Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions
Abstract
1. Introduction
2. Results
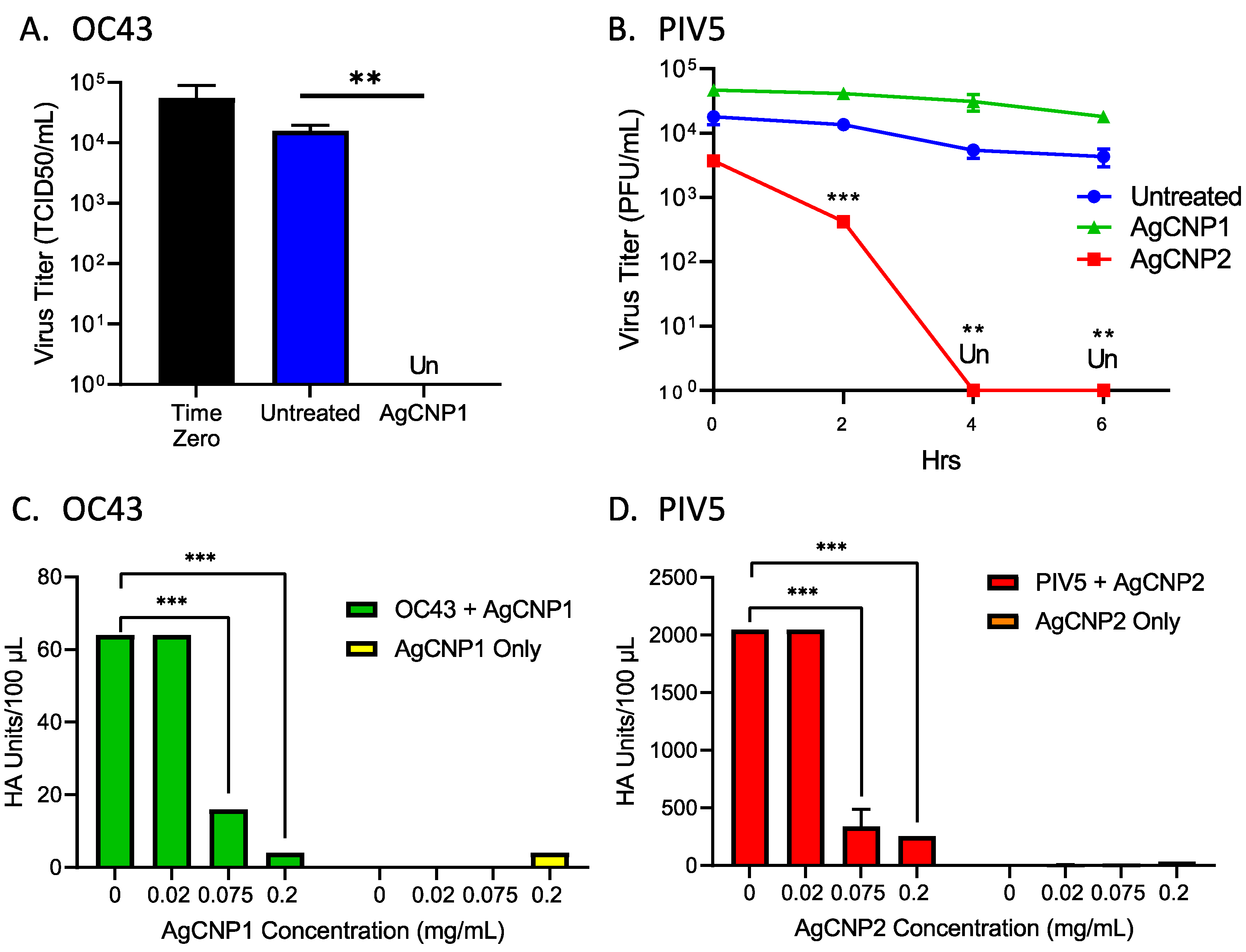
2.1. Silver-Modified Nanoceria Inactivates Distinct Enveloped RNA Viruses through the Disruption of Virus–Receptor Interactions and Virus Aggregation
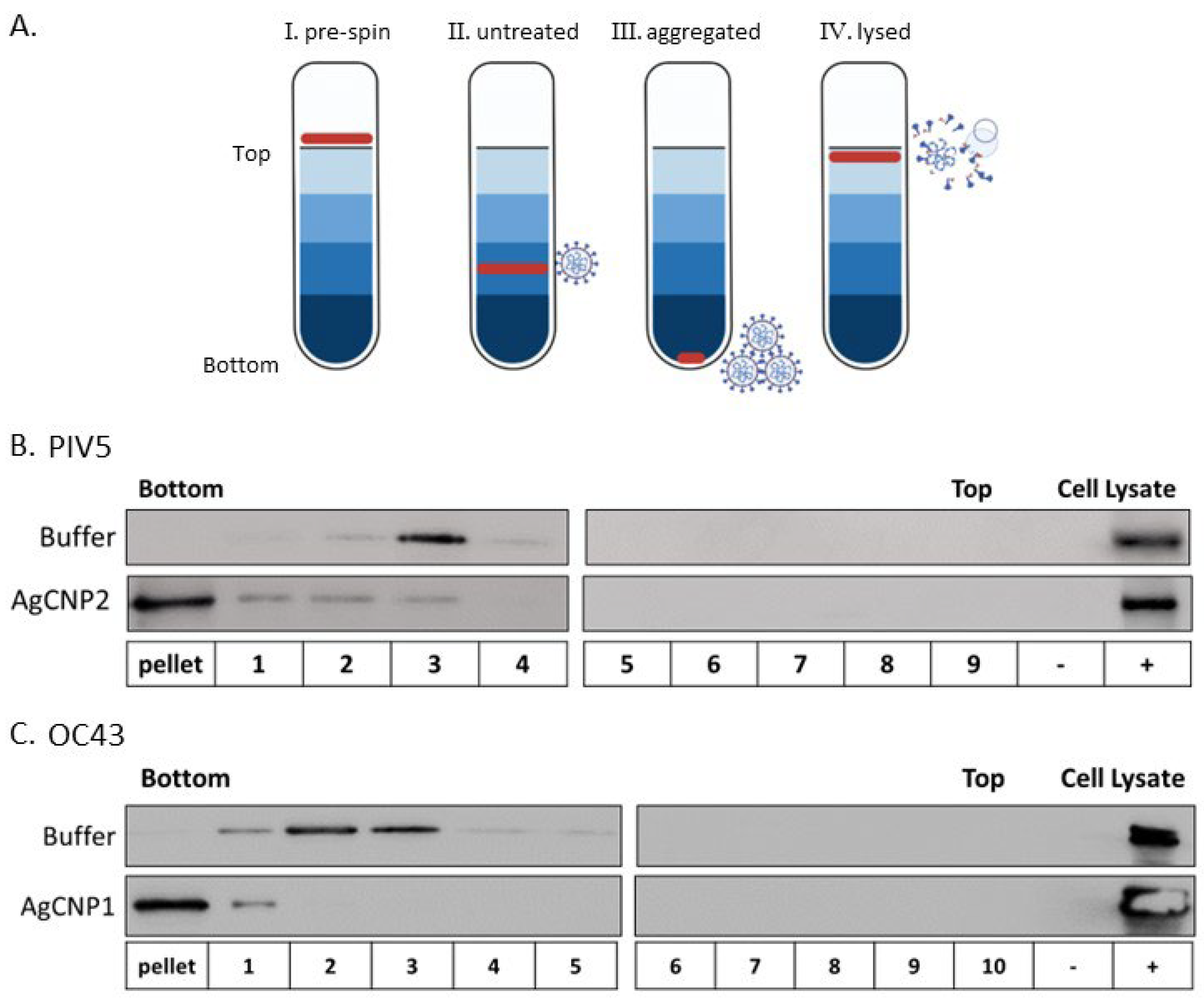
2.2. Silver-Modified Nanoceria Induces Aggregation of Distinct Enveloped RNA Viruses
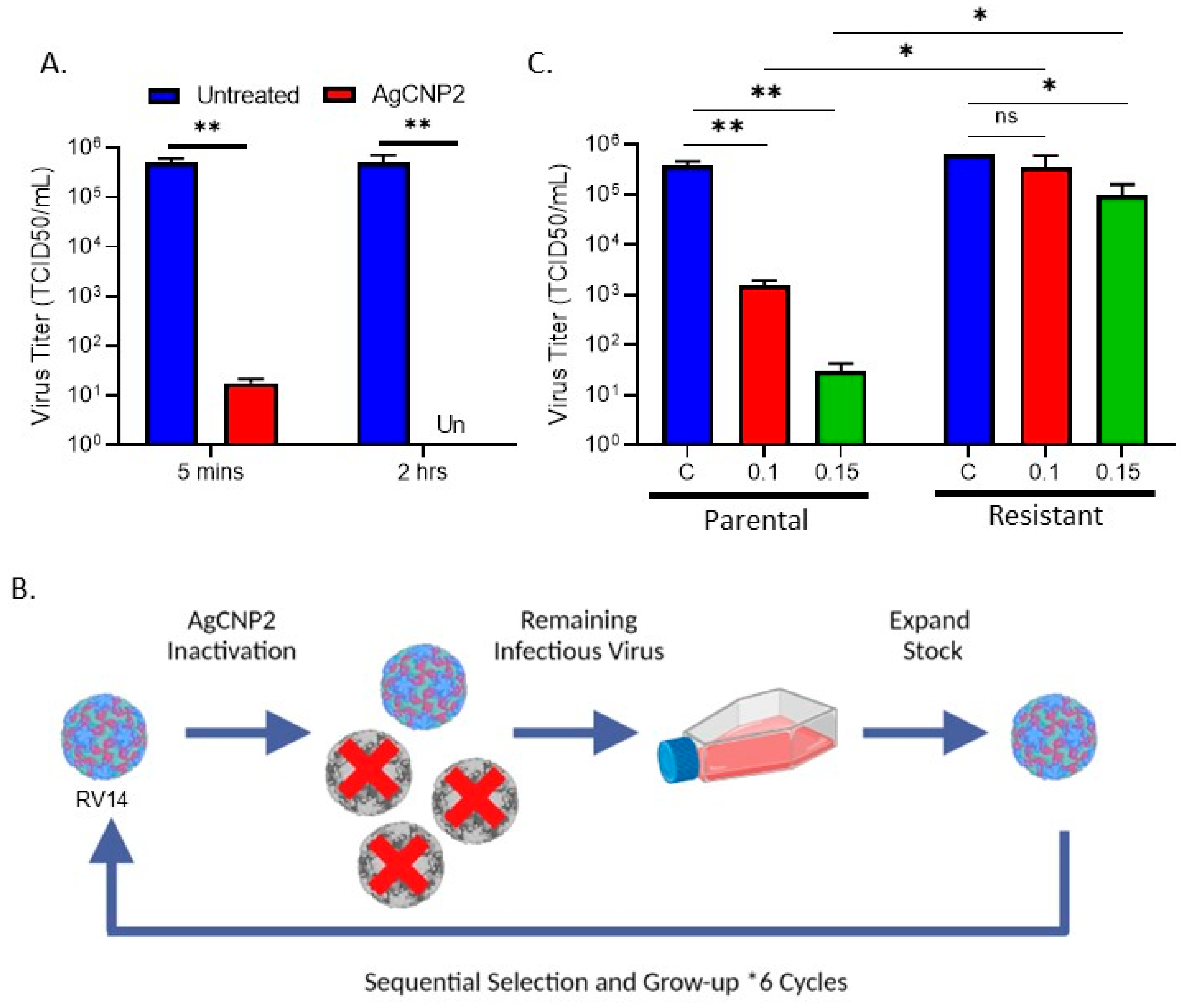
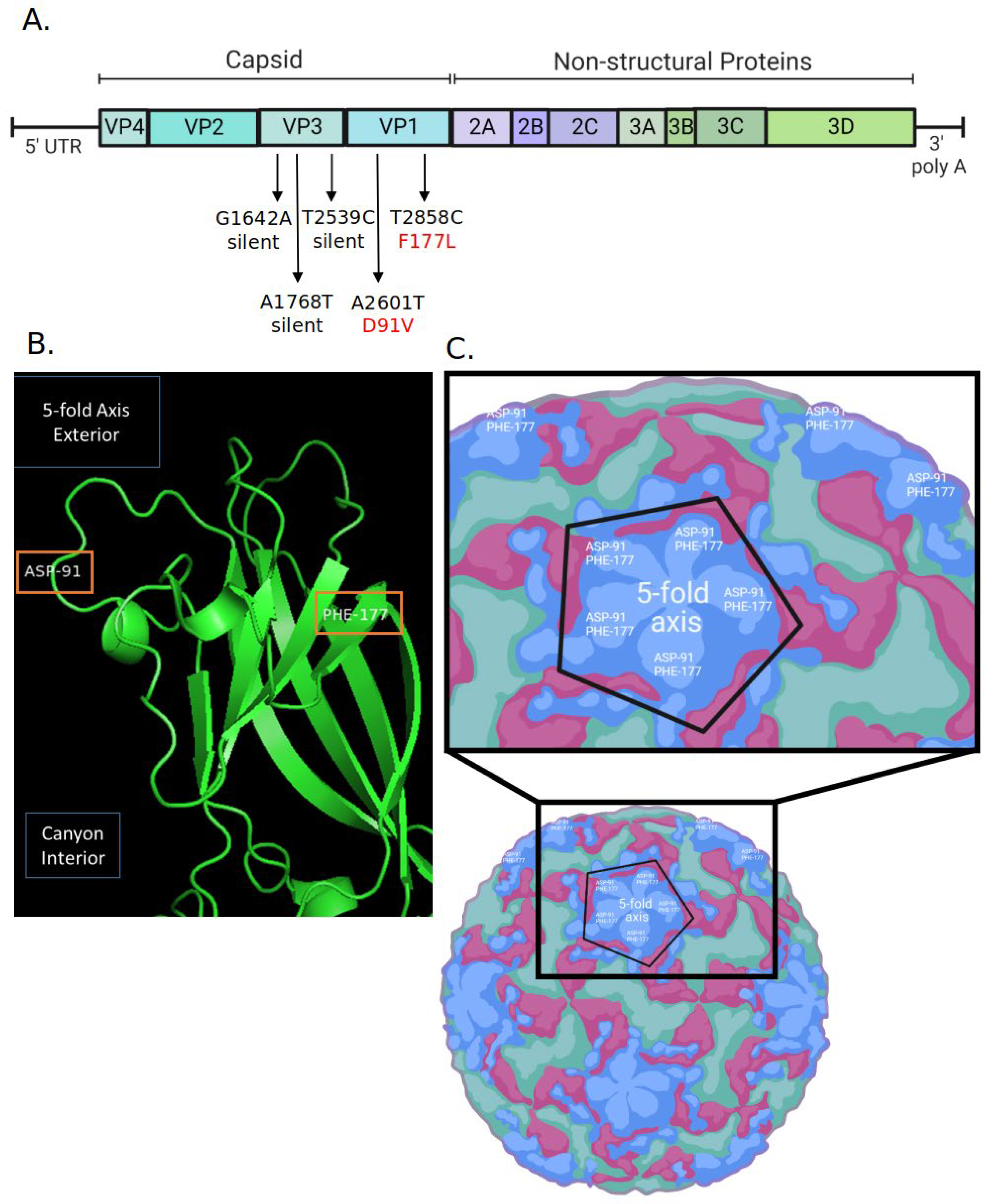
2.3. Generation of RV14 That Is Resistant to AgCNP2 Inactivation and Identification of Resulting Genomic Changes
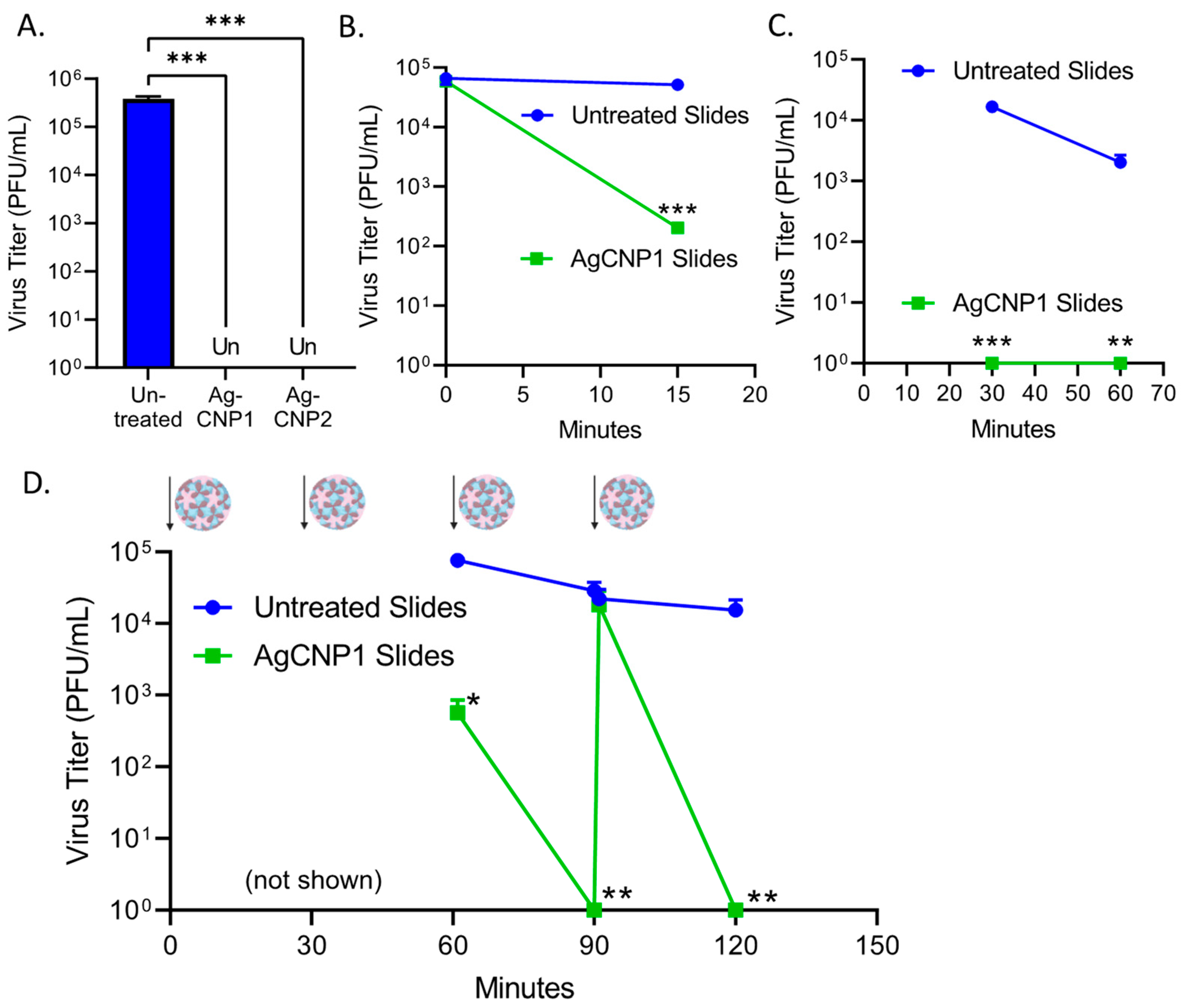
2.4. Surface-Dried Silver-Modified Nanoceria Inactivated Structurally Distinct Enveloped RNA Viruses
2.5. A Single Coating of Silver-Modified Nanoceria Inactivates Multiple Rounds of RV14 Challenge
2.6. A Single Coating of Silver-Modified Nanoceria Inactivates Multiple Rounds of FCV Challenge
2.7. AgCNP2-Coated Slides Inactivate Both FCV and RV14 in a Mixed Virus Inoculum as Effectively as in Individual Virus Challenges
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Nanoparticle Preparation and Virus Inactivation Studies
4.2.1. Materials
4.2.2. AgCNPs Syntheses
4.3. Hemagglutination Assays
4.4. Sucrose Gradient Centrifugation
4.5. Western Blotting
4.6. AgCNP2 Resistant Rhinovirus Selection and RNA Genome Sequencing
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Howley, P. Fields Virology; LWW: Philadelphia, PA, USA, 2013; Volumes 1 and 2. [Google Scholar]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View (Beijing) 2020, 1, e16. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.A.; Coulliette, A.D.; Rose, L.J.; Shams, A.M.; Edwards, J.R.; Noble-Wang, J.A. Persistence of Influenza A (H1N1) Virus on Stainless Steel Surfaces. Appl. Environ. Microbiol. 2016, 82, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, N.; Savolainen-Kopra, C.; Enstone, J.E.; Kulmala, I.; Pasanen, P.; Salmela, A.; Salo, S.; Nguyen-Van-Tam, J.S.; Ruutu, P.; PANDHUB consortium. Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect. Dis. 2018, 18, 437. [Google Scholar] [CrossRef] [PubMed]
- Sizun, J.; Yu, M.W.; Talbot, P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: A possible source of hospital-acquired infections. J. Hosp. Infect. 2000, 46, 55–60. [Google Scholar] [CrossRef]
- Chan, K.H.; Sridhar, S.; Zhang, R.R.; Chu, H.; Fung, A.Y.; Chan, G.; Chan, J.F.; To, K.K.; Hung, I.F.; Cheng, V.C.; et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020, 106, 226–231. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A.; Rivard, S.; Rahman, M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J. Clin. Microbiol. 1991, 29, 2115–2119. [Google Scholar] [CrossRef]
- Gern, G.; Palmenberg, A. The Rhinoviruses. In Field’s Virology, 6th ed.; Knipe, D.M., Howley, P., Eds.; Lippencott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 531–548. [Google Scholar]
- Hall, A.J.; Eisenbart, V.G.; Etingüe, A.L.; Gould, L.H.; Lopman, B.A.; Parashar, U.D. Epidemiology of foodborne norovirus outbreaks, United States, 2001-2008. Emerg. Infect. Dis. 2012, 18, 1566–1573. [Google Scholar] [CrossRef]
- CDC. National Center for Immunization and Respiratory Diseases; Diseases, D.o.V. Prevent Norovirus. Available online: https://www.cdc.gov/norovirus/about/prevention.html (accessed on 8 June 2023).
- Takahashi, H.; Ohuchi, A.; Miya, S.; Izawa, Y.; Kimura, B. Effect of food residues on norovirus survival on stainless steel surfaces. PLoS ONE 2011, 6, e21951. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110924. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Lozovski, V.; Lysenko, V.; Piatnytsia, V.; Scherbakov, O.; Zholobak, N.; Spivak, M. Physical Point of View for Antiviral Effect Caused by the Interaction Between the Viruses and Nanoparticles. J. Bionanosci. 2012, 6, 109–112. [Google Scholar] [CrossRef]
- Kolanthai, E.; Neal, C.J.; Kumar, U.; Fu, Y.; Seal, S. Antiviral nanopharmaceuticals: Engineered surface interactions and virus-selective activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1823. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Stellacci, F. Broad-Spectrum Antiviral Agents Based on Multivalent Inhibitors of Viral Infectivity. Adv. Healthc. Mater. 2021, 10, e2001433. [Google Scholar] [CrossRef]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Maeda; Kazuhiko; Domen, K. New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Nefedova, A.; Rausalu, K.; Zusinaite, E.; Vanetsev, A.; Rosenberg, M.; Koppel, K.; Lilla, S.; Visnapuu, M.; Smits, K.; Kisand, V.; et al. Antiviral efficacy of cerium oxide nanoparticles. Sci. Rep. 2022, 12, 18746. [Google Scholar] [CrossRef] [PubMed]
- Derevianko, S.; Vasylchenko, A.; Kaplunenko, V.; Kharchuk, M.; Demchenko, O.; Spivak, M. Antiviral Properties of Cerium Nanoparticles. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 187–204. [Google Scholar] [CrossRef]
- Neal, C.J.; Kolanthai, E.; Wei, F.; Coathup, M.; Seal, S. Surface Chemistry of Biologically-Active Reducible Oxide Nanozymes. Adv. Mater. 2023, e2211261. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Jeyaranjan, A.; Neal, C.J.; Kumar, U.; Sakthivel, T.S.; Sayle, D.C. Engineered defects in cerium oxides: Tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale 2020, 12, 6879–6899. [Google Scholar] [CrossRef]
- Neal, C.J.; Fox, C.R.; Sakthivel, T.S.; Kumar, U.; Fu, Y.; Drake, C.; Parks, G.D.; Seal, S. Metal-Mediated Nanoscale Cerium Oxide Inactivates Human Coronavirus and Rhinovirus by Surface Disruption. ACS Nano 2021, 15, 14544–14556. [Google Scholar] [CrossRef]
- Robinson, R.; Dowdle, W. Influenza viruses. In Diagnostic Procedures for Viral and Rickettsial Diseases, 4th ed.; Lennette, E.H., Schmidt, N.J., Eds.; American Public Health Association: New York, NY, USA, 1969; pp. 414–433. [Google Scholar]
- Hulswit, R.J.G.; Lang, Y.; Bakkers, M.J.G.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; van Kuppeveld, F.J.M.; Boons, G.J.; Bosch, B.J.; et al. Human coronaviruses OC43 and HKU1 bind to 9-. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Walls, A.C.; Lang, Y.; Wang, C.; Li, Z.; Koerhuis, D.; Boons, G.J.; Bosch, B.J.; Rey, F.A.; de Groot, R.J.; et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019, 26, 481–489. [Google Scholar] [CrossRef]
- Parks, G.; Manuse, M.; Johnson, J. The Parainfluenza Virus Simian Virus 5. In The Biology of Paramyxoviruses; Samal, S.K., Ed.; Caister Academic Press: Poole, UK, 2011. [Google Scholar]
- Johnson, J.B.; Capraro, G.A.; Parks, G.D. Differential mechanisms of complement-mediated neutralization of the closely related paramyxoviruses simian virus 5 and mumps virus. Virology 2008, 376, 112–123. [Google Scholar] [CrossRef]
- Paterson, R.G.; Leser, G.P.; Shaughnessy, M.A.; Lamb, R.A. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 1995, 208, 121–131. [Google Scholar] [CrossRef]
- Stanway, G.; Hughes, P.J.; Mountford, R.C.; Minor, P.D.; Almond, J.W. The complete nucleotide sequence of a common cold virus: Human rhinovirus 14. Nucleic Acids Res. 1984, 12, 7859–7875. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Lamson, D.M.; St George, K.; Walsh, T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC: New York, NY, USA, 2002. [Google Scholar]
- Touabi, L.; Aflatouni, F.; McLean, G.R. Mechanisms of Rhinovirus Neutralisation by Antibodies. Viruses 2021, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Mosser, A.G.; Colonno, R.J.; Rueckert, R.R. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J. Virol. 1986, 57, 246–257. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, M.; Wang, K.; Estes, M.K. Sequence and genomic organization of Norwalk virus. Virology 1993, 195, 51–61. [Google Scholar] [CrossRef]
- Urakami, H.; Ikarashi, K.; Okamoto, K.; Abe, Y.; Ikarashi, T.; Kono, T.; Konagaya, Y.; Tanaka, N. Chlorine sensitivity of feline calicivirus, a norovirus surrogate. Appl. Environ. Microbiol. 2007, 73, 5679–5682. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Obj. 2020, 24, 100620. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hien Thanh, N.T.; Thuy, N.T.; Chung, P.V.; Hung, P.N.; Le, A.T.; Hong Hanh, N.T. Cytotoxicity and antiviral activity of electrochemical—Synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Johnson, J.B.; Lyles, D.S.; Alexander-Miller, M.A.; Parks, G.D. Virion-associated complement regulator CD55 is more potent than CD46 in mediating resistance of mumps virus and vesicular stomatitis virus to neutralization. J. Virol. 2012, 86, 9929–9940. [Google Scholar] [CrossRef]
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, A.O.; Smith, H.Q.; Svoboda, S.A.; Lewis, M.S.; Sherman, M.B.; Lynch, G.C.; Pettitt, B.M.; Smith, T.J.; Wobus, C.E. Norovirus Escape from Broadly Neutralizing Antibodies Is Limited to Allostery-Like Mechanisms. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Palmenberg, A.C.; Spiro, D.; Kuzmickas, R.; Wang, S.; Djikeng, A.; Rathe, J.A.; Fraser-Liggett, C.M.; Liggett, S.B. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 2009, 324, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Shimojima, M.; Miyazawa, T.; Kato, K.; Tohya, Y.; Akashi, H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2006, 80, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Dreschers, S.; Dumitru, C.A.; Adams, C.; Gulbins, E. The cold case: Are rhinoviruses perfectly adapted pathogens? Cell. Mol. Life Sci. 2007, 64, 181–191. [Google Scholar] [CrossRef]
- Sosnovtsev, S.V.; Belliot, G.; Chang, K.O.; Onwudiwe, O.; Green, K.Y. Feline calicivirus VP2 is essential for the production of infectious virions. J. Virol. 2005, 79, 4012–4024. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.M.; Van Ranst, M. Complete genomic sequence of human coronavirus OC43: Molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Fox, C.R.; Parks, G.D. Complement Inhibitors Vitronectin and Clusterin Are Recruited from Human Serum to the Surface of Coronavirus OC43-Infected Lung Cells through Antibody-Dependent Mechanisms. Viruses 2021, 14, 29. [Google Scholar] [CrossRef]
- Hierholzer, J.C.; Killington, R.A. Virus isolation and quantitation. Virol. Methods Man. 1996, 25–46. [Google Scholar] [CrossRef]
- Reznikov, L.R.; Norris, M.H.; Vashisht, R.; Bluhm, A.P.; Li, D.; Liao, Y.J.; Brown, A.; Butte, A.J.; Ostrov, D.A. Identification of antiviral antihistamines for COVID-19 repurposing. Biochem. Biophys. Res. Commun. 2021, 538, 173–179. [Google Scholar] [CrossRef]
- Arimilli, S.; Johnson, J.B.; Clark, K.M.; Graff, A.H.; Alexander-Miller, M.A.; Mizel, S.B.; Parks, G.D. Engineered expression of the TLR5 ligand flagellin enhances paramyxovirus activation of human dendritic cell function. J. Virol. 2008, 82, 10975–10985. [Google Scholar] [CrossRef]
- Dillon, P.J.; Parks, G.D. Role for the phosphoprotein P subunit of the paramyxovirus polymerase in limiting induction of host cell antiviral responses. J. Virol. 2007, 81, 11116–11127. [Google Scholar] [CrossRef]
- Parks, G.D.; Young, V.A.; Koumenis, C.; Wansley, E.K.; Layer, J.L.; Cooke, K.M. Controlled Cell Killing by a Recombinant Nonsegmented Negative-Strand RNA Virus. Virology 2002, 293, 192–203. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
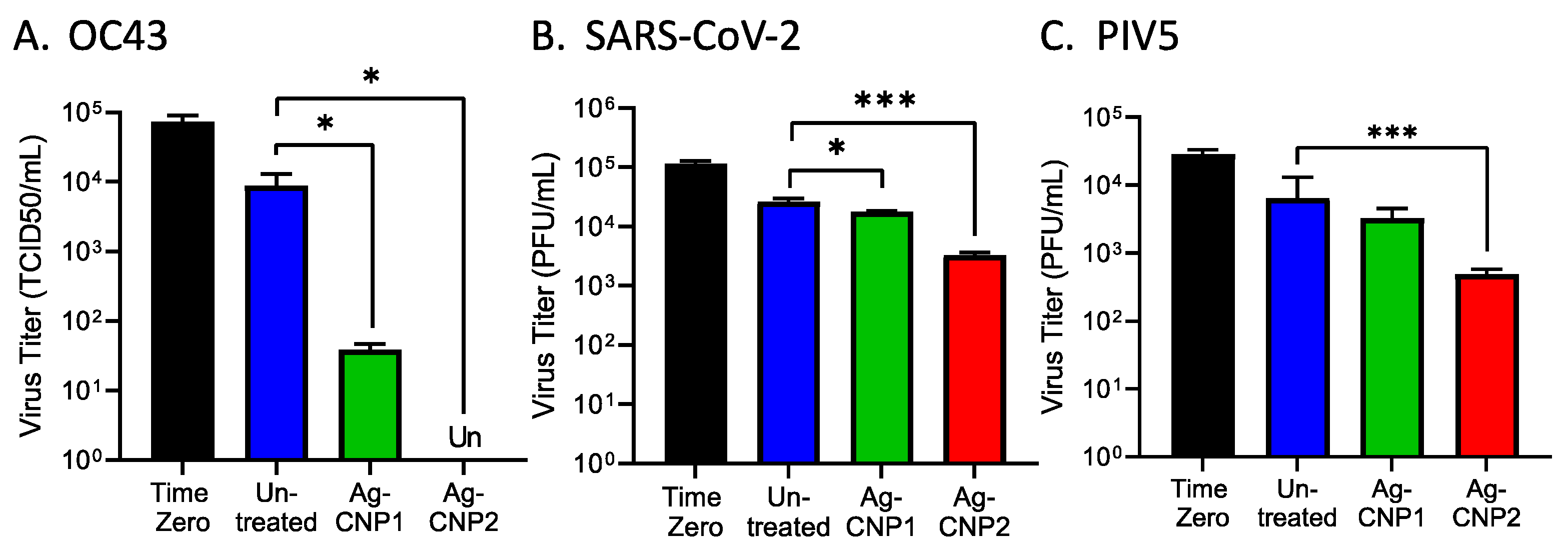


| Structure | Virus | Receptor a | Particle Size Diameter (nm) b | Dried AgCNP1 Sensitivity | Dried AgCNP2 Sensitivity |
|---|---|---|---|---|---|
| Non-enveloped + ssRNA | RV14 | ICAM1 LDLR | 15-30 | - | +++ |
| Non-enveloped + ssRNA | FCV | JAM-A | 27-40 | +++ | +++ |
| Enveloped + ssRNA | OC43 | Sialic acid and others | 120–160 | ++ | +++ |
| Enveloped + ssRNA | SARS-CoV-2 | ACE2 | 100 | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fox, C.R.; Kedarinath, K.; Neal, C.J.; Sheiber, J.; Kolanthai, E.; Kumar, U.; Drake, C.; Seal, S.; Parks, G.D. Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions. Molecules 2023, 28, 5190. https://doi.org/10.3390/molecules28135190
Fox CR, Kedarinath K, Neal CJ, Sheiber J, Kolanthai E, Kumar U, Drake C, Seal S, Parks GD. Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions. Molecules. 2023; 28(13):5190. https://doi.org/10.3390/molecules28135190
Chicago/Turabian StyleFox, Candace R., Kritika Kedarinath, Craig J. Neal, Jeremy Sheiber, Elayaraja Kolanthai, Udit Kumar, Christina Drake, Sudipta Seal, and Griffith D. Parks. 2023. "Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions" Molecules 28, no. 13: 5190. https://doi.org/10.3390/molecules28135190
APA StyleFox, C. R., Kedarinath, K., Neal, C. J., Sheiber, J., Kolanthai, E., Kumar, U., Drake, C., Seal, S., & Parks, G. D. (2023). Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions. Molecules, 28(13), 5190. https://doi.org/10.3390/molecules28135190








