Abstract
Astragalus membranaceus (A. membranaceus), a well-known traditional herbal medicine, has been widely used in ailments for more than 2000 years. The main bioactive compounds including flavonoids, triterpene saponins and polysaccharides obtained from A. membranaceus have shown a wide range of biological activities and pharmacological effects. These bioactive compounds have a significant role in protecting the liver, immunomodulation, anticancer, antidiabetic, antiviral, antiinflammatory, antioxidant and anti-cardiovascular activities. The flavonoids are initially synthesized through the phenylpropanoid pathway, followed by catalysis with corresponding enzymes, while the triterpenoid saponins, especially astragalosides, are synthesized through the universal upstream pathways of mevalonate (MVA) and methylerythritol phosphate (MEP), and the downstream pathway of triterpenoid skeleton formation and modification. Moreover, the Astragalus polysaccharide (APS) possesses multiple pharmacological activities. In this review, we comprehensively discussed the biosynthesis pathway of flavonoids and triterpenoid saponins, and the structural features of polysaccharides in A. membranaceus. We further systematically summarized the pharmacological effects of bioactive ingredients in A. membranaceus, which laid the foundation for the development of clinical candidate agents. Finally, we proposed potential strategies of heterologous biosynthesis to improve the industrialized production and sustainable supply of natural products with pharmacological activities from A. membranaceus, thereby providing an important guide for their future development trend.
1. Introduction
Astragalus membranaceus (A. membranaceus) mainly consists of Astragalus membranaceus (Fisch.) Bge. and Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao is a perennial herbaceous plant of the legume family [1]. The dried root of A. membranaceus, known as Radix Astragali or “Huangqi” in Chinese, has been one of the most commonly used traditional herbal medicines for more than 2000 years in China and other Asian countries, with hepatoprotective, tonic and expectorant properties and as a diuretic [2,3]. The medicinal efficacy of A. membranaceus was firstly documented in the Divine Farmer’s Materia Medica (Shennong Bencao Jing), the earliest extant pharmaceutical monograph in China. Furthermore, modern pharmacological studies have found that A. membranaceus is clinically beneficial for the treatment of respiratory, immunological, cardiovascular and hepatic diseases [4,5,6]. In addition, A. membranaceus has also commonly been used as a dietary supplement and additive in European and American countries; in particular, the United States has classified A. membranaceus as an over-the-counter dietary supplement that can be sold at health food markets [7,8].
A. membranaceus can widely synthesize a variety of bioactive components as secondary metabolites, such as flavonoids, triterpene saponins and polysaccharides, which determine the quality of the medicinal materials to a large extent [9]. Among them, flavonoids are the most abundant and ubiquitous secondary metabolites distributed in A. membranaceus tissues, mainly in the roots, stems, leaves, flowers, fruits and seeds. Although flavonoids have various chemical structures, they share the same structural skeleton in the early stages of biosynthesis, which mainly contains three rings (C6-C3-C6) [10,11]. To date, more than 52 flavonoid components, including flavones, isoflavones, flavanones, flavonols, chalcones and anthocyanidins, have been identified in A. membranaceus [1]. In nature, triterpenes and their saponins are the second largest secondary metabolites with a total of more than 20,000, which are widely distributed in higher plants, dicotyledons, monocotyledons, fungi, pteridophytes and marine organisms [12], while the tetracyclic and pentacyclic triterpenoids are common triterpene saponins compounds in A. membranaceus. The astragalosides, important tetracyclic triterpenoids compounds, have a wide range of biological activities and important pharmacological effects [13]. Astragaloside and calycosin-7-O-β-D-glucoside (CG) are considered to be the most important bioactive ingredients that belong to the triterpene saponins and flavonoids in A. membranaceus, respectively, and are often used as “marker components” in the Chinese Pharmacopoeia [14], and astragaloside is also recorded in the European Pharmacopoeia and British Pharmacopoeia [15,16].
Currently, the interest of numerous scholars around the world has been attracted to A. membranaceus due to its potential pharmacological activities and therapeutic effects. Thus, it is imperative to solve the challenges of the resources and production of A. membranaceus. In particular, the demand for A. membranaceus is rapidly growing in the pharmaceutical, nutraceutical, food and cosmetics industries. Nevertheless, with the recklessly extensive excavation of medicinal resources, wild A. membranaceus has become an endangered species [17]. Therefore, we have to comprehensively understand the biosynthetic pathways and corresponding key genes involved in flavonoids’, triterpene saponins’ and polysaccharides’ biosynthesis in A. membranaceus, which have provided great guidance for the medicinal plant breeding and novelty drug exploration derived from A. membranaceus.
In this review, we systematically summarized the biosynthetic pathways and involved key enzymes, and the bioactivity of flavonoids, triterpene saponins and polysaccharides in A. membranaceus. In addition, we proposed potential strategies of heterologous biosynthesis to improve the industrialized production and sustainable supply of active components with pharmacological activities in A. membranaceus, aiming to offer new insights into the exploration and biomanufacturing of natural products.
2. Biosynthesis of Flavonoids
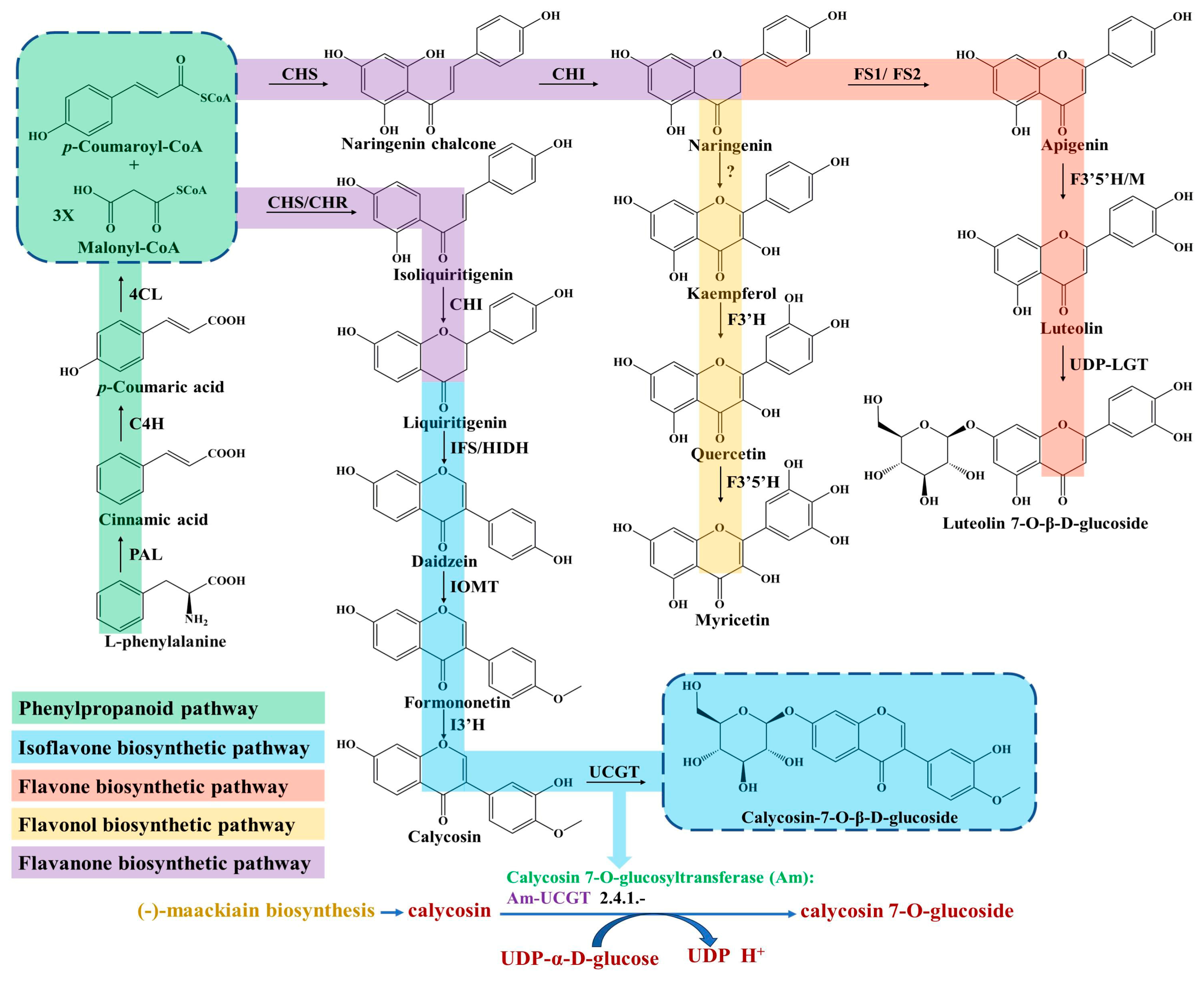
Flavonoids are ubiquitous in Astragalus tissues, including the roots, stems, leaves, flowers, fruits and seeds. The biosynthetic pathway of flavonoids, especially its subclass isoflavonoids, has been widely studied in A. membranaceus and partially elucidated [18,19]. The upstream pathway of flavonoids biosynthesis starts from the L-phenylalanine involved in the phenylpropanoid pathway (Figure 1), which is necessary for the growth of plants and the result of the long-term adaptation of plants to natural conditions [20]. Furthermore, the phenylpropane pathway is one of the main universal routes used to synthesize secondary metabolites in plants [21].
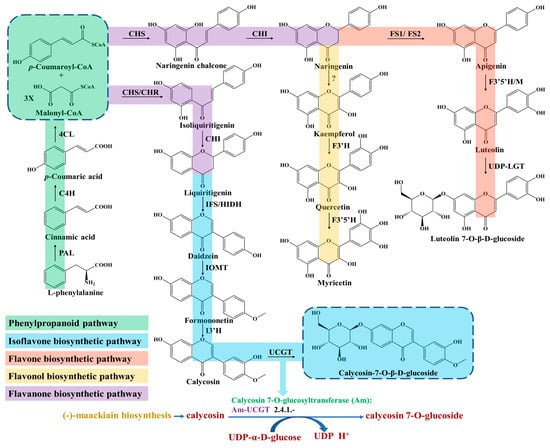
Figure 1.
Biosynthesis pathway of flavonoids in A. membranaceus.
The first committed step is regulated by the phenylalanine ammonia lyase (PAL), which catalyzes the deamination of L-phenylalanine and produces the cinnamic acid (Figure 1). PAL is an important rate-limiting enzyme in phenylpropane metabolism, which is largely expanded and experienced in tandem duplication events in A. membranaceus [22]. Liu et al. isolated an AmPAL1 (GenBank No. AY986506) gene from the A. membranaceus, which was significantly induced by mechanical wounding, UV irradiation and white light irradiation, and expressed universally in various organs [23]. In addition, they found that the contents of flavonoids and quercetin in A. membranaceus of different ages were closely related with the PAL enzymatic activity.
Subsequently, p-coumaric acid is generated through hydroxylation at the C-4 positions by a cytochrome P450 monooxygenase of cinnamate 4-hydroxylase (C4H) [24]. Next, the 4-coumarate-CoA ligase (4CL) catalyzes the conversion of p-coumaric acid into p-coumaroyl-CoA (Figure 1), which acts as an important precursor for various synthetic derivatives involved in the phenylpropanoid pathway, including lignans and flavonoids [25].
The biosynthesis of all flavonoids in Astragalus starts from the p-coumaroyl-CoA and is catalyzed by chalcone synthase/chalcone reductase (CHS/CHR), which catalyzes the condensation of p-coumaroyl-CoA and three molecules of malonyl-CoA to form a chalcone intermediate, such as isoliquiritigenin or naringenin chalcone [26]. In the flavanones biosynthetic pathway, the chalcone isomerase (CHI) catalyzes chalcone substrates to produce flavanones (Figure 1), which are transformed to various isoflavones, flavones, flavonols and dihydorflavonols by the respective enzymes [27]. For isoflavones synthesis, the CHI catalyzes the cyclization of isoliquiritigenin and generates liquiritigenin involved in downstream enzymatic reactions for ultimate isoflavone compounds’ formation in Astragalus [28]. Subsequently, the daidzein is synthesized under the catalyzation of isoflavone synthase (IFS), which converts the flavanones to their corresponding isoflavones through a 2, 3 aryl ring migration. Isoflavones are synthesized almost exclusively in leguminous plants under the catalysis of IFS, which is deficient in most other plant species [29].
Finally, isoflavone 3′-hydroxylase (I3′H) catalyzes the hydroxylation reaction at the 3′-position of formononetin to produce calycosin (Figure 1), which is continuously transformed into the CG under calycosin 7-O-glucosyltransferase (UCGT) [30]. The final step of UGT-catalyzed glycosylation in the isoflavones’ biosynthesis pathway promotes the stability, solubility and specific bioactivity of isoflavones’ secondary metabolites and adaptation to the harsh environment of A. membranaceus plants [31,32,33]. In a recent study, two 7-O-glycosyltransferases of AmUCGT (c303354, GenBank No. MN241498) and AmUFGT (c778119, GenBank No. ON375915), which catalyze calycosin and formononetin, respectively, were predicted by phylogenetic analysis [21]. Kim et al. found that the transcript levels of the main genes (AmPAL, AmC4H, Am4CL, AmCHR, AmCHS, AmCHI, AmIFS, AmI3′H and AmUCGT) related to the biosynthesis of calycosin-7-O-β-D-glucoside and calycosin were higher in the flower than those of other organs (leaf, stem and root) in A. membranaceus; thus, they speculated that the accumulation of calycosin-7-O-β-D-glucoside in roots might originate from the calycosin in the stem and leaf [25]. In addition, most of the UGTs’ genes family were distributed on chromosomes as gene clusters in A. membranaceus [22]. The key enzymes of C4H, IFS and I3′H involved in isoflavone biosynthesis belong to the CYP450 superfamily, which are located on the nine chromosomes and have the largest enrichment (46, 19%) on chromosome 5 in A. membranaceus [22].
3. Biosynthesis of Triterpenoid Saponins
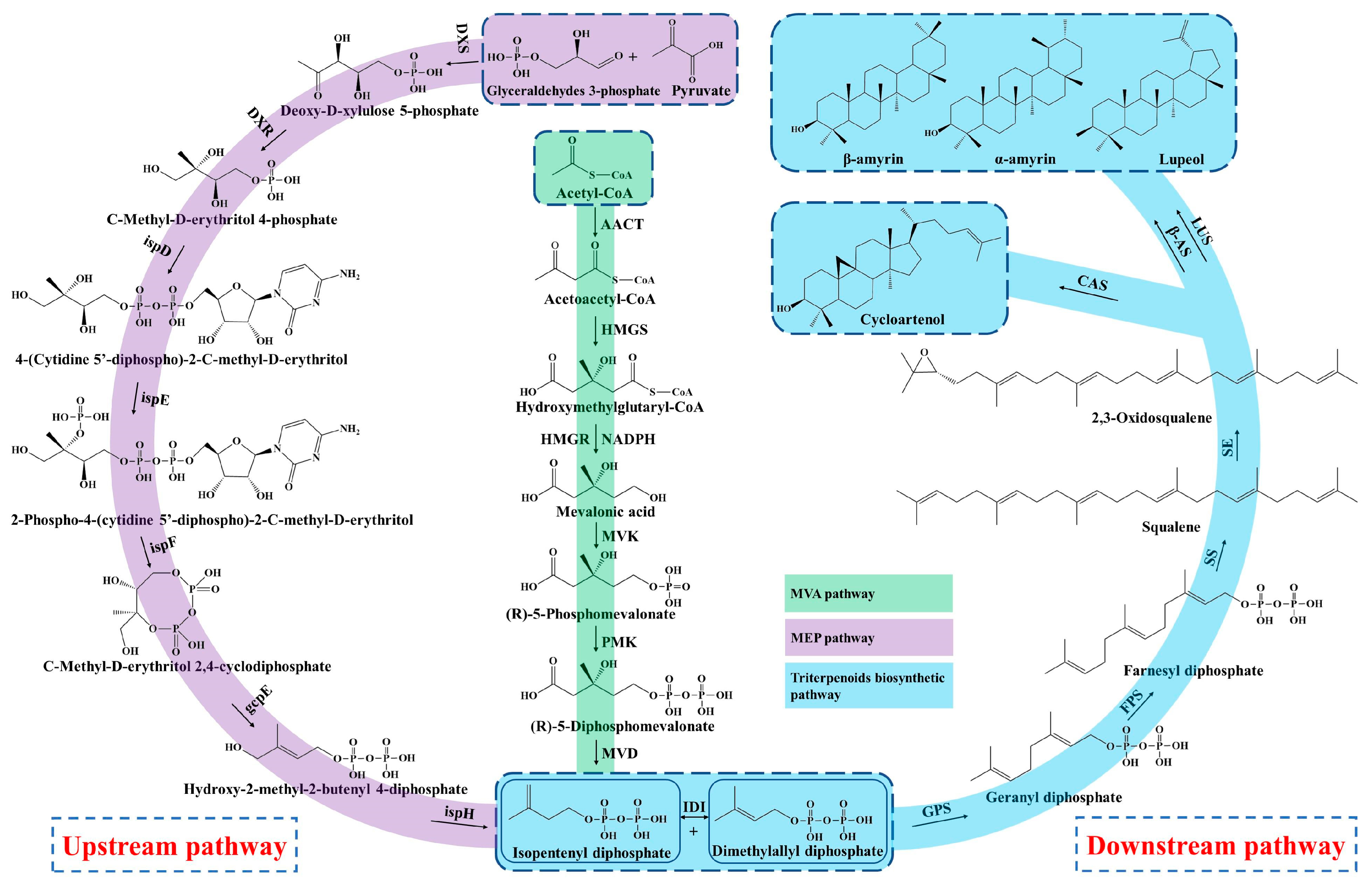
Triterpenoid saponins are a group of commonly natural products with a diverse structure and important bioactivities [34]. Triterpenoids are formed by the condensation of triterpenoid saponins coupled with sugar or other chemical groups, while the tetracyclic triterpenoids (Astragalosides) and pentacyclic triterpenoids (Oleanolic acid) are common compounds in A. membranaceus. Biosynthesis of triterpenoid saponins is a complex multi-step process, mainly including precursors synthesis, chain elongation, isomerization, cyclization, chain coupling, epoxide protonation and glycosylation [35]. In general, the biosynthesis of triterpenoid can be divided into three stages: (1) the synthesis of upstream precursors, such as isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP); (2) the synthesis of the carbocyclic skeleton; and (3) the formation of different kinds of triterpenoid saponins [34]. Triterpenoid saponins are synthesized in plants through the universal upstream pathways of the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways and the downstream pathway of triterpenoid skeleton formation and modification (Figure 2).
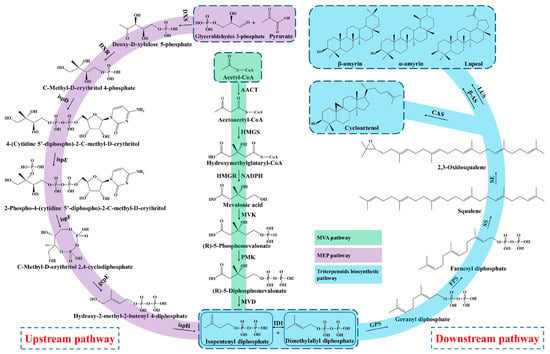
Figure 2.
Biosynthesis pathway of triterpenoid saponins in A. membranaceus.
3.1. Upstream Biosynthesis Pathway
Firstly, the upstream precursor of IPP is synthesized from the initial substrate of acetyl CoA via a six-step condensation reaction in the MVA pathway [12,36]. The MEP pathway also synthesizes the IPP by utilizing the glyceraldehydes 3-phosphate and pyruvate as starting substrates through a seven-step reaction. In addition, the MEP pathway produces polyterpenes along with the chloroplast-bound isoprenoids (b-carotene, prenyl chains of plastoquinone and chlorophylls) in the plastid [37]. Nevertheless, the MVA pathway is well-clarified and plays a dominant role in the biosynthesis of triterpenoid saponins, which mainly synthesizes the isoprenoids, sterols, ubiquinones and sesquiterpenes in the cytoplasm and mitochondria [38]. The upstream precursor of IPP and DMAPP are interconverted under the catalyzation of isopentenyl diphosphate isomerase (IDI).
3.2. Downstream Biosynthesis Pathway
Subsequently, the geranyl diphosphate (GPP, C10) is synthesized from the substrate of IPP and DMAPP through the condensation reaction by geranyl pyrophosphate synthase (GPS), and then converted to farnesyl diphosphate (FPP, C15) by adding the second IPP unit under the action of the farnesyl diphosphate synthase (FPS) [39]. In the stage of triterpenoid skeleton formation, the downstream precursor of squalene is formed from the condensation of two FPPs by squalene synthase (SS), which is considered a rate-limiting enzyme in the triterpenoids’ biosynthesis [40,41]. Next, the squalene is oxidized into 2,3-oxidosqualene under the catalyzation of squalene epoxidase (SE), serving as the key precursor of triterpenoids saponins [12].
Moreover, the most important step of tetracyclic triterpenoids skeletons’ formation is the cyclization of 2,3-oxidosqualene and generation of cycloartenol, which is catalyzed by the cycloartenol synthase (CAS) that belongs to the oxidosqualene cyclase (OSCs) family [42]. Duan et al. (2023) identified a cycloartenol synthase of AmCAS1 from A. membranaceus through the in vivo (in yeast) and in vitro functional identification based on the guidance of transcriptome and phylogenetic analysis [43]. They found that this enzyme catalyzes the cyclization of 2,3-oxidosqualene into cycloartenol A. membranaceus. The cycloartenol is derived from the C-20 protosteryl cation, which is generated from the chair-boat-chair (CBC) conformation of 2,3-Oxidosqualene after folding. In the process of cyclization, the epoxide group of 2,3-Oxidosqualene is initially protonated, which triggers a carbocationic cyclization and rearrangement cascade, and forms diverse triterpene skeletons after deprotonation reactions [44].
On the other hand, the 2,3-Oxidosqualene substrate is cyclized to the chair-chair-chair (CCC) conformation and gives the tetracyclic dammarenyl cation. This cation may undergo further conversion to the pentacyclic triterpenoids by β-amyrin synthases (β-AS) and lupeol synthases (LUS), including lupeol, α-amyrin and β-amyrin [45]. Thus, the OSCs is an important enzyme and branch point to synthesize tetracyclic triterpenoids and pentacyclic triterpenoids. The OSCs mainly includes CAS, α-amyrin synthase (α-AS), β-AS and LUS in plants, which catalyze the cyclization of 2,3-Oxidosqualene and generate more than 100 variations of the triterpenoid and sterol skeleton [45,46]. In addition, the flexible and changeable core site of OSCs maybe causes the various structures of triterpenoid saponins in that the catalytic properties of OSCs mainly rely on its amino acid sequence [47]. Two OSC genes, AmOSC2 and AmOSC3, were identified from A. membranaceus, and their functions were studied by heterologous expression in tobacco and yeast [48]. AmOSC2 is a β-amyrin synthase that is associated with the synthesis of β-amyrin and soyasaponins in vivo and shows higher expression activity in underground parts, while AmOSC3 is a cycloartenol synthase, which is closely related to the production of cycloartane-type astragalosides and cycloartenol.
Then the triterpenoid backbone undergoes diverse structural modifications (such as oxidation, glycosylation and substitution), mainly performed by the cytochrome P450-dependent monooxygenases, glycosyltransferases (UGTs) and other enzymes [49]. Chen et al. found that the key genes involved in triterpenoids biosynthesis, such as OSCs, CYP450 and UGT, were closely linked and clustered in the genome of A. membranaceus, which implied that there was collaborative expression of genes during the triterpenoids’ synthesis [22]. Finally, the diverse triterpenoid saponin compounds are synthesized under the structural modifications by OSCs, CYP450, UGT and other enzymes in A. membranaceus.
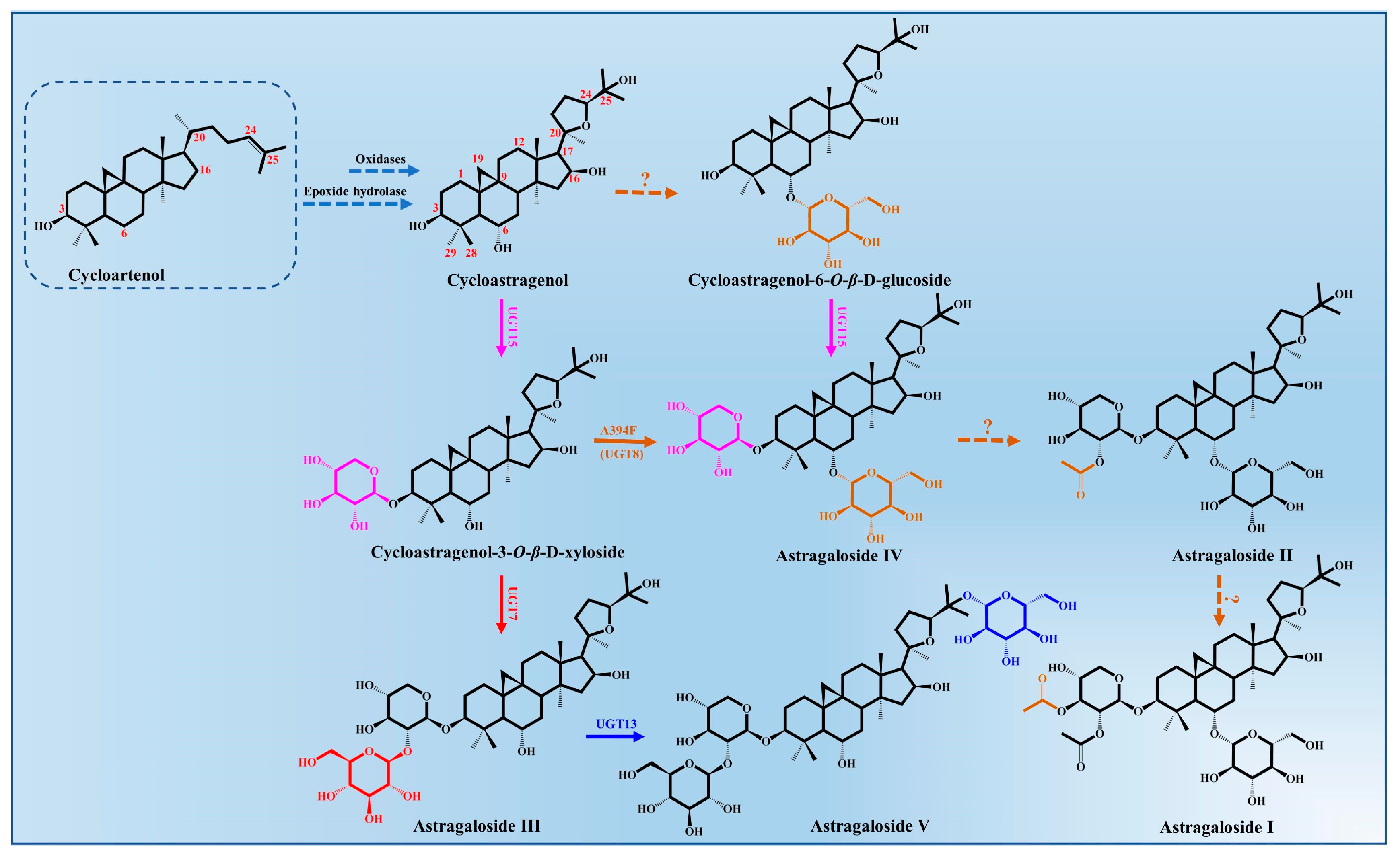
3.3. Biosynthesis Pathway of Astragalosides
Astragalosides, important tetracyclic triterpenoid compounds, are considered to be the main active constituents in A. membranaceus, of which cycloastragenol-type glycosides are the most typically bioactive compounds with pharmacological activities. In nature, cycloastragenol-type glycosides are a rare kind of triterpenoid glycoside, which were derived from the cyclization of 2,3-oxidosqualene to cycloartenol with a typical 9,19-cyclopropane moiety. The biosynthesis of cycloastragenol involved a series of oxidations and furan ring formation under the action of oxidases and epoxide hydrolase (Figure 3). There may be an epoxidation reaction in the terminal olefinic bond of cycloartenol, and then the hydroxyl group at the C20 position attacks the epoxide via an epoxide hydrolase, which forms the 20,24-tetrahydrofuran furan ring in the 5-exo-tet mechanism [50]. Thus, this 5-member cyclic ether in the side chain is a more common representative structure of astragaloside that belongings to the cycloartane-type saponins. Meanwhile, the cycloastragenol was generated from the cycloartenol by forming hydroxyl groups at the C6, C16 and C25 positions under the action of oxidations [43].
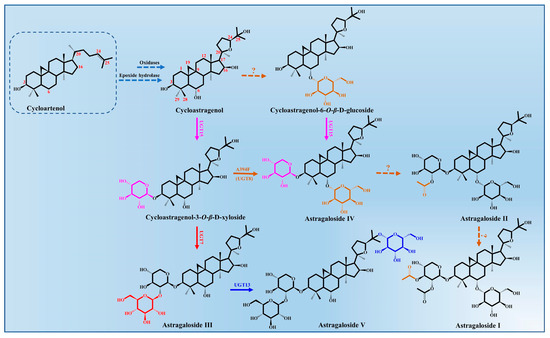
Figure 3.
Astragalosides’ biosynthesis pathways from cycloartenol in A. membranaceus.
The glycosylation of cycloastragenol catalyzed by a series of UGTs is commonly considered to be the last step in the biosynthesis of astragalosides and its derivatives, resulting in the vast structural diversity and important bioactivity of astragalosides [34]. The different sugar units (glucose or xylose) and numbers of glycosyl moieties (mono-, di-, tri-, or branched sugar chains) are mainly transferred to the 3-OH, 6-OH, 25-OH and 2′-OH position of cycloastragenol by UGTs.
To date, only four glycosyltransferases (AmUGT7, AmUGT8, AmUGT13 and AmUGT15) responsible for cycloastragenol glycosides have been identified in A. membranaceus [43,51]. AmUGT15 could catalyze the 3-O-xylosylation of cycloastragenol and cycloastragenol-6-O-β-D-glucoside to form the cycloastragenol-3-O-β-D-xyloside and astragalosides IV, respectively, while astragalosides IV was assigned as the most important triterpenoid saponin in A. membranaceus, which could also be synthesized from the cycloastragenol-3-O-β-D-xyloside by the variants A394F of AmUGT8. AmUGT7, a 2′-O-glucosyltransferase with regioselectivity towards 2-OH of xylosyl moiety at C3 positions, was able to synthesize astragaloside III. During the biosynthesis of astragalosides, AmUGT13 is mainly responsible for the 25-O-glucosylation of astragaloside III and forms astragaloside V [52]. This glycosyltransferase exhibits high regio-specificity and flexibility towards both acceptors and sugar donors. In addition, the other diverse modifications of astragalosides’ structure, including oxygenation, glycosylation and acylation, remain unclear and require further elaboration.
4. Structural Features of Polysaccharides
The astragalus polysaccharide (APS) is one of the major bioactive components in A. membranaceus with a complex and diverse structure, and possesses multiple pharmacological activities [53]. Although the APS is mainly linked by the glycosidic bonds between different monosaccharides, the deep understanding on accurate components is very limited. Moreover, the types of glycosidic bond and corresponding pharmacological activities of the APS in A. membranaceus are also different depending on the basic sources, medicinal part, planting method, place of production and growth year [54]. The polysaccharide components of A. membranaceus mainly include heteropolysaccharide, neutral polysaccharide, dextran and acidic polysaccharide, which were linked by the main glycosidic bond types of 1,4-glucose linkage [55].
Most of the APSs isolated and purified from A. membranaceus are heteropolysaccharides whose molecular weight ranges from 8.7 to 4800 kDa (Table 1). In addition, the heteropolysaccharides are in water-soluble forms and composed of various monosaccharides, including D-glucose, D-galactose, D-mannose, D-ribose, D-xylose, L-rhamnose, L-rabinose, L-xylose, L-ribose, glucuronic acid (GlcA) and galacturonic acid (GalA) [56], while the dextran extracted from A. membranaceus includes α-(1→4) dextran and α-(1→4)(1→6) dextran with water-insoluble and water-soluble forms, respectively [57].
A pure polysaccharide of AX-I-3b (Mw: 7.9 kDa) was extracted with hot water and purified through DEAE-cellulose 52 column and Sephacryl S-400 HR gel column chromatography, which consisted of Ara, Xyl and Glu at the ratio of 10.4, 79.3 and 1.1, respectively, and were linked as follows: →2,3,4)-β-D-Xyl-(1→, →4)-β-D-Ara-(1→, →4)-β-D-Glc-(1→[58]. Jiang et al. obtained a heteropolysaccharide (linked as α and β indicant bonds) of the APS extracted with microwave, which was purified by using ultrafiltration and resin absorbing (DEAE Sepharose FF) [59]. They found that the monosaccharide compositions of the APS were Man, Gal, Fru, Fuc and Xyl. In addition, a cold-water-soluble polysaccharide (Mw: 12.3 kDa) of cAMPs-1A was purified through a DEAE-cellulose 52 anion-exchange column and a Sephadex G-100 column, which consisted of Fuc, Ara, Gal, Glu and Xyl with a molar ratio of 0.01, 0.1, 0.2, 1.0 and 0.1, respectively [60].

Table 1.
The chemical composition, structural properties and pharmacological activities of polysaccharides derived from the A. membranaceus.
Table 1.
The chemical composition, structural properties and pharmacological activities of polysaccharides derived from the A. membranaceus.
| Components | Extraction/Purification | Monosaccharide Composition | Structural Information | Molecular Weight (kDa) | Pharmacological Activities | References |
|---|---|---|---|---|---|---|
| AERP1 | Hot-water/Sephacryl® S-400 column | Man:Rha:GalA:Glu:Gal:Ara with a molar ratio of 1.00:2.59:12.15:2.60:3.07:4.54 | 3/5-α-araf-(1→,T-α-araf,→4,6-β-manp-(1→,→3/3,6-β-galp-(1→,→2/2,4-α-rha-(1→,→-4/4,6-α-glcp-(1→,→4-α-galpA-(1→and→4)-6-OMe-α-galpA-(1→ | 2.01 × 103 | Improved diabetes-related cognitive dysfunction | [61] |
| AERP2 | Hot-water/Sephacryl® S-400 column | Glucan | →4/6-α-glcp-(1→ linkage) | 2.11 | Improved diabetes-related cognitive dysfunction | [61] |
| APSID3 | Hot-water/DEAE Sepharose Fast Flow and Sephacryl S-300 chromatography | Ara:Rha:Gal:Glc with a molar ratio of 2:2:5:6 | The minimal repeat unit: one terminal Ara, one 1,5-linked Ara, one 1,3-linked Rha, one 1,3,4-linked Rha, five 1,4-linked GalA and six 1,4-linked GluA | 5.8 × 102 | [56] | |
| RAP | Boiling water/Buchi Purifier system coupled with a Hiload 26/60 Superdex-200 column | Rha:Ara:Glc:Gal:GalA with a molar ratio of 0.03:1.0:0.3:0.4:0.3 | The backbone:1,2,4-linked Rha, α-1,4-linked Glc, α-1,4-linked GalA6Me, β-1,3,6-linked Gal; The side chains: α-T-Ara and α-1,5-linked Ara; The terminal residues: T-linked Ara, T-linked Glc and T-linked Gal. | 1.3 × 103 | Immunomodulation | [62] |
| APS-I | Sephadex G-100 column | Glu:Gal:Ara:Rha:GalA with a molar ratio of 1.5:1:5.4:0.08:0.1 | 1,4-linked D-Glc, 1,2-linked D-Glc, L-Rha, 1,5-linked D-Ara, 1,2,5-linked D-Ara, 1,4-linked D-Ara, D-Gal | 5 × 102 | Immunomodulation | [63] |
| APS-II | Sephadex G-100 column | Glu:Gal:Ara:Rha:GalA with a molar ratio of 9:1:1.4:0.04:0.001 | 1,4-linked α-D-Glc, 1,6-linked α-D-Glc, 1,4,6-linked α-D-Glc, 1,3,4,6-linked α-D-Glc, 1,2-linked α-D-Glc, α-L-Rha, 1,5-linked α-D-Ara, 1,4-linked α-D-Ara, β-D-Gal | 10 | Immunomodulation | [63] |
| AX-I-3b | Hot-water/DEAE-cellulose 52 column chromatography and Sephacryl S-400 HR gel column | Ara:Xyl:Glu with a molar ratio of 10.4:79.3:1.1 | 1,4-linked β-D-Xyl, 1,4-linked β-D-Ara, β-D-Glc | 7.9 | Immunomodulation and antitumor | [58] |
| APS | - | Glu:Ara:Xyl:Man:Gal with a molar ratio 95:2.9:0.7:0.7:0.6 | - | 17.4 | Antitumor | [64] |
| AMA-1-b-PS2 | Ara:Fuc:Gal:Glu:Man:Rha:Xyl:GalA:GluA with a molar ratio of 12.8:4.5:25.6:23.6:24.8:5.1:0.7:1.5:1.4. | The backbone: β-D-(1→3) linked galactans | Immunomodulation | [65] | ||
| APS-II | DEAE-32 anion-exchange chromatography and Sephacryl S-300 high resolution column chromatography | Xyl:Glu:Ara:Rha:Man:Gal with a molar ratio of 9.2:77.9:1:5.2:4.5:2.2 | 11.4 | Immunomodulation | [66] | |
| AMP | Hot-water/cationic exchange column (Dowex 50 W-x8) | Glu:Ara:Gal with a molar ratio of 91:6.2:2.8 | 6.9–9.2 × 102 | Immunomodulation | [67] | |
| AMon-S | Hot-water/DEAE Sephadex A-25, Con A-Sepharose chromatography, Toyopearl HW60F | Ara:Gal:GalA:Glc with a molar ratio of 18:18:1:1 | Structural units: α-Arabino-β-3,6-galactan type | 76 | Reticuloendothelial system-potentiating activity | [68] |
| APS | Hot-water/DEAE-Sepharose CL-6B | Glu:Gal:Ara with a molar ratio of 1.75:1.63:1 | 36 | Hepatoprotection | [69] | |
| APS | Hot-water/DEAE-cellulose column and Sephacryl-S400 column | Glc | The repeat units: a (1→4)-linked backbone with a (1→6)-linked branch every 10 residues | 20.1 | Antioxidant and immunomodulation | [53] |
| APS2 | Boiling water/precipitation with 40% ethanol | Ara | 40 | Immunomodulation | [70] | |
| APS3 | Boiling water/precipitation with 60% ethanol | Rha:Glu:Gal:Ara with a molar ratio of 1:10.8:6.6:12 | 15.3 | Immunomodulation | [70] | |
| APS | Microwave/ultrafiltration and resin absorbing (DEAE Sepharose FF) | Man, Gal, Fru, Fuc, Xyl | Heteropolysaccharide with α and β indicant bonds | Immunomodulation and antiviral | [59] | |
| APS | Boiling water/Sephadex G-100 column | Rha:Xyl:Glc:Gal with a molar ratio of 1:4:5:1.5 | linear backbone:1,3-linked β-D-Gal residues with insertion of β-Glc, 1,6-linked α-Gal, 1,5-linked β-Xyl, 1,4-linked β-Gal, β-D-Gal, 1,2-linked α-Rha, 1,2,4-linked α-Rha residues | 3.01 × 102 | Immunomodulation | [71] |
| APS | Hot water | Man:Glu:Xyl:Ara:GluA:Rha with a molar ratio of 0.3:12.8:1.6:0.7:1.0:0.6 | 2.04 × 103 | Antiinflammatory | [72] | |
| cAMPs-1A | Cold-water/DEAE-cellulose 52 anion-exchange column and a Sephadex G-100 column | Fuc:Ara:Gal:Glu:Xyl with a molar ratio of 0.01:0.1:0.2:1.0:0.1 | 12.3 | Antitumor | [60] | |
| APS | Hot-water/anion-exchange and gel permeation chromatography | Glc | α-(1→4)-D-glucan, with a single α-D-glucose at the C-6 position every nine residue, on average, along the main chain. | 36 | Renal protection | [73] |
| APS | Hot-water/Sephadex G-50 and lyophilized | Ara:Gal:Glu:Man with a molar ratio of 1.00:0.98:3.01:1.52 | pyranose ring and α-type glycosidic linkages | 2.1 | Antitumor | [74] |
5. Pharmacological Activities of Bioactive Ingredients
A. membranaceus has a long history (more than 2000 years) in Chinese herbal medicine for medicinal usage due to its significant bioactivities and pharmacological effects. When compared to other Astragalus genus plants (Table 2), A. membranaceus exhibited the widest range of pharmacological activities, including anticancer, antidiabetic, antiviral, hepatoprotective, immunomodulatory, antiinflammatory, antioxidant and anti-cardiovascular activities (Figure 4).

Table 2.
Comparison of pharmacological potentials between A. membranaceus and other Astragalus genus plants.
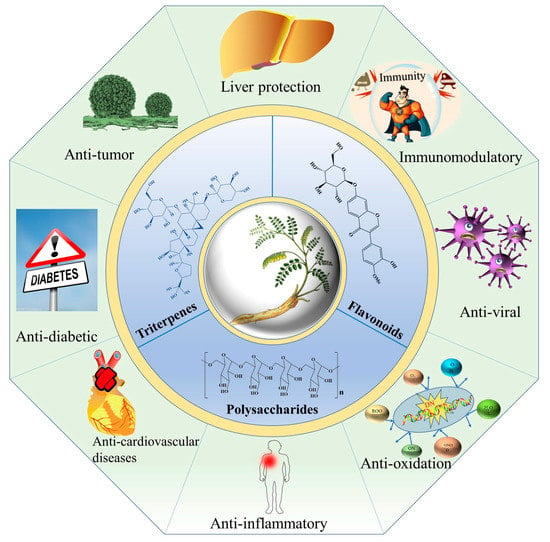
Figure 4.
Pharmacological activities of flavonoids, triterpene saponins and polysaccharides derived from A. membranaceus.
5.1. Immunomodulatory Effects
Modern pharmacological studies have proven that A. membranaceus has immunomodulatory effects by improving the immune system and alleviating the adverse effects of conventional drug treatments [84]. Several studies have proven that the APS (40–400 µg/mL) can efficiently protect the bone marrow mesenchymal stem cells from radiation-induced apoptosis, formaldehyde-induced cytotoxicity and genotoxicity by regulating the relative genes expression, such as B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X (Bax) and xeroderma pigmentosum group A [85,86]. Bao et al. reported that the APS (150 mg/kg) was able to protect the hematopoietic stem cells by improving the bone marrow and hematopoietic stem cell, and increasing the number of CD34+ cells, Lin–c-Kit+cells and Lin–Sca1+c-Kit+ cells [87].
In addition, the APS is a regulator for the secretion and production of cytokines, which could improve splenocytes to induce interferon (IFN), produce interleukin 2 (IL2), enhance the secretion of IL3, IL4 and IL6 [9,88,89], and induce the production of IL8, IL10 and IL12 [90,91]. As for the macrophages, the APS of 12.5–100 µg/mL is able to increase the expression and production of NO, IL-1β, TNF-α and IL-6 by activating the MAPK and NF-κB signal pathways [67]. Similarly, Wei et al. found that the APS is able to activate TLR4-related MAPKs signal pathways, including phosphorylated JNK (p-JNK), phosphorylated ERK (p-ERK) and phosphorylated p38 (p-p38), and induce NF-κB translocation and IκB-α degradation [92]. In short, the APS may induce the production of cytokines in RAW264.7 cells by activating the MAPKs and NF-κB signal pathways mediated with TLR4. In addition, Li et al. proved that the APS and deproteinated APS (DP) stimulated the production of NO and the up-regulation of cytokines’ mRNA expression by activating the NF-κB and MAPKs pathways in RAW264.7 cells, while the desulfated AMP (DS) significantly decreased the activation of RAW264.7 and NK cells [67].
Moreover, the important role of APS in Ig is to regulate immunity by secreting IgA, IgG and IgM. In vivo studies indicated that the APS of 8 mg/kg could improve the immunity by promoting the proliferation of T and B cells, and producing a variety of cytokines in cyclophosphamide-induced immunosuppressive mice, such as IgG, IgA, IgM, TNF-α, IL-6, IL-2 and IFN-γ [93]. In previous studies, the APS of 300–1200 mg/kg improved the percentages of CD3+CD4+ T cells and CD3+, and decreased the ratio of CD3+CD8+/CD3+CD4+ and the expression of IL-10, IL-6 and TNF-α [94].
In an in vivo animal model of BALB/c mice and Wistar rats, the total flavonoids extracted from A. membranaceus promoted the serum hemolysin level and delayed type hypersensitivity, macrophage phagocytic and the immune organ index in mice, while they alleviated mouse ear edema and vascular permeability, and rat paw edema granuloma formation [95].
5.2. Anticancer Effects
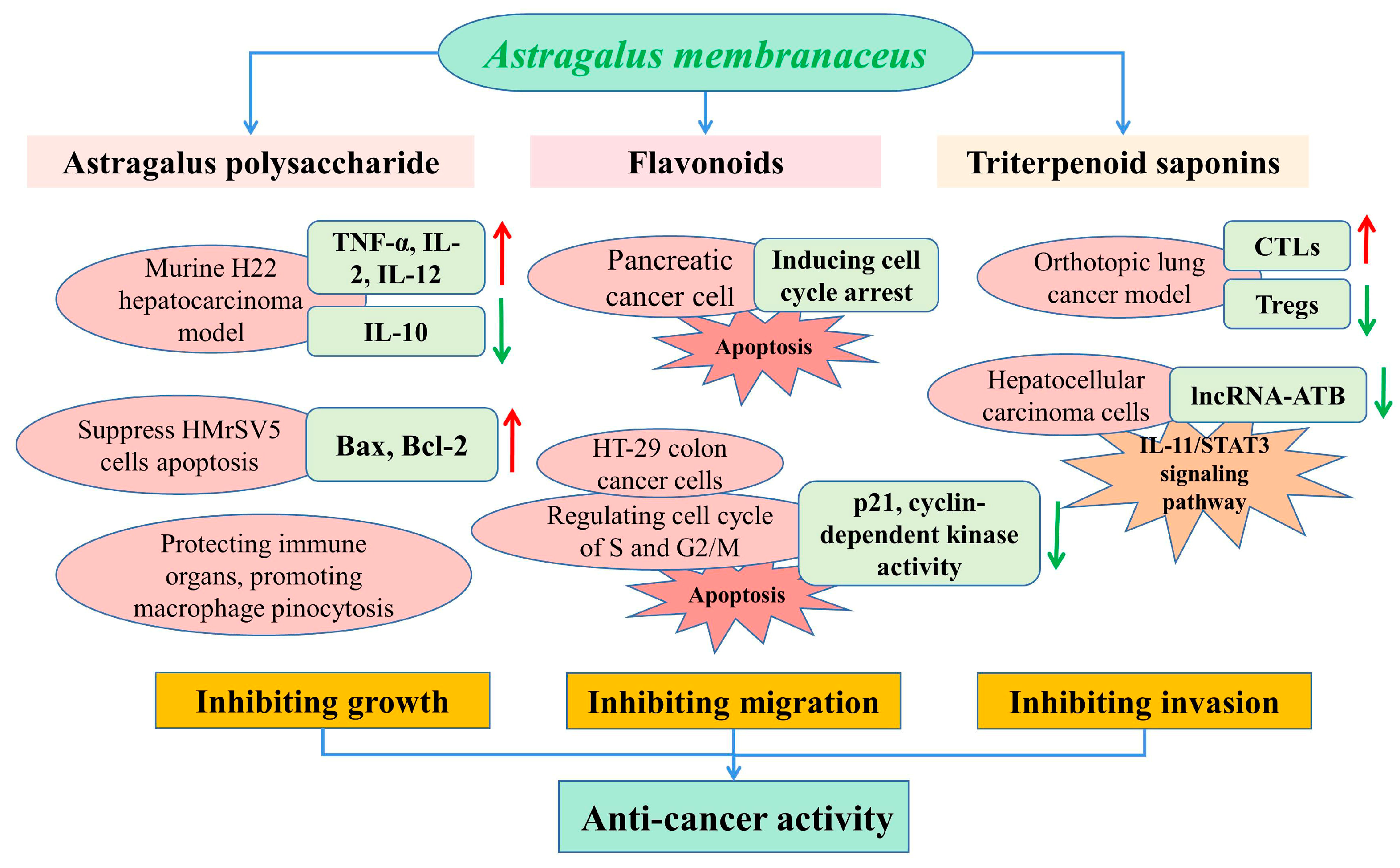
To date, cancer remains the leading cause of death and significantly influences life expectancy [96]. While A. membranaceus has exhibited potential antitumor activities against various tumor types on the basis of inhibiting the tumor growth, migration and invasion [97,98], in recent years, the APS has more commonly been used for the prevention and treatment of various tumors, such as gastric cancer, liver cancer and colon cancer. It was reported that tumor growth and migration were mainly caused by the rapid proliferative capacity of tumor cells [56]. In a murine H22 hepatocarcinoma model, APS treatment (100 and 400 mg/kg) effectively inhibited the growth of a solid tumor transplanted in BALB/c mice, and promoted the expression of TNF-α, IL-2 and IL-12 and decreased the concentration of IL-10 in serum [99]. Liu et al. reported that the oral administration of APS at dosages of 75, 150 and 300 mg/kg significantly inhibited tumor growth and had inhibitory rates of 20.53%, 36.50% and 44.49%, respectively [60]. They also found that the APS protected the immune organs and promoted macrophage pinocytosis in tumor-bearing mice (Figure 5).
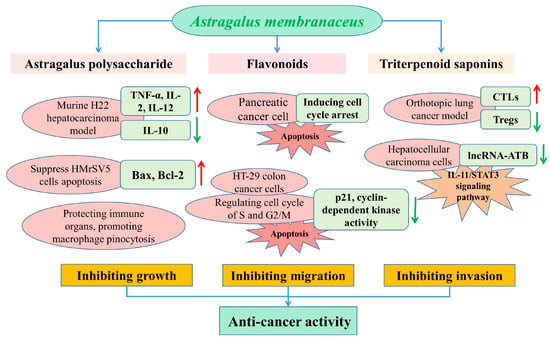
Figure 5.
Schematic presentation of the anticancer mechanisms of main bioactive compounds in A. membranaceus.
The effects of A. membranaceus on gastric-cancer-induced mesothelial cell apoptosis were analyzed in a previous study, which reported that the Astragalus treatment could partly suppress HMrSV5 cells’ apoptosis induced by the supernatant of MKN45 gastric cancer cells, condensation of chromatin and nuclear fragmentations, and regulate the expressions of Bax and Bcl-2 in the HMrSV5 cells [100]. Flavonoids and isoflavonoids are the most important secondary metabolites in a plant, with more than 8000 compounds to date [29], which have been proven to impede the growth of cancer cells by the modulation of apoptosis, and by inhibiting the DNA topoisomerase and tyrosine kinase activities [101]. In a study by Zhang et al., the calycosin inhibited the pancreatic cancer cell growth through the induction of cell cycle arrest induced by p21Waf1/Cip1 and apoptosis in a caspase-dependent manner, which also promoted the migration of MIA PaCa-2 cells via the epithelial–mesenchymal transition and by activation of a matrix metalloproteinase [102].
In addition, astragaloside-IV (AS-IV) remarkably inhibited the growth of a tumor in vivo based on immune enhancement activity by inducing the CTLs activity and inhibiting the Tregs expression in an orthotopic lung cancer model of C57BL/6 mice [103]. It was reported that AS-IV inhibited the migration and invasiveness of hepatocellular carcinoma cells by significantly down-regulating the expression of lncRNA-ATB in a time- and dose-dependent manner by blocking the signaling pathway of IL-11/STAT3 [97]. Astragalus saponins (AST) promoted the apoptosis of HT-29 colon cancer cells in a caspase 3- and polymerase-dependent manner, and inhibited cell proliferation by regulating the cell cycle of the S and G2/M phase, with concomitant inhibition of p21 expression and cyclin-dependent kinase activity. In an in vivo study, the antitumorigenic effects of AST were similar to the conventional chemotherapeutic drug 5-fluorouracil (5-FU), such as the reduction in tumor volume and the pro-apoptotic and antiproliferative effects in a mice xenograft [104]. Therefore, AST could be used for tumor therapy as an effective chemotherapeutic agent, or combined with other orthodox chemical drugs in order to alleviate the systemic side effects of toxic chemotherapeutic compounds.
5.3. Antiinflammatory and Antioxidant Effects
Numerous studies have proven that the most bioactive ingredients derived from A. membranaceus have antiinflammatory and antioxidant effects, and thus have been widely used in clinic. Adesso et al. proved that the extract derived from A. membranaceus reduces the lipopolysaccharide (LPS, derived from E. coli) plus interferon-γ-induced inflammatory response, and decreases the expression of cycloxygenase-2 (COX-2), the formation of nitrotyrosine and the release of TNF-α as well as the activation of NF-κB in the rat intestinal epithelial cells [105]. In an oxidative stress model induced by hydrogen peroxide (H2O2) in an intestinal epithelial cell line, the A. membranaceus extract decreases the ROS levels, and increases the antioxidant cytoprotective factors expression and nuclear factor-like 2 (Nrf2) activation. In addition, AST showed antiinflammatory properties by suppressing the lipopolysaccharide-induced NF-κB pathway, thereby decreasing the expression of inducible nitric oxide synthase (iNOS) in the macrophage RAW264.7 [106]. Similarly, the total flavonoids extracted from A. membranaceus effectively inhibited the production of inflammatory mediators, such as NO and cytokine IL-1β, TNF-α, IL-6 and IFN-γ in lipopolysaccharide-stimulated RAW 264.7 macrophages in a dose-dependent manner, whereas they promoted the production of these inflammatory mediators in unstimulated macrophages [95].
According to the previous study, the flavonoids of A. membranaceus inhibit the PC12 neuronal cell injury induced by glutamate by increasing the antioxidant enzyme activities of superoxide dismutase and glutathione peroxidase [107]. In addition, the flavonoids of A. membranaceus also showed a high scavenging activity to 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals in the cell-free system. Moreover, the APS possessed good antioxidant properties in ferric-reducing antioxidant power (FRAP), hydroxyl radical (•OH), DPPH and superoxide radical (•O2−) scavenging capacity [108]. Therefore, A. membranaceus and its bioactive components could be developed as a novel antioxidant agent against inflammation in various diseases.
5.4. Antidiabetic Effects
Diabetes, characterized by elevated blood glucose, is a globally metabolic disorder disease, which could damage the kidneys, eyes, heart and gastric mucosa, and could also lead to coma and death without proper treatment [109,110]. It was reported that formononetin (7-hydroxy-4′-methoxyisoflavone) had potential to treat diabetic retinopathy by inhibiting the secretion of vascular endothelial growth factor (VEGF) in the HIF-1α/VEGF signaling pathway, and reducing the expressions of PHD, HIF-1α and VEGF proteins [111]. Liu et al. recently found that an APS of AERP (Mw: 2.01 × 103 kDa) has hypoglycemic properties in a db/db diabetic mice model by reducing hyperglycemia and tissue impairment, and promoting cognitive function [61].
In a diabetic model of human umbilical vein endothelial cells (HUVEC), AS-IV effectively protected the HUVEC injury induced by high glucose by promoting the cell proliferation, and suppressing the apoptosis and inflammatory reactions in HUVEC, through the inhibition of the c-Jun Nterminal kinase (JNK) signaling pathway [112]. Zhang et al. studied the effect of the APS on diabetic nephropathy in streptozotocin-induced diabetic male Sprague-Dawley rats, and found that the APS not only decreased the concentration of blood glucose, microalbuminuria and plasma lipid, but improved renal function and reduced the ratio of kidney weight to body weight. In addition, the APS decreased the expression level of NF-κB in the renal cortex and raised the IκB mRNA level, which indicated that the APS has the potential for prevention and treatment of the progress of diabetic nephropathy [113].
5.5. Hepatoprotective Effects
As the largest solid organ in the human body, the liver plays important roles in drug metabolism, detoxification and the production of chemicals, and is easily injured by viral infection, metabolic disorder, overdose of toxin ingestion and immunological insult [114]. Modern pharmacological studies have proved that the bioactive ingredients derived from A. membranaceus are clinically beneficial for hepatoprotection. In a previous study, the authors found the AS-IV has the potential for the treatment of hepatic steatosis with activities in reducing lipid accumulation and insulin resistance in HepG2 cells. In addition, the AS-IV induces the phosphorylation of SREBP-1c at Ser372 in an AMPK-dependent manner in HepG2 cells [115]. Moreover, in an adipose dysfunction model induced by a high-fat diet (HFD) feeding in male ICR mice, AS-IV significantly reduced the accumulation of adipose cAMP by promoting Akt phosphorylation and combination with PDE3B. In addition, AS-IV inhibits the overproduction of hepatic glucose by decreasing the ectopic fat deposition in the liver [116].
Yan et al. investigated the hepatoprotective effect of APS on carbon tetrachloride (CCl4)-induced chronic liver injury in Sprague-Dawley rat models, and found that the APS reduced the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP), and significantly increased the activities of catalase (CAT) and superoxide dismutase (SOD) in the liver [69]. Dang et al. evaluated the protective effects of APS on chronic hepatic injury induced by CCl4 in male Sprague-Dawley rats and found that APS treatment reduced the serum levels of total bilirubin (TBIL) and ALT, and increased the albumin level and SOD activity in the liver [117]. Therefore, the APS could effectively protect hepatocytes and prevent hepatic injury by limiting free radical production, elevating antioxidant enzyme activities and reducing lipid peroxidation.
5.6. Antiviral Effects
Many studies have comprehensively proven that A. membranaceus has the important property of antiviral activity. Influenza is an influenza-virus-caused acute respiratory infection disease, while A. membranaceus exhibited the obvious activity of anti-influenza virus in a previous study described by Liang et al. [118]. They found that A. membranaceus could effectively increase the survival rate of influenza-virus-infected Raw264.7 cells, which were mainly caused by the increase in SOD activity, the reduction in the malondialdehyde level, and the regulation of the TLR3 signaling pathway and cell proliferating cycle.
In addition, hepatitis B virus (HBV) remains the leading cause of acute and chronic hepatitis, cirrhosis and liver cancer, and the current treatment strategy of antivirus drugs (such as lamivudine and interferon) is associated with various drawbacks, including low antiviral potency, side effects and a long treatment period [119]. Therefore, Dang et al. evaluated the inhibitory effects of the APS on HBV replication in HBV transgenic mice [120]. They reported that the administration of APS and emodin decreased the viral DNA levels in the serum, and reduced the serum levels of the surface antigen of hepatitis B virus (HBsAg), hepatitis B e antigen (HBeAg) and hepatitis B core antigen (HBcAg) in the mice, which indicated that the APS and emodin had a persistent inhibitory effect on virus replication in vivo. Du et al. reported that APS effectively increased the T cells’ proliferating activity and improved the HBsAg-related antibody level, thus inhibiting the viral reproduction by inducing the CD4+ and CD8+ T cells to produce cytokines and protecting the body against viruses [121]. In a human liver cell line, HepG2 2.2.15 transfected by HBV, the bioactive ingredient of AS-IV effectively inhibits the secretion of HBsAg and HBeAg, which suggests that AS-IV has potent anti-HBV activity in vitro and deserves to be further developed as an anti-HBV agent [13].
5.7. Anti-Cardiovascular Effects
Cardiovascular diseases are mainly caused by diabetes, hypertension, high cholesterol and dyslipidemia syndrome, and the most notable symptoms are ischemic or hemorrhagic lesions in the heart, brain and whole body [12]. Wang et al. investigated the effects of Astragalus flavonoids on cardiovascular disease in vivo [122]. In a diet-induced atherosclerotic rabbit model, the flavonoids significantly decreased the total cholesterol levels in plasma, reduced the aortic fatty streak area, and effectively scavenged the hydroxyl radicals and superoxide in a concentration-dependent manner.
A study was carried out by Wu et al. to explore the vasorelaxation effects of formononetin on an isolated rat aorta and underlying mechanisms [123]. Formononetin possesses vascular relaxation in a endothelium-independent and endothelium-/NO-dependent manner by activating the adenosine triphosphate (ATP)-dependent K+ (KATP) and Ca2+-activated K+ (BKCa) channels. Similarly, calycosin has proven to be an excellent endothelium-independent vasorelaxant on precontracted thoracic aortic rings in rats due to its function of being a noncompetitive Ca2+ channel blocker [124]. In addition, Zhu et al. evaluated the effects of formononetin derivative (sodium formononetin-3′-sulphonate) on angiogenesis and neuroprotection in a cerebral ischemia and reperfusion injury rat model [125]. They found that sodium formononetin-3′-sulphonate effectively protected the brain from the ischemia and reperfusion injury in vivo, which caused the improvement in neurological function, and an increase in the expression of the vascular endothelial growth factor and platelet endothelial cell adhesion molecule, as well as the suppression of cell apoptosis.
5.8. Toxicity and Clinical Trials
A few studies on the preclinical toxicity of bioactive compounds in A. membranaceus were carried out to verify the clinical safety. It was reported that the AS-IV has fetal toxicity at a dose more than 0.5 mg/kg and maternal toxicity after intravenous administration of 1.0 mg/kg, while it has no teratogenic effects in rats and rabbits [126]. In addition, the AS-IV delayed the fur development, liff parry reflex, and eye opening after birth under 1.0 mg/kg during a reproductive toxicity test in Sprague-Dawley rats, while there was no effect on the memory and learning [127].
In addition, it is necessary to evaluate the therapeutic effects of A. membranaceus on humans in clinical trials. A positive effect in patients with myocardial infarction was reported following the administration of Tongguan Capsules (TGC, composed of: A. membranaceus, Borneolum syntheticum, Salvia miltiorrhiza and Grasshopper), at a dose of 4.5 g/day for 6 months, who demonstrated a reduced left ventricular end-systolic volume index as well as decreased myocardial markers of fibrosis and apoptosis and reduced circulating levels of inflammatory cytokines [128]. Lee et al. reported a study in children with growth retardation syndrome who received HT042, containing a mixture of A. membranaceus roots, Eleutherococcus senticosus stems and Phlomis umbrosa roots, twice a day for 24 weeks; the HT042-receiving patients showed a significant increase in insulin-like growth factor binding protein-3 (IGFBP-3) and IGF-1, as well as an increase in the height and weight of children [129].
6. Future Perspectives
6.1. Future Market Prospects of A. membranaceus
With the improvement in people’s living standards and emphasis on health, the demand for A. membranaceus has increased due to its vital role in the prevention and early interventional treatment of diseases. It is worth noting that HSBD (Huashibaidu granules, A. membranaceus as a major component) was clinically proven to be effective for the treatment of COVID-19 patients [130,131]. Thus, the therapeutic effect of A. membranaceus on COVID-19 has promoted the sharp increase in its market price. There is expected to be an increasing demand for A. membranaceus in the future market following the worldwide spread of the epidemic. Nevertheless, the A. membranaceus industry has a long chain with a wide range, including cultivation, processing, acquisition, storage, transportation, as well as product research and development. Moreover, the A. membranaceus industry is more oriented towards quality assurance than production yield. Therefore, it is imperative to promote the establishment of price formation mechanisms oriented by the quality and to alleviate the large fluctuation of market prices and the total output caused by production dispersion, as well as strengthen the guidance practices on the development of the A. membranaceus industry.
6.2. Development Trend of Biotechnology in A. membranaceus
At the current stage, the numerous studies on the biosynthesis of flavonoids and triterpene saponins, and the structural analysis of polysaccharides in A. membranaceus have mainly focused on the discovery of important structural and regulatory genes involved in the biosynthetic pathway, while the key enzyme functions associated with the synthesis of these bioactive ingredients in A. membranaceus are still unknown, especially those involved in structural modification, transcriptional regulation and the catalytic process, thereby restricting the industrialized production and sustainable supply of natural products with pharmacological activities.
Therefore, it is imperative to comprehensively understand the biosynthetic pathway and regulatory mechanism of bioactive ingredients, and an effective way for further addressing these bottlenecks is heterologous biosynthesis by using heterologous plants or microorganisms as the chassis of cell factories that have transformed the metabolic flux and reconstructed the biosynthetic pathway of bioactive ingredients. Moreover, it is currently still a challenge to improve the accumulation of target products and realize the efficient and large-scale production by optimizing the chassis cell system, the regulatory factors and the fermentation conditions on the basis of heterologous biosynthesis. At present, there has not been much study on the synthetic biology in A. membranaceus, which still needs more in-depth exploration and breakthroughs in the future.
7. Conclusions
With increasing demand for traditional Chinese medicine, the sustainable development of the A. membranaceus industry has received more attention. Thus, this review focuses primarily on the biosynthesis pathway of flavonoids and triterpenoid saponins, as well as the structural features of polysaccharides derived from A. membranaceus. Nevertheless, the biosynthetic processes of flavonoids and astragaloside in A. membranaceus have not been completely resolved, and there is a lack of key steps in the synthesis pathways, which thwarts the large-scale production of bioactive ingredients. In addition, the pharmacological activities of these bioactive components were also summarized, which provided a more comprehensive understanding for the traditional Chinese medicine development and clinical applications. Finally, we also discussed the future market prospects and development trend of the bioengineering technology of A. membranaceus, hoping to lay a foundation for the in-depth study and utilization of A. membranaceus.
Author Contributions
Writing—review and editing, M.D.; conceptualization and funding acquisition, M.L.; validation, J.L.; investigation and visualization, D.Y.; resources and supervision, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Key Laboratory of Aridland Crop Science/Gansu Agricultural University Key (GSCS-2022-Z04), the National Key R&D Program of China (2022YFC3501501), the Young S&T Foundation of Gansu Province (23JRRA1432), the Young Scholars Science Foundation of Gansu Agricultural University (GAU-KYQD-2022-22), and the Earmarked Funding for CARS (CARS-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, P.; Zhao, M.; Chen, S.; Wu, X.; Wan, Q. Transcriptional regulation mechanism of flavonoids biosynthesis gene during fruit development in Astragalus membranaceus. Front. Genet. 2022, 13, 972990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS. J. Pharm. Anal. 2019, 9, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, Y.; Wang, Y.; Long, C. Phytochemical analysis of an antiviral fraction of Radix astragali using HPLC-DAD-ESI-MS/MS. J. Nat. Med. 2010, 64, 182–186. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef]
- Shan, H.; Zheng, X.; Li, M. The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, L.; Xia, Q.; Tian, L.; Qi, J.; Cao, M. Radix Astragali and Radix Angelicae Sinensis in the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Front. Pharm. 2020, 11, 415. [Google Scholar] [CrossRef]
- Chu, C.; Qi, L.-W.; Liu, E.H.; Li, B.; Gao, W.; Li, P. Radix Astragali (Astragalus): Latest advancements and trends in chemistry, analysis, pharmacology and pharmacokinetics. Curr. Org. Chem. 2010, 14, 1792–1807. [Google Scholar] [CrossRef]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.Y.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Ren, D.; Qu, D.; Sun, Y. The structure features and improving effects of polysaccharide from Astragalus membranaceus on antibiotic-associated diarrhea. Antibiotics 2019, 9, 8. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2022, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Li, J.Y.; Huang, H.; Gao, W.; Zhuang, C.L. Anti-hepatitis B virus activities of astragaloside IV isolated from Radix Astragali. Biol. Pharm. Bull. 2009, 32, 132–135. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission (CPC). Pharmacopoeia of the People’s Republic of China: 2020; Chinese Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Maisonneuve, S.A. European Pharmacopoeia. 1993. Available online: https://www.bmj.com/content/2/5402/192.5 (accessed on 1 June 2023).
- Paterson, G.R. British Pharmacopoeia 1980. Can. Med. Assoc. J. 1982, 126, 514. [Google Scholar]
- Jin, H.; Yu, Y.; Quan, X.; Wu, S. Promising strategy for improving calycosin-7-O-β-D-glucoside production in Astragalus membranaceus adventitious root cultures. Ind. Crop. Prod. 2019, 141, 111792. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Wang, W.; Shan, Y.; Wang, C.; Zhu, M.; La, Q.; Zhong, Y.; Xu, Y.; Nan, P.; et al. Integrated metabolomics and transcriptomics study of traditional herb Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao reveals global metabolic profile and novel phytochemical ingredients. BMC Genom. 2020, 21, 697. [Google Scholar] [CrossRef]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol. 2000, 124, 781–793. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Luo, Y.; Li, H.; Qin, X. Biosynthetic mechanisms of isoflavone accumulation affected by different growth patterns in Astragalus mongholicus products. BMC Plant Biol. 2022, 22, 410. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, T.; Su, H.; Duan, S.; Ma, R.; Wang, P.; Wu, L.; Sun, W.; Hu, Q.; Zhao, M.; et al. A reference-grade genome assembly for Astragalus mongholicus and insights into the biosynthesis and high accumulation of triterpenoids and flavonoids in its roots. Plant Commun. 2022, 4, 100469. [Google Scholar] [CrossRef]
- Liu, R.; Xu, S.; Li, J.; Hu, Y.; Lin, Z. Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep. 2006, 25, 705–710. [Google Scholar] [CrossRef]
- Russell, D.W. The metabolism of aromatic compounds in higer plants. X. Properties of the cinnamic acid 4-hydroxylase of pea seedlings and some aspects of its metabolic and developmental control. J. Biolog. Chem. 1971, 246, 3870–3878. [Google Scholar] [CrossRef]
- Kim, Y.B.; Thwe, A.A.; Li, X.; Tuan, P.A.; Zhao, S.; Park, C.G.; Lee, J.W.; Park, S.U. Accumulation of flavonoids and related gene expressions in different organs of Astragalus membranaceus Bge. Appl. Biochem. Biotechnol. 2014, 173, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Jia, X.; Sun, S.; Su, Y.; Chen, G. Differential expression of calycosin-7-O-beta-D-glucoside biosynthesis genes and accumulation of related metabolites in different organs of Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao under drought stress. Appl. Biochem. Biotechnol. 2022, 194, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, C.; Li, C.; Zhao, Q.; Liu, L.; Fang, X.; Chen, X.Y. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016, 61, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chu, S.; Shan, T.; Zha, L.; Peng, H. Full-length transcriptome sequences by a combination of sequencing platforms applied to isoflavonoid and triterpenoid saponin biosynthesis of Astragalus mongholicus Bunge. Plant Methods 2021, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pang, Y.; Dixon, R.A. Biosynthesis and genetic engineering of proanthocyanidins and (iso)flavonoids. Phytochem. Rev. 2007, 7, 445–465. [Google Scholar] [CrossRef]
- Xu, R.Y.; Nan, P.; Yang, Y.; Pan, H.; Zhou, T.; Chen, J. Ultraviolet irradiation induces accumulation of isoflavonoids and transcription of genes of enzymes involved in the calycosin-7-O-beta-d-glucoside pathway in Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao. Physiol. Plant 2011, 142, 265–273. [Google Scholar] [CrossRef]
- Vogt, T.; Jones, P. Glycosyltransferases in plant natural product synthesis: Characterization of a supergene family. Trends Plant Sci. 2000, 5, 380–386. [Google Scholar] [CrossRef]
- Bowles, D.; Lim, E.-K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of lipophilic small molecules. Ann. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef]
- Hirotani, M.; Kuroda, R.; Suzuki, H.; Yoshikawa, T. Cloning and expression of UDP-glucose: Flavonoid 7-O-glucosyltransferase from hairy root cultures of Scutellaria baicalensis. Planta 2000, 210, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochem 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Yin, X.; Wang, X.; Qi, X.; Xue, Z. Diverse triterpene skeletons are derived from the expansion and divergent evolution of 2,3-oxidosqualene cyclases in plants. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, X.-T.; Xu, Y.-Q.; Zhong, Y.; Li, Y.-X.; Chen, J.-K.; Li, X.; Nan, P. Global transcriptome analysis profiles metabolic pathways in traditional herb Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao. BMC Genom. 2015, 16, S15. [Google Scholar] [CrossRef]
- Oldfield, P.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Edit. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef]
- Ding, Y.X.; Xiang, O.Y.; Shang, C.H. Molecular cloning, characterization, and differential expression of a farnesyl-diphosphate synthase gene from the basidiomycetous fungus ganoderma lucidum. Biosci. Biotechnol. Biochem. 2008, 72, 1571–1579. [Google Scholar] [CrossRef]
- Kim, Y.B.; Thwe, A.A.; Li, X.; Tuan, P.A.; Lee, S.; Lee, J.W.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Accumulation of astragalosides and related gene expression in different organs of Astragalus membranaceus Bge. var mongholicus (Bge.). Molecules 2014, 19, 922–935. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeong, J.H.; Seo, J.W.; Shin, C.G.; Kim, Y.S. Enhanced triterpene and phytosterol biosynthesis in panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef]
- Xue, Z.; Duan, L.; Liu, D.; Guo, J.; Song, G.; Dicks, J.; Ómáille, P.; Osbourn, A.; Qi, X. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012, 193, 1022–1038. [Google Scholar] [CrossRef]
- Duan, Y.; Du, W.; Song, Z.; Chen, R.; Xie, K.; Liu, J.; Chen, D.; Dai, J. Functional characterization of a cycloartenol synthase and four glycosyltransferases in the biosynthesis of cycloastragenol-type astragalosides from Astragalus membranaceus. Acta Pharm. Sin. B 2023, 13, 271–283. [Google Scholar] [CrossRef]
- Tian, B.X.; Eriksson, L.A. Catalytic mechanism and product specificity of oxidosqualene-lanosterol cyclase: A QM/MM study. J. Phys. Chem. B 2012, 116, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kosmas Haralampidis, M.T.; Anne, E. Osbourn, Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of structural diversity of triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Xu, L.; Yi, Y.; Wang, L.; Wang, H.; Wang, Z.; Xing, J.; Li, P.; Zhang, X.; et al. Identification of oxidosqualene cyclases associated with saponin biosynthesis from Astragalus membranaceus reveals a conserved motif important for catalytic function. J. Adv. Res. 2023, 43, 247–257. [Google Scholar] [CrossRef]
- Sandeep; Misra, R.C.; Chanotiya, C.S.; Mukhopadhyay, P.; Ghosh, S. Oxidosqualene cyclase and CYP716 enzymes contribute to triterpene structural diversity in the medicinal tree banaba. New Phytol. 2019, 222, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef]
- Zhang, M.; Yi, Y.; Gao, B.H.; Su, H.F.; Bao, Y.O.; Shi, X.M.; Wang, H.D.; Li, F.D.; Ye, M.; Qiao, X. Functional characterization and protein engineering of a triterpene 3-/6-/2′-O-Glycosyltransferase reveal a conserved residue critical for the regiospecificity. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113587. [Google Scholar]
- Kitagawa, I.; Wang, H.; Saito, M.; Yoshikawa, M. Saponin and Sapogenol. XXXVI. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus BUNGE. (3). Astragalosides III, V, and VI. Chem. Pharm. Bull. 1983, 31, 709–715. [Google Scholar] [CrossRef][Green Version]
- Niu, Y.; Wang, H.; Xie, Z.; Whent, M.; Gao, X.; Zhang, X.; Zou, S.; Yao, W.; Yu, L. Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge. var. mongolicus (Bge.) Hsiao. Food Chem. 2011, 128, 620–626. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A review of the pharmacological action of astragalus polysaccharide. Front. Pharm. 2020, 11, 349. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; An, J.H.; Wang, Y.D.; Jin, H.Y.; Ma, S.C.; Ni, J. Study on standardization method of Astragalus polysaccharide reference substance. Chin. Tradit. Herb. Drugs 2017, 48, 4897–4903. [Google Scholar]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Gao, N. Research progress on physiological active components and pharmacological effects of Astragalus membranaceus. New Agric. 2017, 1, 20–21. [Google Scholar]
- Li, K.; Li, S.; Wang, D.; Li, X.; Wu, X.; Liu, X.; Du, G.; Li, X.; Qin, X.; Du, Y. Extraction, characterization, antitumor and immunological activities of hemicellulose polysaccharide from Astragalus radix herb residue. Molecules 2019, 24, 3644. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, C.; Gao, H.; Song, J.; Li, H. Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine 2010, 28, 5614–5616. [Google Scholar] [CrossRef]
- Liu, A.J.; Yu, J.; Ji, H.Y.; Zhang, H.C.; Zhang, Y.; Liu, H.P. Extraction of a novel cold-water-soluble polysaccharide from Astragalus membranaceus and its antitumor and immunological activities. Molecules 2017, 23, 62. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Li, J.; Tang, S.; Wang, M.; Huang, W.; Yao, W.; Gao, X. A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydr. Polym. 2019, 205, 500–512. [Google Scholar] [CrossRef]
- Yin, J.Y.; Chan, B.C.; Yu, H.; Lau, I.Y.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, C.B.; Xie, M.Y.; Fung, K.P.; et al. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, S.; Du, Y.; Qin, X. Screening and structure study of active components of Astragalus polysaccharide for injection based on different molecular weights. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1152, 122255. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zhang, W.; Dhananjay, Y.; An, E.K.; Kwak, M.; You, S.; Lee, P.C.; Jin, J.O. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int. J. Biol. Macromol. 2021, 182, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Structural characterization of an intestinal immune system-modulating arabino-3,6-galactan-like polysaccharide from the above-ground part of Astragalus membranaceus (Bunge). Carbohydr. Polym. 2016, 136, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, D.; Yang, L.; Zhu, N.; Li, J.; Zhao, J.; Hu, Z.; Wang, F.J.; Zhang, L.W. Comparative studies on the immunoregulatory effects of three polysaccharides using high content imaging system. Int. J. Biol. Macromol. 2016, 86, 28–42. [Google Scholar] [CrossRef]
- Li, C.; Talapphet, N.; Palanisamy, S.; Ma, N.; Cho, M.L.; You, S. The relationship between structural properties and activation of RAW264.7 and natural killer (NK) cells by sulfated polysaccharides extracted from Astragalus membranaceus roots. Process Biochem. 2020, 97, 140–148. [Google Scholar] [CrossRef]
- Shimizu, N.; Kanari, M.T.; Gonda, M.R. An acidic polysaccharide having activity on the reticuloendothelial system from the root of Astragalus mongholicus. Chem. Pharm. Bull. 1991, 39, 2969–2972. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, Q.Y.; Jiao, L.; Han, T.; Zhang, H.; Qin, L.P.; Khalid, R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomedicine 2009, 16, 805–813. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, X.; Gao, K.; Liu, W.; Li, N.; Cheng, N.; Ding, G.; Huang, W.; Wang, Z.; Xiao, W. Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconj. J. 2016, 33, 755–761. [Google Scholar] [CrossRef]
- Fu, J.; Huang, L.; Zhang, H.; Yang, S.; Chen, S. Structural features of a polysaccharide from Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. J. Asian Nat. Prod. Res. 2013, 15, 687–692. [Google Scholar] [CrossRef]
- Liao, J.; Li, C.; Huang, J.; Liu, W.; Chen, H.; Liao, S.; Chen, H.; Rui, W. Structure characterization of honey-processed astragalus polysaccharides and its anti-inflammatory activity in vitro. Molecules 2018, 23, 168. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohyd. Polym. 2009, 78, 343–348. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.Y.; Liu, A.J. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef]
- Ikbal, A.M.A.; Rajkhowa, A.; Debnath, B.; Singh, W.S.; Manna, K.; Bhattacharjee, B.; Goswami, S. Pharmacological Review on Astragalus membranaceus: Chinese Traditional Herb. Pharmacogn. Rev. 2022, 16, 90–94. [Google Scholar] [CrossRef]
- El Dib, R.A.; Soliman, H.S.M.; Hussein, M.H.; Attia, H.G. Two new flavonoids and biological activity of Astragalus abyssinicus (Hochst.) Steud. ex A. Rich. Aerial Parts. Drug Res. 2014, 65, 259–265. [Google Scholar] [CrossRef]
- Krasteva, I.; Bratkov, V.; Bucar, F.; Kunert, O.; Kollroser, M.; Kondeva-Burdina, M.; Ionkova, I. Flavoalkaloids and flavonoids from Astragalus monspessulanus. J. Nat. Prod. 2015, 78, 2565–2571. [Google Scholar] [CrossRef]
- Guo, K.; He, X.; Zhang, Y.; Li, X.; Yan, Z.; Pan, L.; Qin, B. Flavoniods from aerial parts of Astragalus hoantchy. Fitoterapia 2016, 114, 34–39. [Google Scholar] [CrossRef]
- Li, Y.; Wei, W.; Zhang, J.; Li, G.; Gao, K. Structures and antipathogenic fungi activities of flavonoids from pathogen-infected Astragalus adsurgens. Nat. Prod. Res. 2019, 33, 822–826. [Google Scholar] [CrossRef]
- Aslanipour, B.; Gülcemal, D.; Nalbantsoy, A.; Yusufoglu, H.; Bedir, E. Cycloartane-type glycosides from Astragalus brachycalyx FISCHER and their effects on cytokine release and hemolysis. Phytochem. Lett. 2017, 21, 66–73. [Google Scholar] [CrossRef]
- Denizli, N.; Horo, I.; Gülcemal, D.; Masullo, M.; Festa, M.; Capasso, A.; Alankuş-Çalışkan, Ö. Cycloartane glycosides from Astragalus plumosus var. krugianus and evaluation of their antioxidant potential. Fitoterapia 2014, 92, 211–218. [Google Scholar] [CrossRef]
- Graziani, V.; Esposito, A.; Scognamiglio, M.; Chambery, A.; Russo, R.; Ciardiello, F.; D’Abrosca, B. Spectroscopic characterization and cytotoxicity assessment towards human colon cancer cell lines of acylated cycloartane glycosides from Astragalus boeticus L. Molecules 2019, 24, 1725. [Google Scholar] [CrossRef]
- Un, R.; Horo, I.; Masullo, M.; Falco, A.; Senol, S.G.; Piacente, S.; Alankuş-Çalıskan, Ö. Cycloartane and oleanane-type glycosides from Astragalus pennatulus. Fitoterapia 2016, 109, 254–260. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, L.-Y.; Zhou, H.; Li, Y.-Y.; Wei, K.-X.; Li, C.-H.; Zhou, T.; Wang, J.-F.; Wei, W.-J.; Hua, J.-R.; et al. Astragalus polysaccharide inhibits radiation-induced bystander effects by regulating apoptosis in Bone Mesenchymal Stem Cells (BMSCs). Cell Cycle 2020, 19, 3195–3207. [Google Scholar] [CrossRef]
- She, Y.; Zhao, X.; Wu, P.; Xue, L.; Liu, Z.; Zhu, M.; Yang, J.; Li, Y. Astragalus polysaccharide protects formaldehyde-induced toxicity by promoting NER pathway in bone marrow mesenchymal stem cells. Folia Histochem. Cytobiol. 2021, 59, 124–133. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Q.; Zheng, H.; Li, L.; Liu, M.; Cheng, H.; Wong, T.; Zhang, G.; Lu, A.; Bian, Z.; et al. Radix Astragali polysaccharide RAP directly protects hematopoietic stem cells from chemotherapy-induced myelosuppression by increasing FOS expression. Int. J. Biol. Macromol. 2021, 183, 1715–1722. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yao, Y.M.; Zhang, S.W.; Sheng, Z.Y. Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J. Ethnopharmacol. 2011, 136, 457–464. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Tian, Z.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κB activation. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef]
- Yang, M.; Lin, H.-B.; Gong, S.; Chen, P.-Y.; Geng, L.-L.; Zeng, Y.-M.; Li, D.-Y. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine 2014, 70, 81–86. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yang, X.; Yao, J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef]
- Wei, W.; Xiao, H.T.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Han, X.Q.; Ko, C.H.; Lau, C.B.-S.; Wong, C.K.; Fung, K.P.; et al. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharmacol. 2016, 179, 243–252. [Google Scholar] [CrossRef]
- Meng, F.; Xu, P.; Wang, X.; Huang, Y.; Wu, L.; Chen, Y.; Teng, L.; Wang, D. Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saud. Pharm. J. 2017, 25, 460–463. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, L.; Cui, N.; Ji, X.; Jiang, H.; Deng, T.; Wang, S. Polysaccharides from radix astragali exert immunostimulatory effects to attenuate the dampness stagnancy due to spleen deficiency syndrome. Pharmacogn. Mag. 2019, 15, 500–506. [Google Scholar]
- Guo, Z.; Xu, H.Y.; Xu, L.; Wang, S.S.; Zhang, X.M. In vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 60–73. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Chen, H. Astragaloside IV inhibits cell migration and viability of hepatocellular carcinoma cells via suppressing long noncoding RNA ATB. Biomed. Pharmacother. 2018, 99, 134–141. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, W.; Zhang, C.; Wang, H.; Cheng, W.; Li, Z.; Qian, Y.; Li, X.; Yu, Y. Disrupted topological organization of the motor execution network in alcohol dependence. Psychiatry Res.-Neuroimaging 2018, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef]
- Na, D.; Liu, F.N.; Miao, Z.F.; Du, Z.M.; Xu, H.M. Astragalus extract inhibits destruction of gastric cancer cells to mesothelial cells by anti-apoptosis. World J. Gastroenterol. 2009, 15, 570–577. [Google Scholar] [CrossRef]
- Boersma, B.J.; Barnes, S.; Kirk, M.; Wang, C.C.; Darley-Usmar, V.M. Soy isoflavonoids and cancer—Metabolism at the target site. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2001, 480–481, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Auyeung, K.K.; Sze, S.C.; Zhang, S.; Yung, K.K.; Ko, J.K. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: A “TGF-beta paradox”. Phytomedicine 2020, 68, 153177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Zheng, Y.H.; Que, Z.J.; Zhang, L.L.; Lin, S.C. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J. Cancer Res. Clin. 2014, 140, 1883–1890. [Google Scholar] [CrossRef]
- Tin, M.M.Y.; Cho, C.-H.; Chan, K.; James, A.E.; Ko, J.K.S. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis 2007, 28, 1347–1355. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-kappaB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, T.; Zheng, L.; Chen, H.; Ko, J.K.; Auyeung, K.K. Astragalus saponins inhibits lipopolysaccharide-induced inflammation in mouse macrophages. Am. J. Chin. Med. 2016, 44, 579–593. [Google Scholar] [CrossRef]
- Yu, D.; Duan, Y.; Bao, Y.; Wei, C.; An, L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J. Ethnopharmacol. 2005, 98, 89–94. [Google Scholar] [CrossRef]
- Chen, R.-Z.; Tan, L.; Jin, C.-G.; Lu, J.; Tian, L.; Chang, Q.-Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crop. Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Ju, Y.; Su, Y.; Chen, Q.; Ma, K.; Ji, T.; Wang, Z.; Li, W.; Li, W. Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother. 2019, 109, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Siu, F.; Zhang, Y. Effect of astragaloside IV on diabetic gastric mucosa in vivo and in vitro. Am. J. Trans. Res. 2017, 9, 4902–4913. [Google Scholar]
- Jianming, W.; Xiao, K.; Na, M.; Wei, W.; Wei, F.; Hongcheng, Z.; Manxi, Z.; Xiaoping, G.; Zhirong, Z. Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathway. Drug Des. Dev. Ther. 2016, 10, 3071–3081. [Google Scholar]
- You, L.; Fang, Z.; Shen, G.; Wang, Q.; He, Y.; Ye, S.; Wang, L.; Hu, M.; Lin, Y.; Liu, M.; et al. Astragaloside IV prevents high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK pathway in human umbilical vein endothelial cells. Mol. Med. Rep. 2019, 19, 1603–1612. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wu, C.Y.; Cheng, J.T. Merit of Astragalus polysaccharide in the improvement of early diabetic nephropathy with an effect on mRNA expressions of NF-kappaB and IkappaB in renal cortex of streptozotoxin-induced diabetic rats. J. Ethnopharmacol. 2007, 114, 387–392. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar]
- Wang, C.; Li, Y.; Hao, M.; Li, W. Astragaloside IV inhibits triglyceride accumulation in insulin-resistant HepG2 cells via AMPK-induced SREBP-1c phosphorylation. Front. Pharmacol. 2018, 9, 338–345. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, S.; Li, A.; Mohammad, I.S.; Liu, B.; Li, Y. Astragaloside IV inhibits adipose lipolysis and reduces hepatic glucose production via Akt dependent PDE3B expression in HFD-Fed mice. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Zhang, X.; Jia, X.; Cheng, Y.; Song, P.; Liu, E.; He, Q.; Li, Z. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin. Med. J. 2008, 121, 1010–1014. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Q.; Zhang, L.; Wang, R.; Xu, X.; Hu, X. Astragalus Membranaceus treatment protects raw264.7 cells from influenza virus by regulating G1 phase and the TLR3-mediated signaling pathway. Evid. Based Complement. Altern. Med. 2019, 2019, 2971604. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, E.B.; Dieterich, D.T.; Pawlotsky, J.M.; Benhamou, Y. Chronic hepatitis B: Preventing, detecting, and managing viral resistance. Clin. Gastroenterol. Hepatol. 2008, 6, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.S.; Jia, X.L.; Song, P.; Cheng, Y.A.; Zhang, X.; Sun, M.Z.; Liu, E.Q. Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J. Gastroenterol. 2009, 15, 5669–5673. [Google Scholar] [CrossRef]
- Du, X.; Zhao, B.; Li, J.; Cao, X.; Diao, M.; Feng, H.; Chen, X.; Chen, Z.; Zeng, X. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int. Immunopharmacol. 2012, 14, 463–470. [Google Scholar] [CrossRef]
- Wang, D.; Zhuang, Y.; Tian, Y.; Thomas, G.N.; Ying, M.; Tomlinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, Q.; Wu, M.Y.; Guo, D.J.; Chen, H.L.; Chen, S.L.; Seto, S.W.; Au, A.L.; Poon, C.C.; Leung, G.P.; et al. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J. Nutr. Biochem. 2010, 21, 613–620. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, Y.Y.; Cheng, J.; Zhao, Y.Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol. Sin. 2006, 27, 1007–1012. [Google Scholar] [CrossRef]
- Zhu, H.; Zou, L.; Tian, J.; Lin, F.; He, J.; Hou, J. Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Planta Med. 2014, 80, 262–268. [Google Scholar] [CrossRef]
- Zhu, J.B.; Wan, X.Y.; Zhu, Y.P.; Ma, X.L.; Zheng, Y.W.; Zhang, T.B. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J. Appl. Toxicol. 2009, 29, 381–385. [Google Scholar]
- Wan, X.Y.; Zhu, J.B.; Zhu, Y.P.; Ma, X.L.; Zheng, Y.W.; Zhang, T.B. Effect of astragaloside IV on the general and peripartum reproductive toxicity in Sprague-Dawley rats. Int. J. Toxicol. 2010, 29, 505–516. [Google Scholar]
- Mao, S.; Ouyang, W.; Zhou, Y.; Zeng, R.; Zhao, X.; Chen, Q.; Zhang, M.; Hinek, A. Addition of Chinese herbal remedy, Tongguan Capsules, to the standard treatment in patients with myocardial infarction improve the ventricular reperfusion and remodeling: Proteomic analysis of possible signaling pathways. J. Ethnopharmacol. 2020, 257, 112794. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.H.; Song, J.; Jee, H.J.; Cha, S.H.; Chang, G.T. Effects of Astragalus extract mixture HT042 on height growth in children with mild short stature: A multicenter randomized controlled trial. Phytother. Res. 2018, 32, 49–57. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, P.; Zhang, Z.; Youn, J.Y.; Wang, C.; Zhang, H.; Cai, H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol. Ther. 2021, 225, 107843. [Google Scholar] [CrossRef]
- Leung, E.L.H.; Pan, H.D.; Huang, Y.F.; Fan, X.X.; Wang, W.Y.; He, F.; Liu, L. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering 2020, 6, 1099–1107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).