ligand Preparation
4.1.4. Molecular Docking
4.2. Molecular Dynamics Simulation
4.3. Prediction of ADME Properties
4.4. Toxicity Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterson, R.R.M.; Lima, N. Filamentous Fungal Human Pathogens from Food Emphasising Aspergillus, Fusarium and Mucor. Microorganisms 2017, 5, 44. [Google Scholar] [CrossRef]
- Russell, M.R.; Paterson, N.L. Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bouakline, A.; Lacroix, C.; Roux, N.; Gangneux, J.P.; Derouin, F. Fungal Contamination of Food in Hematology Units. J. Clin. Microbiol. 2000, 38, 4272–4273. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Bow, E.; Chiller, T.; Maschmeyer, G.; Ribaud, P.; Segal, B.; Steinbach, W.; Wingard, J.R.; Nucci, M. Fungal infection prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009, 44, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med. Mycol. 2009, 47 (Suppl. S1), S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Trown, P.W.; Bilello, J.A. Mechanism of Action of Gliotoxin: Elimination of Activity by Sulfhydryl Compounds. Antimicrob. Agents Chemother. 1972, 2, 261–266. [Google Scholar] [CrossRef]
- Patel, R.; Hossain, M.A.; German, N.; Al-Ahmad, A.J. Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res. 2018, 34, 257–268. [Google Scholar] [CrossRef]
- Corrier, D. Mycotoxicosis: Mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 1991, 30, 73–87. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P.; Chi, J.; Han, X.Y.; Komanduri, K.V.; Kontoyiannis, D.P.; Prince, R.A. Detection of Gliotoxin in Experimental and Human Aspergillosis. Infect. Immun. 2005, 73, 635–637. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- Perrin, R.M.; Fedorova, N.D.; Bok, J.W.; Cramer, R.A.; Wortman, J.R.; Kim, H.S.; Nierman, W.C.; Keller, N.P. Transcriptional Regulation of Chemical Diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007, 3, e50. [Google Scholar] [CrossRef]
- Hanson, F.R.; Eble, T.E. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Guo, C.-J.; Palmer, J.M.; Sekonyela, R.; Wang, C.C.C.; Keller, N.P. Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. USA 2013, 110, 17065–17070. [Google Scholar] [CrossRef]
- Sin, N.; Meng, L.; Wang, M.Q.W.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.P.; Reeves, E.P.; Kavanagh, K. Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J. Med. Microbiol. 2010, 59 Pt 6, 625–633. [Google Scholar] [CrossRef]
- Zbidah, M.; Lupescu, A.; Jilani, K.; Lang, F. Stimulation of Suicidal Erythrocyte Death by Fumagillin. Basic Clin. Pharmacol. Toxicol. 2013, 112, 346–351. [Google Scholar] [CrossRef]
- Guruceaga, X.; Ezpeleta, G.; Mayayo, E.; Sueiro-Olivares, M.; Abad-Diaz-De-Cerio, A.; Urízar, J.M.A.; Liu, H.G.; Wiemann, P.; Bok, J.W.; Filler, S.G.; et al. A possible role for fumagillin in cellular damage during host infection by Aspergillus fumigatus. Virulence 2018, 9, 1548–1561. [Google Scholar] [CrossRef]
- Chitty, J.L.; Fraser, J.A. Purine Acquisition and Synthesis by Human Fungal Pathogens. Microorganisms 2017, 5, 33. [Google Scholar] [CrossRef]
- Chitty, J.L.; Tatzenko, T.L.; Williams, S.J.; Koh, Y.Q.A.E.; Corfield, E.C.; Butler, M.S.; Robertson, A.A.; Cooper, M.A.; Kappler, U.; Kobe, B.; et al. GMP Synthase Is Required for Virulence Factor Production and Infection by Cryptococcus neoformans. J. Biol. Chem. 2017, 292, 3049–3059. [Google Scholar] [CrossRef]
- Nguyen, S.; Jovcevski, B.; Pukala, T.L.; Bruning, J.B. Nucleoside selectivity of Aspergillus fumigatus nucleoside-diphosphate kinase. FEBS J. 2021, 288, 2398–2417. [Google Scholar] [CrossRef]
- The Coffee Report and Outlook. April 2023. Available online: https://icocoffee.org/documents/cy2022-23/Coffee_Report_and_Outlook_April_2023_-_ICO.pdf (accessed on 21 June 2023).
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and Antiradical Activity of Coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R.; Fernandes, C.; Gonçalves, T. Antifungal Activity of Spent Coffee Ground Extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Nonthakaew, A.; Matan, N.; Aewsiri, T.; Matan, N. Antifungal Activity of Crude Extracts of Coffee and Spent Coffee Ground on Areca Palm Leaf Sheath (Areca catechu) Based Food Packaging. Packag. Technol. Sci. 2015, 28, 633–645. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of Polyphenolic Fraction from Arabica Coffee Pulp and Its Antifungal Applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Farr-Jones, S.; Sopchak, L.; Boggs, A.; Nicely, H.W.; Khoury, R.; Biros, M. High-Throughput Screening: Update on Practices and Success. SLAS Discov. Adv. Sci. Drug Discov. 2006, 11, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Prasad, S.K. In silico Screening of Violacein as an epidermal growth factor receptor inhibitor. Int. J. Health Allied Sci. 2022, 11, 6. [Google Scholar] [CrossRef]
- Bhat, S.S.; Mahapatra, S.D.; Sommano, S.R.; Prasad, S.K. Virtual Screening and Quantitative Structure–Activity Relationship of Moringa oleifera with Melanoma Antigen A (MAGE-A) Genes against the Therapeutics of Non-Small Cell Lung Cancers (NSCLCs). Cancers 2022, 14, 5052. [Google Scholar] [CrossRef]
- Bhat, S.S.; Pradeep, S.; Patil, S.S.; Flores-Holguín, N.; Glossman-Mitnik, D.; Frau, J.; Sommano, S.R.; Ali, N.; Mohany, M.; Shivamallu, C.; et al. Preliminary Evaluation of Lablab purpureus Phytochemicals for Anti-BoHV-1 Activity Using In Vitro and In Silico Approaches. ACS Omega 2023, 8, 22684–22697. [Google Scholar] [CrossRef]
- Bhat, S.S.; Pradeep, S.; Jadhav, G.; Devegowda, D.; Sommano, S.R.; Shivamallu, C.; Kollur, S.P.; Prasad, S.K. In silico examination of peptides containing selenium and ebselen Backbone to Assess Their Tumoricidal Potential. Int. J. Health Allied Sci. 2022, 11, 9. Available online: https://rescon.jssuni.edu.in/ijhas/vol11/iss2/9 (accessed on 1 April 2023).
- Shiragannavar, V.D.; Gowda, N.G.S.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023, 14, 1135952. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2023.1135952 (accessed on 1 April 2023). [CrossRef]
- Gowda, N.G.S.; Shiragannavar, V.D.; Puttahanumantharayappa, L.D.; Shivakumar, A.T.; Dallavalasa, S.; Basavaraju, C.G.; Bhat, S.S.; Prasad, S.K.; Vamadevaiah, R.M.; Madhunapantula, S.V.; et al. Quercetin activates vitamin D receptor and ameliorates breast cancer induced hepatic inflammation and fibrosis. Front. Nutr. 2023, 10, 1158633. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, M.; Nuti, M. Valorisation of the Residues of Coffee Agro-industry: Perspectives and Limitations. Open Waste Manag. J. 2017, 10, 13–22. [Google Scholar] [CrossRef]
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; da Rocha, S.J.S.S.; Coimbra, J.S. New sustainable perspectives for “coffee wastewater” and other by-products: A critical review. Futur. Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Muzaifa, M.; Rahmi, F.; Syarifudin. Utilization of Coffee By-Products as Profitable Foods—A Mini Review. In Proceedings of the 3rd International Conference on Food and Agriculture, Jember, East Java, Indonesia, 7–8 November 2020. [Google Scholar]
- Kim, J.H.; Tam, C.C.; Chan, K.L.; Cheng, L.W.; Land, K.M.; Friedman, M.; Chang, P.-K. Antifungal Efficacy of Redox-Active Natamycin against Some Foodborne Fungi—Comparison with Aspergillus fumigatus. Foods 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Cockroaches and Food-borne Pathogens. Environ. Health Insights 2020, 14, 1178630220913365. [Google Scholar] [CrossRef]
- Langfeldt, A.; Gold, J.A.W.; Chiller, T. Emerging Fungal Infections: From the Fields to the Clinic, Resistant Aspergillus fumigatus and Dermatophyte Species: A One Health Perspective on an Urgent Public Health Problem. Curr. Clin. Microbiol. Rep. 2022, 9, 46–51. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 2022, 7, 663–674. [Google Scholar] [CrossRef]
- Vermeulen, E.; Lagrou, K.; Verweij, P.E. Azole resistance in Aspergillus fumigatus: A growing public health concern. Curr. Opin. Infect. Dis. 2013, 26, 493–500. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Prasad, S.K.; Pradeep, S.; Shimavallu, C.; Kollur, S.P.; Syed, A.; Marraiki, N.; Egbuna, C.; Gaman, M.A.; Kosakowska, O.; Cho, W.C.; et al. Evaluation of Annona muricata Acetogenins as Potential Anti-SARS-CoV-2 Agents Through Computational Approaches. Front. Chem. 2021, 8, 1281. [Google Scholar] [CrossRef]
- Chen, M.; Peng, W.; Hu, S.; Deng, J. miR-126/VCAM-1 regulation by naringin suppresses cell growth of human non-small cell lung cancer. Oncol. Lett. 2018, 16, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.G. Naringin-generated ROS promotes mitochondria-mediated apoptosis in Candida albicans. IUBMB Life 2021, 73, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, Z.; Lamer-Zarawska, E. Investigation of antifungal effect of flavonoids. Herba Polonica 1984, 30, 53–57. [Google Scholar]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Guan, X.; Liu, B.; Wang, Y.; Xu, K.; Peng, W.; Su, W.; Zhang, K. Acute and 13 weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem. Toxicol. 2013, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, S.; Guan, X.; Cen, X.; Hu, C.; Peng, W.; Wang, Y.; Su, W. Six months chronic toxicological evaluation of naringin in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 66, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, H.; Wang, Y.; Peng, W.; Su, W. Toxicological evaluation of naringin: Acute, subchronic, and chronic toxicity in Beagle dogs. Regul. Toxicol. Pharmacol. 2020, 111, 104580. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of naringin when used as a sensory additive for all animal species. EFSA J. 2011, 9, 2416. [Google Scholar]
- Kasuya, M.C.; Luz, J.; Dias Nunes, M.; Silva, M.; Carvalho, D.; de Assunção, L.; de Almeida Paula, T.; Moura, C.; Braga Pereira Bento, C. Production of Selenium-Enriched Mushrooms in Coffee Husks and Use of This Colonized Residue. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 301–309. [Google Scholar]
- Oliveira, L.; Franca, A. An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 283–291. [Google Scholar]
- LLC. Schrödinger Release 2021-4, Glide, Schrödinger; LLC: New York, NY, USA, 2021. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, S.K.; Bhat, S.S.; Koskowska, O.; Sangta, J.; Ahmad, S.F.; Nadeem, A.; Sommano, S.R. Naringin from Coffee Inhibits Foodborne Aspergillus fumigatus via the NDK Pathway: Evidence from an In Silico Study. Molecules 2023, 28, 5189. https://doi.org/10.3390/molecules28135189
Prasad SK, Bhat SS, Koskowska O, Sangta J, Ahmad SF, Nadeem A, Sommano SR. Naringin from Coffee Inhibits Foodborne Aspergillus fumigatus via the NDK Pathway: Evidence from an In Silico Study. Molecules. 2023; 28(13):5189. https://doi.org/10.3390/molecules28135189
Chicago/Turabian StylePrasad, Shashanka K., Smitha S. Bhat, Olga Koskowska, Jiraporn Sangta, Sheikh F. Ahmad, Ahmed Nadeem, and Sarana Rose Sommano. 2023. "Naringin from Coffee Inhibits Foodborne Aspergillus fumigatus via the NDK Pathway: Evidence from an In Silico Study" Molecules 28, no. 13: 5189. https://doi.org/10.3390/molecules28135189
APA StylePrasad, S. K., Bhat, S. S., Koskowska, O., Sangta, J., Ahmad, S. F., Nadeem, A., & Sommano, S. R. (2023). Naringin from Coffee Inhibits Foodborne Aspergillus fumigatus via the NDK Pathway: Evidence from an In Silico Study. Molecules, 28(13), 5189. https://doi.org/10.3390/molecules28135189






