Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions
Abstract
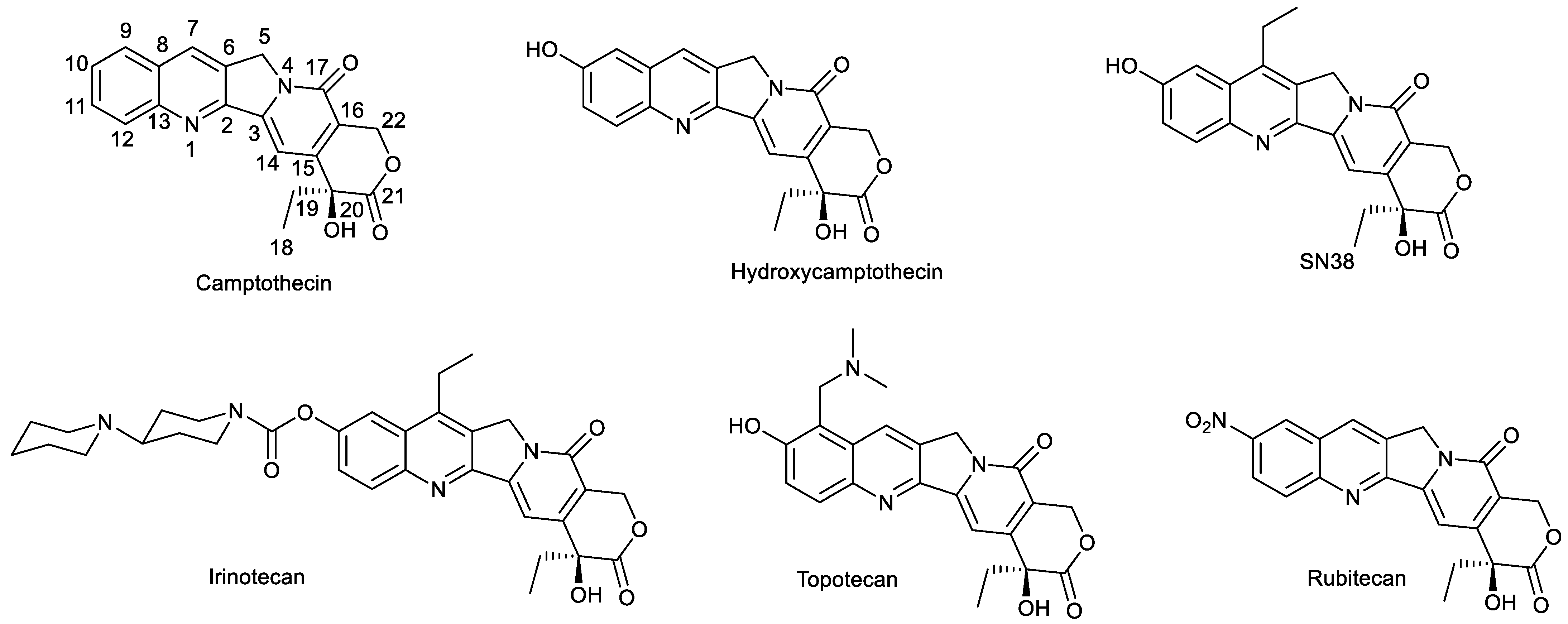
1. Introduction
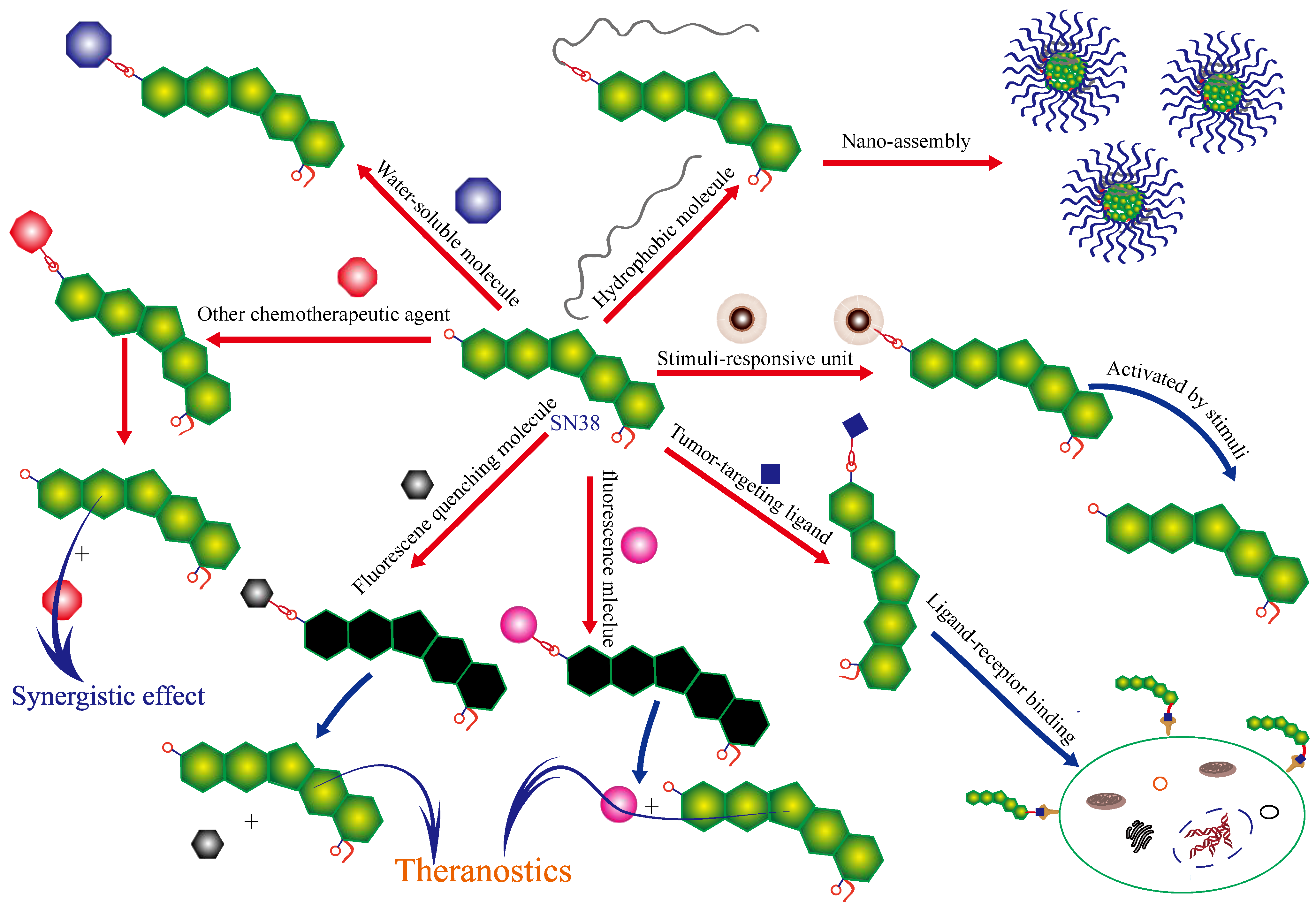
2. Modification of SN38 at C-10 Position
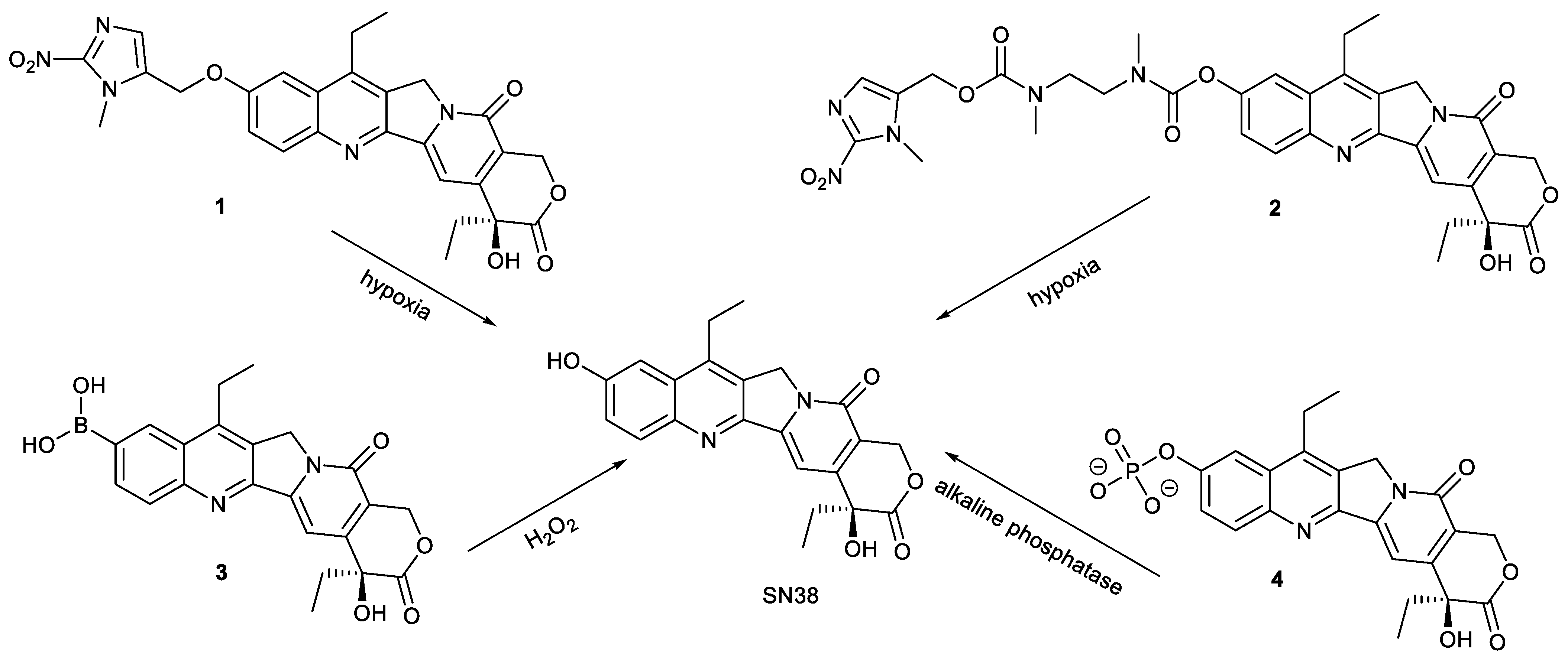
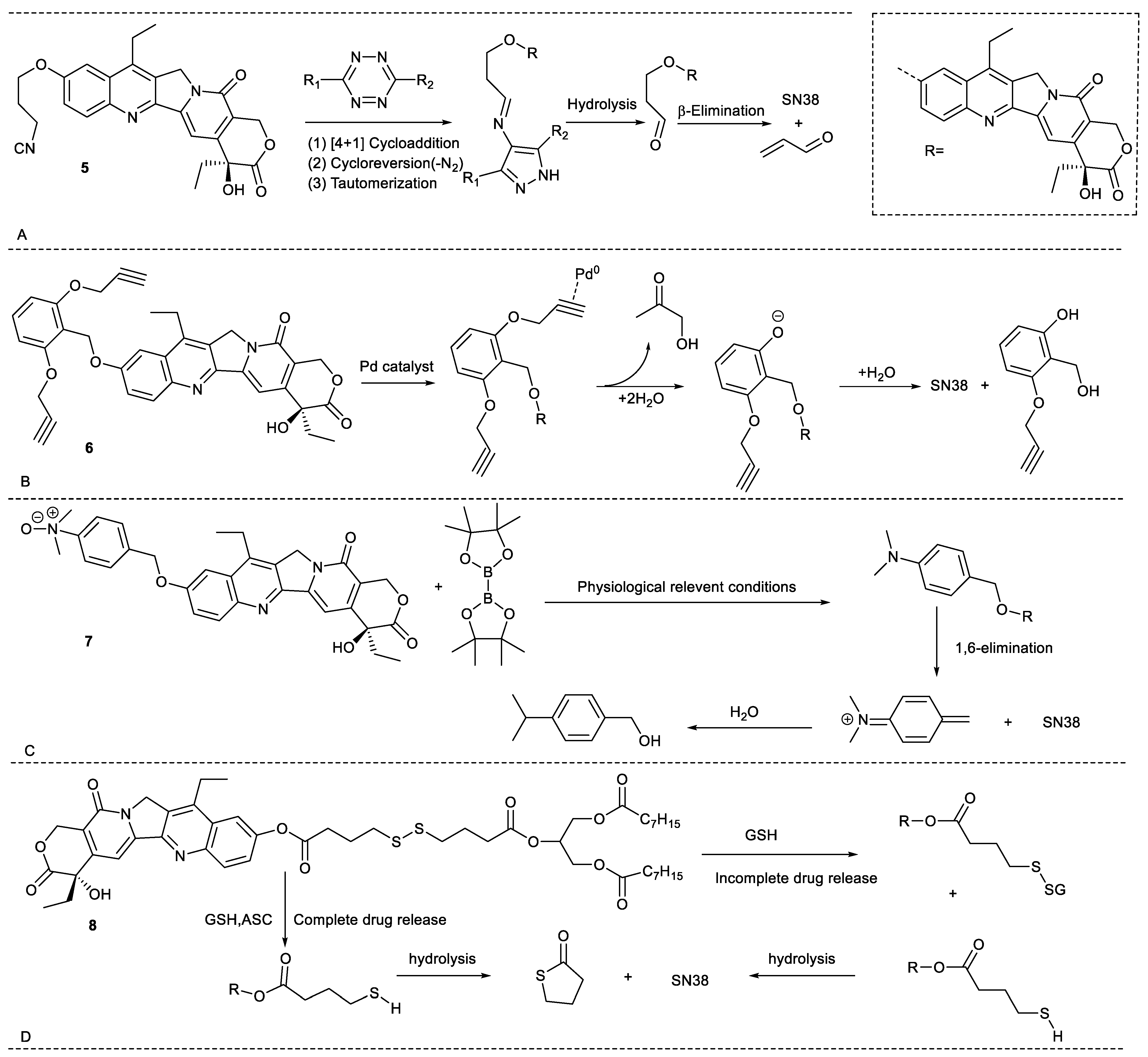
2.1. Stimuli-Responsive Release of Drugs
2.2. Tumor Targeting
2.3. Improvement of Water Solubility
2.4. Synergism between Different Drugs
2.5. Theranostics
2.6. Other Functions
3. Modification of SN38 at C-20 Position
3.1. Synergism between Different Drugs
3.2. Tumor Targeting
3.3. Improvement of Solubility
4. Modification of SN38 at Both C-10 Position and C-20 Position
4.1. Tumor Targeting
4.2. Theranostics
4.3. Other Functions
5. Modification of SN38 at C-9 Position and the Opening of E-Ring
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gusakov, E.A.; Topchu, I.A.; Mazitova, A.M.; Dorogan, I.V.; Bulatov, E.R.; Serebriiskii, I.G.; Abramova, Z.I.; Tupaeva, I.O.; Demidov, O.P.; Toan, D.N.; et al. Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents. RSC Adv. 2021, 11, 4555–4571. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Nagy, N.; Keglevich, P.; Szigetvari, A.; Dekany, M.; Szantay, C.; Hazai, L. Synthesis of Novel Vindoline-Chrysin Hybrids. Chem. Biodivers. 2022, 19, e2100725. [Google Scholar] [CrossRef]
- Keglevich, A.; Danyi, L.; Rieder, A.; Horvath, D.; Szigetvari, A.; Dekany, M.; Szantay, C.; Latif, A.D.; Hunyadi, A.; Zupko, I.; et al. Synthesis and Cytotoxic Activity of New Vindoline Derivatives Coupled to Natural and Synthetic Pharmacophores. Molecules 2020, 25, 1010. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Das, I.; Debnath, S.; Parveen, S.; Das, T. Synthesis of Substituted Tropones and Advancement for the Construction of Structurally Significant Skeletons. Chemistryselect 2022, 7, e202200440. [Google Scholar] [CrossRef]
- Guan, Y.F.; Liu, X.J.; Pang, X.J.; Liu, W.B.; Yu, G.X.; Li, Y.R.; Zhang, Y.B.; Song, J.; Zhang, S.Y. Recent progress of oridonin and its derivatives for cancer therapy and drug resistance. Med. Chem. Res. 2021, 30, 1795–1821. [Google Scholar] [CrossRef]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef]
- Si, J.; Zhao, X.; Gao, S.; Huang, D.; Sui, M. Advances in delivery of Irinotecan (CPT-11) active metabolite 7-ethyl-10-hydroxycamptothecin. Int. J. Pharm. 2019, 568, 118499. [Google Scholar] [CrossRef]
- Tanizawa, A.; Kohn, K.W.; Kohlhagen, G.; Leteurtre, F.O.; Pommier, Y. Differential Stabilization of Eukaryotic DNA Topoisomerase I Cleavable Complexes by Camptothecin Derivatives. Biochemistry 1995, 34, 7200–7206. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Boyd, B.J.; Prestidge, C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J. Control. Release 2013, 172, 48–61. [Google Scholar] [CrossRef]
- Wu, F.; Qiu, F.; Wai-Keong, S.A.; Diao, Y. The Smart Dual-Stimuli Responsive Nanoparticles for Controlled Anti-Tumor Drug Release and Cancer Therapy. Anticancer Agents Med. Chem. 2021, 21, 1202–1215. [Google Scholar] [CrossRef]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Guo, Z. Endogenous Stimuli-responsive Nanocarriers for Drug Delivery. Chem. Lett. 2016, 45, 242–249. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Wang, C.; He, F.; Yang, D.; Gai, S.; Yang, P.; Lin, J.; Jin, D.; Xing, B. Tumour microenvironment responsive nanoconstructs for cancer theranostic. Nano Today 2019, 26, 16–56. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, Q.; Lu, W. Synthesis and biological evaluation of hypoxia-activated prodrugs of SN-38. Eur. J. Med. Chem. 2017, 132, 135–141. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Ma, L.; Chen, Y.; Lu, W. 10-Boronic acid substituted camptothecin as prodrug of SN-38. Eur. J. Med. Chem. 2016, 116, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Quintanilla, R.H.; Grecian, S.; Gee, K.R.; Rao, M.S.; Lakshmipathy, U. Novel live alkaline phosphatase substrate for identification of pluripotent stem cells. Stem Cell Rev. Rep. 2012, 8, 1021–1029. [Google Scholar] [CrossRef]
- Mao, D.; Chung, X.K.W.; Andoh-Noda, T.; Qin, Y.; Sato, S.I.; Takemoto, Y.; Akamatsu, W.; Okano, H.; Uesugi, M. Chemical decontamination of iPS cell-derived neural cell mixtures. Chem. Commun. 2018, 54, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, M.; Parvez, S.; Peterson, R.T.; Franzini, R.M. Bioorthogonal Removal of 3-Isocyanopropyl Groups Enables the Controlled Release of Fluorophores and Drugs in Vivo. J. Am. Chem. Soc. 2018, 140, 8410–8414. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Perez-Lopez, A.M.; Hamilton, L.; Rubio-Ruiz, B.; Bray, T.L.; Sieger, D.; Brennan, P.M.; Unciti-Broceta, A. Bioorthogonal Uncaging of the Active Metabolite of Irinotecan by Palladium-Functionalized Microdevices. Chemistry 2018, 24, 16783–16790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Feng, S.; Zhou, J.; Ji, X.; Long, Y.Q. On-Demand Activation of a Bioorthogonal Prodrug of SN-38 with Fast Reaction Kinetics and High Releasing Efficiency In Vivo. J. Med. Chem. 2022, 65, 333–342. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Miao, Y.; Song, J.; Zhang, M.; Wang, Z.; Zhang, H.; He, Z.; Tian, C.; Sun, J. Boosting SN38-based oral chemotherapy to combine reduction-bioactivated structured lipid-mimetic prodrug with ascorbic acid. Nano Res. 2022, 15, 9092–9104. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Gu, W.; Meng, F.; Haag, R.; Zhong, Z. Actively targeted nanomedicines for precision cancer therapy: Concept, construction, challenges and clinical translation. J. Control. Release 2021, 329, 676–695. [Google Scholar] [CrossRef]
- Pearce, O.M.; Laubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef]
- Bull, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Zhang, Q.; Che, R.; Lu, W. Enhanced cellular uptake efficiency of DCM probes or SN38 conjugating with phenylboronic acids. Bioorganic Med. Chem. 2020, 28, 115377. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Jin, X.; Liu, L.; Tian, X. Folate Receptor Targeting and Cathepsin B-Sensitive Drug Delivery System for Selective Cancer Cell Death and Imaging. ACS Med. Chem. Lett. 2020, 11, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Ando, S.; Sato, S.I.; Qin, Y.; Hirata, N.; Katsuda, Y.; Kawase, E.; Kuo, T.F.; Minami, I.; Shiba, Y.; et al. A Synthetic Hybrid Molecule for the Selective Removal of Human Pluripotent Stem Cells from Cell Mixtures. Angew. Chem. 2017, 56, 1765–1770. [Google Scholar] [CrossRef]
- Walther, R.; Jarlstad Olesen, M.T.; Zelikin, A.N. Extended scaffold glucuronides: En route to the universal synthesis of O-aryl glucuronide prodrugs. Org. Biomol. Chem. 2019, 17, 6970–6974. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhao, H.Y.; Lei, H.; Yuan, B.; Mao, S.; Xin, M.; Zhang, S.Q. Synthesis and Biological Evaluation of 10-Substituted Camptothecin Derivatives with Improved Water Solubility and Activity. ChemMedChem 2021, 16, 1000–1010. [Google Scholar] [CrossRef]
- Doi, H.; Kida, T.; Nishino, K.; Nakatsuji, M.; Sakamoto, S.; Shimizu, S.; Teraoka, Y.; Tamura, Y.; Kataoka, Y.; Inui, T. Solubility-Improved 10-O-Substituted SN-38 Derivatives with Antitumor Activity. ChemMedChem 2017, 12, 1715–1722. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, X.; Wang, Y.; Duan, X.; Wang, X.; Chen, C.; Tian, D.; Luo, Z.; Zhang, Z.; Zeng, Y. Hydrophobized SN38 to redox-hypersensitive nanorods for cancer therapy. J. Mater. Chem. B 2019, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, X.; Li, T.; Wu, Y.; Song, L.; Zhang, W.; Yin, L.; Wu, Y.; Han, W.; Yang, Y. An esterase-activatable prodrug formulated liposome strategy: Potentiating the anticancer therapeutic efficacy and drug safety. Nanoscale Adv. 2022, 4, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, M.; Xing, J.; Liu, F.; Dong, P.; Tian, X.; Xu, H.; Zhang, L.; Gu, H.; Yang, L.; et al. TQ-B3203, a potent proliferation inhibitor derived from camptothecin. Med. Chem. Res. 2017, 26, 3395–3406. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Q.; Bi, Y.; Chen, X.; Liu, L.; Ruan, C.; Zhao, Z.; Jiang, C. Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs. J. Mater. Chem. B 2017, 5, 2644–2654. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, X.; Shen, Q.; Zhang, Q.; Su, M.; Zhou, Y.; Li, J.; Chen, Y.; Lu, W. A series of camptothecin prodrugs exhibit HDAC inhibition activity. Bioorganic Med. Chem. 2018, 26, 4706–4715. [Google Scholar] [CrossRef]
- Botta, L.; Filippi, S.; Zippilli, C.; Cesarini, S.; Bizzarri, B.M.; Cirigliano, A.; Rinaldi, T.; Paiardini, A.; Fiorucci, D.; Saladino, R.; et al. Artemisinin Derivatives with Antimelanoma Activity Show Inhibitory Effect against Human DNA Topoisomerase 1. ACS Med. Chem. Lett. 2020, 11, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Jangili, P.; Won, M.; Kim, S.J.; Chun, J.; Shim, I.; Kang, C.; Ren, W.X.; Kim, J.S. Binary Drug Reinforced First Small-Molecule-Based Prodrug for Synergistic Anticancer Effects. ACS Appl. Bio Mater. 2019, 2, 3532–3539. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.M.; Jung, E.; Lee, J.; Han, J.; Kim, Y.J.; Kim, J.Y.; Seo, J.H.; Kim, J.S. A synchronized dual drug delivery molecule targeting cancer stem cells in tumor heterogeneity and metastasis. Biomaterials 2022, 289, 121781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, J.; Xie, K.; Fang, T.; Chen, C.; Xie, H.; Zhou, L.; Zheng, S. Precise Engineering of Prodrug Cocktails into Single Polymeric Nanoparticles for Combination Cancer Therapy: Extended and Sequentially Controllable Drug Release. ACS Appl Mater Interfaces 2017, 9, 10567–10576. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiang, K.; Xu, M.; Li, Y.; Cui, J.; Zhang, L.; Tang, X.; Zhu, X.; Qian, L.; Zhang, M.; et al. Prodrug Nanomedicine Inhibits Chemotherapy-Induced Proliferative Burst by Altering the Deleterious Intercellular Communication. ACS Nano 2021, 15, 781–796. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, W.S.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Bhuniya, S.; Kim, J.S. Small conjugate-based theranostic agents: An encouraging approach for cancer therapy. Chem. Soc. Rev. 2015, 44, 6670–6683. [Google Scholar] [CrossRef]
- Kijewska, M.; Sharfalddin, A.A.; Jaremko, L.; Cal, M.; Setner, B.; Siczek, M.; Stefanowicz, P.; Hussien, M.A.; Emwas, A.H.; Jaremko, M. Lossen Rearrangement of p-Toluenesulfonates of N-Oxyimides in Basic Condition, Theoretical Study, and Molecular Docking. Front. Chem. 2021, 9, 662533. [Google Scholar] [CrossRef]
- Whang, C.-H.; Yoo, E.; Hur, S.K.; Kim, K.S.; Kim, D.; Jo, S. A highly GSH-sensitive SN-38 prodrug with an “OFF-to-ON” fluorescence switch as a bifunctional anticancer agent. Chem. Commun. 2018, 54, 9031–9034. [Google Scholar] [CrossRef]
- Huang, K.; Xie, S.; Wang, W.; Wu, Z.-S.; Wu, J.; Jiang, L.; Chen, J.; Li, J. A hydrogen sulfide-responsive prodrug for monitoring real-time release and improving therapeutic effects of anticancer drug SN-38. Sens. Actuators B Chem. 2022, 373, 132750. [Google Scholar] [CrossRef]
- Shin, W.S.; Han, J.; Verwilst, P.; Kumar, R.; Kim, J.H.; Kim, J.S. Cancer Targeted Enzymatic Theranostic Prodrug: Precise Diagnosis and Chemotherapy. Bioconjugate Chem. 2016, 27, 1419–1426. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, E.J.; Han, J.; Lee, H.; Shin, W.S.; Kim, H.M.; Bhuniya, S.; Kim, J.S.; Hong, K.S. Hypoxia-directed and activated theranostic agent: Imaging and treatment of solid tumor. Biomaterials 2016, 104, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Bobba, K.N.; Cho, M.Y.; Park, H.S.; Won, M.; Velusamy, N.; Hong, K.S.; Bhuniya, S.; Kim, J.S. Molecular Theranostic Agent with Programmed Activation for Hypoxic Tumors. ACS Appl. Bio Mater. 2019, 2, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Alex, S.M.; Bobba, K.N.; Maiti, K.K.; Bhuniya, S. New Insight into a Cancer Theranostic Probe: Efficient Cell-Specific Delivery of SN-38 Guided by Biotinylated Poly(vinyl alcohol). ACS Appl. Mater. Interfaces 2016, 8, 33430–33438. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Cao, Y.-X.; Li, G.-Y.; Lei, H.; Attiogbe, M.K.I.; Yao, J.-C.; Yang, X.-Y.; Liu, Y.-J.; Hei, Y.-Y.; Zhang, H.; et al. F10, a new camptothecin derivative, was identified as a new orally–bioavailable, potent antitumor agent. Eur. J. Med. Chem. 2020, 202, 112528. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, W.; Zhu, L.-Z.; Liu, X.-F.; Li, L.; Peng, L.-Z.; Kai, G.-Y.; Liu, Y.-Q.; Zhang, Z.-J.; Xu, C.-R. Anti-tumor effects and mechanism of a novel camptothecin derivative YCJ100. Life Sci. 2022, 311 Pt A, 121105. [Google Scholar] [CrossRef]
- Liang, D.; Wu, X.; Hasinoff, B.B.; Herbert, D.E.; Tranmer, G.K. Evaluation of Nitrobenzyl Derivatives of Camptothecin as Anti-Cancer Agents and Potential Hypoxia Targeting Prodrugs. Molecules 2018, 23, 2041. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, D.; Zhang, G.; Li, Y.; Shi, W.; Zhong, B.; Yu, H. A novel irinotecan derivative ZBH-1207 with different anti-tumor mechanism from CPT-11 against colon cancer cells. Mol. Biol. Rep. 2022, 49, 8359–8368. [Google Scholar] [CrossRef]
- Dal Corso, A.; Borlandelli, V.; Corno, C.; Perego, P.; Belvisi, L.; Pignataro, L.; Gennari, C. Fast Cyclization of a Proline-Derived Self-Immolative Spacer Improves the Efficacy of Carbamate Prodrugs. Angew. Chem. Int. Ed. 2020, 59, 4176–4181. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Huang, Y.; Dong, F.; Li, L.; Song, J.; Zuo, S.; Zhu, Z.; Kamei, K.I.; He, Z.; et al. Critical roles of linker length in determining the chemical and self-assembly stability of SN38 homodimeric nanoprodrugs. Nanoscale Horiz. 2023, 8, 235–244. [Google Scholar] [CrossRef]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorganic Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef]
- de Groot, F.; Busscher, G.; Aben, R.; Scheeren, H. Novel 20-carbonate linked prodrugs of camptothecin and 9-aminocamptothecin designed for activation by tumour-associated plasmin. Bioorganic Med. Chem. Lett. 2002, 12, 2371–2376. [Google Scholar] [CrossRef]
- Guerrant, W.; Patil, V.; Canzoneri, J.C.; Yao, L.P.; Hood, R.; Oyelere, A.K. Dual-acting histone deacetylase-topoisomerase I inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Musso, L.; Artali, R.; Guglielmi, M.B.; La Porta, I.; Melito, C.; Colelli, F.; Cardile, F.; Signorino, G.; Fucci, A.; et al. Hybrid topoisomerase I and HDAC inhibitors as dual action anticancer agents. PLoS ONE 2018, 13, e0205018. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Z.; Zhang, Q.; Zhu, Q.; Chen, Y.; Lu, W. Co-Prodrugs of 7-Ethyl-10-hydroxycamptothecin and Vorinostat with in Vitro Hydrolysis and Anticancer Effects. ACS Omega 2019, 5, 350–357. [Google Scholar] [CrossRef]
- Andrikopoulou, A.; Zografos, E.; Liontos, M.; Koutsoukos, K.; Dimopoulos, M.A.; Zagouri, F. Trastuzumab Deruxtecan (DS-8201a): The Latest Research and Advances in Breast Cancer. Clin. Breast Cancer 2021, 21, e212–e219. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [PubMed]
- Sugahara, K.N.; Braun, G.B.; de Mendoza, T.H.; Kotamraju, V.R.; French, R.P.; Lowy, A.M.; Teesalu, T.; Ruoslahti, E. Tumor-penetrating iRGD peptide inhibits metastasis. Mol. Cancer Ther. 2015, 14, 120–128. [Google Scholar] [CrossRef]
- Xie, H.; Xu, X.; Chen, J.; Li, L.; Wang, J.; Fang, T.; Zhou, L.; Wang, H.; Zheng, S. Rational design of multifunctional small-molecule prodrugs for simultaneous suppression of cancer cell growth and metastasis in vitro and in vivo. Chem. Commun. 2016, 52, 5601–5604. [Google Scholar] [CrossRef]
- Zhu, S.; Shen, Q.; Gao, Y.; Wang, L.; Fang, Y.; Chen, Y.; Lu, W. Design, Synthesis, and Biological Evaluation of HSP90 Inhibitor-SN38 Conjugates for Targeted Drug Accumulation. J. Med. Chem. 2020, 63, 5421–5441. [Google Scholar] [CrossRef]
- Yang, C.; Xia, A.J.; Du, C.H.; Hu, M.X.; Gong, Y.L.; Tian, R.; Jiang, X.; Xie, Y.M. Discovery of highly potent and selective 7-ethyl-10-hydroxycamptothecin-glucose conjugates as potential anti-colorectal cancer agents. Front. Pharmacol. 2022, 13, 1014854. [Google Scholar] [CrossRef]
- Song, Z.-L.; Wang, M.-J.; Li, L.; Wu, D.; Wang, Y.-H.; Yan, L.-T.; Morris-Natschke, S.L.; Liu, Y.-Q.; Zhao, Y.-L.; Wang, C.-Y.; et al. Design, synthesis, cytotoxic activity and molecular docking studies of new 20(S)-sulfonylamidine camptothecin derivatives. Eur. J. Med. Chem. 2016, 115, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, W.; He, R.; Ismail, M.; Ling, L.; Yao, C.; Fu, Z.; Li, X. Dual 7-ethyl-10-hydroxycamptothecin conjugated phospholipid prodrug assembled liposomes with in vitro anticancer effects. Bioorganic Med. Chem. 2017, 25, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Bala, V.; Rao, S.; Li, P.; Wang, S.; Prestidge, C.A. Lipophilic Prodrugs of SN38: Synthesis and in Vitro Characterization toward Oral Chemotherapy. Mol. Pharm. 2016, 13, 287–294. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Mao, W.; Xiang, J.; Zhou, Z.; Liu, X.; Tang, J.; Shen, Y. Assemblies of Peptide-Cytotoxin Conjugates for Tumor-Homing Chemotherapy. Adv. Funct. Mater. 2019, 29, 1807446. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, J.; Sun, Y.; Yang, C.; Zhang, S.; Wang, R.; Tan, W. Programmable Repurposing of Existing Drugs as Pharmaceutical Elements for the Construction of Aptamer-Drug Conjugates. ACS Appl Mater Interfaces 2021, 13, 9457–9463. [Google Scholar] [CrossRef]
- Sun, G.; He, Z.; Hao, M.; Xu, Z.; Hu, X.Y.; Zhu, J.J.; Wang, L. Bifunctional supramolecular prodrug vesicles constructed from a camptothecin derivative with a water-soluble pillar [5]arene for cancer diagnosis and therapy. Chem. Commun. 2019, 55, 10892–10895. [Google Scholar] [CrossRef]
- Tian, X.; Zuo, M.; Niu, P.; Velmurugan, K.; Wang, K.; Zhao, Y.; Wang, L.; Hu, X.Y. Orthogonal Design of a Water-Soluble meso-Tetraphenylethene-Functionalized Pillar [5]arene with Aggregation-Induced Emission Property and Its Therapeutic Application. ACS Appl. Mater. Interfaces 2021, 13, 37466–37474. [Google Scholar] [CrossRef]
- Jones, K.A.; Kentala, K.; Beck, M.W.; An, W.; Lippert, A.R.; Lewis, J.C.; Dickinson, B.C. Development of a Split Esterase for Protein-Protein Interaction-Dependent Small-Molecule Activation. ACS Cent. Sci. 2019, 5, 1768–1776. [Google Scholar] [CrossRef]
- Bai, Y.P.; Yang, C.J.; Deng, N.; Zhang, M.; Zhang, Z.J.; Li, L.; Zhou, Y.; Luo, X.F.; Xu, C.R.; Zhang, B.Q.; et al. Design and synthesis of novel 7-ethyl-10-fluoro-20-O-(cinnamic acid ester)-camptothecin derivatives as potential high selectivity and low toxicity topoisomerase I inhibitors for hepatocellular carcinoma. Biochem Pharm. 2022, 200, 115049. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Li, X.Z.; Liu, J.T.; Qin, N.; Duan, H.Q.; Duan, X.C. Disulfide Bond-Based SN38 Prodrug Nanoassemblies with High Drug Loading and Reduction-Triggered Drug Release for Pancreatic Cancer Therapy. Int. J. Nanomed. 2023, 18, 1281–1298. [Google Scholar] [CrossRef]
- Naumczuk, B.; Wiktorska, K.; Lubelska, K.; Kawęcki, R.; Bocian, W.; Bednarek, E.; Sitkowski, J.; Chilmonczyk, Z.; Kozerski, L. New generation of camptothecin derivatives spontaneously alkylating DNA. New J. Chem. 2016, 40, 7978–7985. [Google Scholar] [CrossRef]
- Yan, M.; Peng, W.; Wang, H.; Zhang, D.; Li, Z. Supramolecular Organic Framework Loading for Camptothecin Open-Ring Carboxylates and Their Lactonization Kinetics. Chin. J. Org. Chem. 2019, 39, 2567. [Google Scholar] [CrossRef]
- Zheng, C.; Li, M.Z.; You, T.P.; Tang, W.P.; Lou, L.G. Synthesis and antitumor activity of a series of lactone-opened camptothecin derivatives. J. Asian Nat. Prod. Res. 2019, 21, 51–61. [Google Scholar] [CrossRef] [PubMed]


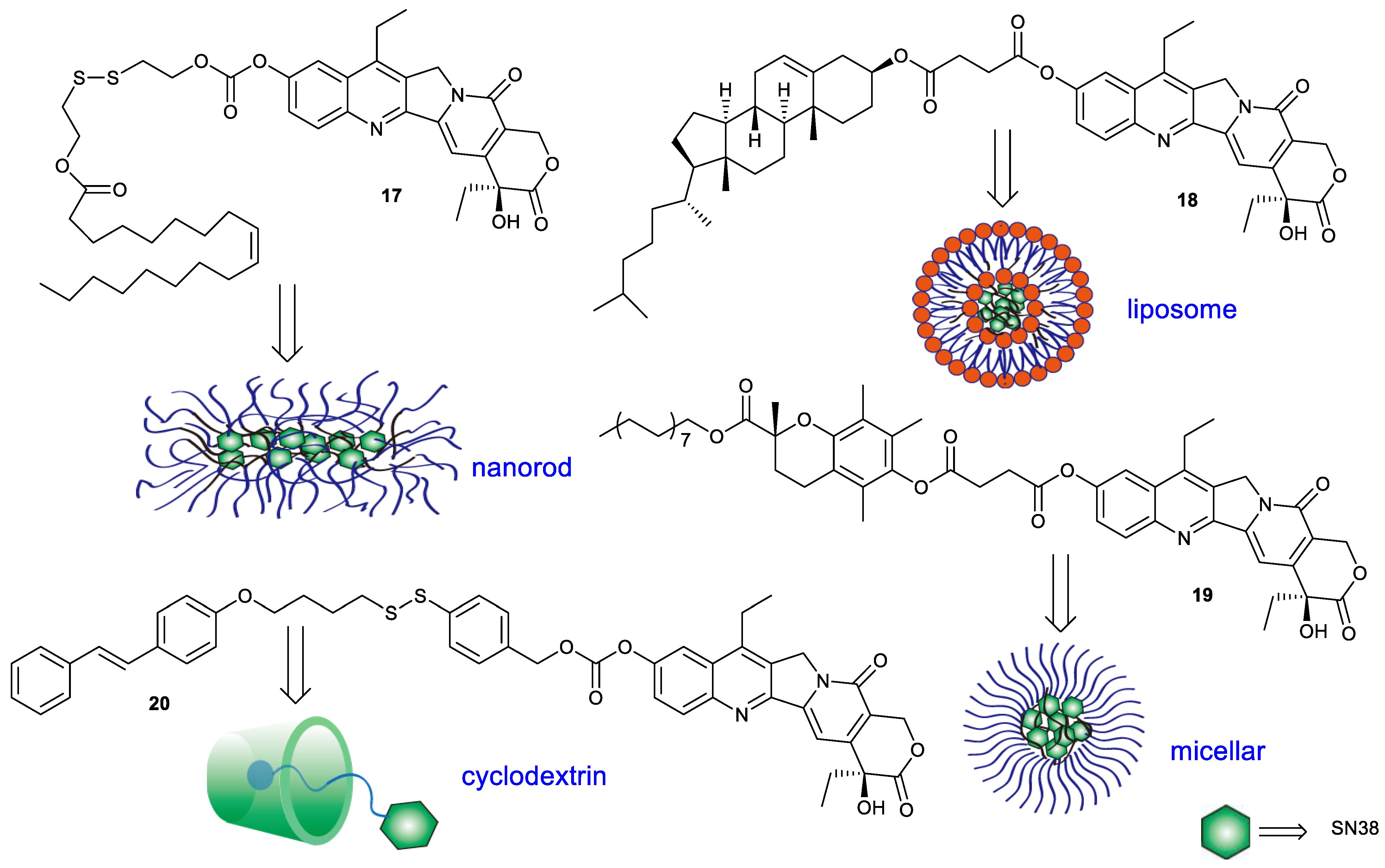
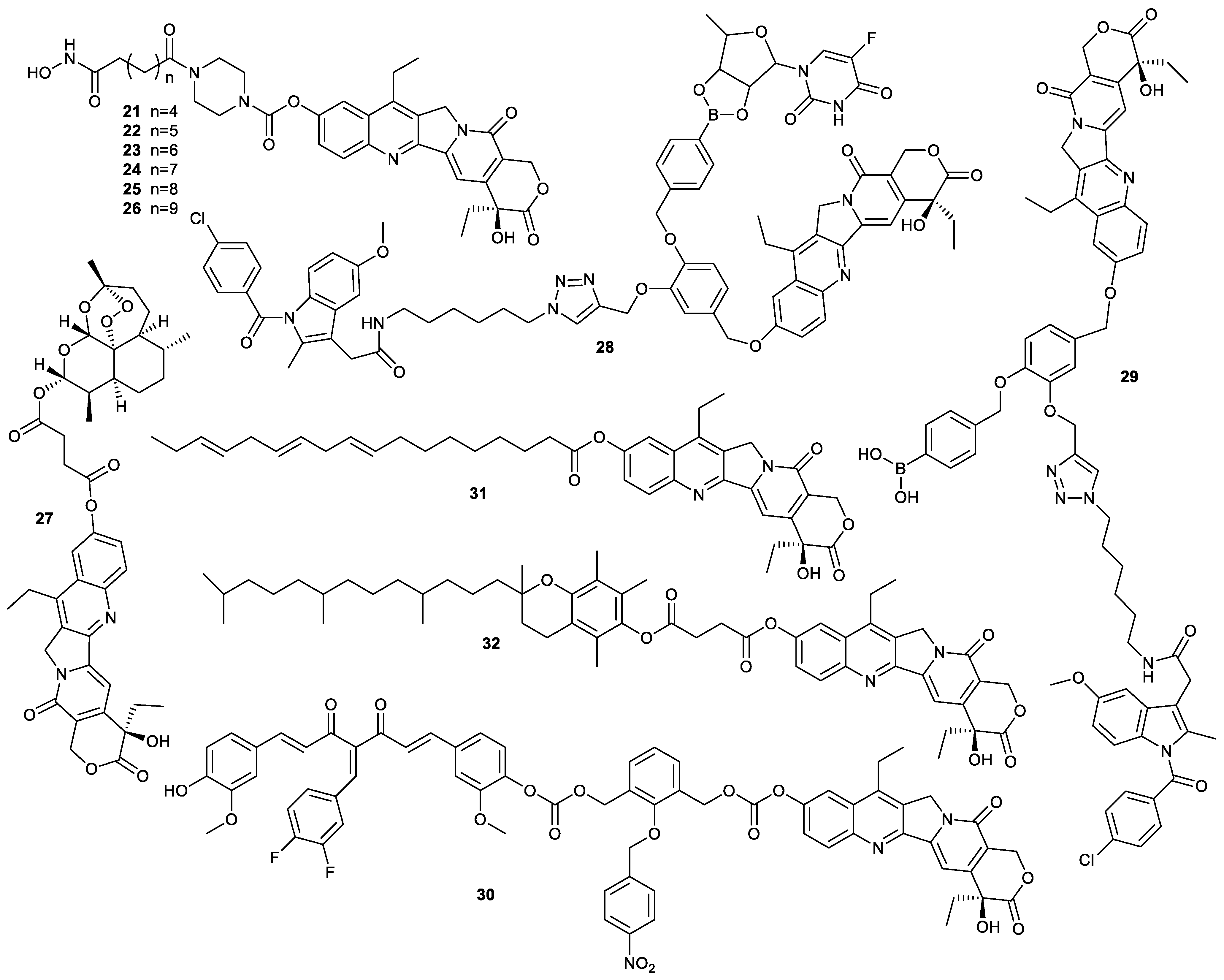

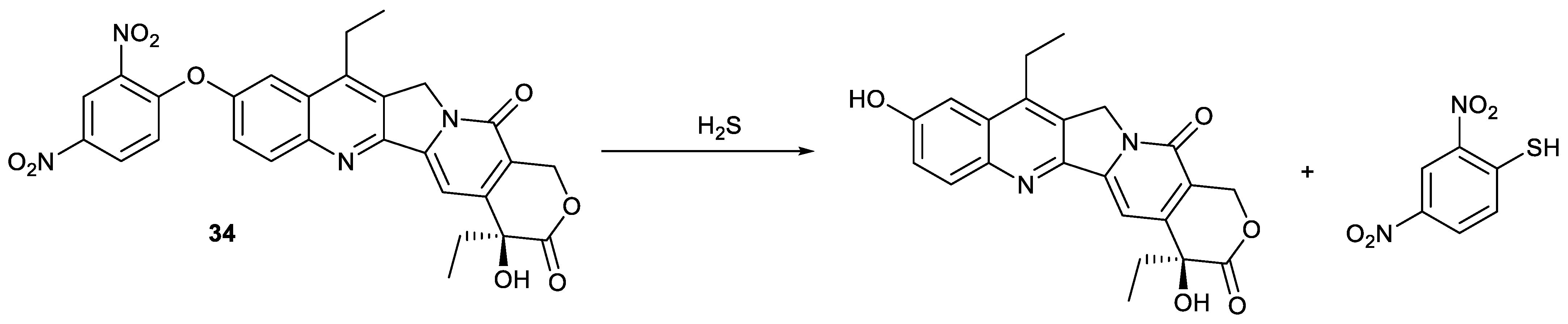
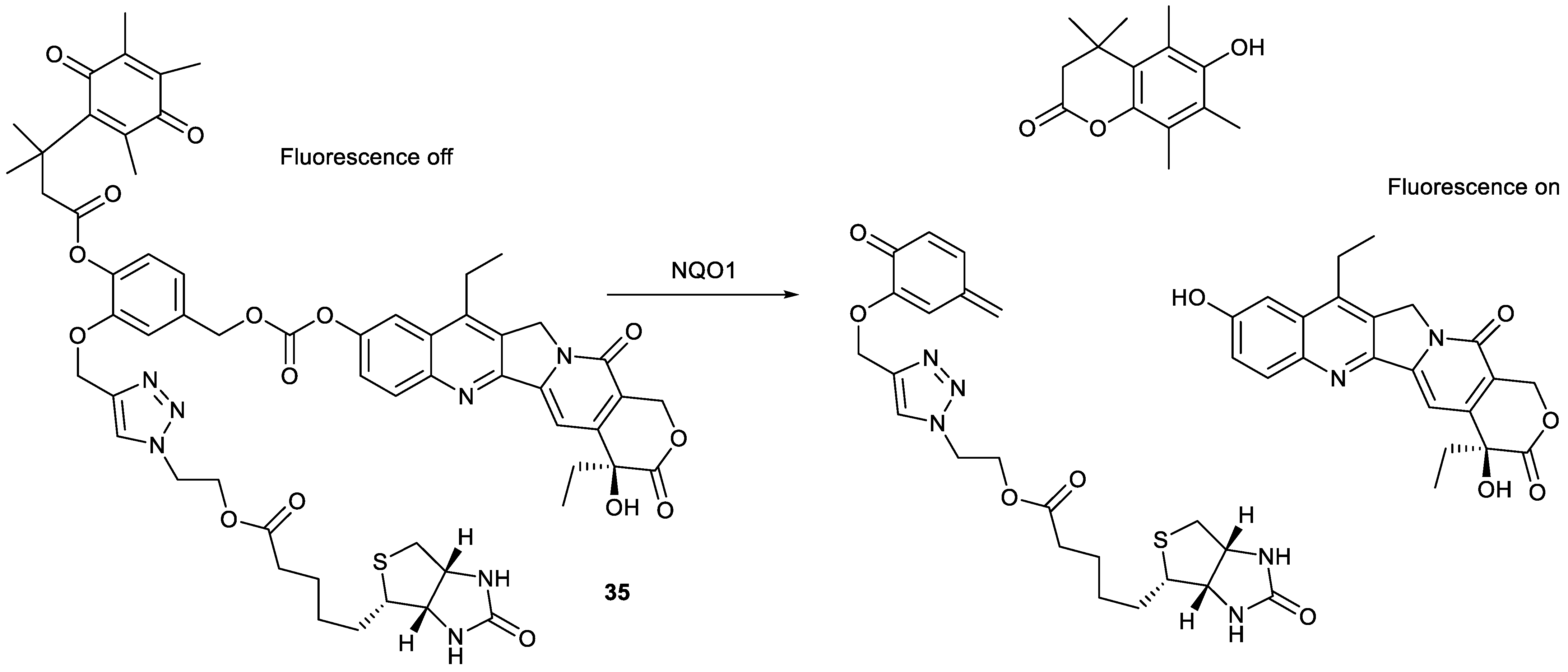
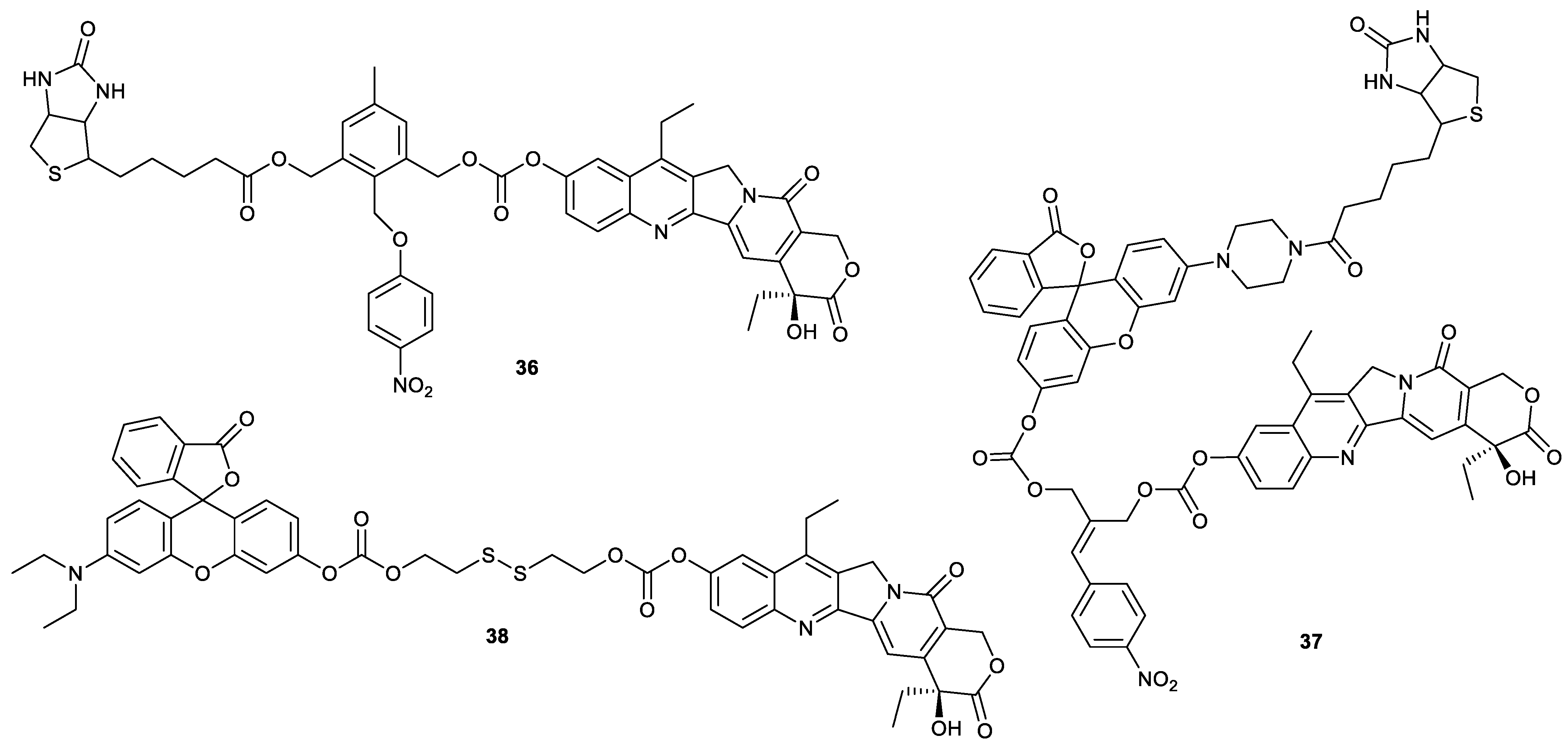

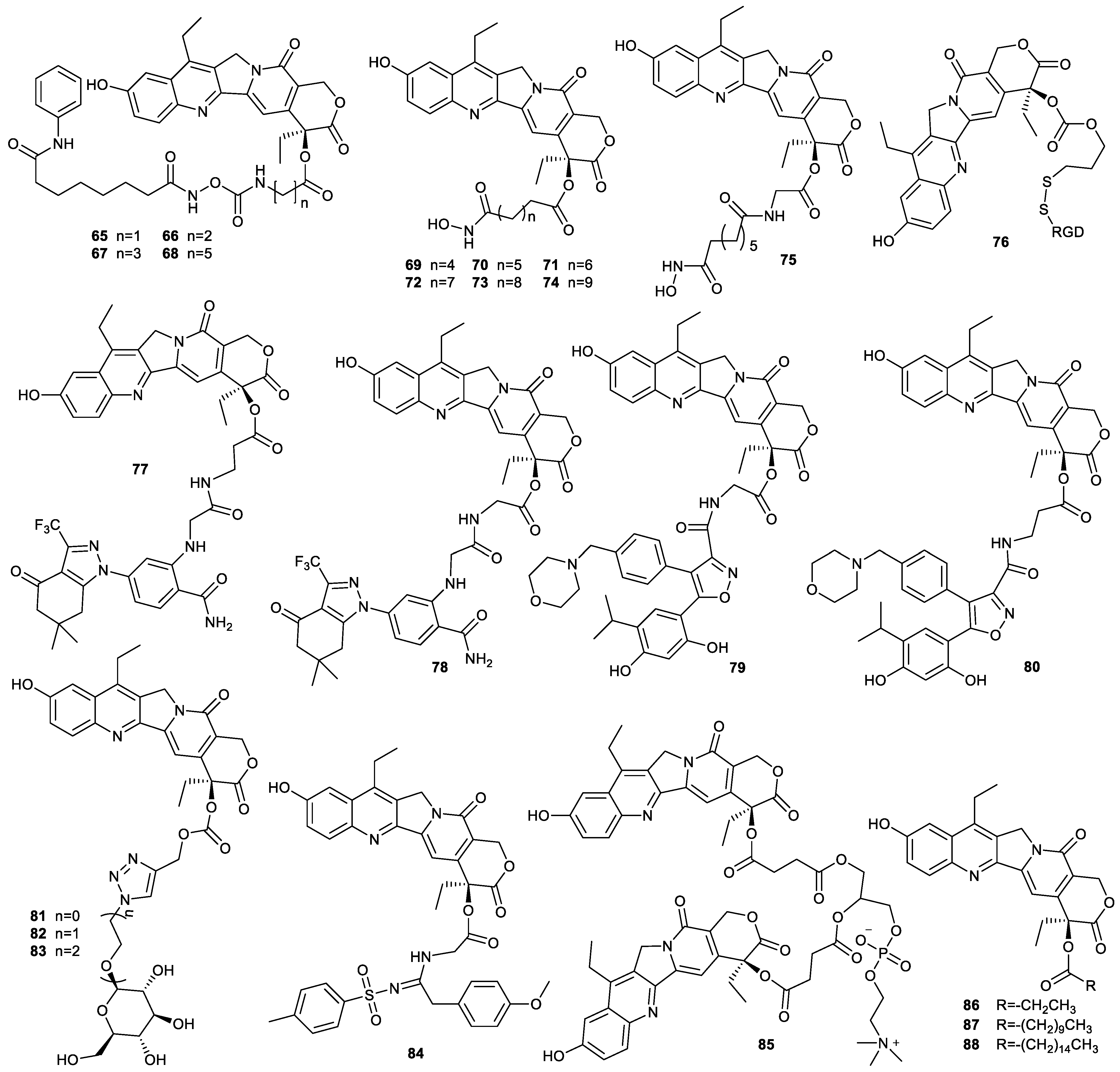
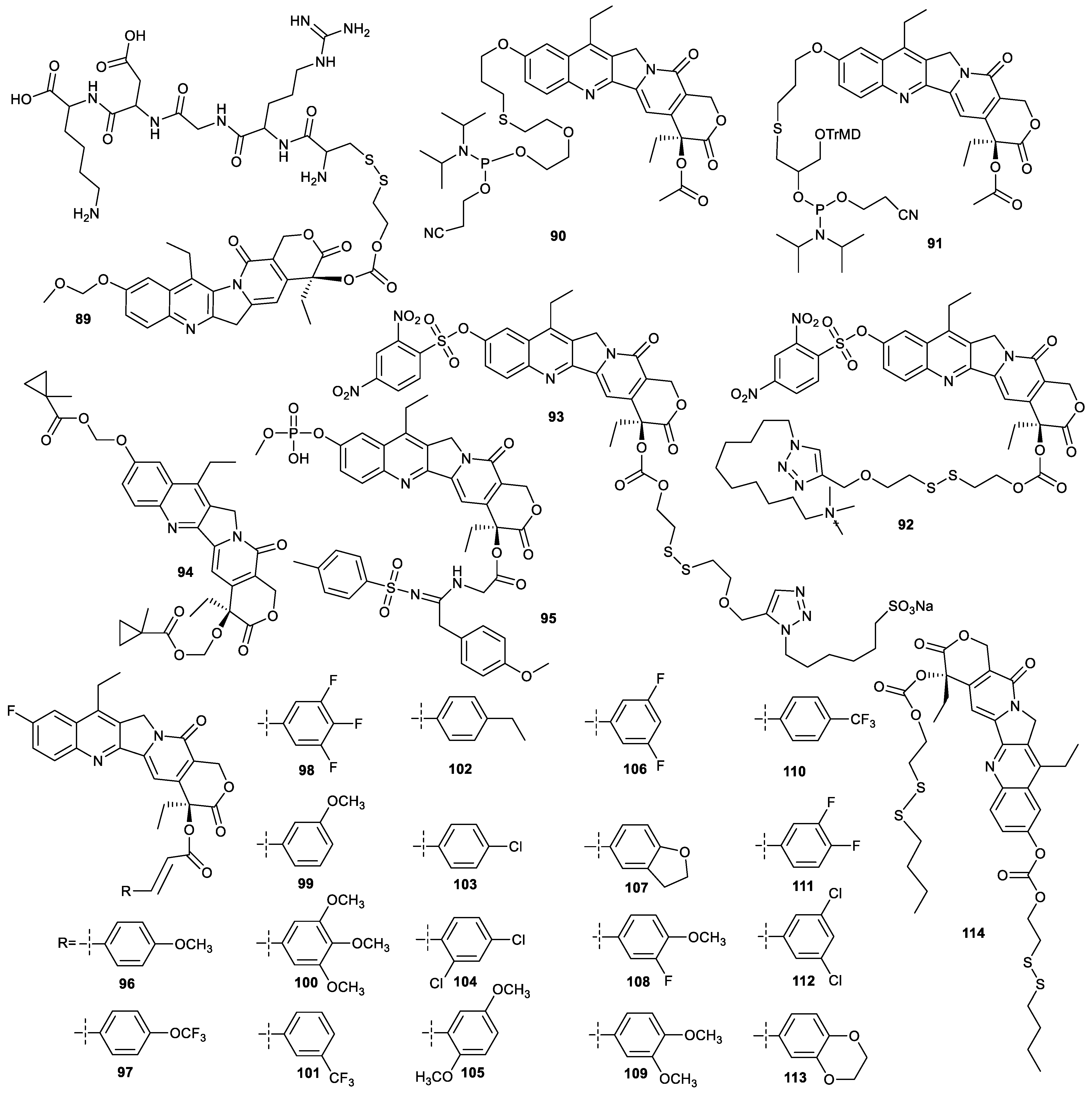
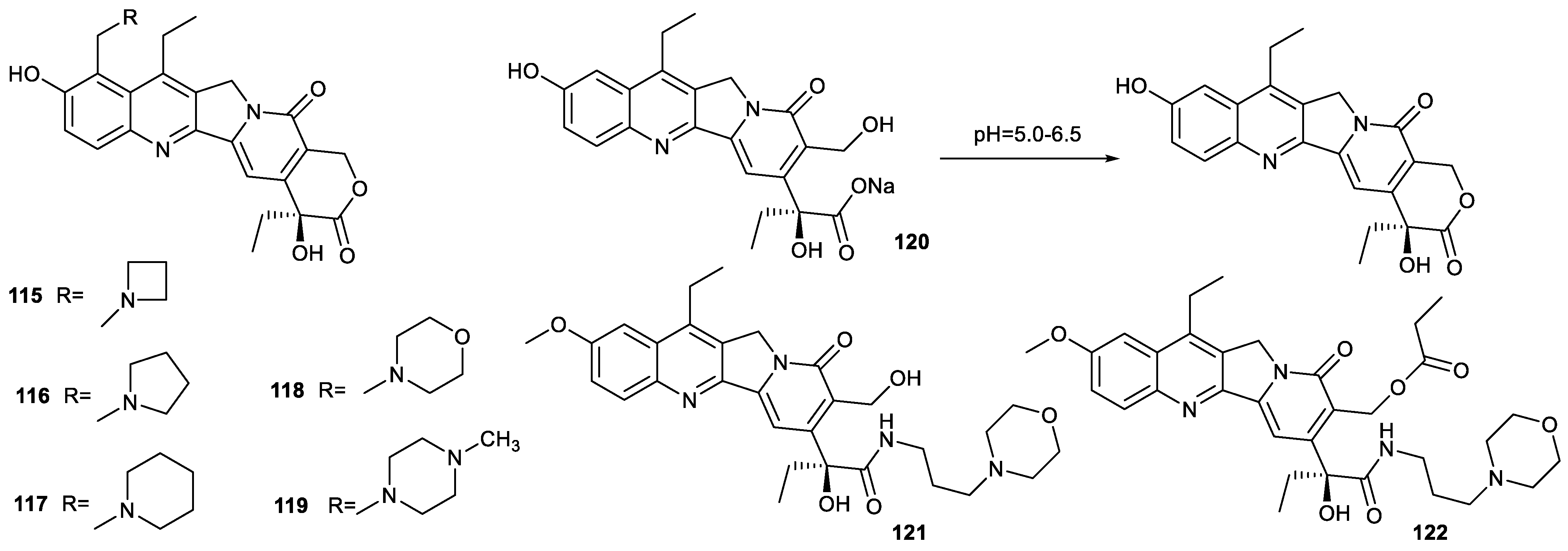
| Types of Structural Modification | Achievable Functions | Current Disadvantages |
|---|---|---|
| Modification of C-10 Position | Stimuli-responsive Release of Drugs | Dependent on the type of cell lines or exogenous stimuli |
| Tumor Targeting | Dependent on the type of cell lines | |
| Improvement of Water Solubility | The occuring bottleneck of designing the novel small-molecular SN38 derivatives with improvement of water solubility | |
| Synergism between Different Drugs | Little consideration of the ratio between drugs | |
| Theranostics | Weak tissue penetration due to short excitation wavelength; photobleaching | |
| Other Functions | Null | |
| Modification of C-20 Position | Synergism between Different Drugs | Little consideration of the ratio between drugs |
| Tumor Targeting | Dependent on the type of cell lines | |
| Improvement of Solubility | The occuring bottleneck of designing the novel small-molecular SN38 derivatives with improvement of water solubility | |
| Modification of C-10 Position and C-20 Position | Tumor Targeting | Dependent on the type of cell lines |
| Theranostics | Weak tissue penetration due to short excitation wavelength; photobleaching | |
| Oher Functions | Null | |
| Modification of C-9 Position | Multitarget Inhibition of Cancer Cells | A relatively challenging modification |
| The Opening of E-ring | Maintenance of Anticancer Activity | The usual reduced anticancer activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Qian, M.; Li, Y. Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions. Molecules 2023, 28, 4931. https://doi.org/10.3390/molecules28134931
Dai Y, Qian M, Li Y. Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions. Molecules. 2023; 28(13):4931. https://doi.org/10.3390/molecules28134931
Chicago/Turabian StyleDai, Yi, Meng Qian, and Yan Li. 2023. "Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions" Molecules 28, no. 13: 4931. https://doi.org/10.3390/molecules28134931
APA StyleDai, Y., Qian, M., & Li, Y. (2023). Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions. Molecules, 28(13), 4931. https://doi.org/10.3390/molecules28134931




