Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania
Abstract
1. Introduction
- (i)
- Main soil characteristics (conductivity, pH and major elements) of fibre hemp cultivation habitat;
- (ii)
- Chemical composition of cultivated hemp (C. sativa ssp. sativa) EOs obtained from inflorescences, leaves (during various growth stages) and stems;
- (iii)
- Chemical composition of volatile organic compounds (VOCs) in C. sativa extracts of flowers, leaves, unshelled seeds and roots (collected at different hemp vegetation stages: before flowering, at flowering and at seed maturation);
- (iv)
- Main cannabinoids in hemp inflorescence and leaf (during various growth periods) extracts;
- (v)
- Total phenolic content (TPC) in hemp inflorescence, leaf (during various growth phases), unshelled seed and root aqueous extracts;
- (vi)
- AA of fibre hemp root (material collected at various growth stages) extracts and EOs obtained from leaves (in different plant vegetation periods), inflorescences and unshelled seeds evaluated by the spectrophotometric DPPH● scavenging assay;
- (vii)
- AA of fibre hemp inflorescence, leaf and seed extracts by electrochemical methods, such as cyclic and square wave voltammetry;
- (viii)
- H2O2 scavenging activity of fibre hemp roots and stems extracts.
2. Results
2.1. Soil Characteristics (Conductivity, pH and Major Elements)
2.2. Chemical Composition of Cultivated Fibre Hemp (C. sativa) EOs
2.3. Chemical Composition of VOCs in Cultivated Fibre Hemp (C. sativa) Extracts
2.4. Main Cannabinoids in Fibre Hemp (C. sativa) Extracts
2.5. TPC in Fibre Hemp (C. sativa) Extracts
3. Antioxidant Activity (AA) of Fibre Hemp (C. sativa) Extracts
3.1. AA of Fibre Hemp Root (Material Collected at Various Growing Stages) Extracts and Inflorescence, Leaf and Unshelled Seed EOs Tested by Spectrophotometric DPPH● Scavenging
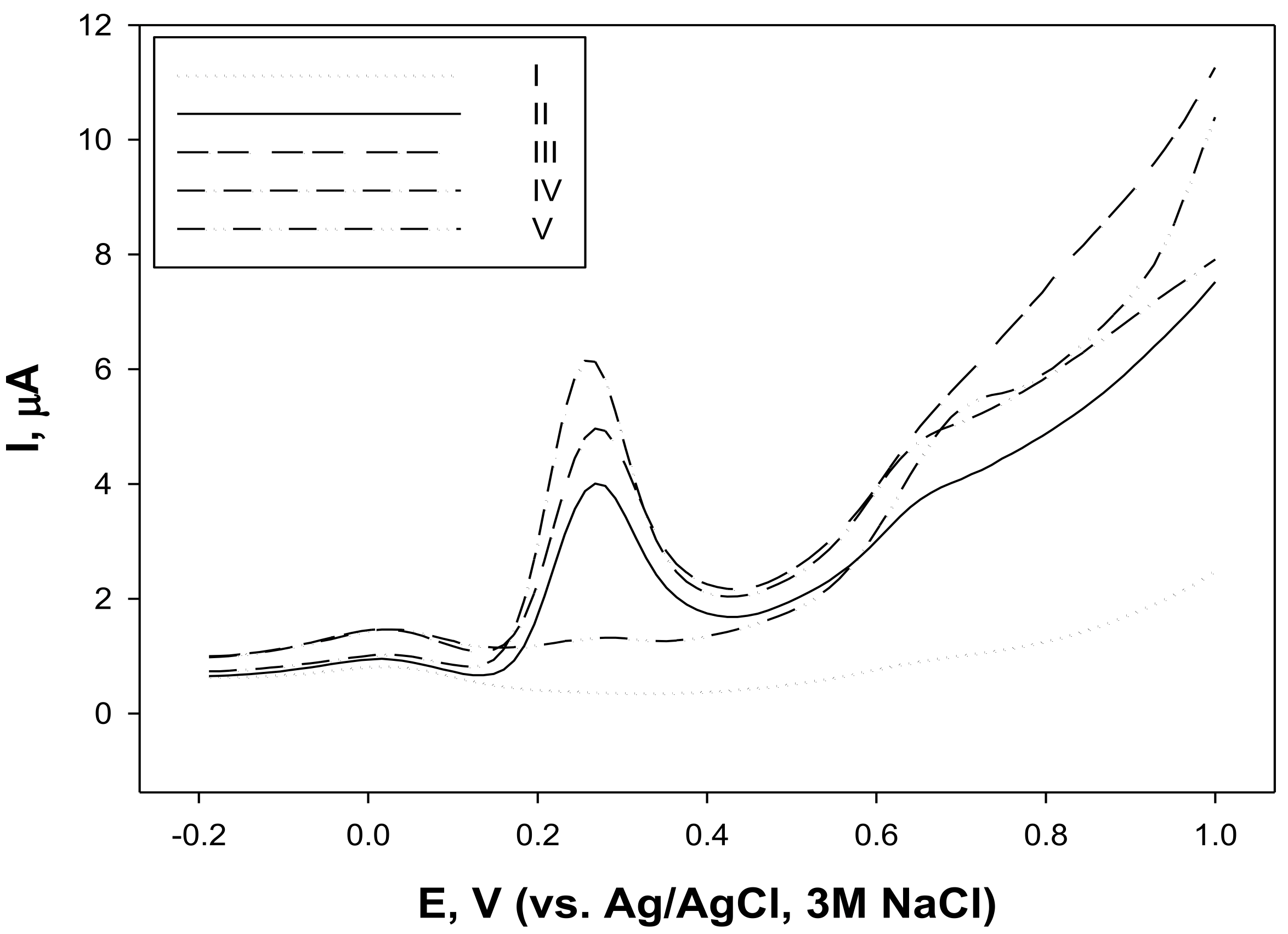
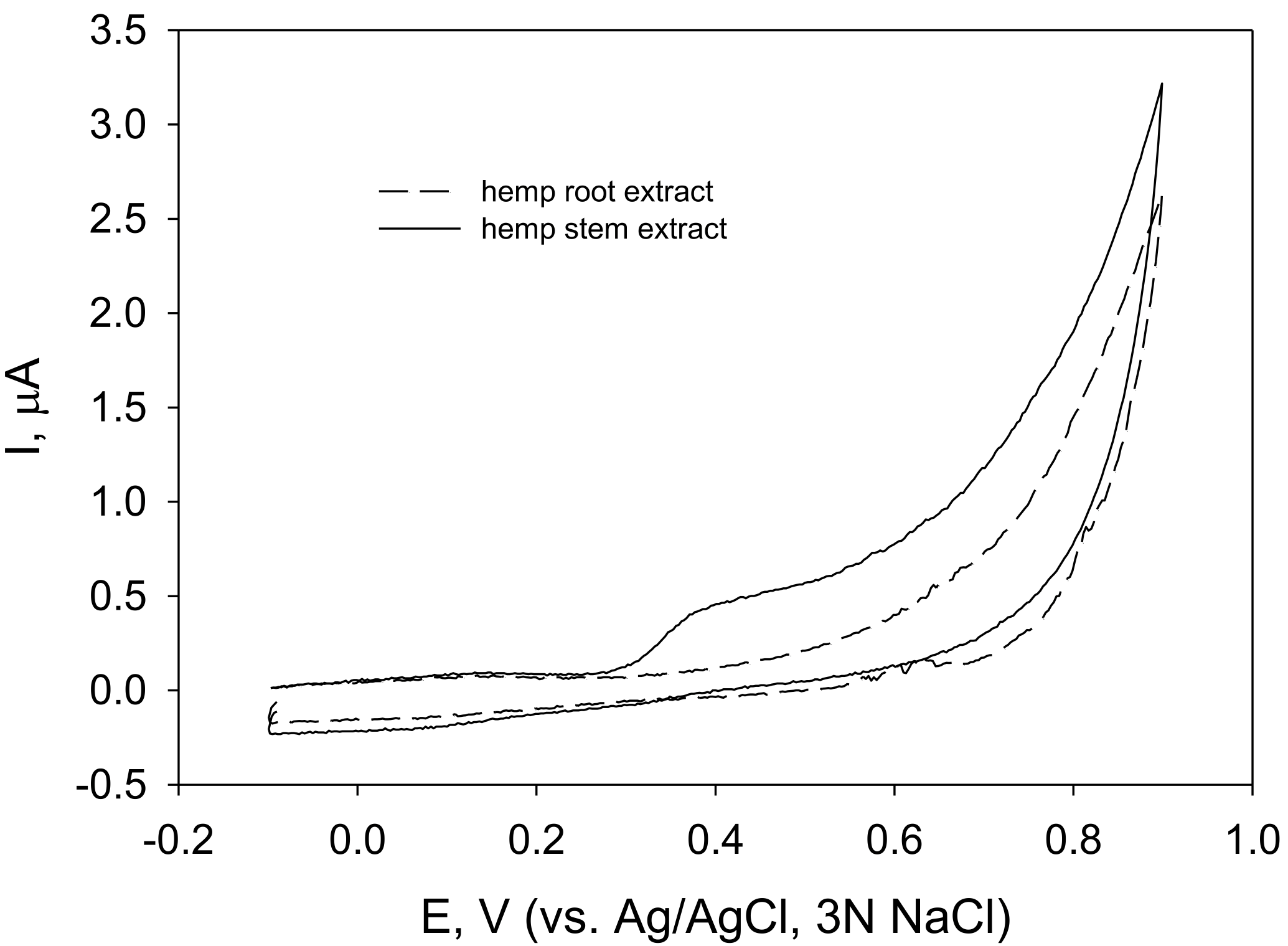
3.2. AA of Fibre Hemp (C. sativa) Inflorescence, Leaf and Seed Extracts Determined by Electrochemical Methods (Cyclic and Square Wave Voltammetry)
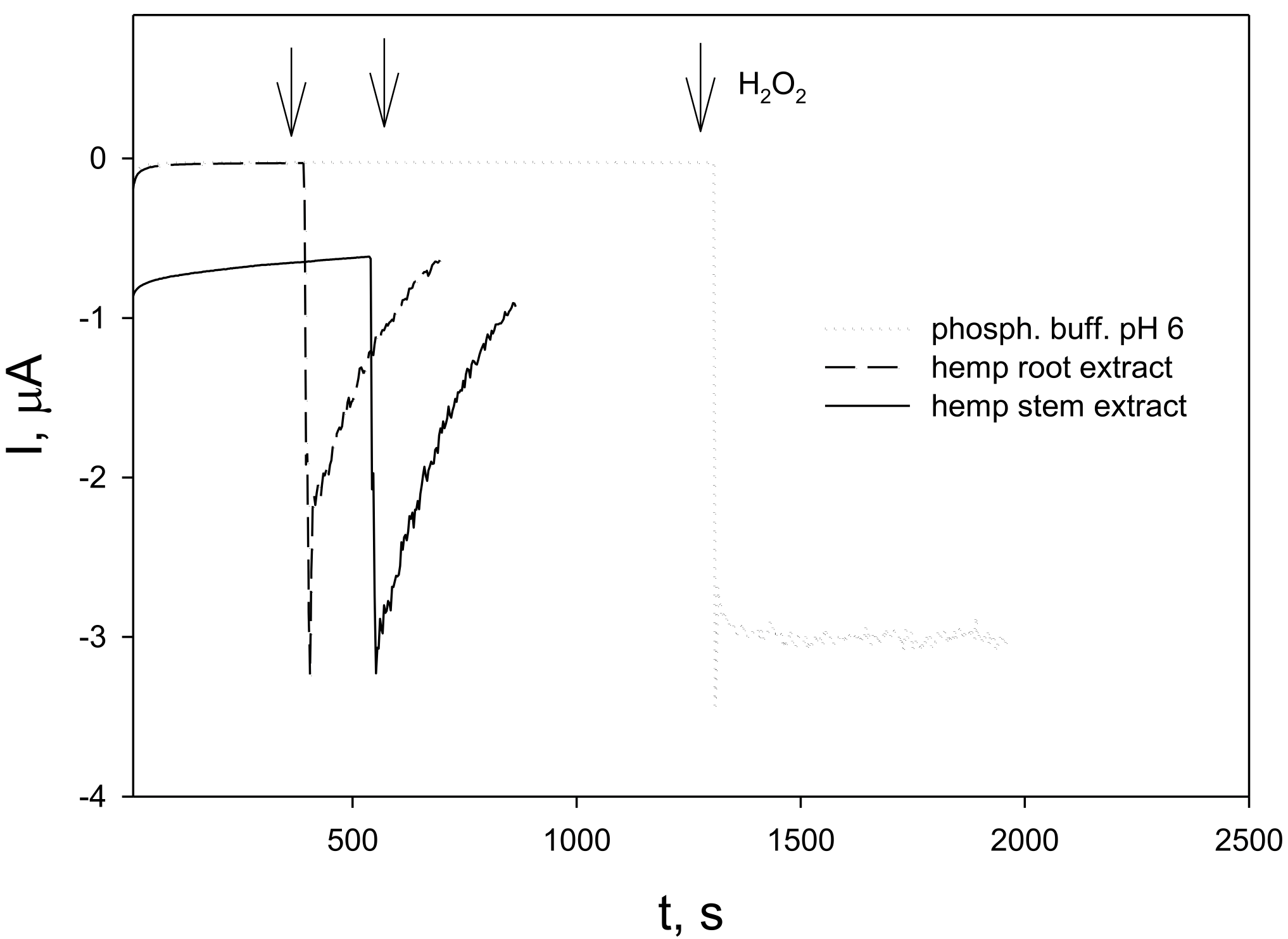
3.3. H2O2 Scavenging Activity of Fibre Hemp (C. sativa) Root and Stem Extracts
4. Discussion
5. Materials and Methods
5.1. Soil Analysis
5.2. Plant Material
5.3. EO Isolation from Different Parts of Fibre Hemp (C. sativa)
5.4. Preparation of Various Fibre Hemp (C. sativa) Extracts for Chemical Analysis
5.4.1. Extraction Procedure for GC/MS Analysis of VOCs in Hemp Methanolic Extracts
5.4.2. Preparation of Hemp Extracts for HPLC-DAD-TOF Analysis
5.4.3. Procedure of Preparation of Hemp Extracts for TPC and Free Radical Scavenging Capacity Measurements
5.4.4. Extraction Procedure for AA Tests by Electrochemical Measurements
5.5. GC Analysis of Fibre Hemp (C. sativa) Eos and Extracts
5.5.1. GC/FID (Flame-Ionization Detector) Analysis
5.5.2. GC-MS Analysis of Hemp EOs
5.5.3. GC-MS Analysis of Hemp Methanolic Extracts
5.5.4. Identification of Individual Components
5.6. HPLC-DAD-MS (TOF) Analysis of Fibre Hemp (C. sativa) Extracts
5.7. TPC in Hemp (C. sativa) Extracts
5.8. Spectrophotometric DPPH Radical Scavenging Assay
5.9. Electrochemical (Cyclic and Square Wave Voltammetry) Analysis
5.10. Hydrogen Peroxide Scavenging Test
6. Statistical Analysis
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Koren, A.; Sikora, V.; Kiprovski, B.; Brdar-Jokanović, M.; Aćimović, M.; Konstantinović, B.; Latković, D. Controversial taxonomy of hemp. Genetika 2020, 52, 1–13. [Google Scholar] [CrossRef]
- Small, E. Cannabis: A Complete Guide; CRC Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Flajšman, M.; Kocjan Ačko, D. Chapter 2—Industrial hemp breeding and genetics. In Industrial Hemp: Food and Nutraceutical Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 37–57. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Gimbutienė, M. Old Europa; Mokslo ir Enciklopedijų Leidykla: Vilnius, Lithuania, 1996. (In Lithuanian) [Google Scholar]
- Nath, M.K. Benefits of cultivating industrial hemp (Cannabis sativa ssp. sativa)—A versatile plant for a sustainable future. Chem. Proc. 2022, 10, 14. [Google Scholar] [CrossRef]
- European Commission. Agriculture and Rural Development. Hemp. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/hemp_en?ref=nangmantujapateuneoseu (accessed on 24 April 2023).
- European Industrial Hemp Association (EIHA). Hemp Cultivation & Production in Europe in 2018. 2020. Available online: https://eiha.org/wp-content/uploads/2020/06/2018-Hemp-agri-report.pdf (accessed on 12 April 2023).
- Jankauskiene, Z. Varieties of fiber hemp and their possibilities. Mano ūkis 2017, 10, 1–3. (In Lithuanian). Available online: https://www.manoukis.lt/mano-ukis-zurnalas/2017/10/pluostiniu-kanapiu-veisles-ir-ju-potencialas/ (accessed on 17 April 2023).
- Jonaitienė, V.; Jankauskiene, Z.; Stuogė, I. Towards Industrial Applications from Science to Market: Hemp Cultivation Opportunities and Perspectives in Lithuania. In Natural Fibres: Advances in Science and Technology; Fangueiro, R., Rana, S., Eds.; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2016; Volume 12, pp. 407–414. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Tsaliki, E.; Kalivas, A.; Jankauskiene, Z.; Irakli, M.; Cook, C.; Grigoriadis, I.; Panoras, I.; Vasilakoglou, I.; Dhima, K. Fibre and seed productivity of industrial hemp (Cannabis sativa L.) varieties under Mediterranean conditions. Agronomy 2021, 11, 171. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial hemp (Cannabis sativa L.) agronomy and utilization: A review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Mass, E. Hemp: The new, old fiber makes a comeback for clothes, fabrics, and home furnishings. Nat. Life 2009, 127, 36. [Google Scholar]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crop. Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Industrial Hemp. Chapter 10—Industrial hemp foods and beverages and product properties. In Industrial Hemp Food and Nutraceutical Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 219–246. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceut. Funct. Med. Foods 2000, 2, 36–53. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Mileti, O.; Aiello, D.; Lupi, F.R.; Napoli, A.; Gabriele, D. Hemp seed oil extraction and stable emulsion formulation with hemp protein isolates. Appl. Sci. 2022, 12, 11921. [Google Scholar] [CrossRef]
- Vitorović, J.; Joković, N.; Radulović, N.; Mihajilov-Krstev, T.; Cvetković, V.J.; Jovanović, N.; Mitrović, T.; Aleksić, A.; Stanković, N.; Bernstein, N. Antioxidant activity of hemp (Cannabis sativa L.) seed oil in Drosophila melanogaster larvae under non-stress and H2O2-induced oxidative stress conditions. Antioxidants 2021, 10, 830. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef]
- Miao, C.; Hui, L.-F.; Liu, Z.; Tang, X. Evaluation of hemp root bast as a new material for papermaking. BioResources 2014, 9, 132–142. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Fournier, G.; Richez-Dumanois, C.; Duvezin, J.; Mathieu, J.-P.; Paris, M. Identification of a new chemotype in Cannabis sativa: Cannabigerol—dominant plants, biogenetic and agronomic prospects. Planta Med. 1987, 53, 277–280. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and fractionation during distillation alter hemp essential oil profile and its antimicrobial activity. Molecules 2020, 25, 3943. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Noller, J.S.; Maggi, F.; Dale, R. Terpenes and cannabinoids yields and profile from direct-seeded and transplanted CBD-Cannabis sativa. J. Agric. Food Chem. 2022, 70, 10417–10428. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and wild hemp: How different are their essential oil profile and antimicrobial activity? Molecules 2020, 25, 4631. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Palmieri, S.; Maggio, F.; Pellegrini, M.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. Effect of the distillation time on the chemical composition, antioxidant potential and antimicrobial activity of essential oils from different Cannabis sativa L. cultivars. Molecules 2021, 26, 4770. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Bruno Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xu, J.; Zhou, H.; Seeram, N.P.; Ma, H.; Gu, Q. Chemical constituents of industrial hemp roots and their anti-inflammatory activities. J. Cannabis Res. 2023, 5, 1. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, J.; Kang, Q.; Huang, J.; Cui, Y.; Zhang, G.; Wang, Z.; Zhang, L.; Zheng, Z.; Lu, J.; et al. Anti-fatigue activity of hemp leaves water extract and the related biochemical changes in mice. Food Chem. Toxicol. 2021, 150, 112054. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Eržen, M.; Košir, I.J.; Ocvirk, M.; Kreft, S.; Čerenak, A. Metabolomic analysis of cannabinoid and essential oil profiles in different hemp (Cannabis sativa L.) phenotypes. Plants 2021, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yu, W.; Wang, C.; Liu, L.; Li, F.; Tan, Z. Green extraction of cannabidiol from industrial hemp (Cannabis sativa L.) using deep eutectic solvents coupled with further enrichment and recovery by macroporous resin. J. Mol. Liq. 2019, 287, 110957. [Google Scholar] [CrossRef]
- Čalkaitė, I.; Kundrotaitė, A.; Tilvikienė, V.; Barčauskaitė, K. The effect of sowing rate on the bioaccumulation of biologically active compounds and their radical scavenging activity in Cannabis sativa L. Chemija 2022, 33, 190–200. [Google Scholar] [CrossRef]
- Gabotti, D.; Locatelli, F.; Cusano, E.; Baldoni, E.; Genga, A.; Pucci, L.; Consonni, R.; Mattana, M. Cell suspensions of Cannabis sativa (var. Futura): Effect of elicitation on metabolite content and antioxidant activity. Molecules 2019, 24, 4056. [Google Scholar] [CrossRef]
- Kubilienė, A.; Marksa, M.; Baranauskaitė, J.; Ragažinskienė, O.; Ivanauskas, L. Comparative evaluation of antioxidant activity of Cannabis sativa L. using FRAP and CUPRAC assays. Chemija 2020, 31, 156–161. [Google Scholar] [CrossRef]
- Lesma, G.; Consonni, R.; Gambaro, V.; Remuzzi, C.; Roda, G.; Silvani, A.; Vece, V.; Visconti, G.L. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014, 28, 1801–1807. [Google Scholar] [CrossRef]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schroder, K.; Lofke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Moccia, S.; Siano, F.; Russo, G.L.; Volpe, M.G.; La Cara, F.; Pacifico, S.; Piccolella, S.; Picariello, G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L. Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2019, 71, 410–423. [Google Scholar]
- Drinić, Z.; Vidović, S.; Vladić, J.; Koren, A.; Kiprovski, B.; Sikora, V. Effect of extraction solvent on total polyphenols content and antioxidant activity of Cannabis sativa L. Lek. Sirovine 2018, 38, 17–21. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Synowiec, A.; Rys, M.; Bocianowski, J.; Wielgusz, K.; Byczyñska, M.; Heller, K.; Kalemba, D. Phytotoxic effect of fiber hemp essential oil on germination of some weeds and crops. J. Essent. Oil-Bear. Plants 2016, 19, 262–276. [Google Scholar]
- De Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef] [PubMed]
- De Meijer, E.P.M.; Hammond, K.M. The inheritance of chemical phenotype in Cannabis sativa L. (II): Cannabigerol predominant plants. Euphytica 2005, 145, 189–198. [Google Scholar] [CrossRef]
- Onofri, C.; de Meijer, E.P.M.; Mandolino, G. Sequence heterogeneity of cannabidiolic- and tetrahydrocannabinolic acid-synthase in Cannabis sativa L. and its relationship with chemical phenotype. Phytochemistry 2015, 116, 57–68. [Google Scholar] [CrossRef]
- Hillig, K.W.A. Chemotaxonomic analysis of terpenoid variation in Cannabis. Biochem. Syst. Ecol. 2004, 32, 875–891. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.Y.; Li, S.H.; Ma, W.; Wu, D.T.; Li, H.B.; Xiao, A.P.; Liu, L.L.; Zhu, F.; Gan, R.Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. Trends Analyt. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanisms of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Cosio, M.S.; Burratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Blasco, A.J.; Crevillen, A.G.; Gonzalez, M.C.; Escarpa, A. Direct electrochemical sensing of natural antioxidants and antioxidant capacity in vitro systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Simic, A.; Manojlovic, D.; Segan, D.; Todorovic, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Piljac-Zegarac, J.; Valek, L.; Stipcevic, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Seruga, M.; Novak, I.; Jakobek, L. Determination of polyphenol content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of chemotypic markers in three chemotype categories of Cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in Cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Adams, R.P. Essential Oil Components by Quadrupole Gas Chromatography/Mass Spectrometry, 3rd ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2001. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley-WCH: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation optimization and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crop Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A review on the current state of knowledge of growing conditions, agronomic soil health practices and utilities of hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.H.; Deng, G. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut. Res. 2021, 28, 52832–52843. [Google Scholar] [CrossRef] [PubMed]
- Buivydaite, V.V. Soil survey and available soil data in Lithuania. Eur. Soil Bureau. Res. Rep. 2005, 9, 211–223. [Google Scholar]
- Anonymous. Regarding the Approval of the Lithuanian Hygiene Norm HN 60:2004. Maximum Permissible Concentration of Dangerous Chemical Substances in Soil. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.228693/asr (accessed on 18 April 2023).
- Happyana, S.; Agnolet, R.; Muntendam, A.; Dam, V.; Schneider, B. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mahlberg, P.; Kim, E.S. Accumulation of cannabinoids in glandular trichomes of Cannabis (Cannabaceae). J. Ind. Hemp 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Fetterman, P.S.; Keith, E.S.; Waller, C.W.; Guerrero, O.; Doorendos, N.J.; Quimby, M.W. Mississippi-grown Cannabis sativa L: Preliminary observation on chemical definition of phenotype and variations in tetrahydrocannabinol content versus age, sex, and plant part. J. Pharm. Sci. 1971, 60, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Gil, E.S.; Couto, R.O. Flavonoid electrochemistry: A review on the electroanalytical applications. Braz. J. Pharmacogn. 2013, 23, 542–558. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Bilteanu, L.; Serban, A.I. Antioxidant determination with the use of carbon-based electrodes. Chemosensors 2021, 9, 72. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef]
- Garjonyte, R.; Budiene, J.; Labanauskas, L.; Judzentiene, A. In vitro antioxidant and prooxidant activities of red raspberry (Rubus idaeus L.) stem extracts. Molecules 2022, 27, 4073. [Google Scholar] [CrossRef]

| Sampling Site | Conductivity, µS/cm, SD | pH Value, SD |
|---|---|---|
| I | 79.14 (2.23) | 5.78 (0.31) |
| II | 143.13 (3.56) | 6.04 (0.21) |
| III | 119.22 (2.23) | 6.09 (0.05) |
| IV | 114.09 (4.2) | 6.16 (0.14) |
| V | 131.78 (3.24) | 6.41 (0.13) |
| VI | 89.55 (4.07) | 5.34 (0.14) |
| VII | 100.55 (3.22) | 5.14 (0.31) |
| VIII | 97.49 (1.20) | 5.83 (0.22) |
| IX | 103.45 (5.45) | 5.45 (0.12) |
| mg/kg | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Sites | Ca | Mg | K | Na | Al | Mn | Cu | Cd | Cr | Ni | Pb | Zn | P |
| λ, nm | 317.93 | 285.21 | 766.49 | 589.59 | 396.15 | 257.61 | 327.39 | 228.80 | 267.72 | 231.60 | 220.35 | 213.86 | 231.67 |
| I | 1057.0 | 142.2 | 111.5 | 58.7 | 1626.6 | 324.7 | 7.1 | 0.0 | 0.0 | 0.0 | 5.0 | 15.3 | 365.2 |
| SD | 27.0 | 2.1 | 3.4 | 1.0 | 31.1 | 4.4 | 1.2 | 0.0 | 0.0 | 0.0 | 1.3 | 0.6 | 26.9 |
| II | 928.6 | 142.1 | 100.8 | 71.6 | 1592.3 | 284.0 | 4.3 | 0.0 | 0.1 | 0.0 | 2.9 | 13.8 | 297.2 |
| SD | 11.7 | 1.7 | 3.3 | 3.4 | 10.0 | 7.3 | 0.4 | 0.0 | 0.0 | 0.0 | 0.8 | 1.3 | 11.6 |
| III | 1071.0 | 136.9 | 85.9 | 58.4 | 1728.6 | 333.3 | 6.0 | 0.0 | 0.1 | 0.0 | 3.6 | 14.2 | 373.6 |
| SD | 29.5 | 2.4 | 6.0 | 2.2 | 5.5 | 1.0 | 1.0 | 0.0 | 0.1 | 0.0 | 1.9 | 3.7 | 15.6 |
| IV | 1479.0 | 179.0 | 77.0 | 83.6 | 1831.3 | 421.9 | 5.9 | 0.0 | 0.0 | 0.0 | 6.0 | 14.8 | 414.0 |
| SD | 47.1 | 4.5 | 1.5 | 3.1 | 50.1 | 9.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 17.7 |
| V | 2343.0 | 211.1 | 58.0 | 62.1 | 1664.6 | 376.2 | 14.3 | 0.0 | 0.2 | 0.0 | 3.7 | 20.9 | 556.7 |
| SD | 62.8 | 9.2 | 4.1 | 2.8 | 61.6 | 9.3 | 1.3 | 0.0 | 0.1 | 0.0 | 1.3 | 1.8 | 6.1 |
| VI | 1094.0 | 217.8 | 132.5 | 82.6 | 1694.0 | 299.1 | 12.9 | 0.0 | 1.9 | 0.0 | 5.1 | 11.5 | 369.6 |
| SD | 48.2 | 4.9 | 8.6 | 4.2 | 20.2 | 2.4 | 2.6 | 0.0 | 0.1 | 0.0 | 1.1 | 2.3 | 14.6 |
| VII | 1525.0 | 277.8 | 156.6 | 73.4 | 2116.3 | 390.4 | 9.4 | 0.0 | 2.2 | 0.0 | 7.3 | 16.1 | 489.9 |
| SD | 75.3 | 3.6 | 8.2 | 2.9 | 39.5 | 5.0 | 1.6 | 0.0 | 0.4 | 0.0 | 1.9 | 2.0 | 74.9 |
| VIII | 1501.6 | 262.7 | 77.8 | 60.3 | 2144.6 | 402.2 | 8.5 | 0.1 | 2.2 | 0.0 | 9.1 | 16.3 | 513.6 |
| SD | 34.7 | 4.2 | 7.9 | 3.1 | 84.8 | 9.0 | 0.6 | 0.0 | 0.2 | 0.0 | 1.8 | 1.7 | 32.6 |
| IX | 1439.0 | 249.3 | 37.2 | 42.3 | 2261.3 | 436.1 | 7.8 | 0.0 | 2.7 | 0.0 | 7.5 | 21.3 | 516.2 |
| SD | 23.1 | 2.9 | 6.3 | 3.7 | 71.1 | 7.3 | 0.7 | 0.0 | 0.3 | 0.0 | 0.5 | 1.8 | 18.1 |
| % | |||||||
|---|---|---|---|---|---|---|---|
| Compound a | b RILit | c RIExp | Flowers | Leaves (in June) | Leaves (in August) | Leaves (in September) | Stems |
| α-Pinene * | 939 | 938 | 12.12 ± 5.81 | 2.94 ± 0.28 | 1.73 ± 0.84 | 4.76 ± 1.60 | 20.25 ± 3.38 |
| β-Pinene * | 980 | 984 | 1.89 ± 1.00 | 0.70 ± 0.06 | 5.31 ± 3.30 | 1.59 ± 0.97 | 4.82 ± 1.51 |
| β-Myrcene | 991 | 990 | 5.49 ± 3.40 | 0.38 ± 0.30 | 0.85 ± 0.25 | 0.95 ± 0.05 | 4.31 ± 0.04 |
| β-Caryophyllene * | 1419 | 1415 | 39.81 ± 7.31 | 30.37± 4.25 | 46.64 ± 4.25 | 46.54 ± 3.67 | 12.55 ± 2.04 |
| α-trans-Bergamotene | 1436 | 1439 | 3.14 ± 0.04 | 2.85 ± 0.41 | 1.00 ± 0.03 | 3.58 ± 0.05 | 0.01 ± 0.01 |
| β-trans-Farnesene | 1443 | 1445 | 4.76 ± 0.42 | 2.39 ± 0.95 | 0.67 ± 0.33 | 4.55± 0.21 | |
| α-Humulene * | 1455 | 1461 | 11.48 ± 1.82 | 10.78 ± 1.71 | 10.76 ± 5.42 | 10.40 ± 1.67 | 3.56 ± 1.01 |
| allo-Aromadendrene | 1461 | 1465 | 0.64 ± 0.58 | 1.25 ± 0.58 | 0.94 ± 0.50 | 0.57 ± 0.33 | 5.31 ± 2.07 |
| α-Selinene | 1498 | 1503 | 1.37± 0.38 | 2.68 ± 0.26 | 3.26 ± 0.77 | 4.07 ± 4.79 | 0.02 ± 0.01 |
| Caryophyllene oxide * | 1580 | 1586 | 4.13 ± 0.32 | 14.53 ± 2.96 | 10.24 ± 1.36 | 4.64 ± 2.66 | 3.14 ± 0.59 |
| Humulene epoxide II | 1606 | 1615 | 1.23 ± 0.14 | 5.86 ± 0.72 | 4.23 ± 1.25 | 3.23 ± 3.09 | 1.64 ± 1.54 |
| Caryophyla-4(12),8(13)-dien-5-α-ol | 1640 | 1639 | 0.71± 0.14 | 2.50 ± 0.76 | 3.70 ± 0.70 | 2.01 ± 0.94 | 0.04 ± 0.02 |
| Caryophyla-4(12),8(13)-dien-5-β-ol | 1640 | 1641 | 0.75± 0.08 | 3.08 ± 0.52 | 4.70 ± 0.20 | 2.75 ± 1.02 | 0.05 ± 0.01 |
| allo-Himachalol | 1662 | 1660 | 0.01 ± 0.01 | 3.50 ± 0.45 | 0.01 ± 0.01 | 2.54 ± 0.42 | 0.21 ± 0.11 |
| 14-hydroxy- cis-Caryophyllene | 1667 | 1668 | 1.48 ± 0.01 | 3.54 ± 0.38 | 3.12 ± 1.02 | 2.85 ± 0.02 | 0.01 ± 0.01 |
| Cannabidiol (CBD) * | - | 2383 | 4.05 ± 0.56 | 0.41 ± 0.10 | 3.05 ± 0.05 | 3.08 ± 0.02 | 0.04 ± 0.00 |
| Caryophyllene derivatives (average sum) | 46.88 | 54.02 | 68.40 | 58.79 | 15.79 | ||
| % | ||||
|---|---|---|---|---|
| Compound a | b RIExp | Flowers | Leaves (in August) | Unshelled Seeds |
| Heptanal | 908 | 1.75 ± 0.25 | ||
| β-Caryophyllene | 1415 | 4.67 ± 1.01 | 3.77 ± 2.25 | |
| β-trans-Farnesene | 1445 | 0.86 ± 0.22 | 0.58 ± 0.33 | |
| α-Humulene | 1461 | 1.18 ± 0.80 | 0.83 ± 0.44 | |
| Caryophyllene oxide | 1586 | 1.14 ± 0.66 | ||
| Humulene epoxide II | 1615 | 0.53 ± 0.25 | ||
| 14-hydroxy-cis-Caryophyllene | 1666 | 0.69 ± 0.41 | 1.10 ± 0.83 | |
| epi-α-Bisabolol | 1686 | 0.77 ± 0.53 | ||
| Neophytadiene | 1840 | 1.01 ± 0.80 | 0.93 ± 0.33 | |
| Heptadecanoic acid | 2084 | 2.15 ± 1.02 | ||
| 9,12-Octadecadienoic acid methyl ester | 2102 | 0.77 ± 0.05 | ||
| Methyl linoleate | 2109 | 0.50 ± 0.21 | ||
| Phytol | 2117 | 1.91 ± 0.83 | 2.28 ± 0.32 | 1.92 ± 1.00 |
| Oleic acid | 2140 | 0.94 ± 0.58 | ||
| Canabichromene | 2368 | 0.50 ± 0.32 | 0.46 ± 0.28 | |
| Cannabidiol | 2383 | 64.56 ± 2.58 | 48.41 ± 4.05 | 26.09 ± 2.75 |
| 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester | 2423 | 3.12 ± 1.01 | ||
| Dronabinol | 2470 | 1.98 ± 0.41 | 2.21 ± 0.98 | 0.54 ± 0.41 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | 2498 | 3.75 ± 0.97 | ||
| 2-Octyl-1-dodecanol | 2512 | 0.97 ± 0.08 | ||
| 2-Monolinolein | 2606 | 11.31 ± 1.92 | ||
| (Z)-5,11,14,17-Methyl eicosatet-raenoate | 2674 | 0.89 ± 0.28 | 9.70 ± 2.25 | |
| 2,3-Dihydroxypropyl-octadecanoic acid | 2714 | 1.47 ± 0.67 | ||
| Cannabigerol | 2748 | 0.36 ± 0.08 | ||
| α-Tocopherol | 3109 | 0.71 ± 0.63 | 2.70 ± 0.15 | |
| Campesterol | 3110 | 2.12 ± 0.33 | ||
| γ-Sitosterol | 3341 | 2.16 ± 0.88 | 1.61 ± 0.09 | 8.99 ± 2.71 |
| Fucosterol | 3345 | 0.88 ± 0.10 | ||
| β-Amirine | 3355 | 0.63 ± 0.28 | 2.71 ± 0.88 | |
| α-Amirine | 3376 | 0.60 ± 0.19 | 1.74 ± 0.96 | |
| Stigmast-4-en-3-one | 3458 | 1.06 ± 0.03 |
| % | ||||
|---|---|---|---|---|
| Compound a | b RIExp | Roots (Plants before Flowering) | Roots (at Flowering Stage) | Roots (at Seeding Stage) |
| Piranone | 988 | 2.61 ± 1.22 | 1.09 ± 0.93 | 3.07 ± 0.91 |
| 2,3-Dihydrobenzofuran | 1225 | 17.07 ± 2.54 | 9.01 ± 1.04 | 14.19 ± 1.08 |
| (E)-Coniferyl alcohol | 1734 | 6.35 ± 0.92 | 1.75 ± 1.07 | 4.47 ± 0.78 |
| Palmitic (hexadecanoic) acid | 1974 | 4.64 ± 1.73 | 1.26 ± 0.04 | 0.04 ± 0.01 |
| Campesterol | 3110 | 1.87 ± 0.55 | 1.11 ± 0.44 | 7.08 ± 2.66 |
| Stigmasterol | 3310 | 1.36 ± 0.22 | 0.76 ± 0.33 | 2.98 ± 1.45 |
| γ-Sitosterol | 3341 | 6.64 ± 1.77 | 14.03 ± 1.08 | 13.99 ± 2.42 |
| Stigmastanol | 3349 | 3.09 ± 0.41 | 0.11 ± 0.04 | 0.01 ± 0.01 |
| β-Amirine | 3355 | 3.06 ± 0.47 | 2.25 ± 0.55 | 1.26 ± 0.42 |
| Phytol acetate * | 3488 | 0.21 ± 0.21 | 8.35 ± 1.01 | 0.09 ± 0.05 |
| Friedelan-3-one | 3510 | 21.49 ± 3.03 | 16.39 ± 2.82 | 16.96 ± 3.01 |
| (Z,Z)- 9-Octadecenyl ester 9-hexadecenoic acid | 3515 | 10.04 ± 0.77 | 31.18 ± 2.33 | 31.27 ± 1.77 |
| % | ||||
|---|---|---|---|---|
| Plant Organ | CBN Observed m/z [M+H]+ 311.43, Da | CBDA Observed m/z [M+H]+ 359.22, Da | CBD * Observed m/z [M+H]+ 315.23, Da | Total Sum of THC Isomers |
| Leaves before flowering | n.d. | 24.11 ± 2.04 | 3.24 ± 0.43 | 0.10 ± 0.01 |
| Flowering tops | n.d. | 16.31 ± 1.85 | 32.73 ± 2.70 | 22.43 ± 2.04 |
| Leaves in flowering stage | n.d. | 18.20 ± 1.31 | 26.84 ± 1.61 | 10.53 ± 0.72 |
| Leaves in seed maturing stage | n.d. | 24.21 ± 3.02 | 26.54 ± 2.03 | 3.45 ± 0.98 |
| PLANT ORGAN | Leaves before Flowering | Flowering Tops | Leaves in Flowering Stage | Leaves in Seed Maturing Stage | Unshelled SEEDS | Roots |
|---|---|---|---|---|---|---|
| TPC, mg/L GAE | 422.2 (16.6) | 924.7 (5.5) | 922.2 (32.6) | 573.9 (31.7) | 187.9 (3.1) | 223.2 (10.8) |
| Root Extract | DPPH● Scavenging Activity TROLOX (mmol/L) | Essential Oil | DPPH● Scavenging Activity TROLOX (mmol/L) |
|---|---|---|---|
| Before blooming (June) | 0.290 ± 0.116 | Leaf (before blooming/June) | 15.034 ± 0.408 |
| Before blooming (Jully) | 0.562 ± 0.166 | Leaf (before blooming/Jully) | 21.662 ± 0.772 |
| Flowering stage (August) | 1.023 ± 0.005 | Inflorescences (August) | 16.683 ± 0.384 |
| Seed maturation stage (September) | 1.556 ± 0.004 | Leaf (blooming/August) | 35.036 ± 0.355 |
| Leaf (seed maturation stage/September) | 20.311 ± 0.171 | ||
| Unshelled seeds (September) | 13.187 ± 0.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judžentienė, A.; Garjonytė, R.; Būdienė, J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania. Molecules 2023, 28, 4928. https://doi.org/10.3390/molecules28134928
Judžentienė A, Garjonytė R, Būdienė J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania. Molecules. 2023; 28(13):4928. https://doi.org/10.3390/molecules28134928
Chicago/Turabian StyleJudžentienė, Asta, Rasa Garjonytė, and Jurga Būdienė. 2023. "Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania" Molecules 28, no. 13: 4928. https://doi.org/10.3390/molecules28134928
APA StyleJudžentienė, A., Garjonytė, R., & Būdienė, J. (2023). Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania. Molecules, 28(13), 4928. https://doi.org/10.3390/molecules28134928







