Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions
Abstract
1. Introduction
2. Results
2.1. One- and Two-Component Model Solutions
2.2. Multicomponent Model Solutions
2.3. Multicomponent Real Solutions
2.4. Catalytic Reactions
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Synthesis of Nanoparticles
3.3. Catalytic Reaction

3.4. Apparatus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
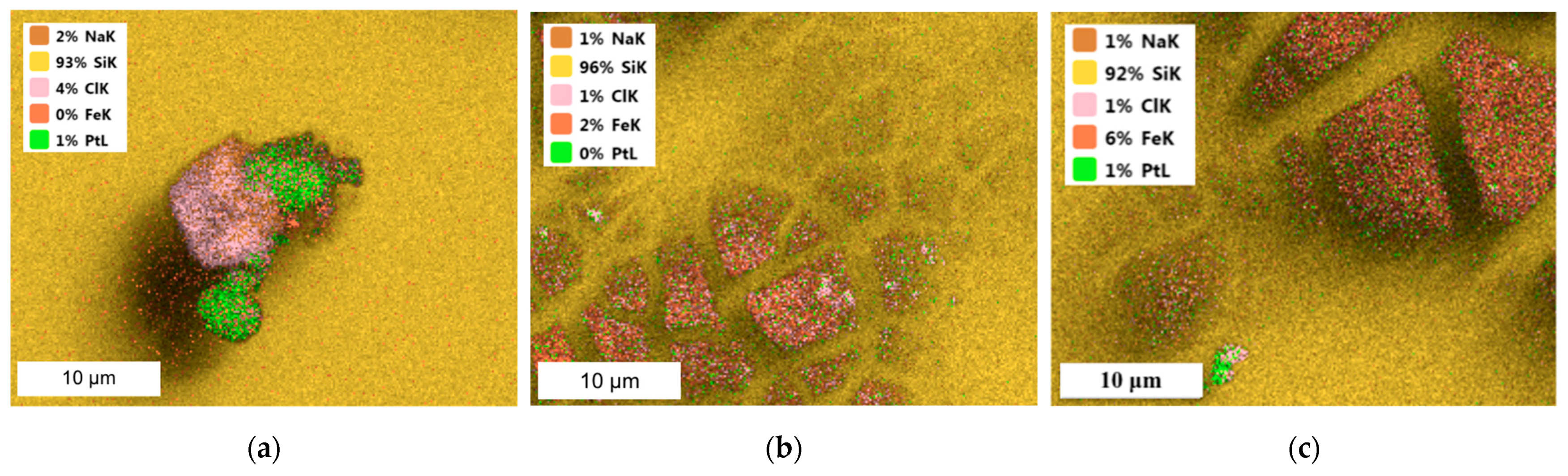
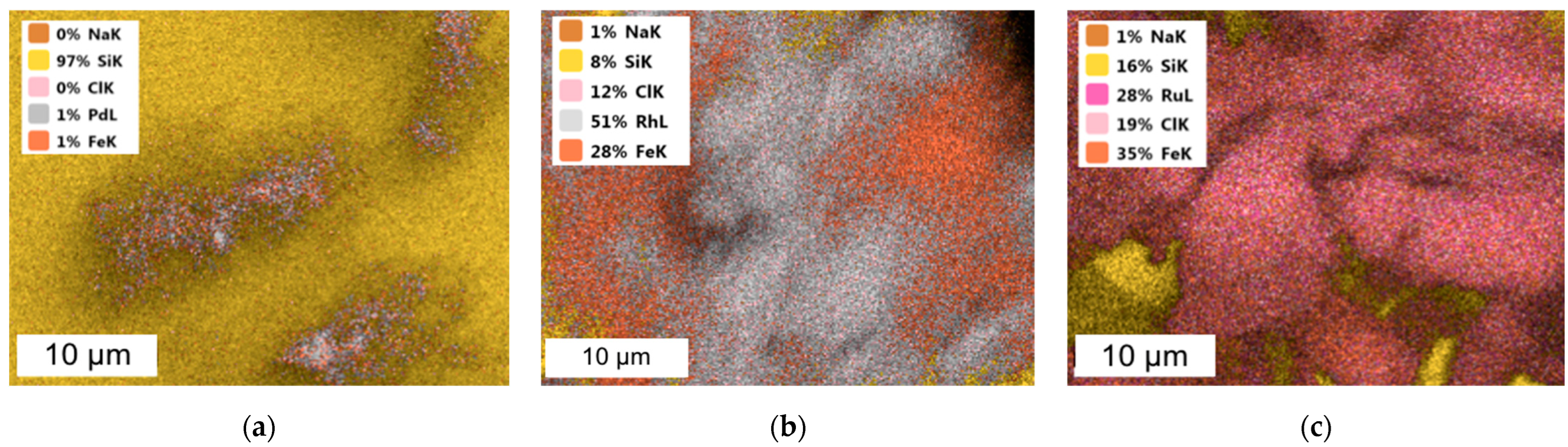
| Chemical Element | Concentration of Metal Ions, mg/dm3 | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Pt(IV) | 247.0 | 19.0 | 89.4 |
| Pd(II) | - | 80.0 | 67.6 |
| Rh(III) | 0 | 0 | 0 |
| Fe ions | 30.1 | 61.0 | 80.0 |
| Mg(II) | 0.64 | 0.5 | 1.0 |
| Zn(II) | 6.9 | 0.2 | 4.3 |
| Cu(II) | 0.6 | 0.9 | 0.9 |
| Reducer | Conversions of NPh, % at pH 11/14 | |||
|---|---|---|---|---|
| Fe | Cu | Mg | Zn | |
| AA | - | 56.6/8.4 | - | 0.0/0.8 |
| NaBH4 | 5/7.7 | 93.9/9.2 | - | 0.5/3.0 |
| SF | 3.3/1.6 | 46.8/0.0 | - | 0.0/15.6 |
| FA | 12.3/12.0 | 87.5/0.0 | - | 0.0/3.0 |
| Amount of Catalyst, mg | Conversions of NPh, % at pH 11/14 | |||
|---|---|---|---|---|
| Pt/Fe | Pd/Fe | Ru/Fe | Rh/Fe | |
| 1 | 63.8/95.5 | 100/100 | - | 100/96.6 |
| 2 | 100/95.5 | 100/96.4 | - | 100/95.0 |
| 3 | 100/95.8 | 100/96.2 | - | 100/96.4 |
| Reducer | Conversion of NPh, % at pH 11/14 |
|---|---|
| Pt/Pd/Rh | |
| AA | 88.7/88.2 |
| NaBH4 | 92.8/94.2 |
| FA | 80.1/79.7 |
| Pt/Pd/Rh/Ru | |
| AA | 92.0/91.9 |
| NaBH4 | 78.8/97.4 |
| SF | 88.1/69.1 |
| FA | 85.5/97.0 |
| Amount of Catalyst, mg | Conversions of NPh, % at pH 11/14 | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| 1 | 39.3/- | 0.0/0.0 | 13.3/96.7 |
| 3 | 42.2/- | 0.0/0.0 | 86.0/97.6 |
References
- Żelechowska, K. Nanotechnologia w Chemii i Medycynie; Wydawnictwo Politechniki Gdańskiej: Gdańsk, Poland, 2014; ISBN 9788373485457. [Google Scholar]
- Murty, B.S.; Shankar, P.; Raj, B.; Rath, B.B.; Murday, J. Textbook of Nanoscience and Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642280306. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Husin, M.H.M.; Nordin, M.R.; Mohamad, I.S.; Yong, L.K. Pt–Cu Bimetallic Nanoparticles Synthesized by Polyol Method under Different Reduction Conditions. J. Mech. Eng. Technol. 2022, 6, 77–86. [Google Scholar]
- Viñes, F.; Görling, A. Explaining Cu@Pt Bimetallic Nanoparticles Activity Based on NO Adsorption. Chem. A Eur. J. 2020, 26, 11478–11491. [Google Scholar] [CrossRef]
- Hansen, H.E.; Fakhri, D.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Sonochemical Synthesis of Cu@Pt Bimetallic Nanoparticles. Molecules 2022, 27, 5281. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Félix, R.M.; Reynoso, E.A.; Gochi-Ponce, Y.; Gómez, Y.V.; Moyado, S.F.; Alonso-Núñez, G. Synthesis of Pt and Pt-Fe Nanoparticles Supported on MWCNTs Used as Electrocatalysts in the Methanol Oxidation Reaction. J. Energy Chem. 2014, 23, 483–490. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.L.; Qin, X.; Hwang, I.; Sun, C.J.; Liu, M.; Hua, W.; Wu, H.-w.; Zhu, S.; et al. Atomically Dispersed Pt and Fe Sites and Pt–Fe Nanoparticles for Durable Proton Exchange Membrane Fuel Cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Bigarre, J.; Lecas, T.; Brault, P.; Caillard, A.; Bigarre, J.; Lecas, T.; Brault, P.; Pdpt, A.C.; Bigarre, J.; Lecas, T.; et al. PdPt Nanocatalyst Synthesis via Liquid Medium Sputtering. In Proceedings of the 24th International Symposium on Plasma Chemistry, Naples, Italy, 9–14 June 2019. Id: Hal-02394305. [Google Scholar]
- Rzelewska-Piekut, M.; Wiecka, Z.; Regel-Rosocka, M. Studies on the Formation of Catalytically Active PGM Nanoparticles from Model Solutions as a Basis for the Recycling of Spent Catalysts. Molecules 2022, 27, 390. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and Design of Ag–Fe Bimetallic Nanoparticles as Antimicrobial Synergistic Combination Therapies against Clinically Relevant Pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef]
- Wiecka, Z.; Cota, I.; Tylkowski, B.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters and Their Conversion into Efficient Recyclable Nanocatalysts. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, S.; Sasaki, K. Selective extraction of precious metals from simulated automotive catalyst waste and their conversion to carbon supported PdPt nanoparticle catalyst. Colloids Surf. A 2023, 665, 131179. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, X.; Wang, J.; Wu, Z.; Han, L.; Lin, R.; Xin, H.L.; Wang, D. Structurally Ordered Pt-Zn/C Series Nanoparticles as Efficient Anode Catalysts for Formic Acid Electrooxidation. J. Mater. Chem. A 2015, 3, 22129–22135. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, C.; Wang, C.; Liang, H.; Chen, W.; Li, W. Effect of TiO2 Nanoparticles and SDBS on Corrosion Behavior of 3003 Aluminum Alloy in Aqueous Ethylene Glycol Containing Chloride Ions at High Temperature. J. Alloys Compd. 2021, 873, 159820. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Alirezaie, A.; Toghraie, D. Thermal Conductivity of Ethylene Glycol Based Nanofluids Containing Hybrid Nanoparticles of SWCNT and Fe3O4 and Its Price-Performance Analysis for Energy Management. J. Mater. Res. Technol. 2021, 14, 1754–1760. [Google Scholar] [CrossRef]
- Tian, S.; Wang, B.; Gong, W.; He, Z.; Xu, Q.; Chen, W.; Zhang, Q.; Zhu, Y.; Yang, J.; Fu, Q.; et al. Dual-Atom Pt Heterogeneous Catalyst with Excellent Catalytic Performances for the Selective Hydrogenation and Epoxidation. Nat. Commun. 2021, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Wang, Z.; Chen, J.; Li, Q.; Liu, P.; Chen, F.; Zhang, S.; Li, J. Design Strategy and Recent Progress of Fluorescent Probe for Noble Metal Ions (Ag, Au, Pd, and Pt). Coord. Chem. Rev. 2021, 432, 213712. [Google Scholar] [CrossRef]
- Fernández-Delgado, E.; Estirado, S.; Espino, J.; Viñuelas-Zahínos, E.; Luna-Giles, F.; Rodríguez Moratinos, A.B.; Pariente, J.A. Influence of Ligand Lipophilicity in Pt(II) Complexes on Their Antiproliferative and Apoptotic Activities in Tumour Cell Lines. J. Inorg. Biochem. 2022, 227, 111688. [Google Scholar] [CrossRef]
- Barnard, B.C.F.J. Platinum Anti-Cancer. Plotinurn Metals Rev. 1989, 33, 162–167. [Google Scholar]
- Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Recovery of Platinum Group Metals from Spent Automotive Catalysts by Leaching and Solvent Extraction. J. Chem. Eng. Jpn. 2019, 52, 835–842. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. An Innovative Hybrid Hydrometallurgical Approach for Precious Metals Recovery from Secondary Resources. J. Environ. Manag. 2022, 307, 114567. [Google Scholar] [CrossRef]
- Karim, S.; Ting, Y.P. Recycling Pathways for Platinum Group Metals from Spent Automotive Catalyst: A Review on Conventional Approaches and Bio-Processes. Resour. Conserv. Recycl. 2021, 170, 105588. [Google Scholar] [CrossRef]
- Méndez, A.; Nogueira, C.A.; Paiva, A.P. Recovery of Platinum from a Spent Automotive Catalyst through Chloride Leaching and Solvent Extraction. Recycling 2021, 6, 27. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Cao, H.; Ning, P.; Jin, W.; Yang, Z. Understanding the Features of PGMs in Spent Ternary Automobile Catalysts for Development of Cleaner Recovery Technology. J. Clean. Prod. 2019, 239, 118031. [Google Scholar] [CrossRef]
- Hasani, M. Selective Recovery of Pt, Pd, and Rh from Spent Catalysts by Functionalized Magnetite Nanoparticles. J. Min. Environ. 2022, 13, 483–493. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Yamada, M.; Kondo, Y.; Shibayama, A.; Hamada, F. P-Sulfonatothiacalix [6]Arene-Impregnated Resins for the Sorption of Platinum Group Metals and Effective Separation of Palladium from Automotive Catalyst Residue. J. Ind. Eng. Chem. 2015, 30, 20–28. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Riaño, S.; Aktan, E.; Deferm, C.; Fransaer, J.; Binnemans, K. Solvometallurgical Recovery of Platinum Group Metals from Spent Automotive Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 337–350. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from Model Chloride Solutions by Liquid-Liquid Extraction with Phosphonium Ionic Liquids. Sep. Purif. Technol. 2019, 212, 791–801. [Google Scholar] [CrossRef]
- Thirupathi, K.; Bárczy, P.; Somosvári, B.M. Impact of Corrosive Liquid on Trivalent Chromium over Aluminium Alloys. J. Surf. Eng. Mater. Adv. Technol. 2017, 7, 51–60. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Li, Y. Understanding of the Major Reactions in Solution Synthesis of Functional Nanomaterials. Sci. China Mater. 2016, 59, 938–996. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Beolchini, F. Recovery of Indium from Liquid Crystal Displays. J. Clean. Prod. 2016, 116, 299–305. [Google Scholar] [CrossRef]
- Liu, Q.M.; Zhou, D.B.; Yamamoto, Y.; Ichino, R.; Okido, M. Preparation of Cu Nanoparticles with NaBH4 by Aqueous Reduction Method. Trans. Nonferrous Met. Soc. China 2012, 22, 117–123. [Google Scholar] [CrossRef]
- Forte, F.; Riaño, S.; Binnemans, K. Dissolution of Noble Metals in Highly Concentrated Acidic Salt Solutions. Chem. Commun. 2020, 56, 8230–8232. [Google Scholar] [CrossRef] [PubMed]
- Beyribey, B.; Çorbacioǧlu, B.; Altin, Z. Synthesis of Platinum Particles from H2PtCl6 with Hydrazine as Reducing Agent. Gazi Univ. J. Sci. 2009, 22, 351–357. [Google Scholar]
- Awadalla, F.T.; Molnar, R.E.; Ritcey, G.M. Recovery of Platinum Group Metals (PGM) from Acidic Solutions by Reduction Precipitation with Sodium Borohydride. Patent CA2016492A1, 10 November 1991. [Google Scholar]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the Overlooked Critical Role of the PH Value on the Kinetics of the 4-Nitrophenol NaBH 4-Reduction Catalyzed by Noble-Metal Nanoparticles (Pt, Pd, and Au). J. Phys. Chem. C 2020, 124, 2939–2944. [Google Scholar] [CrossRef]

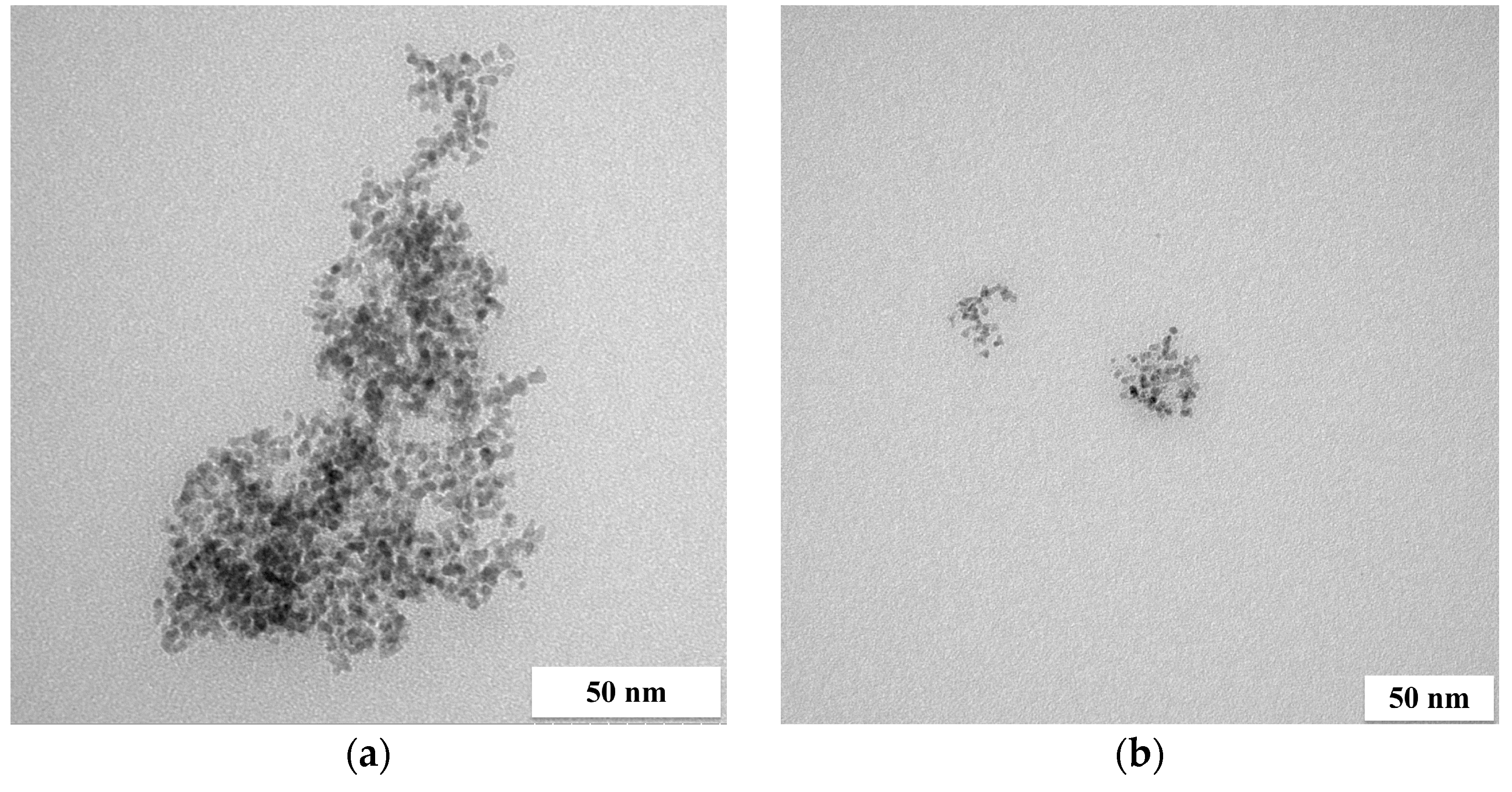

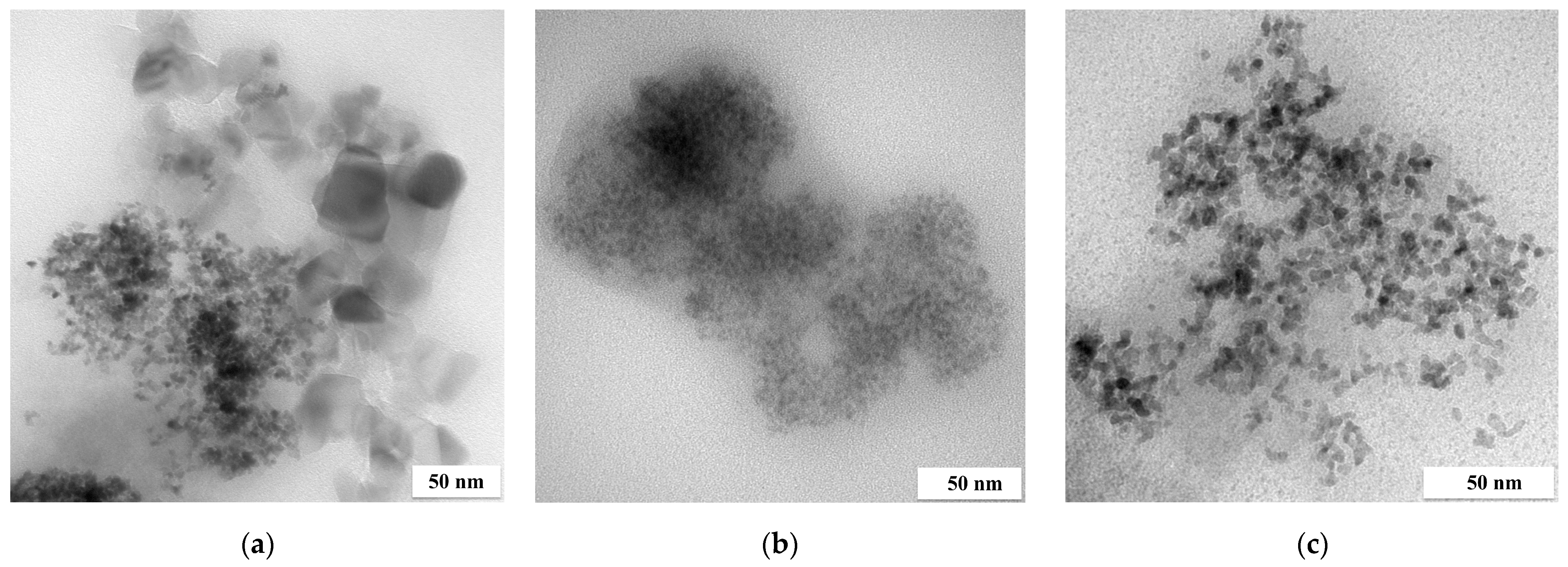

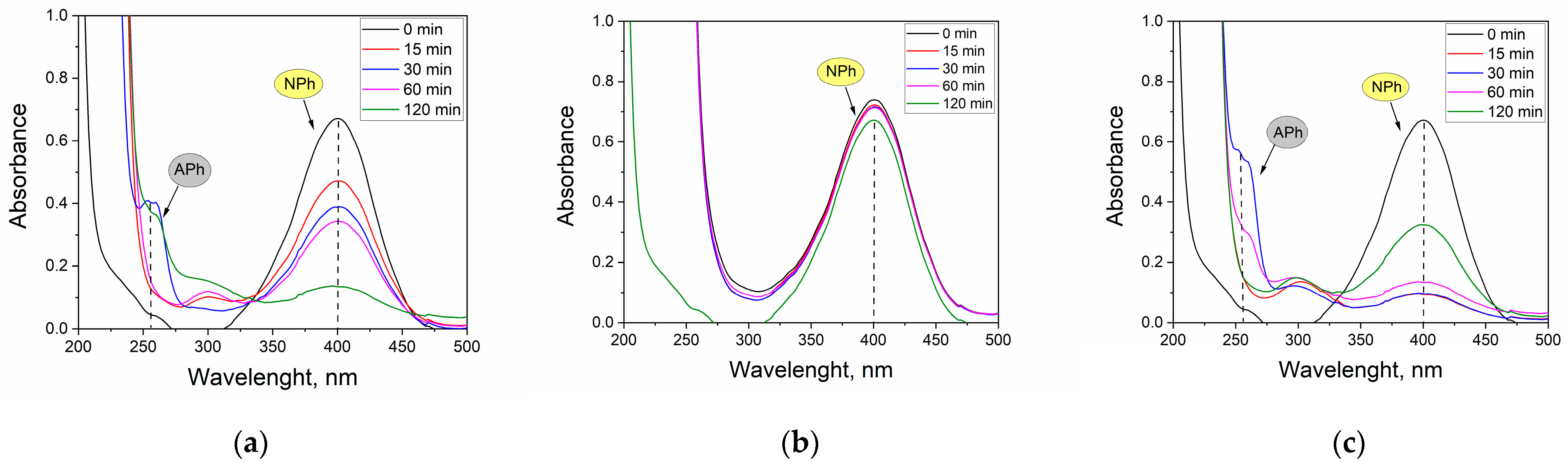
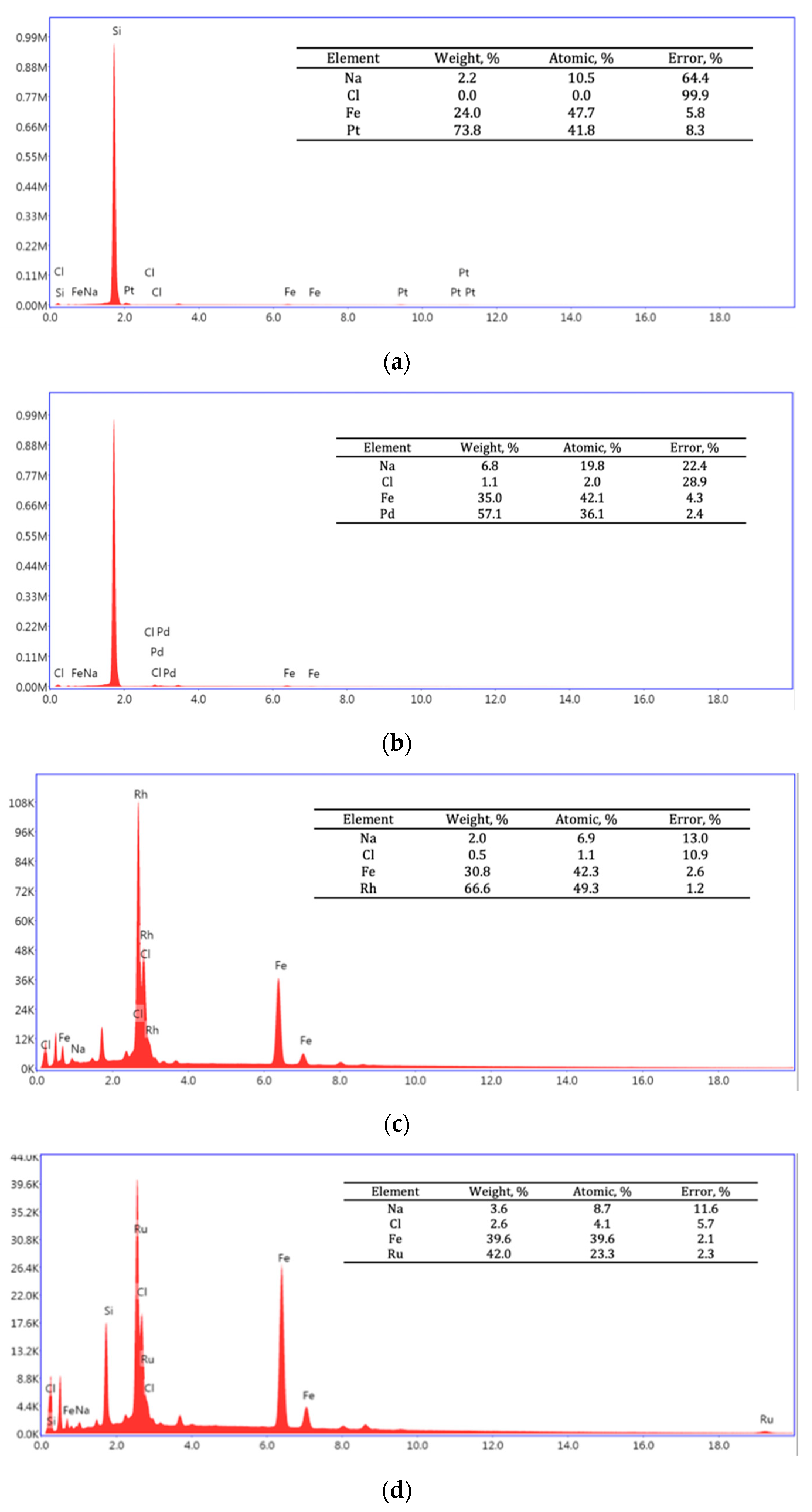
| Reducer | P, % | |||
|---|---|---|---|---|
| Fe | Cu | Zn | Mg | |
| AA | 0 | 0.6 | 46.4 | 8.1 |
| NaBH4 | 100 | 80.7 | 27.2 | 6.3 |
| SF | 100 | 78.7 | 87.8 | 7.8 |
| FA | 85.2 | 69.9 | 93.2 | 11.6 |
| Reducer | P, % | |||
|---|---|---|---|---|
| Pt/Fe | Pd/Fe | Ru/Fe | Rh/Fe | |
| AA | 81.8/11.6 | 0/0 | 40.4/40.5 | 20.1/55.3 |
| NaBH4 | 88.5/100 | 100/100 | 39.7/100 | 99.7/100 |
| SF | 85.4/100 | 74.8/100 | 63.7/100 | 19.8/64.3 |
| FA | 82.7/100 | 93.3/100 | 67.4/100 | 15.2/57.9 |
| Pt/Cu | Pd/Cu | Ru/Cu | Rh/Cu | |
| AA | 62.4/6.5 | 8.7/0 | 67.2/73.1 | 20.4/78.8 |
| NaBH4 | 97.7/90.4 | 29.8/37.2 | 68.4/93.5 | 50.1/95.3 |
| SF | 58.3/91.6 | 100/98.9 | 83.5/95.6 | 38.9/68.2 |
| FA | 67.6/89.8 | 100/27.7 | 85.0/92.6 | 43.0/59.5 |
| Pt/Zn | Pd/Zn | Ru/Zn | Rh/Zn | |
| AA | 59.0/18.7 | 1.8/0 | 64.4/0 | 30.5/76.9 |
| NaBH4 | 91.6/27.1 | 100/51.0 | 63.3/61.1 | 99.1/57.2 |
| SF | 76.1/71.5 | 100/50.3 | 81.1/61.4 | 24.1/29.3 |
| FA | 75.3/68.9 | 100/53.0 | 79.9/54.0 | 26.4/6.8 |
| Pt/Mg | Pd/Mg | Ru/Mg | Rh/Mg | |
| AA | 70.0/6.4 | 0/7.1 | 72.0/1.4 | 0/0 |
| NaBH4 | 76.5/0.9 | 62.1/14.2 | 55.9/0 | 92.7/0 |
| SF | 73.7/16.7 | 94.6/2.4 | 71.6/0.9 | 20.0/0 |
| FA | 70.1/8.9 | 86.7/6.7 | 64.1/0 | 19.6/0 |
| Reducer | P, % | |||
|---|---|---|---|---|
| Three-Component Solution | ||||
| Pt | Pd | Ru | Rh | |
| AA | 93.0 | 40.6 | - | 68.6 |
| NaBH4 | 97.1 | 91.5 | - | 79.9 |
| SF | - | - | - | - |
| FA | 73.7 | 92.7 | - | 83.7 |
| Four-Component Solution | ||||
| Pt | Pd | Ru | Rh | |
| AA | 72.9 | 54.7 | 86.9 | 14.2 |
| NaBH4 | 79.9 | 94.3 | 83.0 | 87.8 |
| SF | 57.5 | 86.4 | 88.8 | 35.0 |
| FA | 57.6 | 78.2 | 83.3 | 34.1 |
| Real Solution | P, % | ||
|---|---|---|---|
| Pt | Pd | Fe | |
| R1 | 98.8 | - | 98.8 |
| R2 | 100 | 100 | 65.2 |
| R3 | 97.6 | 100 | 96.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzelewska-Piekut, M.; Wolańczyk, Z.; Nowicki, M.; Regel-Rosocka, M. Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions. Molecules 2023, 28, 5188. https://doi.org/10.3390/molecules28135188
Rzelewska-Piekut M, Wolańczyk Z, Nowicki M, Regel-Rosocka M. Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions. Molecules. 2023; 28(13):5188. https://doi.org/10.3390/molecules28135188
Chicago/Turabian StyleRzelewska-Piekut, Martyna, Zuzanna Wolańczyk, Marek Nowicki, and Magdalena Regel-Rosocka. 2023. "Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions" Molecules 28, no. 13: 5188. https://doi.org/10.3390/molecules28135188
APA StyleRzelewska-Piekut, M., Wolańczyk, Z., Nowicki, M., & Regel-Rosocka, M. (2023). Precipitation of Pt, Pd, Rh, and Ru Nanoparticles with Non-Precious Metals from Model and Real Multicomponent Solutions. Molecules, 28(13), 5188. https://doi.org/10.3390/molecules28135188







