Insight into the Morphological Properties of Nano-Kaolinite (Nanoscrolls and Nanosheets) on Its Qualification as Delivery Structure of Oxaliplatin: Loading, Release, and Kinetic Studies
Abstract
:1. Introduction
2. Results and Discussion
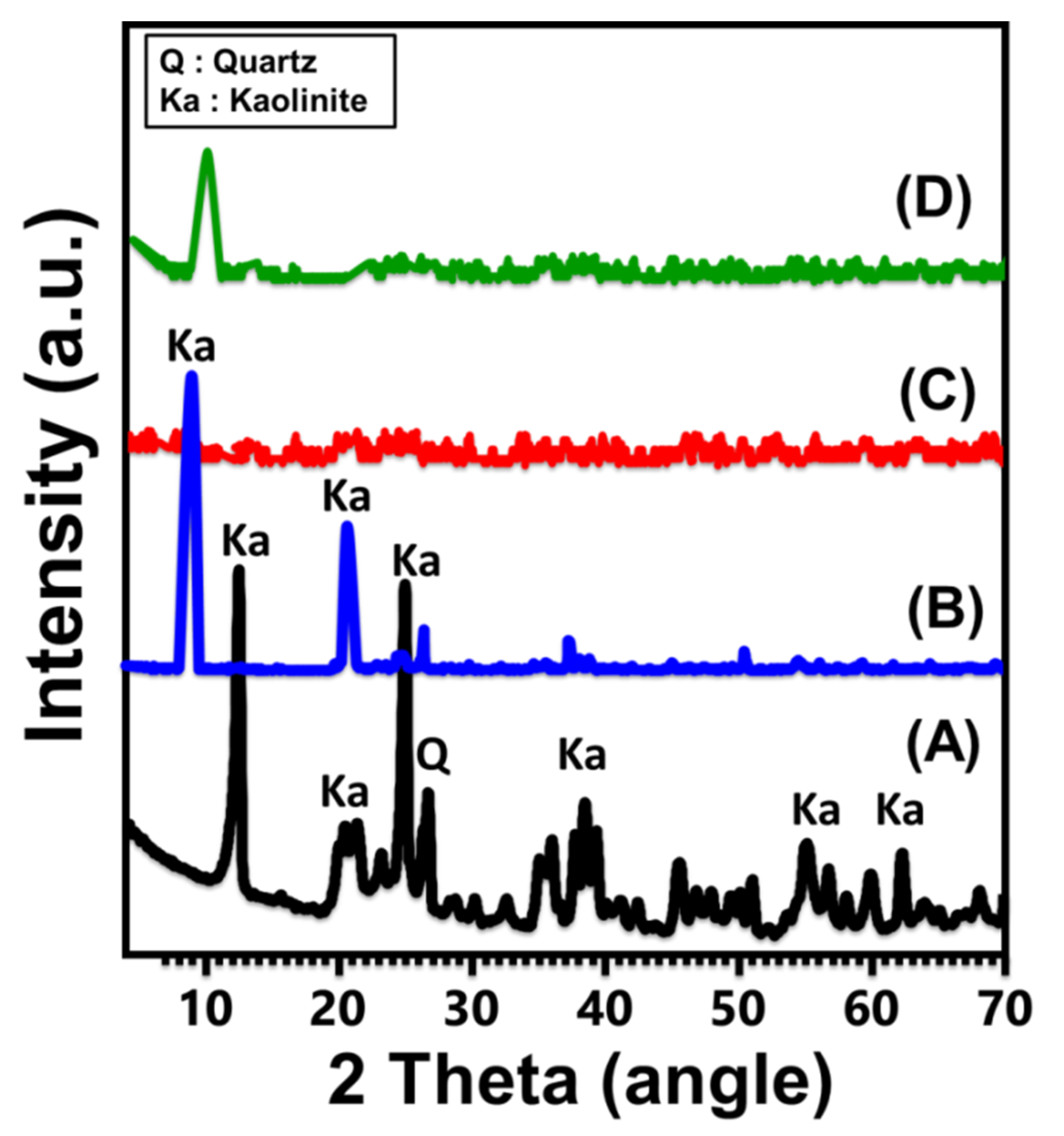
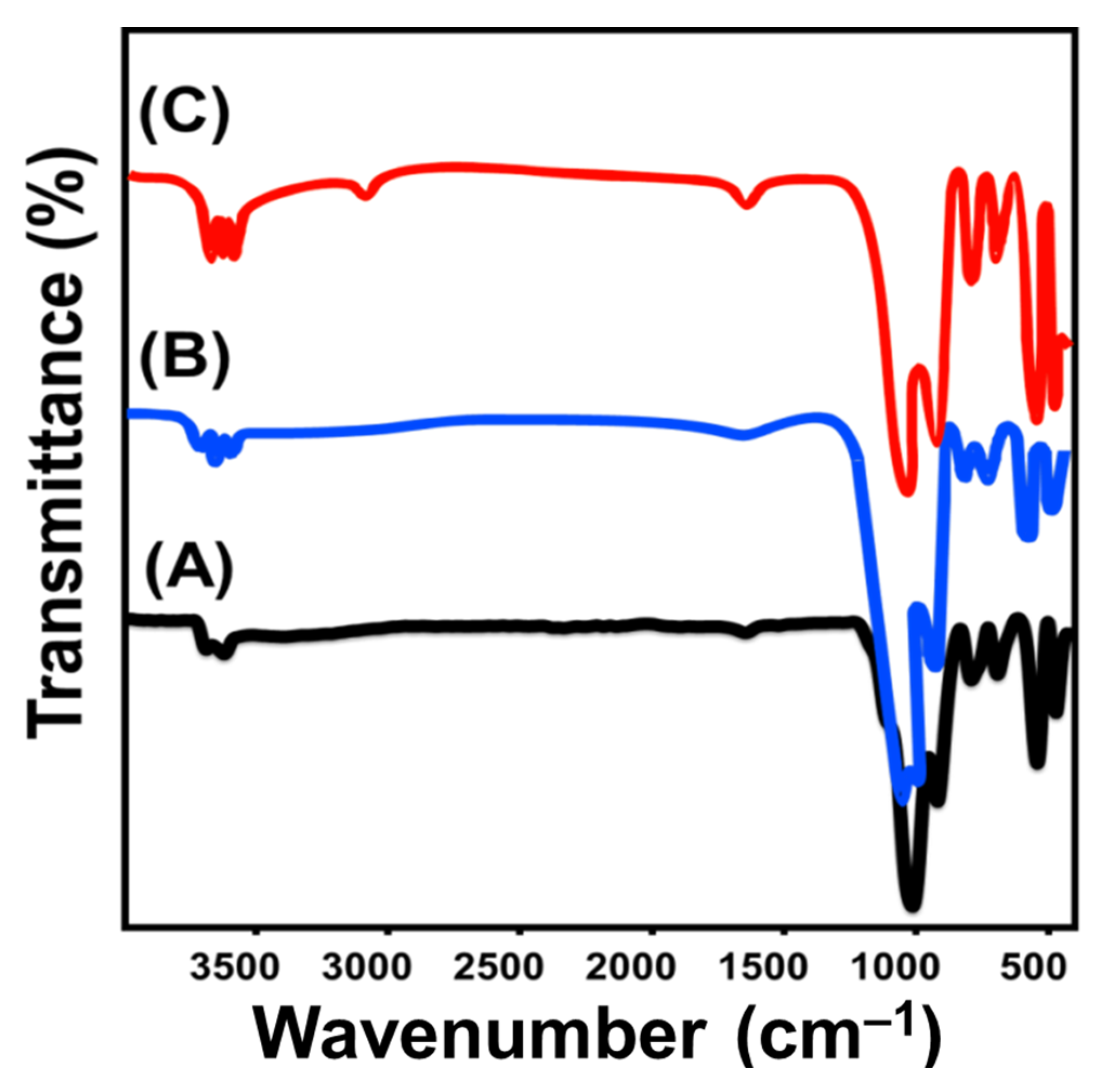
2.1. Characterization of the Carrier
2.2. Encapsulation of OXAP Drug
2.2.1. Influence of the Encapsulation Parameters
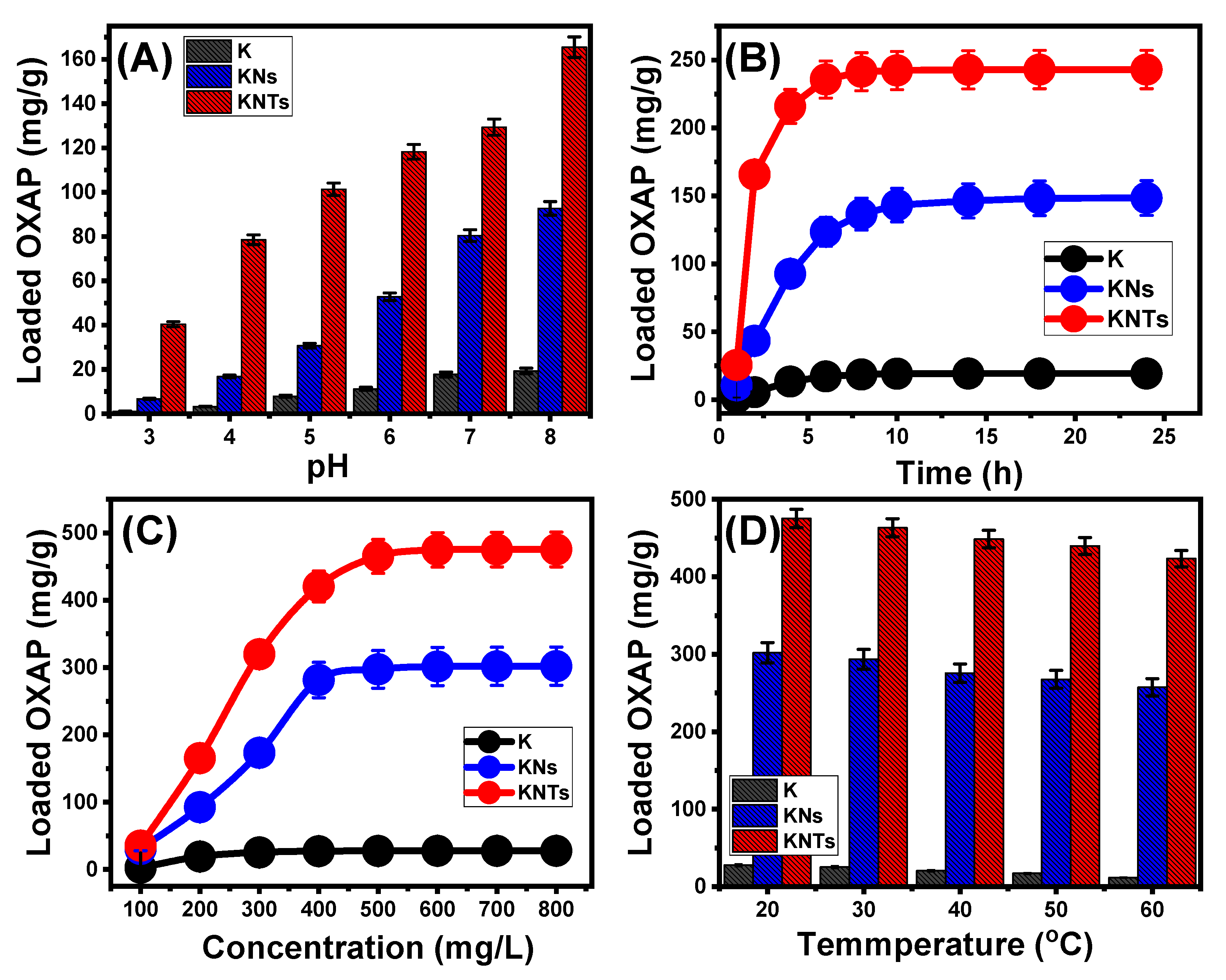
Effect of pH
Encapsulation Interval
OXAP Concentration
Effect of Temperature
2.2.2. Encapsulation Mechanism
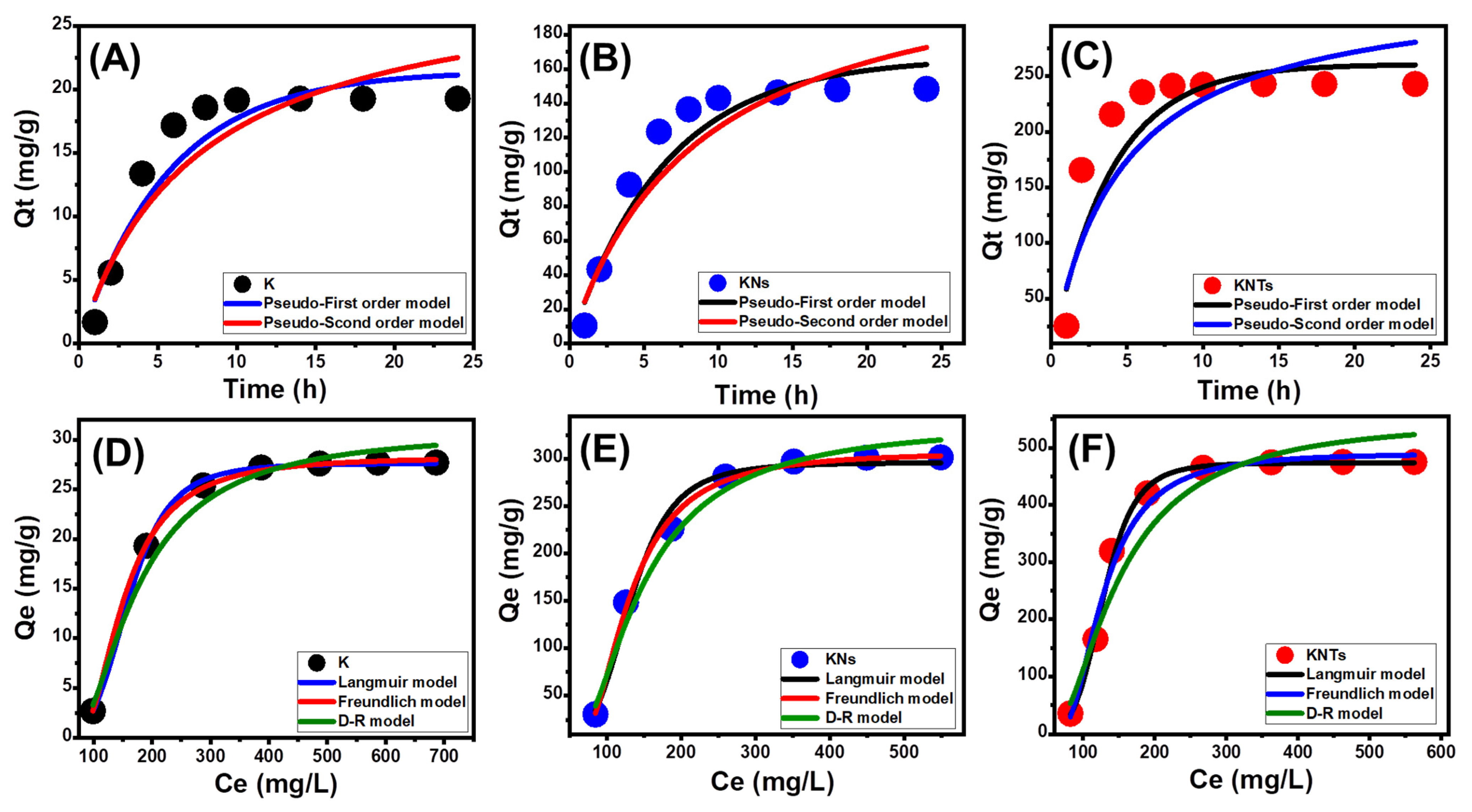
Kinetic Properties
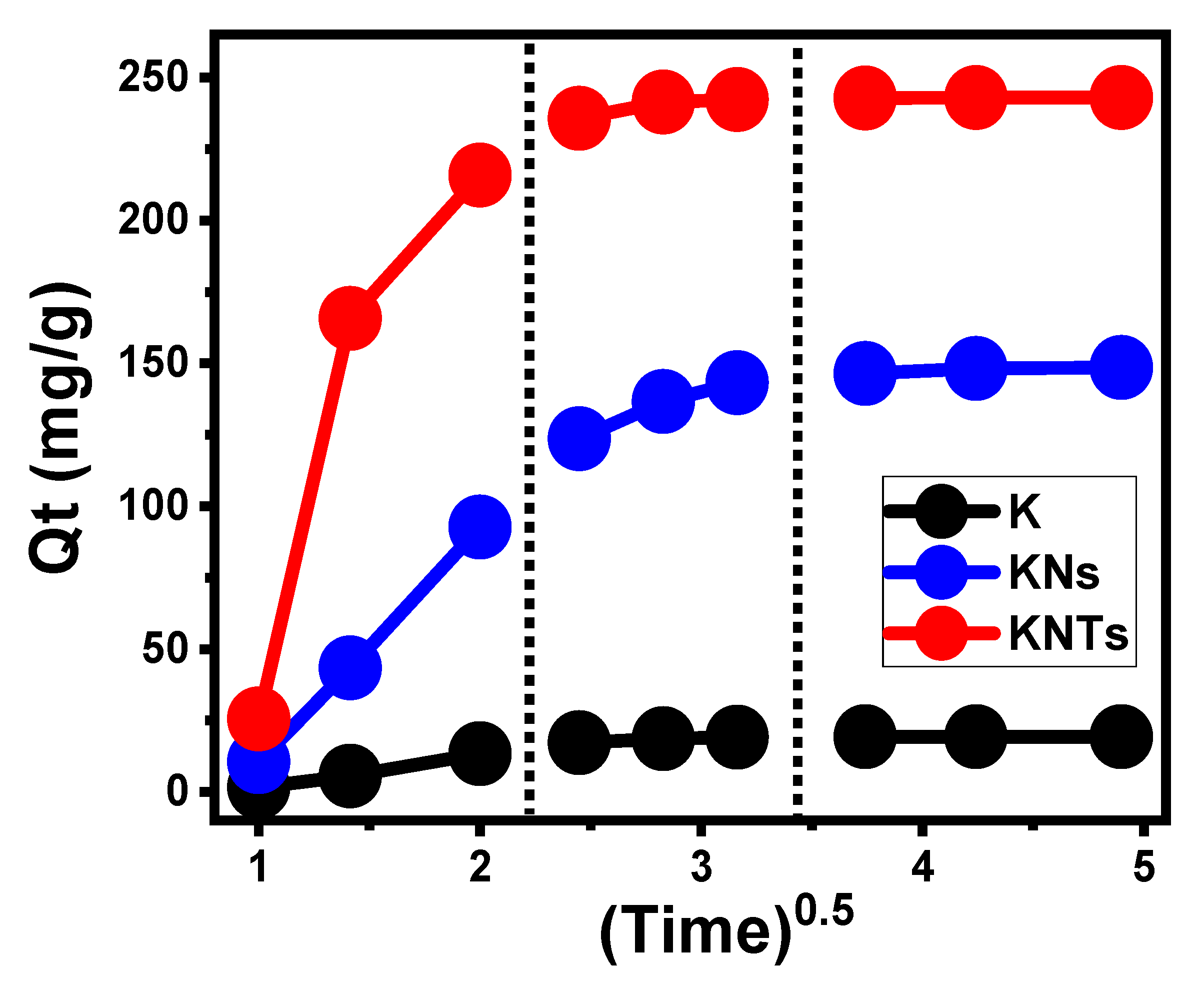
- Intra-Particle Diffusion Behavior
- Kinetic Modeling
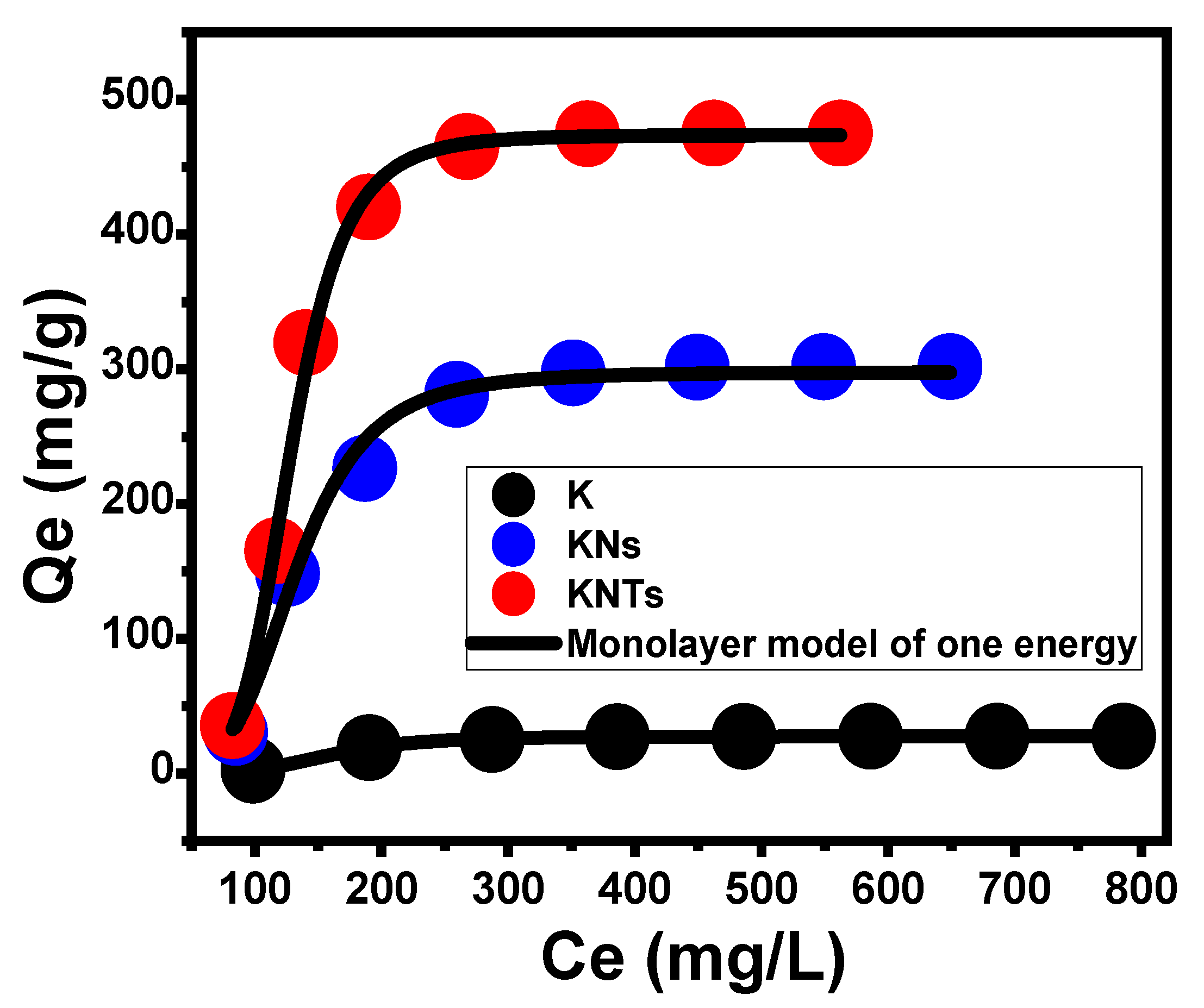
Isotherm Properties
- Classic Isotherm Models
- Advanced Isotherm Models
Thermodynamic Properties
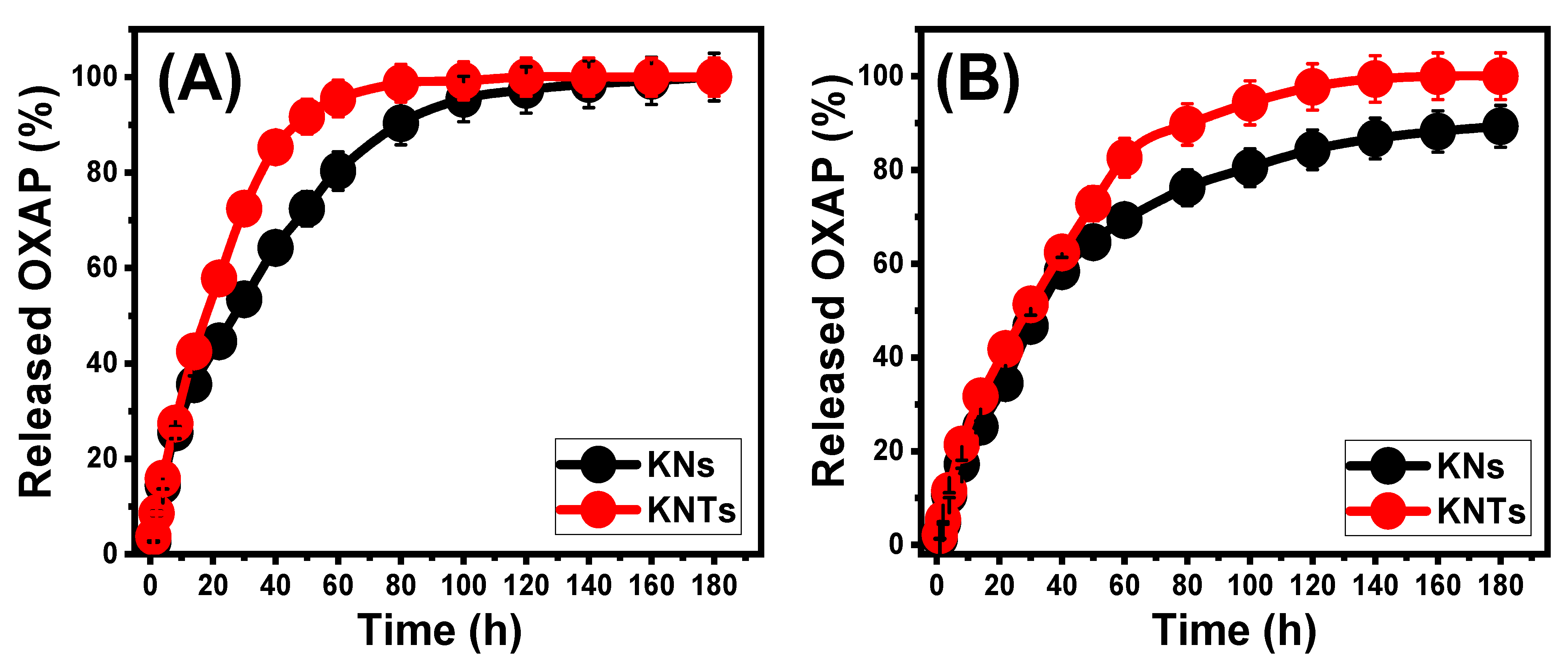
2.3. In Vitro Release Profiles
2.4. Release Kinetic Studies
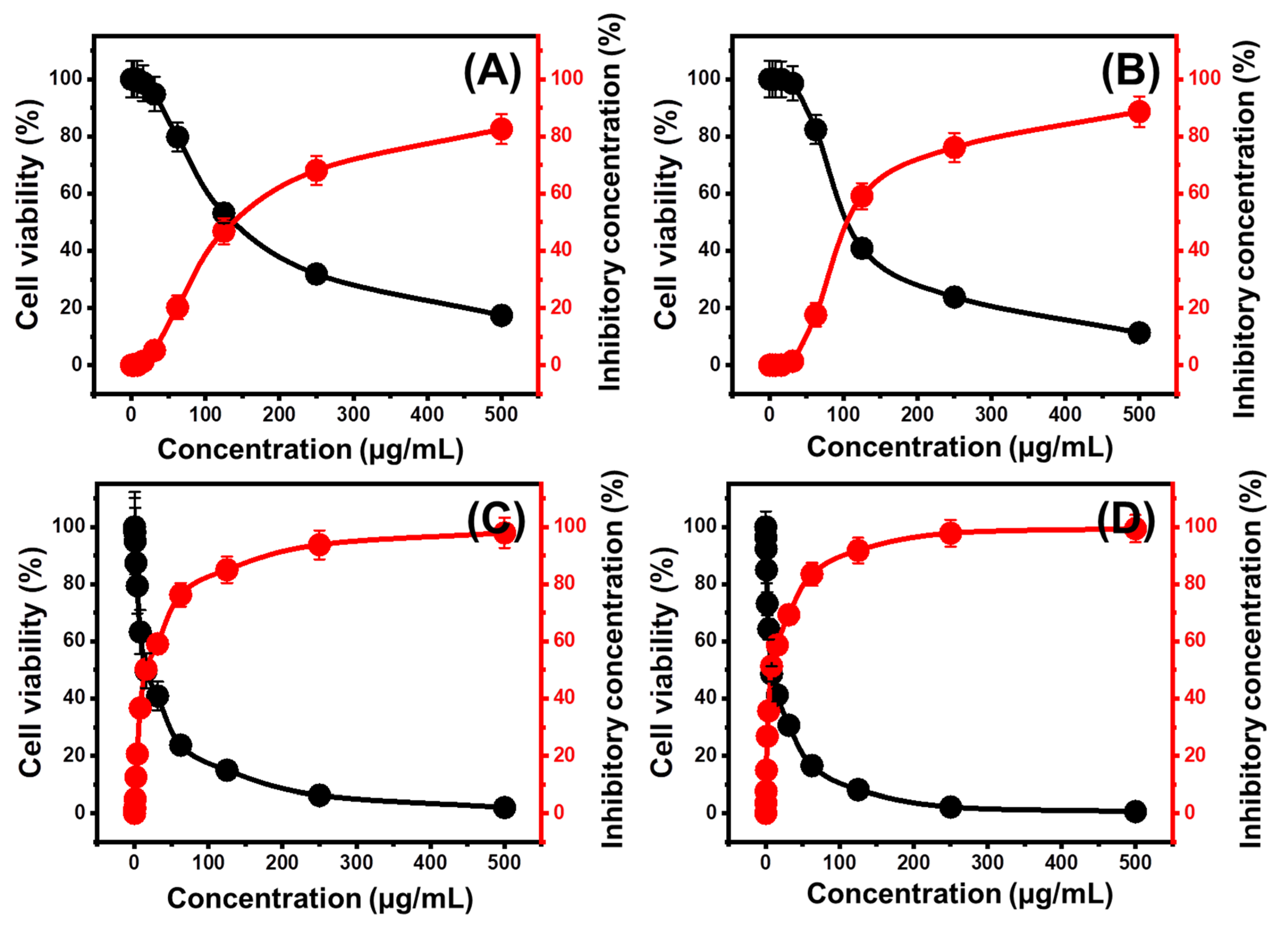
2.5. Cytotoxicity Properties
2.6. Comparison study
3. Experimental Work
3.1. Materials
3.2. Synthesis of Kaolinite Nanosheets (KNs) and Nanotubes (KNTs)
3.3. Analytical Techniques
3.4. OXAP Loading Studies
3.5. The Release Studies
3.6. In Vitro Cytotoxicity
3.6.1. Cell Lines
3.6.2. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Zeiny, H.M.; Abukhadra, M.R.; Sayed, O.M.; Osman, A.H.; Ahmed, S.A. Insight into novel β-cyclodextrin-grafted poly (Nvinylcaprolactam) nanogel structures as advanced carriers for 5-fluorouracil: Equilibrium behavior and pharmacokinetic modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124197. [Google Scholar] [CrossRef]
- Cutrim, E.S.; Vale, A.A.; Manzani, D.; Barud, H.S.; Castellon, E.R.; Santos, A.P.; Alcântara, A.C. Preparation, characterization and in vitro anticancer performance of nanoconjugate based on carbon quantum dots and 5-Fluorouracil. Mater. Sci. Eng. C 2021, 120, 111781. [Google Scholar] [CrossRef] [PubMed]
- Abuzar, S.M.; Park, E.J.; Seo, Y.; Lee, J.; Baik, S.H.; Hwang, S.J. Preparation and evaluation of intraperitoneal long-acting oxaliplatin-loaded multi-vesicular liposomal depot for colorectal cancer treatment. Pharmaceutics 2020, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Altoom, N.; Ashraf, M.T.; Ibrahim, S.M.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Abukhadra, M.R. Insight into the loading, release, and anticancer properties of cellulose/zeolite-A as an enhanced delivery structure for oxaliplatin chemotherapy; characterization and mechanism. J. Sol-Gel Sci. Technol. 2022, 103, 752–765. [Google Scholar] [CrossRef]
- Sayed, M.A.; El-Zeiny, H.M.; Khim, J.S.; Ajarem, J.S.; Allam, A.A.; Abukhadra, M.R. Insight into the loading properties of Na+ green functionalized clinoptilolite as a potential carrier for the 5-fluorouracil drug, its release kinetics, and cytotoxicity. ACS Omega 2022, 7, 6991–7001. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.; Ramasamy, T.; Mishra, S.K.; Jeong, K.Y.; Yong, C.S.; Kim, J.O.; Kim, H.M. Engineering of caveolae-specific self-micellizing anticancer lipid nanoparticles to enhance the chemotherapeutic efficacy of oxaliplatin in colorectal cancer cells. Acta Biomater. 2016, 42, 220–231. [Google Scholar] [CrossRef]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Ghosh, B.; Biswas, S. Oxaliplatin delivery via chitosan/vitamin E conjugate micelles for improved efficacy and MDR-reversal in breast cancer. Carbohydr. Polym. 2022, 282, 119108. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Cui, Y.; Zhang, H.; Zhang, S.; Wang, X.; Liu, S.; Gao, Q. Oxaliplatin derived monofunctional triazole-containing platinum (II) complex counteracts oxaliplatin-induced drug resistance in colorectal cancer. Bioorg. Chem. 2021, 107, 104636. [Google Scholar] [CrossRef]
- Shad, P.M.; Karizi, S.Z.; Javan, R.S.; Mirzaie, A.; Noorbazargan, H.; Akbarzadeh, I.; Rezaie, H. Folate conjugated hyaluronic acid coated alginate nanogels encapsulated oxaliplatin enhance antitumor and apoptosis efficacy on colorectal cancer cells (HT29 cell line). Toxicol. In Vitro 2020, 65, 104756. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Abukhadra, M.R.; Mohamed, A.S.; Nadeem, A.; Ahmad, S.F.; Ibrahim, K.E. Insight into the loading and release properties of an exfoliated kaolinite/cellulose fiber (EXK/CF) composite as a carrier for oxaliplatin drug: Cytotoxicity and release kinetics. ACS Omega 2020, 5, 19165–19173. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.I.; Allam, A.A.; AlFassam, H.; Abu-Taweel, G.M.; Altoom, N.; Abukhadra, M.R. Sonoco green decoration of clinoptilolite with MgO nanoparticles as a potential carrier for 5-fluorouracil drug: Loading behavior, release profile, and cytotoxicity. Inorg. Organomet. Polym. 2021, 31, 4608–4622. [Google Scholar] [CrossRef]
- Rabiee, N.; Atarod, M.; Tavakolizadeh, M.; Asgari, S.; Rezaei, M.; Akhavan, O.; Pourjavadi, A.; Jouyandeh, M.; Lima, E.C.; Mashhadzadeh, A.H.; et al. Green metal-organic frameworks (MOFs) for biomedical applications. Microporous Mesoporous Mater. 2022, 335, 111670. [Google Scholar] [CrossRef]
- Narmani, A.; Kamali, M.; Amini, B.; Salimi, A.; Panahi, Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process. Biochem. 2018, 69, 178–187. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; AlHammadi, A.; El-Sherbeeny, A.M.; Salam, M.A.; El-Meligy, M.A.; Awwad, E.M.; Luqman, M. Enhancing the removal of organic and inorganic selenium ions using an exfoliated kaolinite/cellulose fibres nanocomposite. Carbohydr. Polym. 2021, 252, 117163. [Google Scholar] [CrossRef]
- Almahri, A. The solid-state synthetic performance of bentonite stacked manganese ferrite nanoparticles: Adsorption and photo-fenton degradation of MB dye and antibacterial applications. J. Mater. Res. Technol. 2022, 17, 2935–2949. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Dong, F.; He, H.; Sun, S.; Liu, Z. Selective loading of 5-fluorouracil in the interlayer space of methoxy-modified kaolinite for controlled release. Appl. Clay Sci. 2018, 159, 102–106. [Google Scholar] [CrossRef]
- Dardir, F.M.; Mohamed, A.S.; Abukhadra, M.R.; Ahmed, E.A.; Soliman, M.F. Cosmetic and pharmaceutical qualifications of Egyptian bentonite and its suitability as drug carrier for Praziquantel drug. Eur. J. Pharm. Sci. 2018, 115, 320–329. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption behavior of inorganic-and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; Mostafa, A.M.; Elmeligy, M.A. Facile synthesis of bentonite/biopolymer composites as low-cost carriers for 5-fluorouracil drug; equilibrium studies and pharmacokinetic behavior. Int. J. Biol. Macromol. 2019, 141, 721–731. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Nadeem, A. Instantaneous adsorption of synthetic dyes from an aqueous environment using kaolinite nanotubes: Equilibrium and thermodynamic studies. ACS Omega 2021, 6, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Lee, M.Y.; Kim, J.S. Radio-frequency thermal plasma preparation of nano-sized Ni catalysts supported on MgO nano-rods for partial oxidation of methane. Surf. Coat. Technol. 2013, 228, S91–S96. [Google Scholar] [CrossRef]
- Sabry, R.; AbdulAzeez, O. Hydrothermal growth of ZnO nano rods without catalysts in a single step. Manuf. Lett. 2014, 2, 69–73. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. The role of pH and nitrate concentration in the wet chemical growth of nano-rods shaped ZnO photocatalyst. Nano-Struct. Nano-Objects 2019, 18, 100250. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; El-Meligy, M.A. Insight into the loading and release properties of MCM-48/biopolymer composites as carriers for 5-fluorouracil: Equilibrium modeling and pharmacokinetic studies. ACS Omega 2020, 5, 11745–11755. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Allah, A.F. Synthesis and characterization of kaolinite nanotubes (KNTs) as a novel carrier for 5-fluorouracil of high encapsulation properties and controlled release. Inorg. Chem. Commun. 2019, 103, 30–36. [Google Scholar] [CrossRef]
- Adly, E.R.; Shaban, M.S.; El-Sherbeeny, A.M.; Al Zoubi, W.; Abukhadra, M.R. Enhanced Congo Red Adsorption and Photo-Fenton Oxidation over an Iron-Impeded Geopolymer from Ferruginous Kaolinite: Steric, Energetic, Oxidation, and Synergetic Studies. ACS Omega 2022, 7, 31218–31232. [Google Scholar] [CrossRef]
- Shawky, A.; El-Sheikh, S.M.; Rashed, M.N.; Abdo, S.M.; El-Dosoqy, T.I. Exfoliated kaolinite nanolayers as an alternative photocatalyst with superb activity. J. Environ. Chem. Eng. 2019, 7, 103174. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; Zhang, M.; Cheng, W.; Li, Z.; Nie, J.; Liu, G.; Lian, D.; Xie, Z.; Huang, L.; et al. Self-controlled release of oxaliplatin prodrug from d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) functionalized mesoporous silica nanoparticles for cancer therapy. J. Colloid Interface Sci. 2018, 525, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@ polynaniline/Bentonite Tripartite Structure (G. Zn@ PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. [Google Scholar] [CrossRef]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As (V), Hg (II), and U (VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- El Qada, E. Kinetic Behavior of the Adsorption of Malachite Green Using Jordanian Diatomite as Adsorbent. Jordanian J. Eng. Chem. Ind. (JJECI) 2020, 3. [Google Scholar]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective retention of inorganic Selenium ions (Se (VI) and Se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Dawodu, F.; Akpomie, G.; Abuh, M. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2022, 431, 134312. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; Reinert, L.; Knani, S.; Duclaux, L.; Lamine, A.B. Experimental and theoretical studies of adsorption of ibuprofen on raw and two chemically modified activated carbons: New physicochemical interpretations. RSC Adv. 2016, 6, 12363–12373. [Google Scholar] [CrossRef]
- Ali, R.A.; Mobarak, M.; Badawy, A.M.; Lima, E.C.; Seliem, M.K.; Ramadan, H.S. New insights into the surface oxidation role in enhancing Congo red dye uptake by Egyptian ilmenite ore: Experiments and physicochemical interpretations. Surf. Interface 2021, 26, 101316. [Google Scholar] [CrossRef]
- Ashraf, M.T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Supervison, Y.G.K.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T.; AbuKhadra, M.R. Insight into chitosan/zeolite—A nanocomposite as an advanced carrier for levofloxacin and its anti-inflammatory properties; loading, release, and anti-inflammatory studies. Int. J. Biol. Macromol. 2021, 179, 206–216. [Google Scholar] [CrossRef]
- Ge, M.; Tang, W.; Du, M.; Liang, G.; Hu, G.; Alam, S.J. Research on 5-fluorouracil as a drug carrier materials with its in vitro release properties on organic modified magadiite. Eur. J. Pharm. Sci. 2019, 130, 44–53. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Bin Jumah, M.N.; Othman, S.I.; Alruhaimi, R.S.; Al-Khalawi, N.; Salama, Y.F.; Allam, A.A.; Abukhadra, M.R. Synthesis of Chitosan/Diatomite Composite as an Advanced Delivery System for Ibuprofen Drug; Equilibrium Studies and the Release Profile. ACS Omega 2021, 6, 13406–13416. [Google Scholar] [CrossRef]
- El-Hamshary, H.; El-Newehy, M.H.; Abdulhameed, M.M.; ElFaham, A.; Elsherbiny, A.S. Evaluation of clay-ionene nanocomposite carriers for controlled drug delivery: Synthesis, in vitro drug release, and kinetics. Mater. Chem. Phys. 2019, 225, 122–132. [Google Scholar] [CrossRef]
- Betsiou, M.; Bantsis, G.; Zoi, I.; Sikalidis, C. Adsorption and release of gemcitabine hydrochloride and oxaliplatin by hydroxyapatite. Ceram. Int. 2012, 38, 2719–2724. [Google Scholar] [CrossRef]
- Altoom, N.; Adlii, A.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Synthesis and characterization of β-cyclodextrin functionalized zeolite-A as biocompatible carrier for Levofloxacin drug; loading, release, cytotoxicity, and anti-inflammatory studies. J. Solid State Chem. 2022, 312, 123280. [Google Scholar] [CrossRef]
- Alfassam, H.E.; Ashraf, M.T.; Al Othman, S.I.; Al-Waili, M.A.; Allam, A.A.; Abukhadra, M.R. Characterization of cellulose-functionalized phillipsite biocomposite as an enhanced carrier of oxaliplatin drug during the treatment of colorectal cancer: Loading, release, and cytotoxicity. RSC Adv. 2023, 13, 16327–16341. [Google Scholar] [CrossRef]
- Altoom, N.; Ibrahim, S.M.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Characterization of β-cyclodextrin/phillipsite (β-CD/Ph) composite as a potential carrier for oxaliplatin as therapy for colorectal cancer; loading, release, and cytotoxicity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129144. [Google Scholar] [CrossRef]
- Alfassam, H.E.; Ashraf, M.T.; Al Othman, S.I.; Al-Waili, M.A.; Allam, A.A.; Abukhadra, M.R. Synthesis and characterization of cellulose functionalized zeolitic diatomite as an enhanced carrier of oxaliplatin drug; loading, release, and cytotoxicity. Int. J. Biol. Macromol. 2023, 235, 123825. [Google Scholar] [CrossRef]
- Alfassam, H.E.; Al Othman, S.I.; Al-Waili, M.A.; Allam, A.A.; Abukhadra, M.R. Characterization of β-cyclodextrin Hybridized Diatomite as Potential Delivery Systems of Oxaliplatin and 5-Fluorouracil Drugs; Equilibrium Modeling of Loading and Release Kinetics. J. Macromol. Sci. Part B 2023. accepted. [Google Scholar] [CrossRef]



| Model | Parameters | K | KNs | KNTs | |
|---|---|---|---|---|---|
| Kinetic models | |||||
| Pseudo-first-order | K1 (min−1) | 0.175 | 0.155 | 0.253 | |
| Qe(Cal) (mg/g) | 21.47 | 166.6 | 250.7 | ||
| R2 | 0.94 | 0.94 | 0.90 | ||
| X2 | 0.52 | 4.1 | 5.73 | ||
| Pseudo-second-order | k2 (g mg−1 min−1) | 0.0046 | 4.9 × 10−4 | 6.51 × 10−4 | |
| Qe(Cal) (mg/g) | 29.44 | 234.5 | 334.2 | ||
| R2 | 0.91 | 0.92 | 0.85 | ||
| X2 | 0.73 | 5.4 | 7.3 | ||
| Isotherm models | |||||
| Langmuir | Qmax (mg/g) | 29.63 | 309.3 | 473.8 | |
| b (L/mg) | 0.001 | 0.0057 | 6.93 × 10−4 | ||
| R2 | 0.99 | 0.99 | 0.99 | ||
| X2 | 0.03 | 3.67 | 0.55 | ||
| RL | 0.55–0.90 | 0.18–0.63 | 0.64–0.93 | ||
| Freundlich | 1/n | 0.155 | 0.33 | 0.92 | |
| kF (mg/g) | 0.8 | 6.4 | 1.69 | ||
| R2 | 0.99 | 0.99 | 0.99 | ||
| X2 | 0.08 | 4.2 | 2.11 | ||
| D–R model | β (mol2/KJ2) | 0.0146 | 0.0231 | 0.0077 | |
| Qm (mg/g) | 30.8 | 337.13 | 550.2 | ||
| R2 | 0.99 | 0.98 | 0.96 | ||
| X2 | 0.148 | 1.64 | 7.6 | ||
| E (KJ/mol) | 5.84 | 4.65 | 8.04 | ||
| Monolayer model of one energy | n | 4.6 | 4.7 | 5.85 | |
| Nm (mg/g) | 6.5 | 66.3 | 80.86 | ||
| Q(sat) (mg/g) | 29.9 | 304.9 | 473.07 | ||
| ∆E (kJ/mol) | −5.3 | −7.5 | −4.2 | ||
| Thermodynamics | |||||
| ∆G° (kJ mol−1) | 293.13 | −8.13 | −8.32 | −15.88 | |
| 303.13 | −8.18 | −8.52 | −16.33 | ||
| 313.13 | −7.90 | −8.60 | −16.75 | ||
| 323.13 | −7.67 | −8.77 | −17.21 | ||
| 333.13 | −6.85 | −8.91 | −17.60 | ||
| ΔH° (kJ mol−1) | −4.16 | −3.88 | |||
| ΔS° (J K−1 mol−1) | 14.24 | 18.7 | |||
| Release kinetics | |||||
| Models | KNs | Determination coefficient | |||
| KNTs | |||||
| Acetate buffer (pH 5.5) | Phosphate buffer (pH 7.4) | Acetate buffer (pH 5.5) | Phosphate buffer (pH 7.4) | ||
| Zero-order | 0.75 | 0.79 | 0.61 | 0.78 | |
| First-order | 0.99 | 0.94 | 0.99 | 0.97 | |
| Higuchi | 0.92 | 0.93 | 0.89 | 0.94 | |
| Hixson–Crowell | 0.97 | 0.91 | 0.90 | 0.99 | |
| Korsmeyer–Peppas | 0.94 | 0.93 | 0.93 | 0.91 | |
| n | 0.62 | 0.72 | 0.60 | 0.70 | |
| Carrier | Loading Capacity (mg/g) | References |
|---|---|---|
| Hydroxyapatite | 49.1 | [52] |
| Zeolite-A | 109.03 | [53] |
| Cellulose/zeolite-A | 285.7 | [4] |
| Phillipsite | 79.6 | [54] |
| Β-cyclodextrin/phillipsite | 291.5 | [55] |
| Diatomite | 65.9 | [56] |
| Β-cyclodextrin/diatomite | 238.7 | [57] |
| kaolinite | 29.9 | This study |
| KNs | 304.9 | This study |
| KNTs | 473.07 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.D.; Nasser, N.; Bin Jumah, M.N.; AlZahrani, S.A.; Allam, A.A.; Abukhadra, M.R.; Bellucci, S. Insight into the Morphological Properties of Nano-Kaolinite (Nanoscrolls and Nanosheets) on Its Qualification as Delivery Structure of Oxaliplatin: Loading, Release, and Kinetic Studies. Molecules 2023, 28, 5158. https://doi.org/10.3390/molecules28135158
Alqahtani MD, Nasser N, Bin Jumah MN, AlZahrani SA, Allam AA, Abukhadra MR, Bellucci S. Insight into the Morphological Properties of Nano-Kaolinite (Nanoscrolls and Nanosheets) on Its Qualification as Delivery Structure of Oxaliplatin: Loading, Release, and Kinetic Studies. Molecules. 2023; 28(13):5158. https://doi.org/10.3390/molecules28135158
Chicago/Turabian StyleAlqahtani, Mashael Daghash, Nourhan Nasser, May N. Bin Jumah, Saleha A. AlZahrani, Ahmed A. Allam, Mostafa R. Abukhadra, and Stefano Bellucci. 2023. "Insight into the Morphological Properties of Nano-Kaolinite (Nanoscrolls and Nanosheets) on Its Qualification as Delivery Structure of Oxaliplatin: Loading, Release, and Kinetic Studies" Molecules 28, no. 13: 5158. https://doi.org/10.3390/molecules28135158
APA StyleAlqahtani, M. D., Nasser, N., Bin Jumah, M. N., AlZahrani, S. A., Allam, A. A., Abukhadra, M. R., & Bellucci, S. (2023). Insight into the Morphological Properties of Nano-Kaolinite (Nanoscrolls and Nanosheets) on Its Qualification as Delivery Structure of Oxaliplatin: Loading, Release, and Kinetic Studies. Molecules, 28(13), 5158. https://doi.org/10.3390/molecules28135158









