Impact of UV Irradiation on the Chitosan Bioactivity for Biopesticide Applications
Abstract
1. Introduction
2. Results
2.1. Characterization of Non-Irradiated and Irradiated Chitosans
2.1.1. Physicochemical Characteristics
2.1.2. Spectral Characteristics
- Fourier-Transform Infrared Spectroscopy
- Mass spectrometry
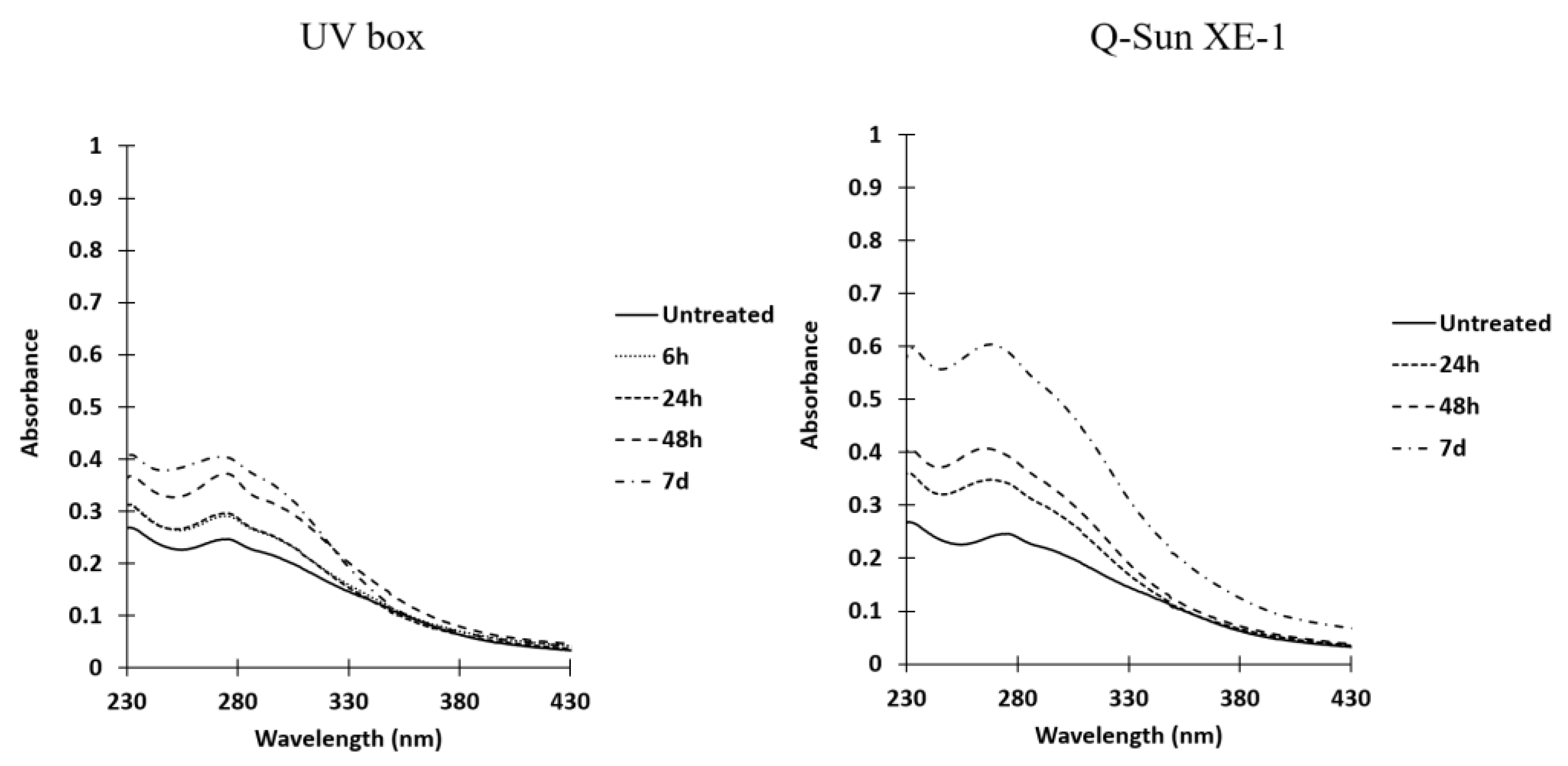
- Comparison of UV spectra of non-irradiated and irradiated chitosans in UV-box.
- Comparison of UV spectra between non-irradiated and irradiated chitosan as a function of irradiation time (Figure 5).
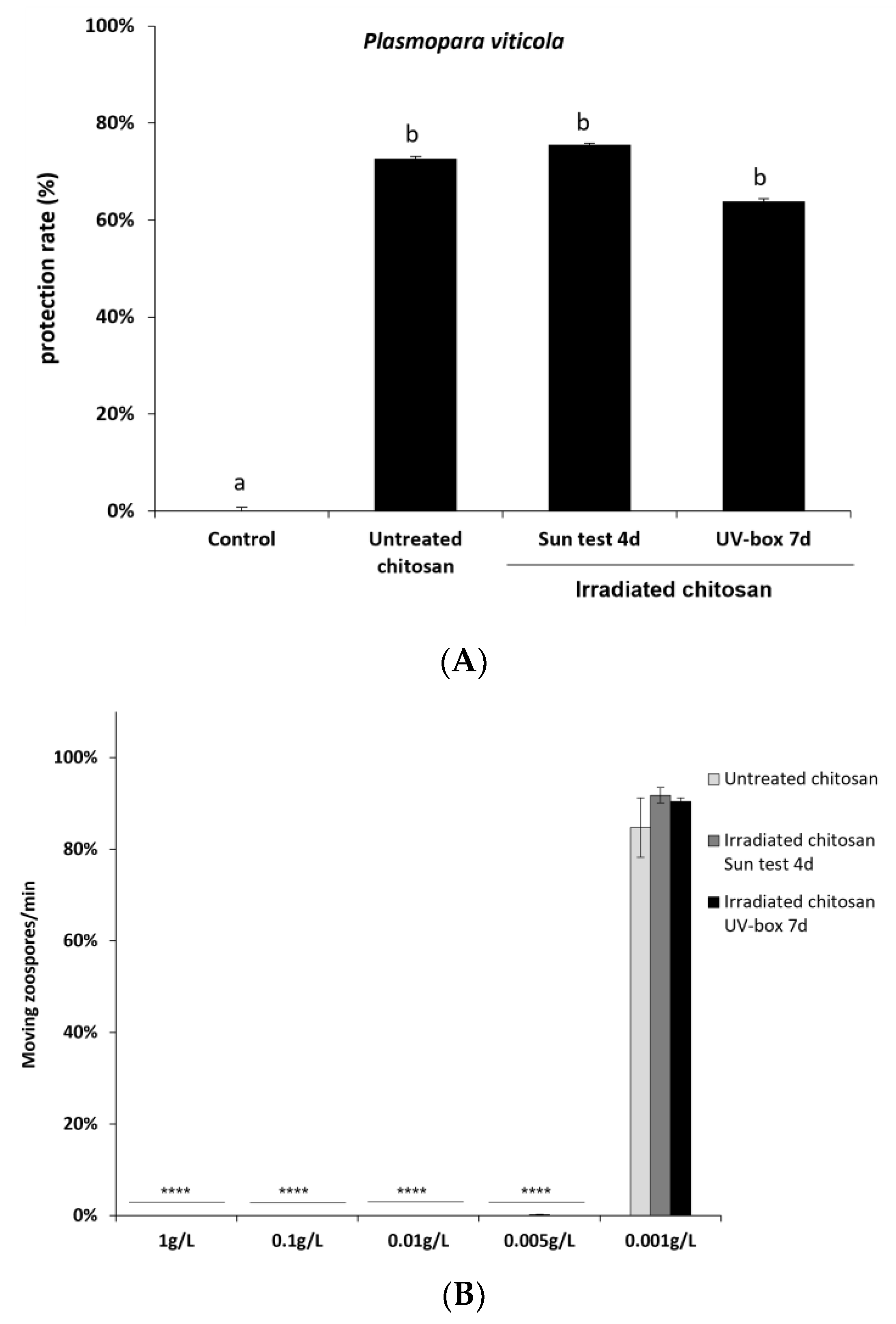
2.2. Bioactivity of Non-Irradiated and Irradiated Chitosan
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Elemental Analyses
4.2.2. Degree of Polymerization (DP) by 1H NMR
4.2.3. Analysis of Chitosan Powder Surface using XPS
4.2.4. Mass Spectrometry
- Electrospray
- MALDI-TOF
4.2.5. ATR-FTIR Spectra
4.2.6. Ultraviolet Irradiation
- Preparation of chitosan acetate from commercial chitosan
- UV irradiation of solid commercial chitosan and chitosan acetate
- (1)
- The Petri dishes were placed in a laboratory-made irradiation chamber (named “UV-box” in the text) equipped with four black light UV lamps (Mazdafluor TFWN 18) emitting mainly at 350 nm. The distance between the lamp and the sample was ~25 cm, the light intensity was 0.25 mW/cm2, and the temperature was maintained at 25 °C using a fan.
- (2)
- The Petri dishes were covered with a borosilicate lid to protect the Q-Sun XE-1 test chamber (Q-Lab Corporation, Westlake, OH, USA) from potential gas and dust that could go outside. The device was equipped with a xenon source and a Daylight-Q filter. The irradiance was equal to 0.47 W/m2 at 340 nm. The chamber was ventilated to maintain the temperature at 50 °C.
- UV–vis analysis of irradiated chitosans
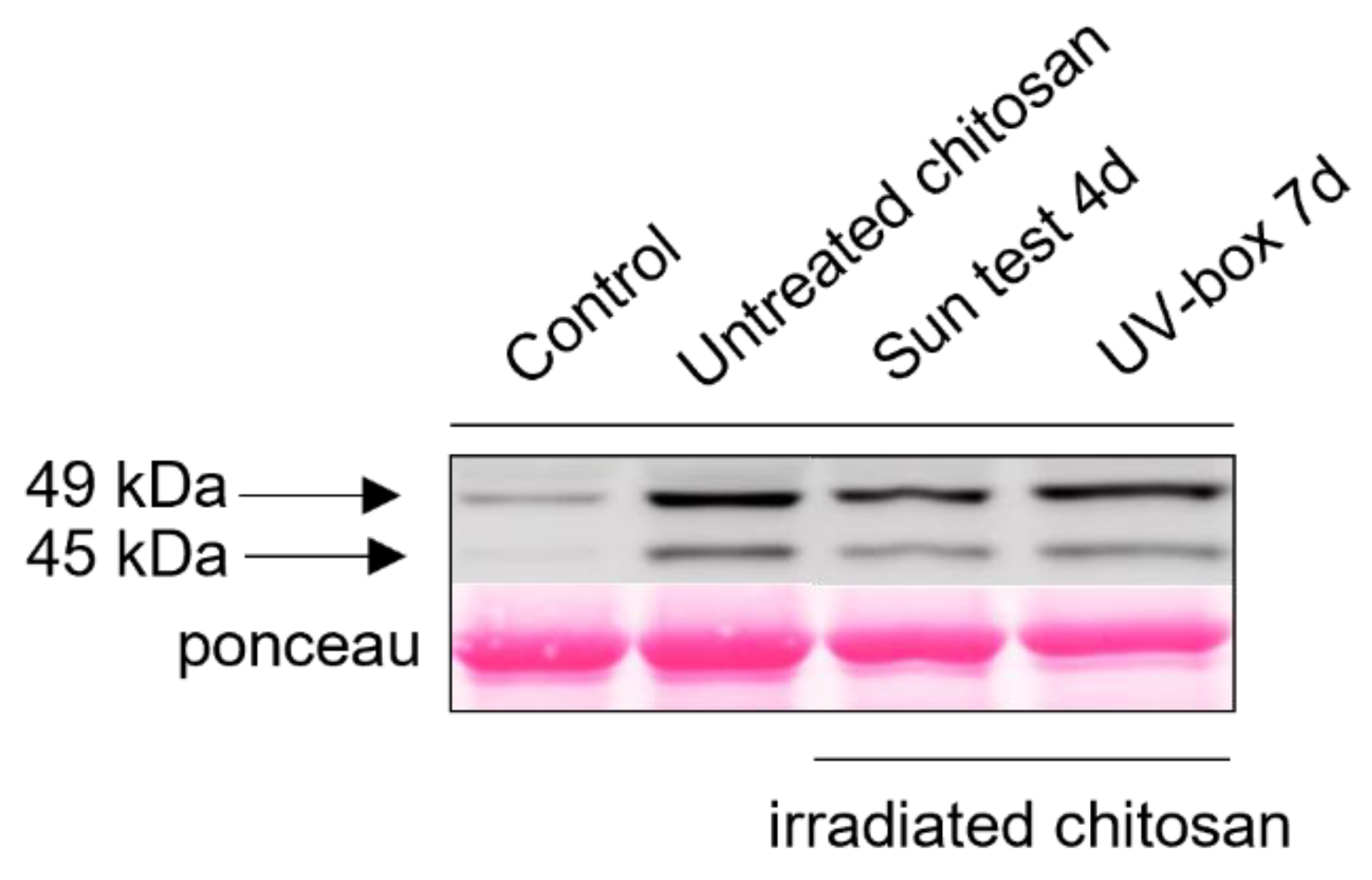
4.2.7. MAPK Activation
4.2.8. Downy Mildew Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; de Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hedge, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Florez, M.; Guerra-Rodriguez, E.; Cazon, P.; Vazquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Lima, R.; Fernandes, C.; Pinto, M.M.M. Molecular modifications, biological activities, and applications of chitosan and derivatives: A recent update. Chirality 2022, 34, 1166–1190. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Biopolymers and their derivatives: Key components of advanced biomedical technologies. Biotechnol. Adv. 2022, 61, 108056. [Google Scholar] [CrossRef]
- Guzman, E.; Ortega, F.; Rubio, R.G. Chitosan: A promising multifunctional cosmetic ingredient for skin and hair care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Sajid, M. Chitosan-based adsorbents for analytical sample preparation and removal of pollutants from aqueous media: Progress, challenges and outlook. Trends Environ. Anal. Chem. 2022, 36, e00815. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- El-Sheikh, E.-S.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.-B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef]
- European Union. Commission Implementing Regulation (EU) 2022/456 approving the basic substance chitosan in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Implementing Regulation (EU) No 540/2011. Off. J. the Eur. Union 2022. [Google Scholar]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Marine Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Panda, K.; Acharya, K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol. Biochem. 2017, 115, 298–307. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy midew. Phytopathology 2006, 96, 1188–1194. [Google Scholar]
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry 2018, 156, 1–8. [Google Scholar] [CrossRef]
- Brulé, D.; Villano, C.; Davies, L.J.; Trdá, L.; Claverie, J.; Héloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The Grapevine (Vitis vinifera) LysM Receptor Kinases VvLYK1-1 and VvLYK1-2 Mediate Chitooligosaccharide-Triggered Immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef]
- Loron, A.; Wang, Y.; Atanasova, V.; Richard-Forget, F.; Gardrat, C.; Coma, V. Chitosan for eco-friendly control of mycotoxinogenic Fusarium graminearum. Food Hydrocoll. 2023, 134, 108067. [Google Scholar] [CrossRef]
- Malausa, T. Le biocontrôle: Introduction, état des lieux, perspectives. In Actes du Colloque Construisons l’Avenir avec le Biocontrôle; Rencontres Régionales de la Recherche, du Développement et de la Formation; Chambres d’agriculture Nouvelle Aquitaine: Libourne Montagne, France, 2017; pp. 9–11. [Google Scholar]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandeka, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Andrady, A.L.; Torikai, A.; Kobatake, T. Spectral sensitivity of chitosan photodegradation. J. Appl. Polym. Sci. 1996, 62, 1465–1471. [Google Scholar] [CrossRef]
- Mucha, M.; Pawlak, A. Complex study on chitosan degradability. Polimery 2002, 47, 509–516. [Google Scholar] [CrossRef]
- Sionkowska, A. Effects of solar radiation on collagen and chitosan films. J. Photochem. Photobiol. B Biol. 2006, 82, 9–15. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kazmarek, H.; Wisniewski, M.; Skopinska, J.; Lazare, S.; Tokarev, V. The influence of UV irradiation on the surface of chitosan films. Surf. Sci. 2006, 600, 3775–3779. [Google Scholar] [CrossRef]
- Sionkowska, A.; Planecka, A.; Kozlowska, J.; Skopinska-Wisniewska, J.; Los, P. Weathering of chitosan films in the presence of low- and high-molecular weight additives. Carbohydr. Polym. 2011, 84, 900–906. [Google Scholar] [CrossRef]
- Sionkowska, A.; Planecka, A.; Lewandowska, K.; Kaczmarek, B.; Szarszewska, P. Influence of UV-irradiation on molecular weight of chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2013, 18, 21–28. [Google Scholar] [CrossRef]
- Bussière, P.O.; Gardette, J.L.; Rapp, G.; Masson, C.; Therias, S. New insights into the mechanism of photodegradation of chitosan. Carbohydr. Polym. 2021, 259, 117715. [Google Scholar] [CrossRef]
- Wang, S.M.; Huang, Q.Z.; Wang, Q.S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res. 2005, 340, 1143–1147. [Google Scholar]
- Kowalonek, J. Studies of chitosan/pectin complexes exposed to UV radiation. Int. J. Biol. Macromol. 2017, 103, 515–524. [Google Scholar] [CrossRef]
- Kowalonek, J. Surface and thermal properties of UV-irradiated chitosan/poly(ethylene oxide) blends. J. Photochem. Photobiol. A Chem. 2017, 348, 209–218. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Vicini, S.; Marsano, E. The influence of UV irradiation on the mechanical properties of chitosan/poly(vinyl pyrrolidone) blends. Polym. Degrad. Stab. 2005, 88, 261–267. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Planecka, A.; Kozlowska, J. The influence of UV irradiation on the properties of chitosan films containing keratin. Polym. Degrad. Stab. 2010, 95, 2486–2491. [Google Scholar] [CrossRef]
- Sionkowska, A.; Planecka, A.; Lewandowska, K.; Michalska, M. The influence of UV-irradiation on thermal and mechanical properties of chitosan and silk fibroin mixtures. J. Photochem. Photobiol. B Biol. 2014, 140, 301–305. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Gnatowska, M.; Kowalonek, J. The influence of UV-irradiation on chitosan modified by the tannic acid addition. J. Photochem. Photobiol. B Biol. 2015, 148, 333–339. [Google Scholar] [CrossRef]
- Infurna, G.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Dintcheva, N.T. Effect of different processing techniques and presence of antioxidant on the chitosan film performance. J. Vinyl Addit. Technol. 2022, 28, 343–351. [Google Scholar] [CrossRef]
- Weisspflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of chitosan with different degree of deacetylation and equal viscosityin dissolved and solid state—Insights by various complimentary methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Gardrat, C.; Al Kharboutly, M.; Castellan, A.; Pianet, I.; Jarmoluk, A.; Coma, V. Chemical characterization, antioxidant and anti-listerial activity of non-animal chitosan-glucan complexes. Food Hydrocoll. 2016, 61, 338.e343. [Google Scholar] [CrossRef]
- Matienzo, L.J.; Winnacker, S.K. Dry processes for surface modification of a biopolymer: Chitosan. Macromol. Mater. Eng. 2002, 287, 871–880. [Google Scholar] [CrossRef]
- Kurozumi, S.; Kiyose, M.; Noguchi, T.; Sato, K. A novel hydrochloride free chitosan oligosaccharide production method to improve taste. Int. J. Biol. Macromol. 2019, 140, 109–118. [Google Scholar] [CrossRef]
- Allison, L.; Lutzke, A.; Reynolds, M.M. Identification of low molecular weight degradation products from chitn and chitosan by electrospray ionization time-of-flight mass spectrometry. Carbohydr. Res. 2020, 493, 108046. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Li, Z.; Guo, X.; Ling, P. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in preparation of chitosan oligosaccharides (COS) with degree of polymerization (DP) 5–12 containing well-distributed acetyl groups. Int. J. Mass Spectrom. 2010, 290, 94–99. [Google Scholar] [CrossRef]
- Kim Khiook, I.L.; Schneider, C.; Heloir, M.C.; Bois, B.; Daire, X.; Adrian, M.; Trouvelot, S. Image analysis methods for assessment of H2O2 production and Plasmopara viticola development in grapevine leaves: Application to the evaluation of resistance to downy mildew. J. Microbiol. Methods 2013, 95, 235–244. [Google Scholar] [CrossRef]
- Yu, D.; Basumatary, I.B.; Kumar, S.; Ye, F.; Dutta, J. Chitosan modified with bio-extract coating with UV filering feature. Int. J. Biol. Macromol. 2023, 230, 123145. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; Abd El-Rehim, H.A.; Elbarbary, A.M.; Hegazy, E.A. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 2010, 79, 555–562. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef]
- Steimetz, E.; Trouvelot, S.; Gindro, K.; Bordier, A.; Poinssot, B.; Adrian, M.; Daire, X. Influence of Leaf Age on Induced Resistance in Grapevine against Plasmopara viticola. Physiol. Mol. Plant Pathol. 2012, 79, 89–96. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Marzaioli, A.M.; Bedini, E.; Lanzetta, R.; Perino, V.; Parilli, M.; De Castro, C. Preparation and NMR characterization of glucosamine oligomers bearing an azide function using chitosan. Carbohydr. Polym. 2012, 90, 847–852. [Google Scholar] [CrossRef]




| Chitosan Sample | DP 1 | XPS Atomic % | Elemental Analysis 2 | DD % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Ca2p | Si2p | Cl2p | %C | %N | |||
| Before irradiation | 9 | 54.32 | 31.86 | 7.03 | 0.24 | 0.40 | 6.16 | 32.46 ± 0.01 | 5.97 ± 0.01 | 83 ± 1 |
| After irradiation | 8 | 53.62 | 32.42 | 7.29 | 0.19 | 0.43 | 6.06 | 32.39 ± 0.04 | 5.95 ± 0.01 | 82 ± 1 |
| Irradiation Time (h) | UV-Box DP | Q-Sun XE-1 DP |
|---|---|---|
| 0 | 9 | 9 |
| 4 | nd * | 8 |
| 6 | 9 | nd * |
| 24 | 9 | 8 |
| 48 | 9 | 8 |
| 96 | nd * | 8 |
| 168 | 9 | nd * |
| Dn | Non-Irradiated Chitosan | Irradiation Over 7 Days UV-Box | Irradiation Over 4 Days Q-Sun XE-1 |
|---|---|---|---|
| D2 | 1000 | 1000 | 1000 |
| D3 | 865 ± 2 | 1034 ± 2 | 1065 ± 2 |
| D4 | 704 ± 5 | 909 ± 3 | 900 ± 2 |
| D5 | 617 ± 7 | 689 ± 6 | 735 ± 4 |
| D6 | 382 ± 10 | 371 ± 9 | 422 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meynaud, S.; Huet, G.; Brulé, D.; Gardrat, C.; Poinssot, B.; Coma, V. Impact of UV Irradiation on the Chitosan Bioactivity for Biopesticide Applications. Molecules 2023, 28, 4954. https://doi.org/10.3390/molecules28134954
Meynaud S, Huet G, Brulé D, Gardrat C, Poinssot B, Coma V. Impact of UV Irradiation on the Chitosan Bioactivity for Biopesticide Applications. Molecules. 2023; 28(13):4954. https://doi.org/10.3390/molecules28134954
Chicago/Turabian StyleMeynaud, Solène, Gaël Huet, Daphnée Brulé, Christian Gardrat, Benoit Poinssot, and Véronique Coma. 2023. "Impact of UV Irradiation on the Chitosan Bioactivity for Biopesticide Applications" Molecules 28, no. 13: 4954. https://doi.org/10.3390/molecules28134954
APA StyleMeynaud, S., Huet, G., Brulé, D., Gardrat, C., Poinssot, B., & Coma, V. (2023). Impact of UV Irradiation on the Chitosan Bioactivity for Biopesticide Applications. Molecules, 28(13), 4954. https://doi.org/10.3390/molecules28134954





