Abstract
Skeletons play an important role in the human body, and can form gaps of varying sizes once damaged. Bone defect healing involves a series of complex physiological processes and requires ideal bone defect implants to accelerate bone defect healing. Traditional grafts are often accompanied by issues such as insufficient donors and disease transmission, while some bone defect implants are made of natural and synthetic polymers, which have characteristics such as good porosity, mechanical properties, high drug loading efficiency, biocompatibility and biodegradability. However, their antibacterial, antioxidant, anti-inflammatory and bone repair promoting abilities are limited. Flavonoids are natural compounds with various biological activities, such as antitumor, anti-inflammatory and analgesic. Their good anti-inflammatory, antibacterial and antioxidant activities make them beneficial for the treatment of bone defects. Several researchers have designed different types of flavonoid-loaded polymer implants for bone defects. These implants have good biocompatibility, and they can effectively promote the expression of angiogenesis factors such as VEGF and CD31, promote angiogenesis, regulate signaling pathways such as Wnt, p38, AKT, Erk and increase the levels of osteogenesis-related factors such as Runx-2, OCN, OPN significantly to accelerate the process of bone defect healing. This article reviews the effectiveness and mechanism of biomaterials loaded with flavonoids in the treatment of bone defects. Flavonoid-loaded biomaterials can effectively promote bone defect repair, but we still need to improve the overall performance of flavonoid-loaded bone repair biomaterials to improve the bioavailability of flavonoids and provide more possibilities for bone defect repair.
1. Introduction
Bone is a complex connective tissue with various physiological functions [1,2,3]. Trauma and tumor-related surgery can lead to bone loss and form bone defects [4]. Small bone defects have the possibility of self-repair, but once they exceed the critical defect area, additional intervention is needed to guide and accelerate the healing process [5]. Bone defect healing is a dynamic and complex biological process, and an ideal bone defect implant should conform to the following standards: (1) good biocompatibility; (2) excellent biodegradability; (3) characteristics of bone induction and bone conduction; (4) suitable porosity; and (5) excellent mechanical performance [6,7,8].
Bone transplantation (such as autologous and allogeneic grafts) is the most commonly used and useful method for clinical treatment of bone defects, but it has certain drawbacks, such as insufficient supply of donor tissue and the need for secondary surgery, which increases the risks of infection and surgical costs [9,10]. Therefore, many researchers have designed various biomaterials for bone defect regeneration to overcome these problems, such as metals, ceramics, natural and synthetic polymers, in recent years [11,12,13,14]. Compared to natural polymers, synthetic polymers have poor biocompatibility, which may lead to aseptic inflammatory reactions in bone defect sites [15]. Natural polymers have good biological adhesion, biocompatibility and biodegradability [13], but their mechanical properties are limited and they need to be used together with synthetic polymers to achieve appropriate mechanical strength. Some natural polymers used to prepare bone defect implants include hyaluronic acid, sodium alginate, cellulose, and chitosan. Some synthetic polymers used to prepare bone defect implants include polycaprolactone (PCL) and poly(lactide-co-glycolide) (PLGA) [16,17,18,19].
The excellent properties of bone defect implants designed with the aforementioned polymers can be further enhanced by adding natural bioactive agents. Flavonoids are a class of natural polyphenolic multifunctional plant derivatives [20]. Several studies have reported that flavonoids exhibit a range of biological activities, such as antioxidant, anti-inflammatory, antibacterial, anticancer, antiviral and antiapoptotic abilities [21,22,23,24,25,26]. Many flavonoids can promote bone formation and have antiosteoporosis effects by stimulating osteogenic differentiation of mesenchymal stem cells (MSCs) [27,28,29]. In addition, European nutrition studies have shown that daily intake of flavonoids contributes to good bone health [30]. Due to their biocompatibility, they have attracted widespread attention from many biomedical researchers and have been used to improve bone health. This article reviews the role and mechanism of flavonoid-loaded biomaterials in bone defect repair.
2. Bone Defects and Healing Process
Bone is the main supporting tissue and plays different roles in the human body, including protecting organs and the nervous system [31]. When suffering from high-energy trauma, bone cancer, osteoporosis, osteomalacia, osteomyelitis, ischemic necrosis and primary tumor resection caused by bone diseases such as atrophic bone non-union, the loss of bone tissue, bone defects will appear in the human body [32]. The repair rate of bone defects depends on different factors (e.g., age, nutrition, infection, the size of the bone defect) [33]. Generally speaking, small bone defects can self-repair and regenerate [34]. On the contrary, when the defect exceeds the critical level, due to issues such as insufficient blood supply and local infection, bone regeneration does not spontaneously occur easily, which can seriously affect the patient’s quality of life. Therefore, additional clinical treatment is needed to promote bone defect healing [6,35].
Bone regeneration relies on an ideal microenvironment. The microenvironment of bone regeneration is very complex. On the one hand, it is the cross action of various cells, including mesenchymal stem cells, immune cells, endothelial cells, osteoblasts and osteoclast, as well as a variety of bioactive factors, which are involved in osteogenesis, angiogenesis and inflammatory regulation [36]. On the other hand, it spans various stages of bone healing (Figure 1), including hematoma, inflammation, fibrous callus formation, intramembrane ossification, endochondral ossification and bone remodeling accompanied by an orderly cascade of anabolism and catabolism [37].
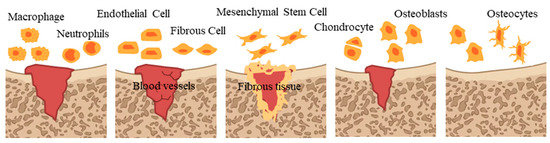
Figure 1.
Bone healing process.
Specifically, at first, blood clots and immune cell migration in the damaged area migrate to remove necrotic components [38]. Next, endothelial cells and fibroblasts gradually infiltrate to form new capillaries, fibrous matrix and granulation tissue [39]. Subsequently, fibroblasts and mesenchymal stem cells proliferate and differentiate into fibrous tissue, which forms soft callus on granulation tissue [40]. Then, bone forms, including intramembrane ossification (IMO) and endochondral ossification (EO). IMO means that mesenchymal stem cells migrate and proliferate to form coagulation, differentiate into osteoblasts and secrete collagen, and blood vessels grow inward to form cortical bone and cancellous bone [41,42], and EO means that mesenchymal stem cells differentiate into chondrocytes and secrete protein matrix, then blood vessels grow inward and osteoblasts invade and replace chondrocytes to form bone tissue [43]. In the final stage, new bone tissue is continuously absorbed and reshaped, forms an orderly bone structure and restores normal function, characterized by replacing mineralized bone and high levels of osteoblast activity, while cartilage tissue develops [44].
3. Classification of Bone Defect Repair Matrices
Bone transplantation is currently the most commonly used method for treating bone defects [45,46]. However, its shortcomings cannot be ignored, for example, the limited amount of donor bone in autologous bone transplantation, and it can easily lead to hematoma, deep infection, inflammation, and uncontrolled absorption rate at the donor site [47,48]. Allogeneic bone grafts have limitations such as immune rejection, disease or virus transmission and require methods such as freeze-drying, radiation and acid washing to avoid immune rejection and eliminate any infections [49,50]. Allogeneic bone transplantation, due to different types of antigens, requires manual handling to avoid possible immune rejection reactions after transplantation [51], and carries the risk of disease, virus transmission and infection [52].
In order to solve the above problems, biomaterials have emerged. According to differences in composition, biomaterials are usually divided into metals, ceramics, natural and synthetic polymers [53]. Compared to natural polymers, synthetic polymers have poor biocompatibility, which may lead to aseptic inflammatory reactions in bone defect sites [15]. Therefore, natural polymers stand out because of their good biocompatibility and biodegradability such as chitosan [54], cellulose [55], hyaluronic acid [56], alginate [56], gelatin [57], etc., which are usually prepared into hydrogels, films and nanomaterials [58,59]. However, their mechanical properties are limited, and they need to be used together with synthetic polymers to achieve appropriate mechanical strength, while their biological activities are still limited.
Bioactive scaffolds are used to transport bioactive molecules such as antibiotics, growth factors and drugs [60,61]. They can continuously supply the required drug concentration at the bone defect site to achieve better treatment effects without obvious secondary adverse reactions. In addition, using biomaterials as drug carriers can protect bioactive molecules from degradation and extend drug circulation and retention time [62,63,64]. Examples of bioactive scaffolds include sponges [65], hydrogels [66], films [67], nanofibers [68], and nanoparticles [69]. These scaffolds are usually designed from natural or synthetic polymers such as cellulose, collagen, sodium alginate, hyaluronic acid and so on [70,71,72,73].
4. The Biological Activity of Flavonoids
Flavonoids are the largest component of phenolic compounds and are abundant in fruit, vegetables, flowers, stems, roots, leaves, bark, grains and certain beverages [74,75,76]. They are divided into different subclasses based on the substitution mode of ring C, the oxidation state of heterocycles and the position of ring B, mainly flavones, flavonols, isoflavones, flavanones, flavanes, anthocyanin, chalcones and isoflavanes [20,77,78]. Their basic chemical structures and representative compounds are shown in Table 1. Flavonoids have various biological activities, such as antioxidant, antitumor, antithrombotic, anti-inflammatory, antiallergic, antiviral, antimicrobial and immune regulation [79,80,81,82,83,84,85]. In addition, flavonoids are famous for their roles in bone synthesis and metabolism and have the ability to effectively regulate bone cell function [86]. According to reports [87], flavonoids can stimulate the expression of osteogenic transcription factors and markers through various signaling pathways, such as Wnt and MAPK pathways, to promote the differentiation of MC3T3-E1 osteoblasts and MSCs into osteoblasts.

Table 1.
Structure and representative compounds of flavonoid subclasses.
The expected bone healing effect of flavonoids may be due to their anti-inflammatory, antioxidant and antibacterial activities [88]. First, it is reported that flavonoids are significant inhibitors of inflammatory mediators (COX or LOX), which can inhibit neutrophil degranulation and prevent bone absorption through their anti-inflammatory properties on osteoclast precursor somatic cell cells [89]. Secondly, flavonoids can participate in the activation of enzymes through various signaling pathways and gene expression to eliminate reactive oxygen species (ROS) or free radicals [74]. ROS have negative effects on osteoblasts in a variety of ways, such as osteoblast apoptosis and activation of osteoclast differentiation by upregulating receptor activator of nuclear factor-κB ligand (RANKL) [90,91]. The antioxidant activity of flavonoids is roughly related to their hydrogen supply capacity [92]. In addition, antibacterial performance is another key role of flavonoids, which is crucial for their application in bone defect healing [93].
5. Application of Flavonoid-Loaded Biomaterials in Bone Defect Repair
Bone defect healing usually requires a longer repair time. The biomaterial loaded with flavonoids not only has the synergistic effect of biomaterials and flavonoids but also can slowly release flavonoids at the bone defect site to prolong drug efficacy. However, there have been few reports on improving the bioavailability of flavonoids through nanoscale composites. The application of polymer biomaterials loaded with flavonoids in bone defect healing is detailed in Table 2.

Table 2.
Application of polymer biomaterials loaded with flavonoids in bone defect healing.
5.1. Hydrogel
Hydrogel is a hydrophilic three-dimensional polymer that can simulate extracellular matrix (ECM) and has good biocompatibility and biodegradability [126]. However, it also has some drawbacks, such as poor mechanical properties in the swelling state, limited biological activity, etc. Its poor mechanical properties are usually overcome by the combination of synthetic polymers and natural polymers. Chenrui Li et al. prepared a composite material for repairing rat skull defects by combining methacrylic acid chondroitin sulfate and gelatin and incorporated baicalin nanocomposites to enhance the biological activity of the composite system. The experimental results showed that the synthesized composite hydrogel had appropriate mechanical properties. Baicalin nanocomposites can significantly regulate the level of sclerotin and enhance osteogenic and angiogenic activities to play a role in bone repair. These effects are achieved by significantly increasing the expression of OPG, OCN, a-SMA and CD31 and inhibiting the levels of sclerotin and RANKL [94]. Therefore, it is necessary to expand the study of flavonoid-loaded hydrogels to provide a study basis for the development of available medical materials.
5.2. Fibrous Membrane
Electrospinning is a simple method for preparing nano- or submicron fiber membranes, and has been widely used in drug delivery [127]. The fiber membrane has a high specific surface area that can promote cell adhesion and continuously and controllably deliver drugs at local points [128]. However, some polymers have encountered some obstacles in electrospinning, such as low mechanical strength, low biocompatibility and low biological activity. Some researchers have reported on polymer fiber membranes loaded with flavonoids. Jung Seung Lee et al. used catechin surface modification of PCL nanofiber membranes to enhance their biological function and prepared a multifunctional matrix for repairing severe skull defects in mice. The research results showed that the deposition of catechin hydrates greatly improved the hydrophilicity and biocompatibility of the matrix. At the same time, the presence of catechin coatings enhanced the antioxidant and calcium binding abilities of the membrane to promote stem cell adhesion, proliferation and osteogenic differentiation, and significantly promoted bone formation in critical size skull defects in vivo [95]. Lihua Yin et al. incorporated icariin as a bone-inducing factor into SF/PLCL nanofiber membranes through electrospinning to enhance the biological activity of the membrane. The study showed that icariin was continuously and controllably released in the nanofiber membrane to effectively increase the expression of ALP and promote bone regeneration in rat skull defects [96]. In addition, Hongbin Zhao et al. prepared a novel core-shell fiber membrane loaded with icariin chitosan microspheres using collagen, polycaprolactone and hydroxyapatite as raw materials for the repair of rabbit tibial defects by electrospinning. The research results showed that the prepared membrane had good mechanical properties and biocompatibility as well as good bone induction and conductivity, and it effectively promoted a large quantity of new bone formation in vivo. These positive effects are achieved by regulating the expression of ALP, COL-1, OC, and OPN [97]. Therefore, these studies indicate that flavonoid-loaded fiber membranes can be successfully used as an effective treatment choice for bone defect repair.
5.3. Sponges
Sponge is a three-dimensional structural network that allows cell attachment, migration and proliferation with excellent biocompatibility, porous structure and biodegradability. Its clinical application has good feasibility [129]. However, most biological materials from multiple sources have microenvironments different from bone tissue, which limits their application in bone regeneration. Some researchers have reported on polymer sponges loaded with flavonoids. Mei Li et al. improved bone induction in the submucosa of the small intestine by incorporating icariin into sponge. Studies showed that icariin can be continuously released in sponges for 30 days. Due to the presence of icariin, the sponge significantly promoted the regulation of osteogenic differentiation markers (ALP, BSP, OCN), improved angiogenic factor (CD31) levels, and resulted in a higher rate of new bone formation in a mouse skull defect model [98]. DetingXue et al. found that hesperidin can promote osteogenic differentiation of human mesenchymal stem cells by regulating the ERK1/2 and Smad1/5/8 signaling pathways. Therefore, it was combined with gelatin sponge to accelerate the healing of tibial fractures in rats [99]. Research has shown that hesperidin can significantly increase the levels of osteogenic factors (ALP, OCN, Runx-2, COL-1) and promote bone regeneration in vivo. In addition, Jeong Eun Song et al. incorporated quercetin into collagen/hydroxyapatite sponge to enhance bone metabolism and osteogenic differentiation of the scaffold. The results showed that the prepared sponge had good compressive strength and high porosity and could significantly increase the expression of COL-1, OCN, and Runx-2, promoting the repair of rat skull defects [100]. R.W.K. Wong et al. used collagen sponge loaded with naringin, quercetin, or puerarin to treat full-thickness parietal bone defects in rabbits. These scaffolds can promote angiogenesis and increase ALP activity to achieve early bone reconstruction and bone formation [101,102,103]. It can be seen that flavonoid-loaded sponges have good application prospects in bone defect repair.
5.4. Microspheres/Nanoparticles
Due to their excellent specific surface area, microspheres/nanoparticles can improve cell adhesion and proliferation [130] and can be used as carriers for drug delivery systems [131]. However, this method often comes with issues such as low encapsulation efficiency. Zuoying Yuan et al. loaded icariin onto MgO/MgCO3 particles and encapsulated them in PLGA microspheres, where Mg2+ and icariin were continuously released. Due to the addition of icariin, microspheres significantly regulated the levels of ALP, Col-1, RunX-2, OPN and OCN and promoted the repair of rat skull defects [104]. Xue Yang et al. used PCL/PEG-b-PCL microspheres to reduce the sudden release of naringin and promote the repair of rat skull defects. Studies showed that the prepared microspheres can increase the expression levels of Runx-2 and OCN to promote the formation of new bones in vivo [105]. In addition, Yuning Zhou et al. prepared hydroxyapatite bioceramic microspheres loaded with quercetin, which proved its ability to induce osteogenesis and angiogenesis in vivo in a severe-size femoral defect model of rats. The results showed that the prepared microspheres could continuously and effectively release quercetin to significantly improve ALP activity and the levels of osteogenic genes (Runx-2, COL-1, BSP, BMP-2, OPN, OCN, and OPG), activate ERK, p38 and AKT signaling pathways, upregulate the expression of VEGF, ANG-1, TGF-β and bFGF, and downregulate the expression of an osteoclast gene (RANKL) to promote the repair of rat femoral defect [106]. Yuqiong Wu et al. prepared new micro/nano hybrid HAp particles, constructed a sustained-release system for icariin and verified its role in promoting bone defect repair in a rat femoral defect model. The results suggested that icariin can obviously increase the expression of osteogenic genes (Runx-2, ALP, Col-1 and OCN) and angiogenic genes (VEGF and ANG-1) and regulate the AKT signaling pathway to enhance angiogenesis and bone formation in vivo [107]. It can be seen that the addition of flavonoids can effectively enhance the bone repair activity of the microsphere/nanoparticle system, and the nanoparticle/microsphere system also improves the bioavailability of flavonoids to provide a new idea for the application of bone defect repair (Figure 2).
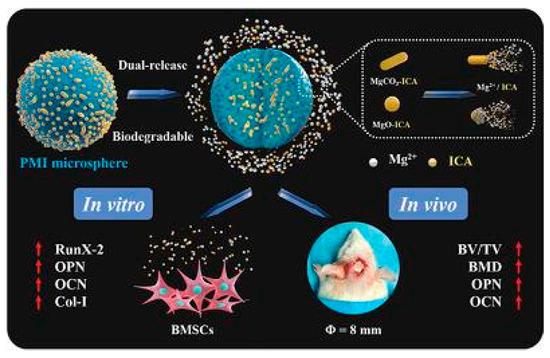
Figure 2.
Repair effect of icariin-loaded microsphere on bone defects [105]. (These figures were reprinted with permission.)
5.5. Bone Cement/Bioglass
Bone cement is an injectable biomaterial with good bone conductivity [132]. Bioglass has excellent specific surface area and its application in bone tissue engineering is rapidly expanding [133]. Bone cement/bioactive glass composed of calcium salts can stimulate new bone formation. However, their biological activities, such as promoting angiogenesis and anti-inflammation, are limited. Some researchers reported on bone cement/bioglass loaded with flavonoids. Jiyuan Zhao et al. promoted the repair of mouse skull defects by loading icariin with calcium phosphate bone cement. This stent can effectively increase the levels of ALP, Runx-2, OC, BSP and promote angiogenesis [108]. Yuqiong Wu et al. constructed icariin-loaded calcium phosphate cement for repairing skull defects in ovariectomized rats. On the one hand, the scaffold improved the level of ALP and promoted osteoblast differentiation; on the other hand, the scaffold upregulated OPG expression and inhibited RANKL expression and the formation of osteoclasts. In addition, the scaffold promoted the expression of angiogenic factors such as VEGF and ANG-1 to promote angiogenesis [109]. In addition, Jianguo Huang et al. prepared a dual drug release system consisting of icariin, vancomycin and injectable calcium phosphate cement for repairing radius defects contaminated by Staphylococcus aureus. The research results showed that the prepared system can release icariin and vancomycin for a long time to endow the system with excellent anti-inflammatory and osteogenic activities and has great potential in the treatment of contaminated bone injury or infectious bone diseases [110]. The icariin-doped bioglass prepared by Xingzhi Jing et al. can significantly increase the expression of osteogenesis-related proteins (COL-1, OPN) and angiogenic factors (CD31, VEGF) and significantly induce new bone formation and new angiogenesis in a rat skull cap bone defect model [111]. These studies indicate that flavonoid-loaded bone cement/bioglass exhibits enormous potential in bone defect repair.
5.6. Scaffolds
In addition to the abovementioned types of scaffolds, some researchers have also reported on many composite scaffolds loaded with flavonoids that have good bone conductivity and biocompatibility.
Tao Wu, Yunlong Xie, and Yunjia Song et al. combined icariin into hydroxyapatite composite scaffolds for bone defect repair. These prepared scaffolds have good biocompatibility and can slowly release icariin for a long time. They have good bone conduction and osteoinduction effects on bone defect models and can fill the bone defect site early to stimulate new bone formation. These positive effects may be attributed to the ability of icariin to upregulate the levels of osteogenic markers Runx-2, ALP and OCN and activate the Wnt signaling pathway [112,113,114]. Xiaowei Xie, Tianlin Lliu and Yuxiao Lai et al. combined icariin into various composite scaffolds and conducted therapeutic studies on bone defect models. These prepared scaffolds can promote bone repair through active angiogenesis, which can be attributed to the regulatory effect of icariin on VEGF. At the same time, these scaffolds can significantly increase the expression of osteogenesis-related proteins such as Bsp, Runx-2, ALP, OCN, OPN, etc. to promote the bone healing process and ultimately stimulate bone defect repair [115,116,117].
Kuoyu Chen, Zhenzhao Guo and Yanping Zuo et al. combined naringin with composite materials to evaluate and compare their potential for repairing bone defects in vivo. Their research results all showed that the addition of naringin enhanced the osteogenic ability of composite scaffolds, with the potential mechanism being to reduce the levels of inflammatory factors such as IL-6 and enhance the expression of osteogenesis-related factors BMP-2, OPN, OCN, Runx-2 and ALP to promote the proliferation of osteoblasts and accelerate bone tissue reconstruction and repair in bone defect models [118,119,120].
The epigallocatechin-3 gallate-loaded β-tricalcium phosphate (β-TCP) scaffold prepared by Reena Rodriguez et al. has excellent anti-inflammatory and antioxidant activities and can effectively promote the repair of critical skull defects in rats [121]. Zhihu Zhao et al. prepared a silk fibroin–hydroxyapatite composite scaffold loaded with naringenin, which can significantly increase the expression of Runx-2, COL-1 and OSX by activating PI3K/AKT, VEGF, and HIF-1 signaling pathways to enhance the osteogenesis and angiogenesis and repair distal femoral defects in rabbits [122]. Shuhei Tsuchiya et al. prepared titanium dioxide implants loaded with kaempferol, which can increase the expression of Runx-2, OCN, ON, OPN, COL-1 and ALP to promote the repair of femoral defects in rats [123]. Henri Granel et al. developed a bioactive glass polycaprolactone mixed scaffold loaded with fisterone, which achieved slow release of fisterone and significantly increased the expression of ALP, Runx-2, and COL-1 to promote the repair of critical skull defects in mice [124]. Jeong Eun Song et al. designed a silk fibroin–hydroxyapatite scaffold loaded with quercetin that exhibited good mechanical strength. The addition of quercetin significantly increased the expression of col1, OCN and Runx-2 to promote the repair of rat skull defects. However, this effect occurred only in low-quercetin-content scaffolds rather than high-quercetin-content scaffolds [125]. It can be seen that flavonoids have various biological activities, such as anti-inflammatory, antioxidant and promoting angiogenesis, that are beneficial for bone defect repair. More types of flavonoids are worth developing more widely to promote the application process of polymer materials in bone repair.
The above studies have shown that polymer biomaterials loaded with flavonoids have a good promoting effect on bone defect healing. This effect is mainly achieved by regulating various signaling pathways, such as Wnt, p38, AKT and Erk signaling pathways, which can effectively promote the expression of angiogenic factors such as VEGF and CD31 and significantly increase the levels of osteogenesis-related factors such as Runx-2, OCN and OPN. Therefore, polymer biomaterials loaded with flavonoids can be widely used to promote bone defect healing.
6. Conclusions
This article reviews the progress of research on flavonoid polymer biomaterials and their mechanisms for promoting bone defect repair. When using flavonoid-loaded scaffolds, the rate of bone repair can be accelerated, and their role involves multiple impact mechanisms.
Although flavonoid-loaded biomaterials can promote bone defect repair, there are still some issues that need to be addressed. Firstly, most of the studies described are in the preclinical stage and their results are very promising. However, these biomaterials require clinical trials. In addition, the bioavailability and solubility of flavonoids are limiting factors in utilizing their characteristics. They can be increased in solubility through microspheres, nanoparticles, self-emulsifying drug delivery systems, liposome vesicles, solid dispersions, inclusion complexes and micelles to improve bioavailability. In fact, only a small number of nanoparticles and microspheres are used to load flavonoids for bone defect repair. At the same time, there are various types of flavonoid-loaded bone repair biomaterials, but the mutual binding of each biomaterial is still rare. For example, microspheres and nanoparticles are widely used to improve the low solubility of flavonoids, and hydrogels and nanofibers are widely used to continuously transport flavonoids. However, there are few reports on combining them as composite carriers to load flavonoids to promote bone repair. Nanogel has the excellent characteristics of both nanoparticles and hydrogels, and it has been widely studied in the field of bone repair, but no report of nanogel combined with flavonoids for bone defect repair has yet appeared. Finally, there are a wide variety of flavonoids. At present, the development and application of flavonoids loaded in bone repair biomaterials are limited to a few types, including flavones, flavonols, isoflavones, and flavanes. However, anthocyanin, chalcones and isoflavanes have not yet been applied. Therefore, the application of flavonoids in bone repair scaffolds needs to be expanded.
Based on the current study, we should improve the overall performance of flavonoid bone repair biomaterials. A flavonoid bone repair biomaterial that meets clinical requirements needs to be prepared by combining multiple research fields such as molecular biology and pharmacology. At the same time, innovation in biomaterials not only needs to improve their promoting effects on bone repair but also needs to enhance the penetration of flavonoids into biofilms and enhance cell phagocytosis of flavonoids.
Author Contributions
Conceptualization and writing—original draft preparation, J.Y. and L.Z.; visualization, S.Z., Q.D. and S.S.; supervision and writing—original draft preparation, J.L. and X.H.; supervision and funding acquisition, W.L. and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jilin Province Science and Technology Development Plan Project, grant 20220508062RC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings are available from the corresponding authors upon reasonable request.
Acknowledgments
The author would like to thank those who have contributed to this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized Connective Tissue: Bone, the Structural Framework of the Upper Extremity. J. Hand Ther. 2012, 25, 123–132. [Google Scholar] [CrossRef]
- Erslev, A. Humoral Regulation of Red Cell Production. Blood 1953, 8, 349–357. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. 2007, 89, 574–580. [Google Scholar] [CrossRef]
- Koolen, M.; Amin Yavari, S.; Lietaert, K.; Wauthle, R.; Zadpoor, A.A.; Weinans, H. Bone Regeneration in Critical-Sized Bone Defects Treated with Additively Manufactured Porous Metallic Biomaterials: The Effects of Inelastic Mechanical Properties. Materials 2020, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Cho, Y.-C.; Liu, C.-M.; Huang, M.-S.; Lan, W.-C.; Huang, B.-H.; Ueno, T.; Tsai, C.-H.; Saito, T.; Chen, M.-S.; et al. An Innovative Bioceramic Bone Graft Substitute for Bone Defect Treatment: In Vivo Evaluation of Bone Healing. Appl. Sci. 2020, 10, 8303. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Jimbo, R.; Gangolli, R.; Perez, L.; Manne, L.; Yoo, D.; Lorenzoni, F.; Witek, L.; Coelho, P.G. Evaluation of bone response to various anorganic bovine bone xenografts: An experimental calvaria defect study. Int. J. Oral Maxillofac. Surg. 2014, 43, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Božić, D.; Ćatović, I.; Badovinac, A.; Musić, L.; Par, M.; Sculean, A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials 2021, 14, 6795. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xia, C.; Mo, J.; Mei, S.; Lin, X.; Fan, S. Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater. Sci. Eng. C 2018, 91, 190–200. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C.; et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 899760. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef]
- Yang, J.-M.; Hung, C.-M.; Fu, C.-N.; Lee, J.-C.; Huang, C.-H.; Yang, M.-H.; Lin, C.-L.; Kao, J.-Y.; Way, T.-D. Hispidulin Sensitizes Human Ovarian Cancer Cells to TRAIL-Induced Apoptosis by AMPK Activation Leading to Mcl-1 Block in Translation. J. Agric. Food Chem. 2010, 58, 10020–10026. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 2008, 31, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Cao, B.; Wang, J.; Wei, H.; Liu, X.; Han, Y.; Yang, S.; Wang, X. Icariin Protects against Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Rats. Cell. Physiol. Biochem. 2018, 47, 694–706. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Rodríguez-Ramos, Á.; Torrecillas-Baena, B.; Dorado, G.; Quesada-Gómez, J.M.; Gálvez-Moreno, M.Á. Flavonoid Phloretin Inhibits Adipogenesis and Increases OPG Expression in Adipocytes Derived from Human Bone-Marrow Mesenchymal Stromal-Cells. Nutrients 2021, 13, 4185. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Miller, G.D.; Groziak, S.M.; DiRienzo, D. Age considerations in nutrient needs for bone health. J. Am. Coll. Nutr. 1996, 15, 553–555. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, H.; Zhao, Y.; Luo, J.; Yang, L.; Liu, W.; He, Q. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: A review and new perspectives. Mater. Sci. Eng. C 2019, 98, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects Carbohydrate. Polymers 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Zimmerman, G.; Marcucio, R. Biological perspectives of delayed fracture healing. Injury 2014, 45, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef]
- Geris, L.; Gerisch, A.; Sloten, J.V.; Weiner, R.; Oosterwyck, H.V. Angiogenesis in bone fracture healing: A bioregulatory model. J. Theor. Biol. 2008, 251, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis: Angiogenesis and Intramembranous Osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36, S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Arasu, M.V.; Arokiyaraj, S.; Viayaraghavan, P.; Kumar TS, J.; Duraipandiyan, V.; Al-Dhabi, N.A.; Kaviyarasu, K. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B Biol. 2019, 190, 154–162. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Hench, L.L. Biomaterials. SCIENCE 1980, 208, 826–831. [Google Scholar] [CrossRef]
- Castanheira, E.J.; Correia, T.R.; Rodrigues JM, M.; Mano, J.F. Novel Biodegradable Laminarin Microparticles for Biomedical Applications. BCSJ 2020, 93, 713–719. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Damiri, F.; Gowda BH, J.; Andra, S.; Balu, S.; Rojekar, S.; Berrada, M. Chitosan Nanocomposites as Scaffolds for Bone Tissue Regeneration. In Chitosan Nanocomposites Biological and Medical Physics, Biomedical Engineering; Swain, S.K., Biswal, A., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 377–394. [Google Scholar]
- Dabbarh, F.; Elbakali-Kassimi, N.; Berrada, M. Chitosan Based Biocomposites for Hard Tissue Engineering. In Chitin and Chitosan—Physicochemical Properties and Industrial Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Zhang, J.; Lei, X.; Tang, J.; Chen, J.; Zhao, Q.; Fang, W.; Zhang, Y.; Li, Y.; Zuo, Y. Effect of Antibacterial Enoxacin on the Properties of Injectable Nano-hydroxyapatite/Polyurethane Cement for Bone Repairing. J. Bionic Eng. 2022, 19, 483–496. [Google Scholar] [CrossRef]
- Lu, S.; Lam, J.; Trachtenberg, J.E.; Lee, E.J.; Seyednejad, H.; Van Den Beucken JJ, J.P.; Tabata, Y.; Wong, M.E.; Jansen, J.A.; Mikos, A.G.; et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 2014, 35, 8829–8839. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Bi, L.; Li, J.; Fan, J. Salvianolic Acid B-Loaded Chitosan/hydroxyapatite Scaffolds Promotes the Repair of Segmental Bone Defect by Angiogenesis and Osteogenesis. IJN 2019, 14, 8271–8284. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Fahmy, H.M.; El-Bisi, M.K.; Ahmed-Farid, O.A. Hyaluronic acid/Na-alginate films as topical bioactive wound dressings. Eur. Polym. J. 2018, 109, 101–109. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Nowaczyk, G.; Jarek, M.; Järvekülg, M.; Kangur, T.; Janiszewska, E.; Jurga, S.; Iatsunskyi, I. Fabrication of Gelatin-ZnO Nanofibers for Antibacterial Applications. Materials 2020, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Ishii, Y. Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. J. Orthop. Sci. 2001, 6, 556–565. [Google Scholar] [CrossRef]
- Woo, B.H.; Fink, B.F.; Page, R.; Schrier, J.A.; Jo, Y.W.; Jiang, G.; DeLuca, M.; Vasconez, H.C.; DeLuca, P.P. Enhancement of Bone Growth by Sustained Delivery of Recombinant Human Bone Morphogenetic Protein-2 in a Polymeric Matrix. Pharm. Res. 2001, 18, 1747–1753. [Google Scholar] [CrossRef]
- Sánchez, E.; Baro, M.; Soriano, I.; Perera, A.; Évora, C. In vivo–in vitro study of biodegradable and osteointegrable gentamicin bone implants. Eur. J. Pharm. Biopharm. 2001, 52, 151–158. [Google Scholar] [CrossRef]
- Cai, B.; Zhong, T.; Chen, P.; Fu, J.; Jin, Y.; Liu, Y.; Huang, R.; Tan, L. Preparation, characterization and in vitro release study of drug-loaded sodium carboxy-methylcellulose/chitosan composite sponge ed Y K Mishra. PLoS ONE 2018, 13, e0206275. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi AO, M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef]
- Pal, P.; Nguyen, Q.C.; Benton, A.H.; Marquart, M.E.; Janorkar, A.V. Drug-Loaded Elastin-Like Polypeptide–Collagen Hydrogels with High Modulus for Bone Tissue Engineering. Macromol. Biosci. 2019, 19, 1900142. [Google Scholar] [CrossRef]
- Qi, J.; Zheng, Z.; Hu, L.; Wang, H.; Tang, B.; Lin, L. Development and characterization of cannabidiol-loaded alginate copper hydrogel for repairing open bone defects in vitro. Colloids Surf. B Biointerfaces 2022, 212, 112339. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Locke, R.C.; Witherel, C.E.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, J.A. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 2022, 14, 014106. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Lin, W.; Kitsberg, D.; Balaguer, P.; Polyak, S.J.; Chung, R.T.; Yarmush, M.L.; Nahmias, Y. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J. Hepatol. 2011, 55, 963–971. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- Lavrador, P.; Gaspar, V.M.; Mano, J.F. Bioinspired bone therapies using naringin: Applications and advances. Drug Discov. Today 2018, 23, 1293–1304. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and osteogenic effects of flavonoids in bone regeneration. Biotech. Bioeng. 2022, 119, 2313–2330. [Google Scholar] [CrossRef]
- Sadat Hosseini, M.; Kamali, B.; Nabid, M.R. Multilayered mucoadhesive hydrogel films based on Ocimum basilicum seed mucilage/thiolated alginate/dopamine-modified hyaluronic acid and PDA coating for sublingual administration of nystatin. Int. J. Biol. Macromol. 2022, 203, 93–104. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Matsumoto, C.; Inoue, H.; Tominari, T.; Watanabe, K.; Hirata, M.; Miyaura, C.; Inada, M. Heptamethoxyflavone, a citrus flavonoid, suppresses inflammatory osteoclastogenesis and alveolar bone resorption. Biosci. Biotechnol. Biochem. 2015, 79, 155–158. [Google Scholar] [CrossRef]
- Tao, H.; Ge, G.; Liang, X.; Zhang, W.; Sun, H.; Li, M.; Geng, D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim. Biophys. Sin. 2020, 52, 1055–1062. [Google Scholar] [CrossRef]
- Thummuri, D.; Jeengar, M.K.; Shrivastava, S.; Nemani, H.; Ramavat, R.N.; Chaudhari, P.; Naidu VG, M. Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-KB and MAPK. Signal. Pharmacol. Res. 2015, 99, 63–73. [Google Scholar] [CrossRef]
- Greeff, J.; Joubert, J.; Malan, S.F.; Van Dyk, S. Antioxidant properties of 4-quinolones and structurally related flavones. Bioorganic Med. Chem. 2012, 20, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Niu, Y.; Zhang, H.; Ouyang, H.; Zhang, G.; Fu, Y. Baicalin Nanocomplexes with an In Situ -Forming Biomimetic Gel Implant for Repair of Calvarial Bone Defects via Localized Sclerostin Inhibition. ACS Appl. Mater. Interfaces 2023, 15, 9044–9057. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.-W. Plant Flavonoid-Mediated Multifunctional Surface Modification Chemistry: Catechin Coating for Enhanced Osteogenesis of Human Stem Cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- Yin, L.; Wang, K.; Lv, X.; Sun, R.; Yang, S.; Yang, Y.; Liu, Y.; Liu, J.; Zhou, J.; Yu, Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 8616. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, J.; Zhou, D.; Weng, Y.; Qin, W.; Liu, C.; Lv, S.; Wang, W.; Zhao, X. Electrospun Icariin-Loaded Core-Shell Collagen, Polycaprolactone, Hydroxyapatite Composite Scaffolds for the Repair of Rabbit Tibia Bone Defects. IJN 2020, 15, 3039–3056. [Google Scholar] [CrossRef]
- Li, M.; Gu, Q.; Chen, M.; Zhang, C.; Chen, S.; Zhao, J. Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater. Sci. Eng. C 2017, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, E.; Zhang, W.; Gao, X.; Wang, S.; Zheng, Q.; Pan, Z.; Li, H.; Liu, L. The role of hesperetin on osteogenesis of human mesenchymal stem cells and its function in bone regeneration. Oncotarget 2017, 8, 21031–21043. [Google Scholar] [CrossRef]
- Song, J.E.; Tian, J.; Kook, Y.J.; Thangavelu, M.; Choi, J.H.; Khang, G. A BMSCs-laden quercetin/duck’s feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J. Biomed. Mater. Res. 2020, 108, 784–794. [Google Scholar] [CrossRef]
- Wong, R.W.K.; Rabie, A.B.M. Effect of naringin collagen graft on bone formation. Biomaterials 2006, 27, 1824–1831. [Google Scholar] [CrossRef]
- Wong, R.W.K.; Rabie, A.B.M. Effect of Quercetin on Preosteoblasts and Bone Defects. Open Orthop. J. 2008, 2, 27–32. [Google Scholar] [CrossRef]
- Wong, R.; Rabie, B. Effect of puerarin on bone formation. Osteoarthr. Cartil. 2007, 15, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wan, Z.; Wei, P.; Lu, X.; Mao, J.; Cai, Q.; Zhang, X.; Yang, X. Dual-Controlled Release of Icariin/Mg2+ from Biodegradable Microspheres and Their Synergistic Upregulation Effect on Bone Regeneration. Adv. Healthcare Mater. 2020, 9, 2000211. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Almassri HN, S.; Zhang, Q.; Ma, Y.; Zhang, D.; Chen, M.; Wu, X. Electrosprayed naringin-loaded microsphere/SAIB hybrid depots enhance bone formation in a mouse calvarial defect model. Drug Deliv. 2019, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Y.; Ma, W.; Jiang, X.; Takemra, A.; Uemura, M.; Xia, L.; Lin, K.; Xu, Y. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B 2017, 5, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, L.; Zhou, Y.; Ma, W.; Zhang, N.; Chang, J.; Lin, K.; Xu, Y.; Jiang, X. Evaluation of osteogenesis and angiogenesis of icariin loaded on micro/nano hybrid structured hydroxyapatite granules as a local drug delivery system for femoral defect repair. J. Mater. Chem. B 2015, 3, 4871–4883. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.; Nagamune, T. Icariin: A Potential Osteoinductive Compound for Bone Tissue Engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 5077. [Google Scholar] [CrossRef]
- Huang, J.-G.; Pang, L.; Chen, Z.-R.; Tan, X.-P. Dual-delivery of vancomycin and icariin from an injectable calcium phosphate cement-release system for controlling infection and improving bone healing. Mol. Med. Rep. 2013, 8, 1221–1227. [Google Scholar] [CrossRef][Green Version]
- Jing, X.; Yin, W.; Tian, H.; Chen, M.; Yao, X.; Zhu, W.; Guo, F.; Ye, Y. Icariin doped bioactive glasses seeded with rat adipose-derived stem cells to promote bone repair via enhanced osteogenic and angiogenic activities. Life Sci. 2018, 202, 52–60. [Google Scholar] [CrossRef]
- Wu, T.; Nan, K.; Chen, J.; Jin, D.; Jiang, S.; Zhao, P.; Xu, J.; Du, H.; Zhang, X.; Li, J.; et al. A new bone repair scaffold combined with chitosan/hydroxyapatite and sustained releasing icariin. Chin. Sci. Bull. 2009, 54, 2953–2961. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, W.; Yan, F.; Liu, H.; Deng, Z.; Cai, L. Icariin-loaded porous scaffolds for bone regeneration through the regulation of the coupling process of osteogenesis and osteoclastic activity. IJN 2019, 14, 6019–6033. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. IJN 2018, 13, 6751–6767. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pei, F.; Wang, H.; Tan, Z.; Yang, Z.; Kang, P. Icariin: A promising osteoinductive compound for repairing bone defect and osteonecrosis. J. Biomater. Appl. 2015, 30, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Luo, Y.; Huang, Y.; Wu, G. Slowly Delivered Icariin/Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells to Promote the Healing of Calvarial Critical-Size Bone Defects. Stem Cells Int. 2016, 2016, 1416047. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Lin, K.-C.; Chen, Y.-S.; Yao, C.-H. A Novel Porous Gelatin Composite Containing Naringin for Bone Repair. Evid. Based Complement. Altern. Med. 2013, 2013, 283941. [Google Scholar] [CrossRef]
- Guo, Z.; Bo, D.; He, P.; Li, H.; Wu, G.; Li, Z.; Zhou, C.; Li, Q. Sequential controlled-released dual-drug loaded scaffold for guided bone regeneration in a rat fenestration defect model. J. Mater. Chem. B 2017, 5, 7701–7710. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Q.; Xiong, Q.; Li, J.; Tang, C.; Zhang, Y.; Wang, D. Naringin Release from a Nano-Hydroxyapatite/Collagen Scaffold Promotes Osteogenesis and Bone Tissue Reconstruction. Polymers 2022, 14, 3260. [Google Scholar] [CrossRef]
- Rodriguez, R.; Kondo, H.; Nyan, M.; Hao, J.; Miyahara, T.; Ohya, K.; Kasugai, S. Implantation of green tea catechin α-tricalcium phosphate combination enhances bone repair in rat skull defects. J. Biomed. Mater. Res. 2011, 98, 263–271. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Zhao, B.; Tian, P.; Ma, J.; Kang, J.; Zhang, Y.; Guo, Y.; Sun, L. Naringin-inlaid silk fibroin/hydroxyapatite scaffold enhances human umbilical cord-derived mesenchymal stem cell-based bone regeneration. Cell Prolif. 2021, 54, e13043. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Sugimoto, K.; Kamio, H.; Okabe, K.; Kuroda, K.; Okido, M.; Hibi, H. Kaempferol-immobilized titanium dioxide promotes formation of new bone: Effects of loading methods on bone marrow stromal cell differentiation in vivo and in vitro. IJN 2018, 13, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Granel, H.; Bossard, C.; Collignon, A.-M.; Wauquier, F.; Lesieur, J.; Rochefort, G.Y.; Jallot, E.; Lao, J.; Wittrant, Y. Osteogenic Effect of Fisetin Doping in Bioactive Glass/Poly(caprolactone) Hybrid Scaffolds. ACS Omega 2022, 7, 22279–22290. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Tripathy, N.; Lee, D.H.; Park, J.H.; Khang, G. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 32955–32964. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Vysloužilová, L.; Buzgo, M.; Pokorný, P.; Chvojka, J.; Míčková, A.; Rampichová, M.; Kula, J.; Pejchar, K.; Bílek, M.; Lukáš, D.; et al. Needleless coaxial electrospinning: A novel approach to mass production of coaxial nanofibers. Int. J. Pharm. 2017, 516, 293–300. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Wang, Z.; Zhang, H.; Yuan, X.; Kong, D. Controlled Release of PDGF-bb by Coaxial Electrospun Dextran/Poly(l-lactide-co-ε-caprolactone) Fibers with an Ultrafine Core/Shell Structure. J. Biomater. Sci. Polym. Ed. 2010, 21, 803–819. [Google Scholar] [CrossRef]
- Imani, S.M.; Rabiee, S.M.; Goudarzi, A.M.; Dardel, M. A novel modification for polymer sponge method to fabricate the highly porous composite bone scaffolds with large aspect ratio suitable for repairing critical-sized bone defects. Vacuum 2020, 176, 109316. [Google Scholar] [CrossRef]
- Pignatello, R.; Pecora TM, G.; Cutuli, G.G.; Catalfo, A.; De Guidi, G.; Ruozi, B.; Tosi, G.; Cianciolo, S.; Musumeci, T. Antioxidant activity and photostability assessment of trans-resveratrol acrylate microspheres. Pharm. Dev. Technol. 2019, 24, 222–234. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, J.; Zheng, L.; Ren, N.; Li, J.; Liu, H.; Miao, J. Sustained delivery of BMP-2 enhanced osteoblastic differentiation of BMSCs based on surface hydroxyapatite nanostructure in chitosan–HAp scaffold. J. Biomater. Sci. Polym. Ed. 2014, 25, 1813–1827. [Google Scholar] [CrossRef]
- Urabe, K.; Naruse, K.; Hattori, H.; Hirano, M.; Uchida, K.; Onuma, K.; Park, H.J.; Itoman, M. In vitro comparison of elution characteristics of vancomycin from calcium phosphate cement and polymethylmethacrylate. J. Orthop. Sci. 2009, 14, 784–793. [Google Scholar] [CrossRef]
- El-Gendy, R.; Kirkham, J.; Newby, P.J.; Mohanram, Y.; Boccaccini, A.R.; Yang, X.B. Investigating the Vascularization of Tissue-Engineered Bone Constructs Using Dental Pulp Cells and 45S5 Bioglass ® Scaffolds. Tissue Eng. Part A 2015, 21, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).