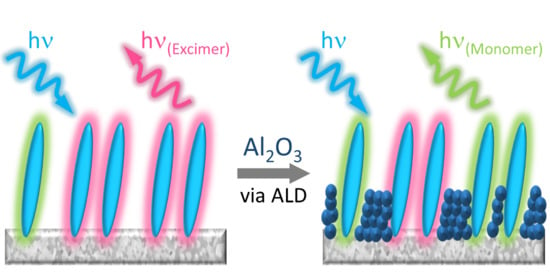
Influence of Al2O3 Overlayers on Intermolecular Interactions between Metal Oxide Bound Molecules
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Loading
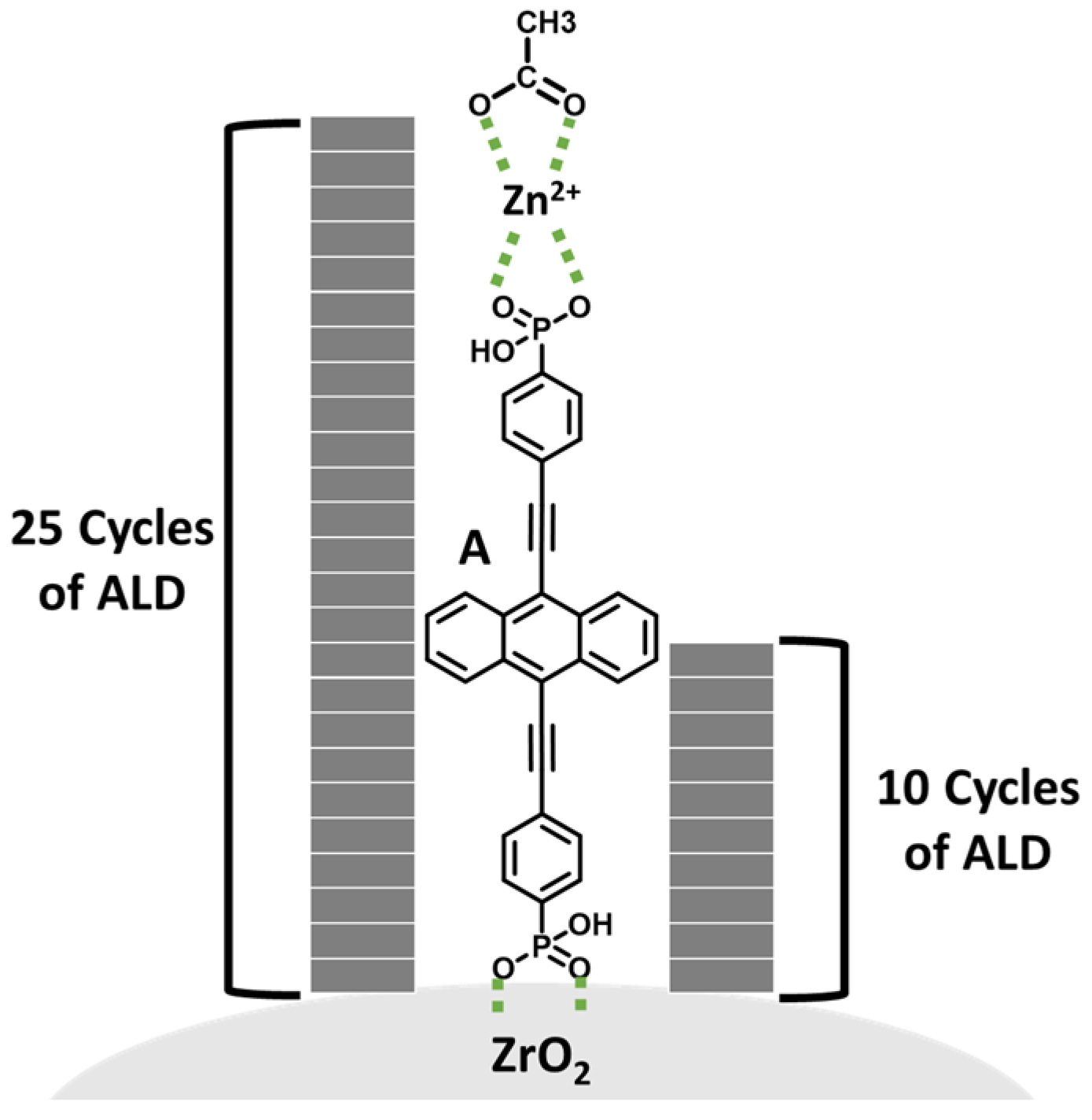
2.1.1. A on ZrO2
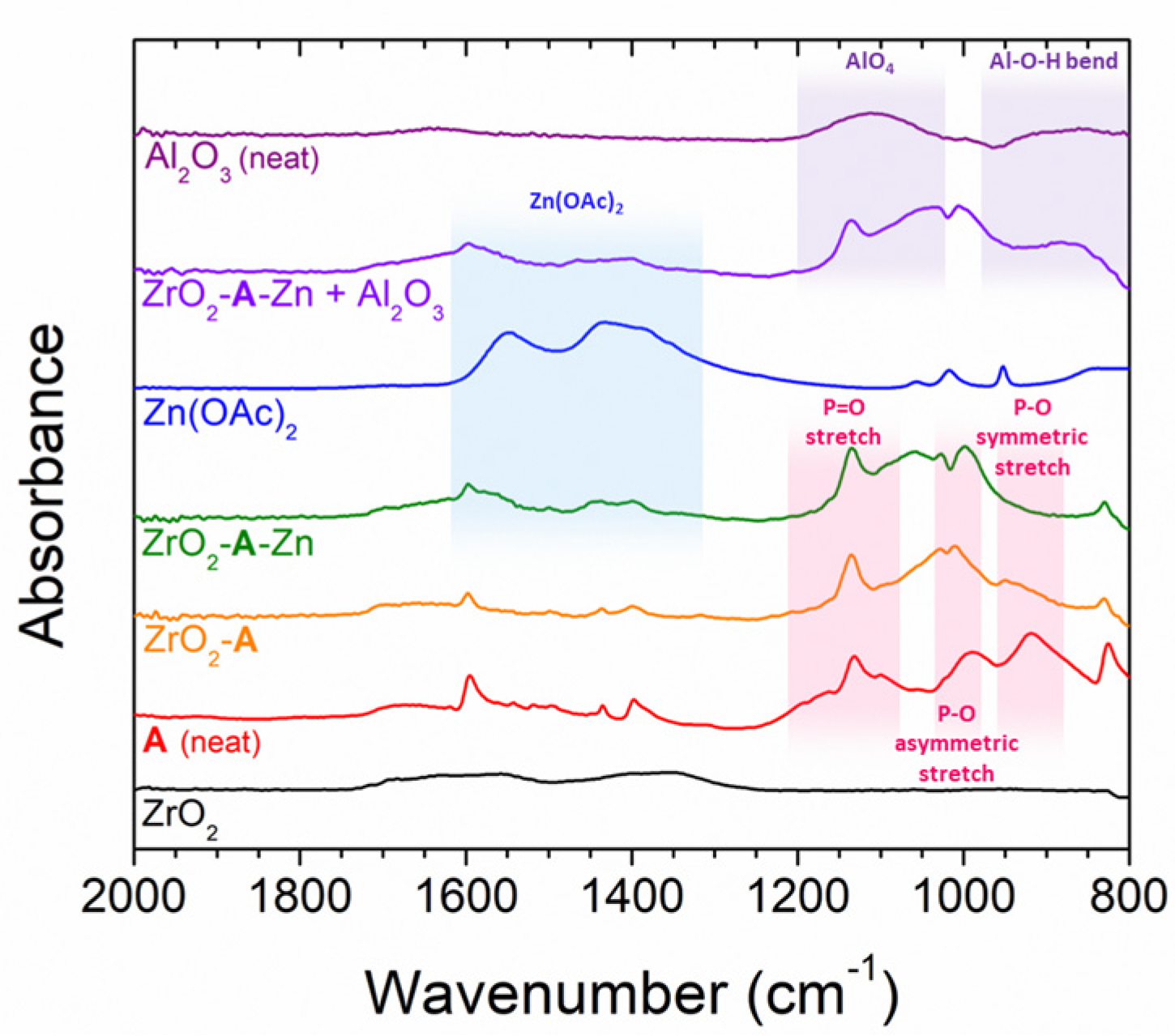
2.1.2. Zn(OAc)2 Treatment
2.1.3. Al2O3 Overlayers
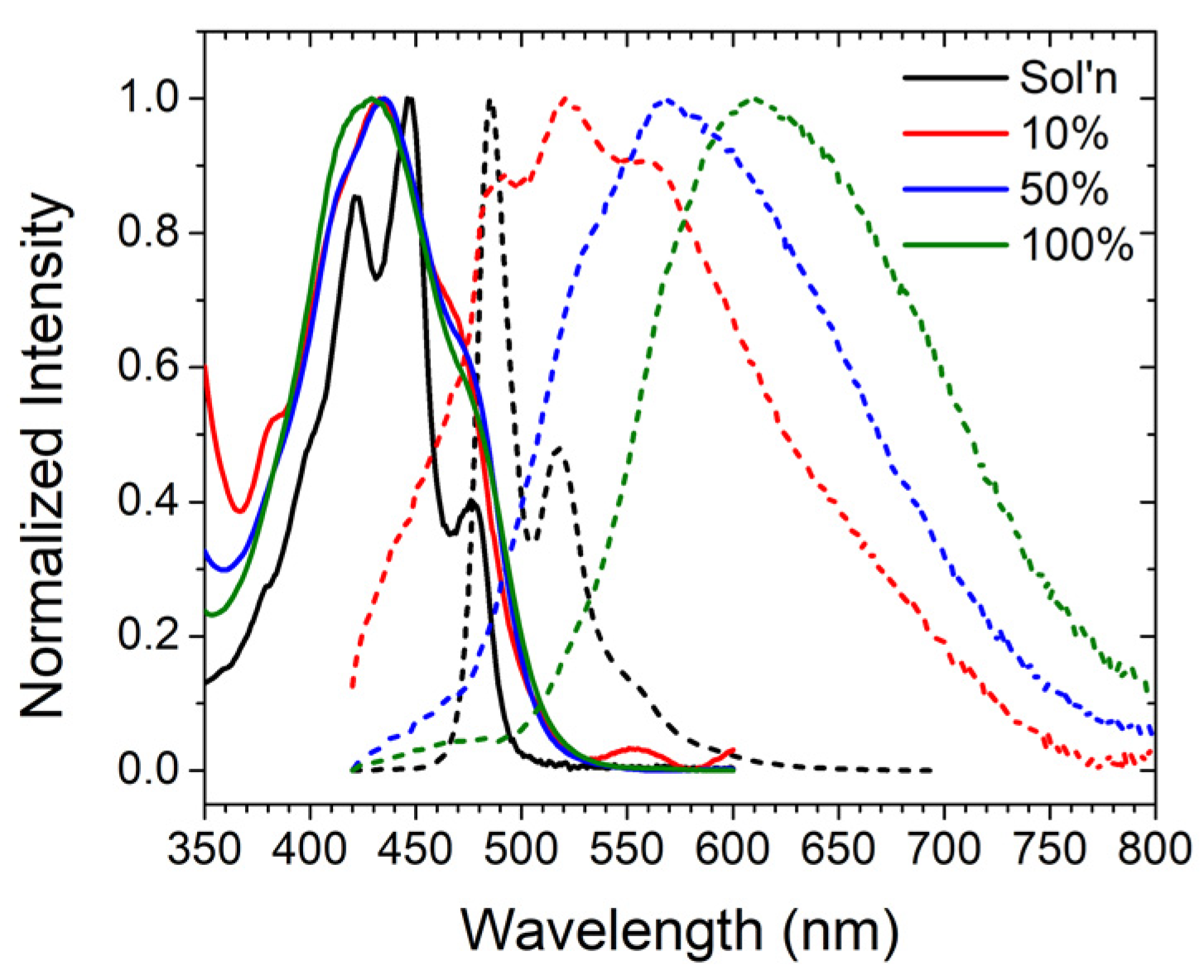
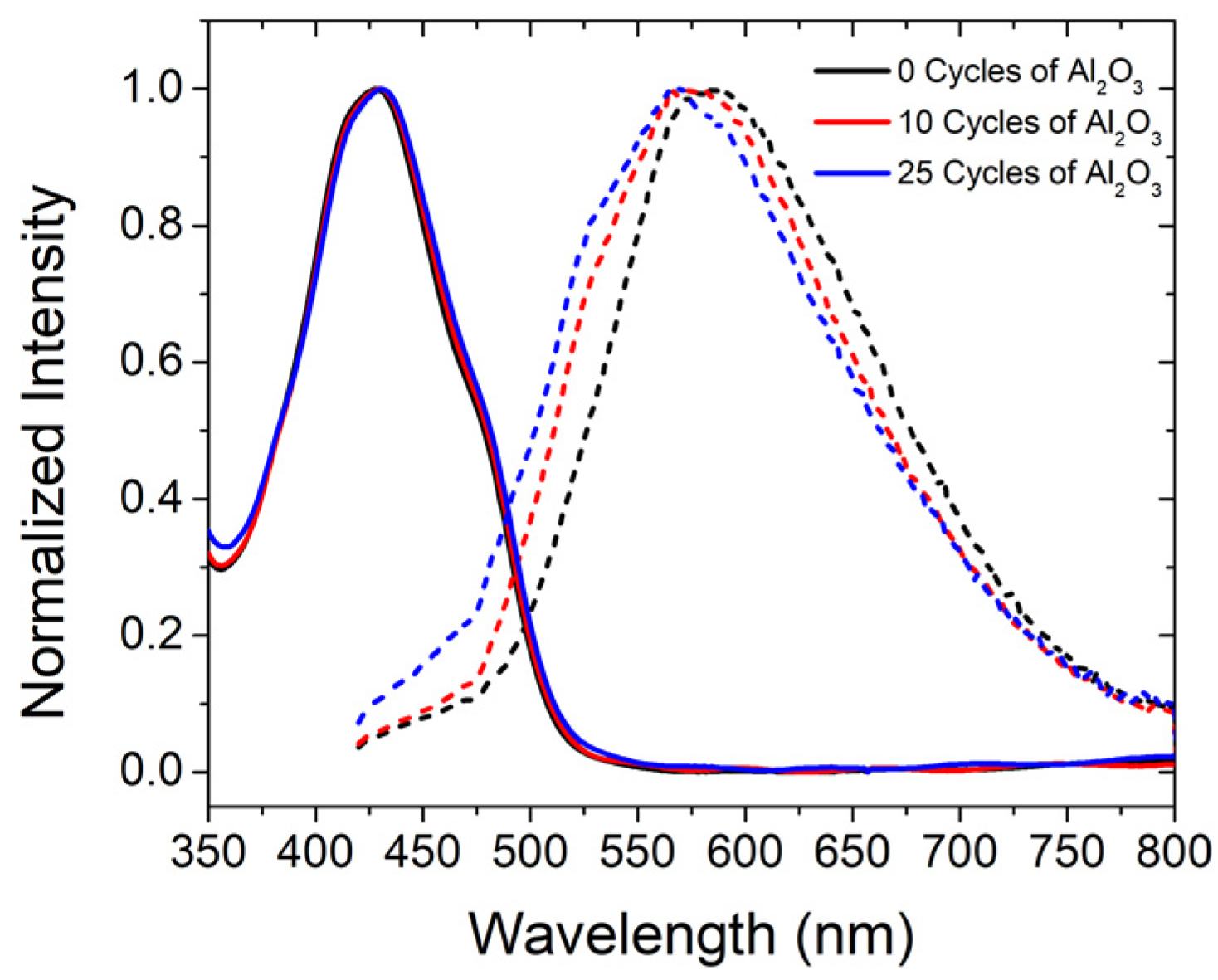
2.2. Photophysical Properties of ZrO2-A
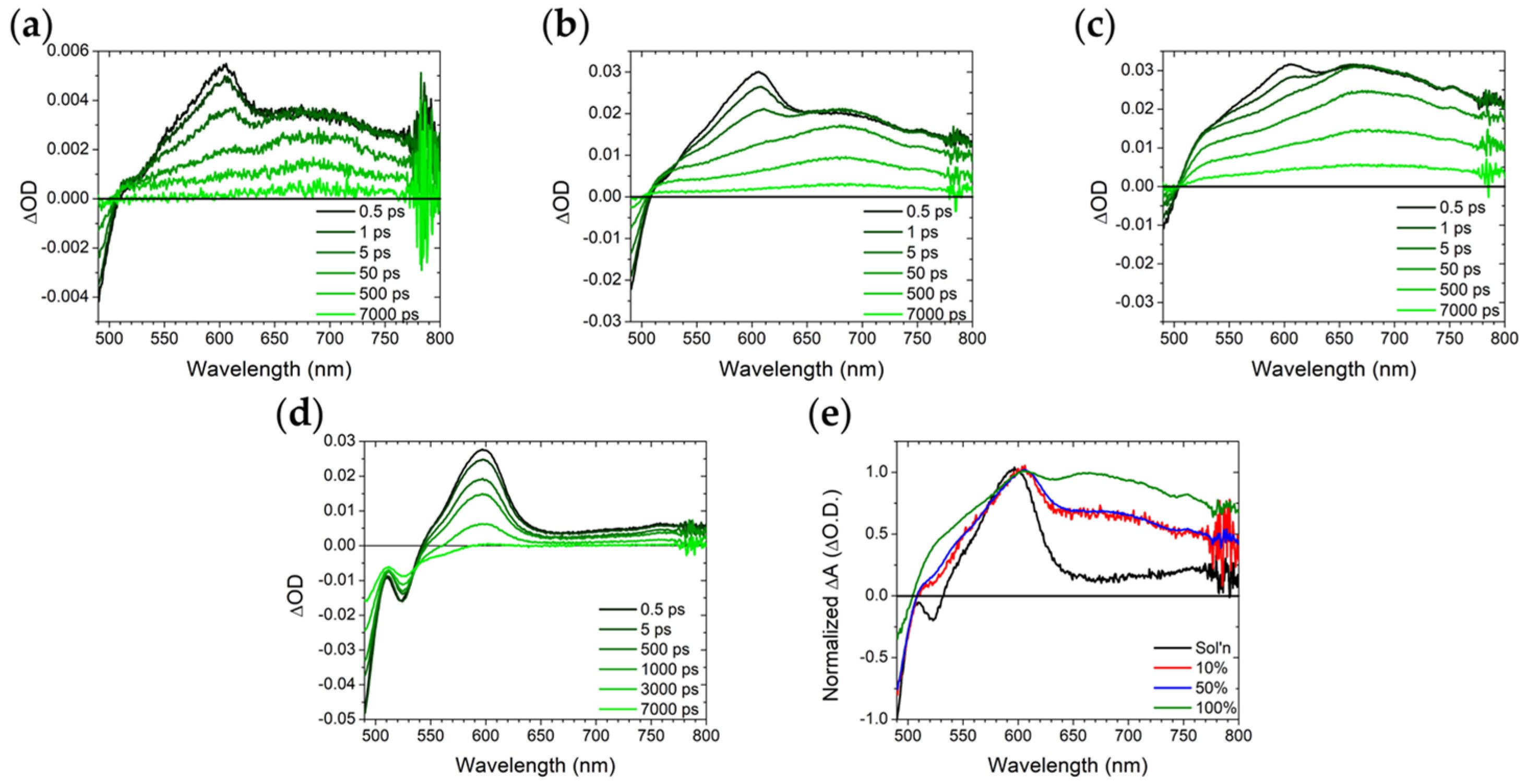
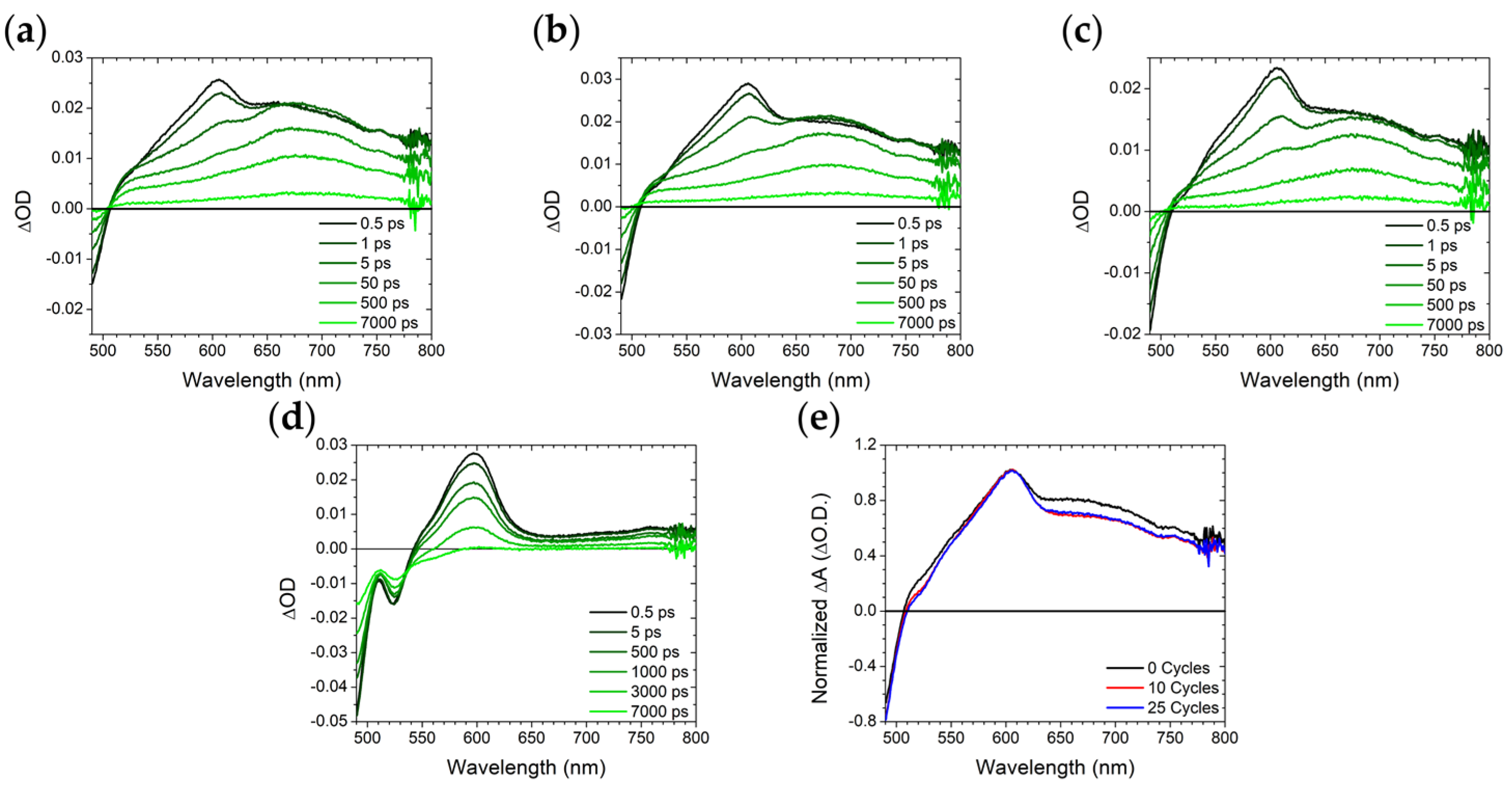
2.3. ZrO2-A-Zn with Al2O3
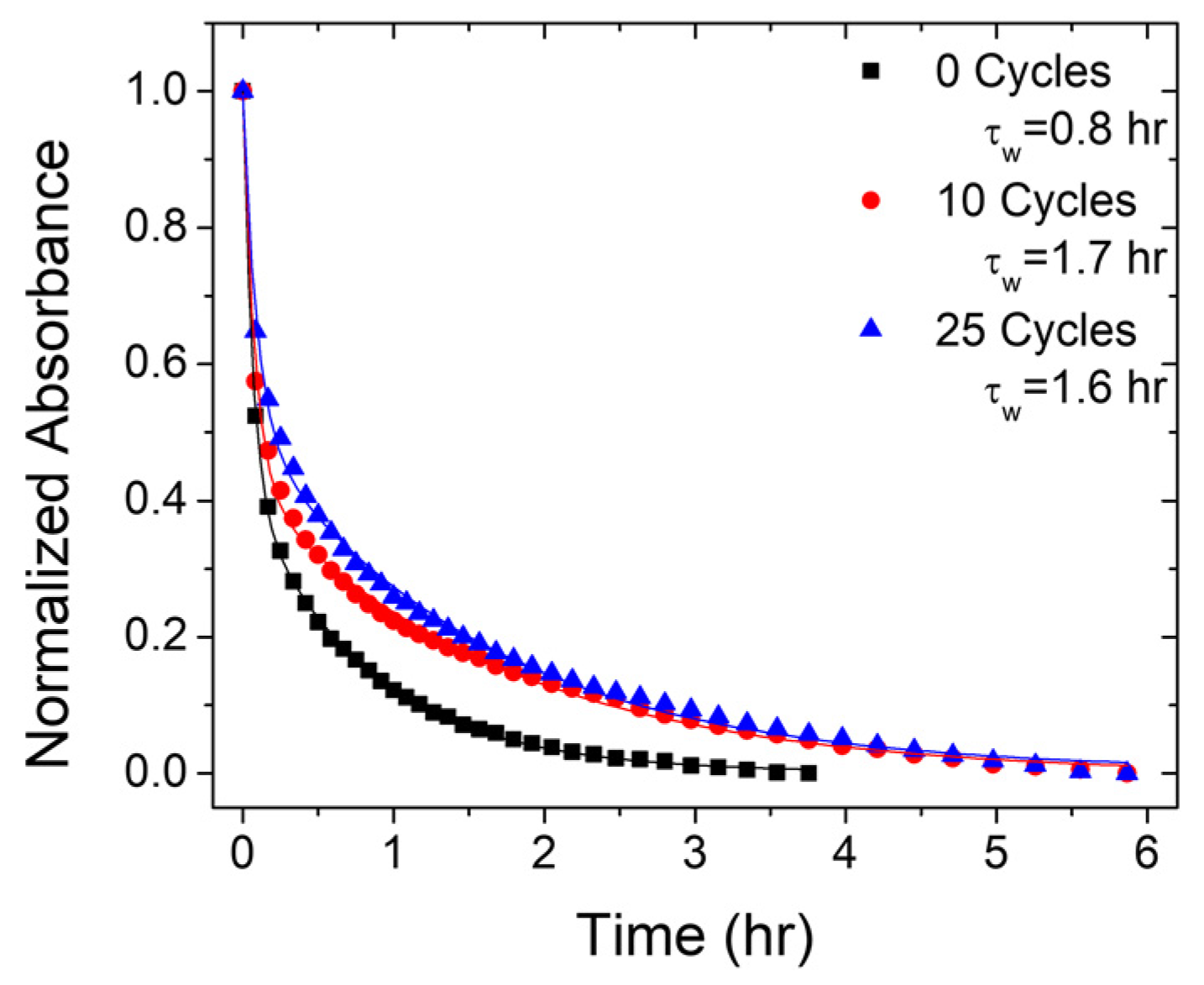
2.4. Photostability
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Thin Film Sample Preparation
3.3. Atomic Layer Deposition
3.4. Attenuated Total Reflectance–Fourier-Transform Infrared Spectroscopy (ATR-IR)
3.5. X-ray Fluorescence
3.6. Absorbance
3.7. Steady-State Emission
3.8. Time Resolved Emission
3.9. Femtosecond Transient Absorption
3.10. Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hestand, N.J.; Spano, F.C. Molecular Aggregate Photophysics beyond the Kasha Model: Novel Design Principles for Organic Materials. Acc. Chem. Res. 2017, 50, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Myong, M.S.; Young, R.M.; Wasielewski, M.R. Excimer Diffusivity in 9,10-Bis(Phenylethynyl)Anthracene Assemblies on Anodic Aluminum Oxide Membranes. J. Phys. Chem. C 2021, 125, 24498–24504. [Google Scholar] [CrossRef]
- Beery, D.; Arcidiacono, A.; Wheeler, J.P.; Chen, J.; Hanson, K. Harnessing Near-Infrared Light via S0 to T1 Sensitizer Excitation in a Molecular Photon Upconversion Solar Cell. J. Mater. Chem. C 2022, 10, 4947–4954. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kang, G.; Malliakas, C.D.; Nelson, J.N.; Zhou, J.; Young, R.M.; Wu, Y.-L.; Van Duyne, R.P.; Schatz, G.C.; Wasielewski, M.R. Singlet Fission in 9,10-Bis(Phenylethynyl)Anthracene Thin Films. J. Am. Chem. Soc. 2018, 140, 15140–15144. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Humphry-Baker, R.; Liska, P.; Grätzel, M. Investigation of Sensitizer Adsorption and the Influence of Protons on Current and Voltage of a Dye-Sensitized Nanocrystalline TiO2 Solar Cell. J. Phys. Chem. B 2003, 107, 8981–8987. [Google Scholar] [CrossRef]
- Wang, J.C.; Hill, S.P.; Dilbeck, T.; Ogunsolu, O.O.; Banerjee, T.; Hanson, K. Multimolecular Assemblies on High Surface Area Metal Oxides and Their Role in Interfacial Energy and Electron Transfer. Chem. Soc. Rev. 2018, 47, 104–148. [Google Scholar] [CrossRef] [PubMed]
- Manthou, V.S.; Pefkianakis, E.K.; Falaras, P.; Vougioukalakis, G.C. Co-Adsorbents: A Key Component in Efficient and Robust Dye-Sensitized Solar Cells. ChemSusChem 2015, 8, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Dover, C.B.; Gallaher, J.K.; Frazer, L.; Tapping, P.C.; Petty, A.J.; Crossley, M.J.; Anthony, J.E.; Kee, T.W.; Schmidt, T.W. Endothermic Singlet Fission Is Hindered by Excimer Formation. Nat. Chem. 2018, 10, 305–310. [Google Scholar] [CrossRef]
- Samanta, S.; Ray, S.K.; Deolka, S.; Saha, S.; Pradeep, K.R.; Bhowal, R.; Ghosh, N.; Chaudhuri, D. Safeguarding Long-Lived Excitons from Excimer Traps in H-Aggregated Dye-Assemblies. Chem. Sci. 2020, 11, 5710–5715. [Google Scholar] [CrossRef]
- Dilbeck, T.; Hanson, K. Molecular Photon Upconversion Solar Cells Using Multilayer Assemblies: Progress and Prospects. J. Phys. Chem. Lett. 2018, 9, 5810–5821. [Google Scholar] [CrossRef]
- Beery, D.; Schmidt, T.W.; Hanson, K. Harnessing Sunlight via Molecular Photon Upconversion. ACS Appl. Mater. Interfaces 2021, 13, 32601–32605. [Google Scholar] [CrossRef]
- Hanna, M.C.; Nozik, A.J. Solar Conversion Efficiency of Photovoltaic and Photoelectrolysis Cells with Carrier Multiplication Absorbers. J. Appl. Phys. 2006, 100, 074510. [Google Scholar] [CrossRef]
- Schrauben, J.N.; Zhao, Y.; Mercado, C.; Dron, P.I.; Ryerson, J.L.; Michl, J.; Zhu, K.; Johnson, J.C. Photocurrent Enhanced by Singlet Fission in a Dye-Sensitized Solar Cell. ACS Appl. Mater. Interfaces 2015, 7, 2286–2293. [Google Scholar] [CrossRef]
- Banerjee, T.; Hill, S.P.; Hermosilla-Palacios, M.A.; Piercy, B.D.; Haney, J.; Casale, B.; DePrince, A.E.I.; Losego, M.D.; Kleiman, V.D.; Hanson, K. Diphenylisobenzofuran Bound to Nanocrystalline Metal Oxides: Excimer Formation, Singlet Fission, Electron Injection, and Low Energy Sensitization. J. Phys. Chem. C 2018, 122, 28478–28490. [Google Scholar] [CrossRef]
- Treat, N.A.; Knorr, F.J.; McHale, J.L. Templated Assembly of Betanin Chromophore on TiO2: Aggregation-Enhanced Light-Harvesting and Efficient Electron Injection in a Natural Dye-Sensitized Solar Cell. J. Phys. Chem. C 2016, 120, 9122–9131. [Google Scholar] [CrossRef]
- Nüesch, F.; Moser, J.E.; Shklover, V.; Grätzel, M. Merocyanine Aggregation in Mesoporous Networks. J. Am. Chem. Soc. 1996, 118, 5420–5431. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-Stacked Perylenediimides as an Alternative Strategy for High Efficiency Nonfullerene Acceptors in Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Margulies, E.A.; Matte, H.S.S.R.; Hersam, M.C.; Marks, T.J.; Wasielewski, M.R. Effects of Crystalline Perylenediimide Acceptor Morphology on Optoelectronic Properties and Device Performance. Chem. Mater. 2016, 28, 3928–3936. [Google Scholar] [CrossRef]
- Mann, J.R.; Gannon, M.K.; Fitzgibbons, T.C.; Detty, M.R.; Watson, D.F. Optimizing the Photocurrent Efficiency of Dye-Sensitized Solar Cells through the Controlled Aggregation of Chalcogenoxanthylium Dyes on Nanocrystalline Titania Films. J. Phys. Chem. C 2008, 112, 13057–13061. [Google Scholar] [CrossRef]
- Arcidiacono, A.; Zhou, Y.; Zhang, W.; Ellison, J.O.; Ayad, S.; Knorr, E.S.; Peters, A.N.; Zheng, L.; Yang, W.; Saavedra, S.S.; et al. Examining the Influence of Bilayer Structure on Energy Transfer and Molecular Photon Upconversion in Metal Ion Linked Multilayers. J. Phys. Chem. C 2020, 124, 23597–23610. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface Chemistry of Atomic Layer Deposition: A Case Study for the Trimethylaluminum/Water Process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Hanson, K.; Losego, M.D.; Kalanyan, B.; Ashford, D.L.; Parsons, G.N.; Meyer, T.J. Stabilization of [Ru(Bpy)2(4,4′-(PO3H2)Bpy)]2+ on Mesoporous TiO2 with Atomic Layer Deposition of Al2O3. Chem. Mater. 2013, 25, 3–5. [Google Scholar] [CrossRef]
- Son, H.-J.; Prasittichai, C.; Mondloch, J.E.; Luo, L.; Wu, J.; Kim, D.W.; Farha, O.K.; Hupp, J.T. Dye Stabilization and Enhanced Photoelectrode Wettability in Water-Based Dye-Sensitized Solar Cells through Post-Assembly Atomic Layer Deposition of TiO2. J. Am. Chem. Soc. 2013, 135, 11529–11532. [Google Scholar] [CrossRef]
- Hanson, K.; Losego, M.D.; Kalanyan, B.; Parsons, G.N.; Meyer, T.J. Stabilizing Small Molecules on Metal Oxide Surfaces Using Atomic Layer Deposition. Nano Lett. 2013, 13, 4802–4809. [Google Scholar] [CrossRef]
- Ayare, P.J.; Gregory, S.A.; Key, R.J.; Short, A.E.; Tillou, J.G.; Sitter, J.D.; Yom, T.; Goodlett, D.W.; Lee, D.-C.; Alamgir, F.M.; et al. Immobilization of Molecular Catalysts on Solid Supports via Atomic Layer Deposition for Chemical Synthesis in Sustainable Solvents. Green Chem. 2021, 23, 9523–9533. [Google Scholar] [CrossRef]
- Brady, M.D.; Troian-Gautier, L.; Motley, T.C.; Turlington, M.D.; Meyer, G.J. An Insulating Al2O3 Overlayer Prevents Lateral Hole Hopping Across Dye-Sensitized TiO2 Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 27453–27463. [Google Scholar] [CrossRef]
- Bae, Y.J.; Shimizu, D.; Schultz, J.D.; Kang, G.; Zhou, J.; Schatz, G.C.; Osuka, A.; Wasielewski, M.R. Balancing Charge Transfer and Frenkel Exciton Coupling Leads to Excimer Formation in Molecular Dimers: Implications for Singlet Fission. J. Phys. Chem. A 2020, 124, 8478–8487. [Google Scholar] [CrossRef]
- Tachibana, Y.; Moser, J.E.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Subpicosecond Interfacial Charge Separation in Dye-Sensitized Nanocrystalline Titanium Dioxide Films. J. Phys. Chem. 1996, 100, 20056–20062. [Google Scholar] [CrossRef]
- Hill, S.P.; Banerjee, T.; Dilbeck, T.; Hanson, K. Photon Upconversion and Photocurrent Generation via Self-Assembly at Organic–Inorganic Interfaces. J. Phys. Chem. Lett. 2015, 6, 4510–4517. [Google Scholar] [CrossRef]
- Gallagher, L.A.; Serron, S.A.; Wen, X.; Hornstein, B.J.; Dattelbaum, D.M.; Schoonover, J.R.; Meyer, T.J. Photoelectrochemistry on RuII-2,2′-Bipyridine-Phosphonate-Derivatized TiO2 with the I3-/I- and Quinone/Hydroquinone Relays. Design of Photoelectrochemical Synthesis Cells. Inorg. Chem. 2005, 44, 2089–2097. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Cabrera, W.; Halls, M.D.; Povey, I.M.; Chabal, Y.J. Surface Oxide Characterization and Interface Evolution in Atomic Layer Deposition of Al2O3 on InP(100) Studied by in Situ Infrared Spectroscopy. J. Phys. Chem. C 2014, 118, 5862–5871. [Google Scholar] [CrossRef]
- Wang, D.; Sheridan, M.V.; Shan, B.; Farnum, B.H.; Marquard, S.L.; Sherman, B.D.; Eberhart, M.S.; Nayak, A.; Dares, C.J.; Das, A.K.; et al. Layer-by-Layer Molecular Assemblies for Dye-Sensitized Photoelectrosynthesis Cells Prepared by Atomic Layer Deposition. J. Am. Chem. Soc. 2017, 139, 14518–14525. [Google Scholar] [CrossRef]
- Gao, W.; Dickinson, L.; Grozinger, C.; Morin, F.G.; Reven, L. Self-Assembled Monolayers of Alkylphosphonic Acids on Metal Oxides. Langmuir 1996, 12, 6429–6435. [Google Scholar] [CrossRef]
- Zhao, R.; Rupper, P.; Gaan, S. Recent Development in Phosphonic Acid-Based Organic Coatings on Aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition Alumina Phases Induced by Heat Treatment of Boehmite: An X-Ray Diffraction and Infrared Spectroscopy Study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Ott, A.W.; Klaus, J.W.; Johnson, J.M.; George, S.M. Al3O3 Thin Film Growth on Si(100) Using Binary Reaction Sequence Chemistry. Thin Solid Films 1997, 292, 135–144. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- van Hemmen, J.L.; Heil, S.B.S.; Klootwijk, J.H.; Roozeboom, F.; Hodson, C.J.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma and Thermal ALD of Al2O3 in a Commercial 200 Mm ALD Reactor. J. Electrochem. Soc. 2007, 154, G165. [Google Scholar] [CrossRef]
- Dingemans, G.; van de Sanden, M.C.M.; Kessels, W.M.M. Influence of the Deposition Temperature on the C-Si Surface Passivation by Al2O3 Films Synthesized by ALD and PECVD. Electrochem. Solid-State Lett. 2009, 13, H76. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.-H.; Jo, I.H.; Seo, J.; Yoo, Y.-E.; Kim, J.H. Influence of Growth Temperature on Dielectric Strength of Al2O3 Thin Films Prepared via Atomic Layer Deposition at Low Temperature. Sci. Rep. 2022, 12, 5124. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.E.; Dillon, R.J.; Nayak, A.; Brogan, S.; Moot, T.; Brennaman, M.K.; Lopez, R.; Meyer, T.J.; Alibabaei, L. Dye-Sensitized Nonstoichiometric Strontium Titanate Core–Shell Photocathodes for Photoelectrosynthesis Applications. ACS Appl. Mater. Interfaces 2021, 13, 15261–15269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, Y.; Cai, B.; Hägglund, C.; Kubart, T.; Boschloo, G.; Tian, H. Atomic Layer Deposition of SnO2 as an Electron Transport Material for Solid-State P-Type Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 12022–12028. [Google Scholar] [CrossRef]
- Durrant, J.R.; Haque, S.A.; Palomares, E. Towards Optimisation of Electron Transfer Processes in Dye Sensitised Solar Cells. Coord. Chem. Rev. 2004, 248, 1247–1257. [Google Scholar] [CrossRef]
- Wirp, C.; Güsten, H.; Brauer, H.-D. Fluorescence Quenching of Meso-Substituted Anthracene Derivatives by Molecular Oxygen. Ber. Der Bunsenges. Für Phys. Chem. 1996, 100, 1217–1225. [Google Scholar] [CrossRef]
- Sagara, Y.; Simon, Y.C.; Tamaoki, N.; Weder, C. A Mechano- and Thermoresponsive Luminescent Cyclophane. Chem. Commun. 2016, 52, 5694–5697. [Google Scholar] [CrossRef]
- Avis, P.; Porter, G. Effect of Concentration on the Absorption and Fluorescence Spectra of Pyrene in a Solid Solution of Poly(Methyl Methacrylate). J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1974, 70, 1057–1065. [Google Scholar] [CrossRef]
- Johnson, G.E. Effect of Concentration on the Fluorescence Spectra and Lifetimes of Pyrene in Polystyrene Films. Macromolecules 1980, 13, 839–844. [Google Scholar] [CrossRef]
- Yamazaki, I.; Tamai, N.; Yamazaki, T. Picosecond Fluorescence Spectroscopy on Excimer Formation and Excitation Energy Transfer of Pyrene in Langmuir-Blodgett Monolayer Films. J. Phys. Chem. 1987, 91, 3572–3577. [Google Scholar] [CrossRef]
- Zachariasse, K.A. Excimer Formation with Pyrenes on Silica Surfaces. In Studies in Surface Science and Catalysis; Anpo, M., Matsuura, T., Eds.; Photochemistry on Solid Surfaces; Elsevier: Amsterdam, The Netherlands, 1989; Volume 47, pp. 48–78. [Google Scholar] [CrossRef]
- Hanson, K.; Brennaman, M.K.; Luo, H.; Glasson, C.R.K.; Concepcion, J.J.; Song, W.; Meyer, T.J. Photostability of Phosphonate-Derivatized, RuII Polypyridyl Complexes on Metal Oxide Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 1462–1469. [Google Scholar] [CrossRef]
- Wei, K.S.; Livingston, R. Reversible Photodimerization of Anthracene and Tetracene. Photochem. Photobiol. 1967, 6, 229–232. [Google Scholar] [CrossRef]
- Li, Y.; Goswami, M.; Zhang, Y.; Liu, T.; Zhang, J.; Kessler, M.R.; Wang, L.; Rios, O. Combined Light- and Heat-Induced Shape Memory Behavior of Anthracene-Based Epoxy Elastomers. Sci. Rep. 2020, 10, 20214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.A.; Abrams, N.M.; Hoertz, P.G.; Barber, G.D.; Halaoui, L.I.; Mallouk, T.E. Coupling of Titania Inverse Opals to Nanocrystalline Titania Layers in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2008, 112, 14415–14421. [Google Scholar] [CrossRef] [PubMed]
- Ogunsolu, O.O.; Wang, J.C.; Hanson, K. Inhibiting Interfacial Recombination Events in Dye-Sensitized Solar Cells Using Self-Assembled Bilayers. ACS Appl. Mater. Interfaces 2015, 7, 27730–27734. [Google Scholar] [CrossRef] [PubMed]
| Sample | 500 nm | 600 nm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | τ1 (ns) | A2 | τ2 (ns) | τw (ns) a | A1 | τ1 (ns) | A2 | τ2 (ns) | τw (ns) a | |
| Sol’n | - | - | - | - | 2.6 ± 0.1 b | - | - | - | - | 2.7 ± 0.1 b |
| 10% | 0.65 | 0.3 | 0.017 | 4.2 | 3.7 ± 0.8 | 0.30 | 0.7 | 0.023 | 10.9 | 10.4 ± 0.3 |
| 50% | 0.94 | 0.2 | 0.008 | 5.8 | 5.2 ± 0.5 | 0.30 | 0.8 | 0.027 | 11.8 | 11.2 ± 0.5 |
| 100% | 0.50 | 0.3 | 0.003 | 6.7 | 5.4 ± 0.5 | 0.32 | 0.6 | 0.029 | 9.2 | 8.8 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knorr, E.S.; Basquill, C.T.; Bertini, I.A.; Arcidiacono, A.; Beery, D.; Wheeler, J.P.; Winfred, J.S.R.V.; Strouse, G.F.; Hanson, K. Influence of Al2O3 Overlayers on Intermolecular Interactions between Metal Oxide Bound Molecules. Molecules 2023, 28, 4835. https://doi.org/10.3390/molecules28124835
Knorr ES, Basquill CT, Bertini IA, Arcidiacono A, Beery D, Wheeler JP, Winfred JSRV, Strouse GF, Hanson K. Influence of Al2O3 Overlayers on Intermolecular Interactions between Metal Oxide Bound Molecules. Molecules. 2023; 28(12):4835. https://doi.org/10.3390/molecules28124835
Chicago/Turabian StyleKnorr, Erica S., Cody T. Basquill, Isabella A. Bertini, Ashley Arcidiacono, Drake Beery, Jonathan P. Wheeler, J. S. Raaj Vellore Winfred, Geoffrey F. Strouse, and Kenneth Hanson. 2023. "Influence of Al2O3 Overlayers on Intermolecular Interactions between Metal Oxide Bound Molecules" Molecules 28, no. 12: 4835. https://doi.org/10.3390/molecules28124835
APA StyleKnorr, E. S., Basquill, C. T., Bertini, I. A., Arcidiacono, A., Beery, D., Wheeler, J. P., Winfred, J. S. R. V., Strouse, G. F., & Hanson, K. (2023). Influence of Al2O3 Overlayers on Intermolecular Interactions between Metal Oxide Bound Molecules. Molecules, 28(12), 4835. https://doi.org/10.3390/molecules28124835