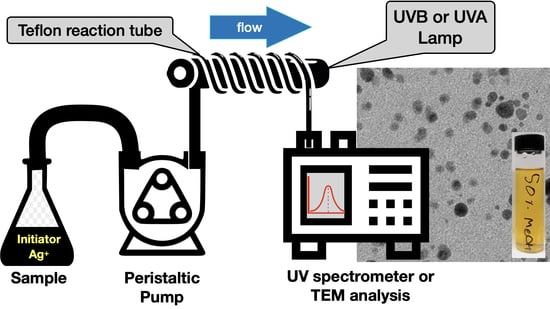
Facile Scale-Up of the Flow Synthesis of Silver Nanostructures Based on Norrish Type I Photoinitiators
Abstract
1. Introduction
2. Results and Discussion
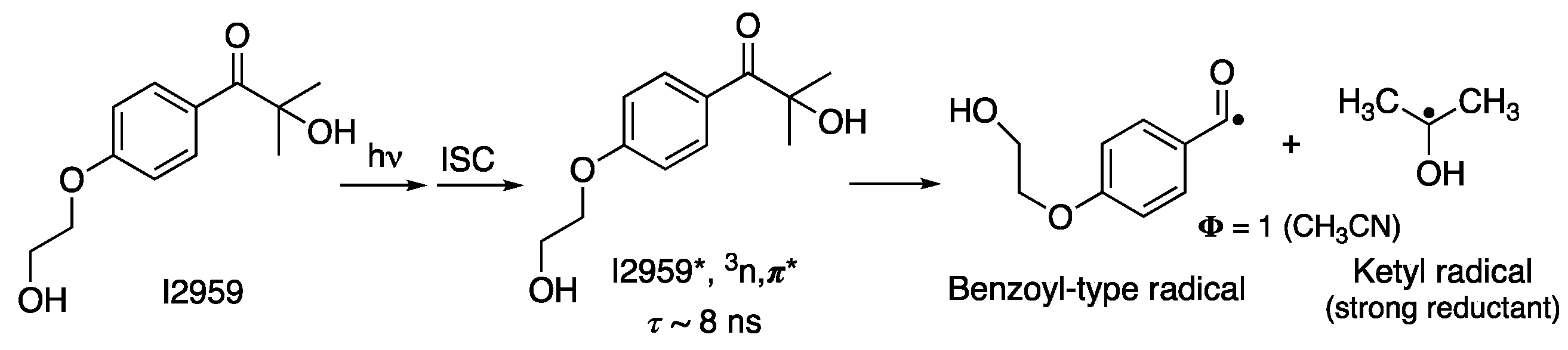
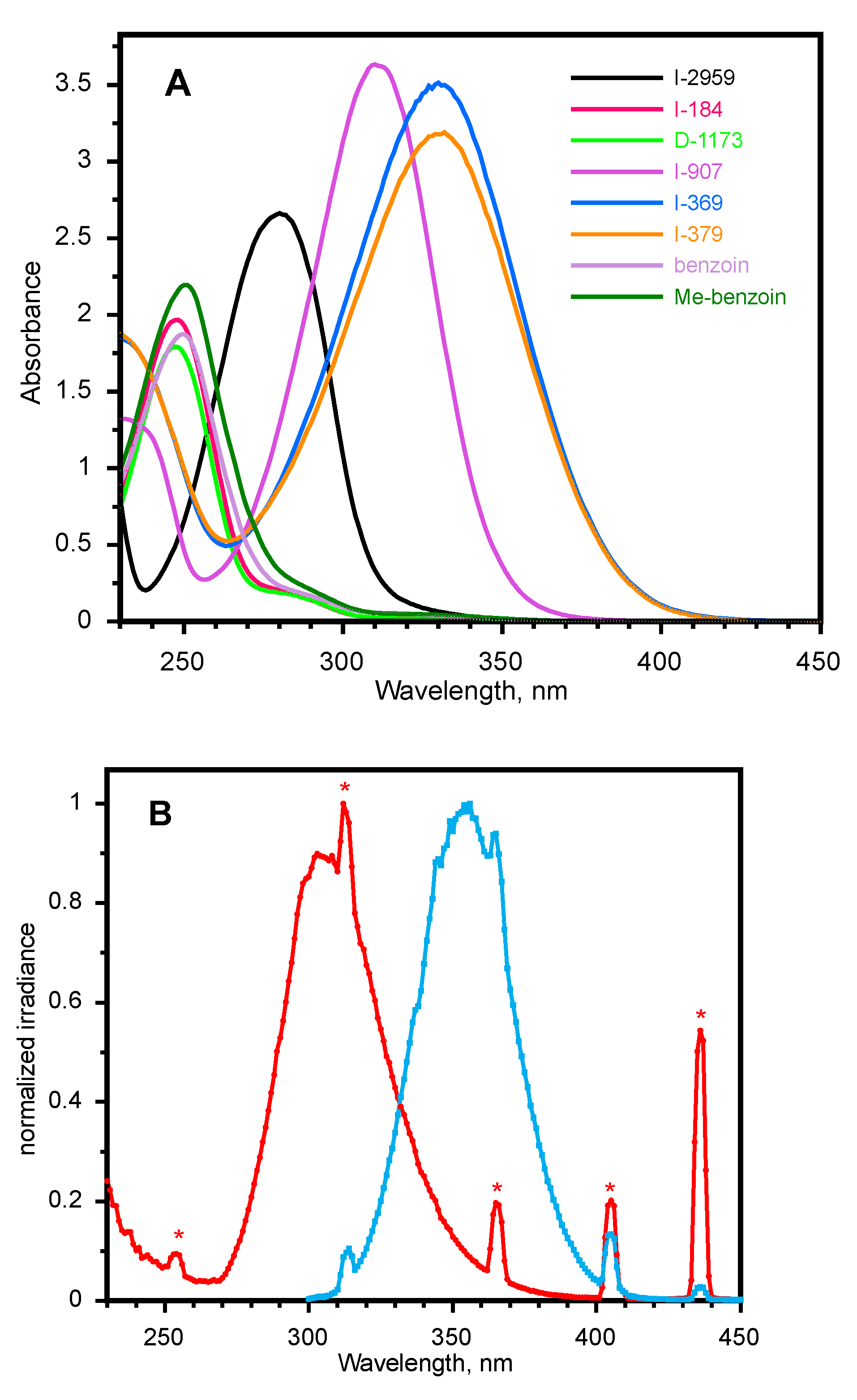
2.1. Spectroscopy of Initiators and Lamps Used
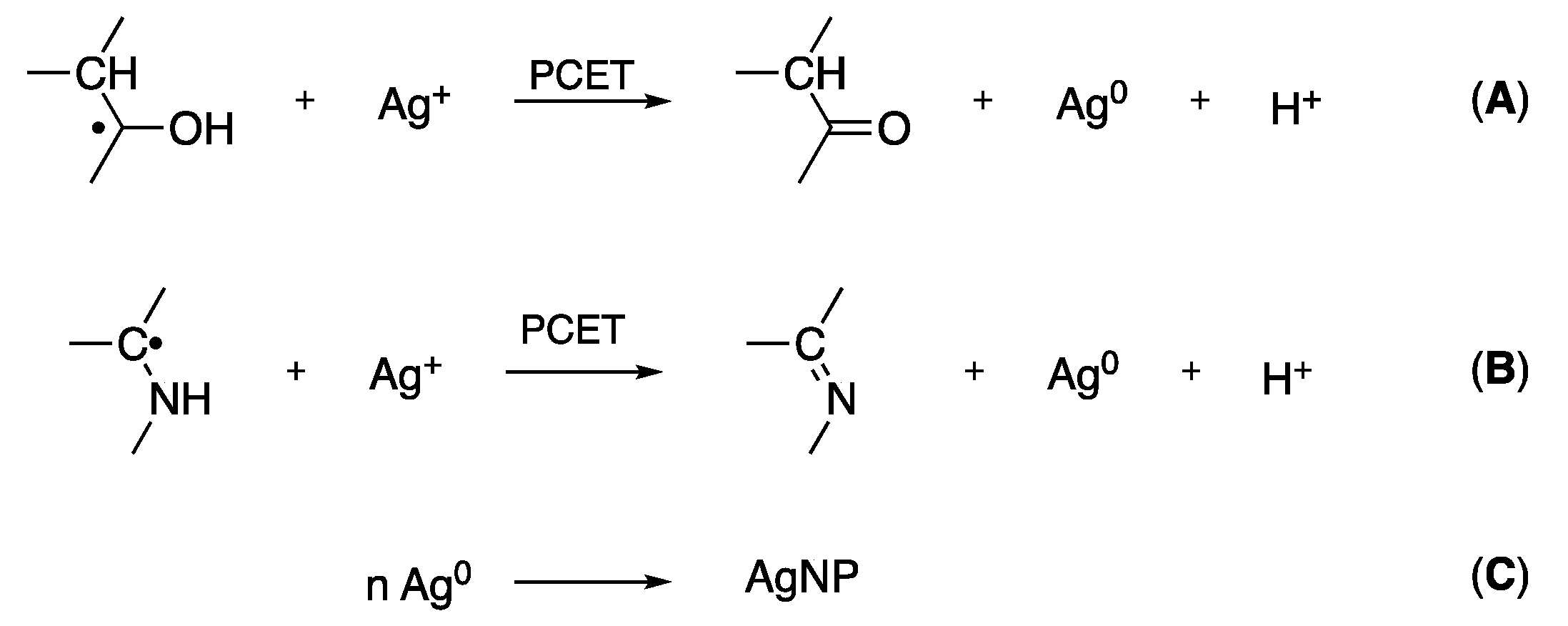
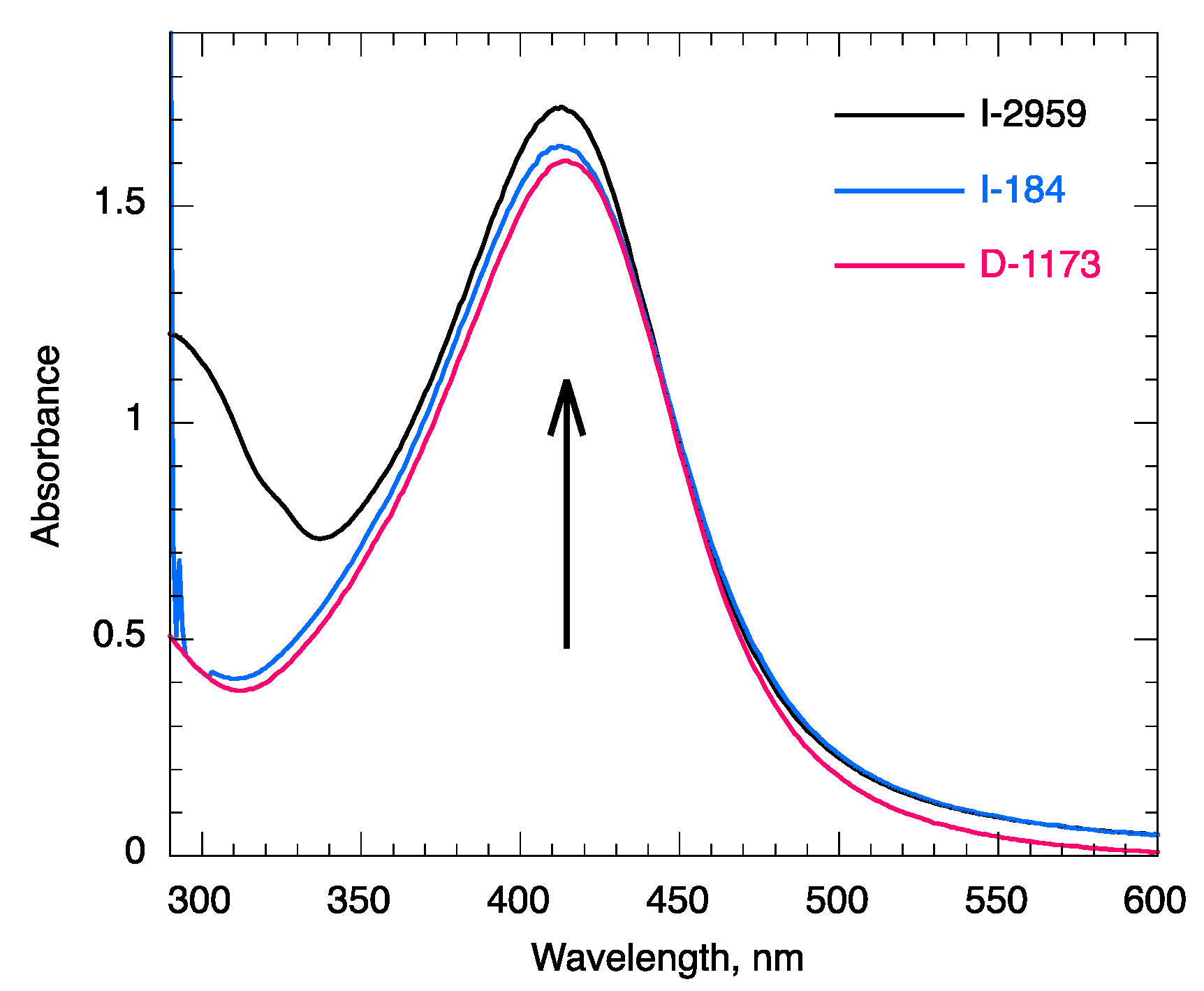
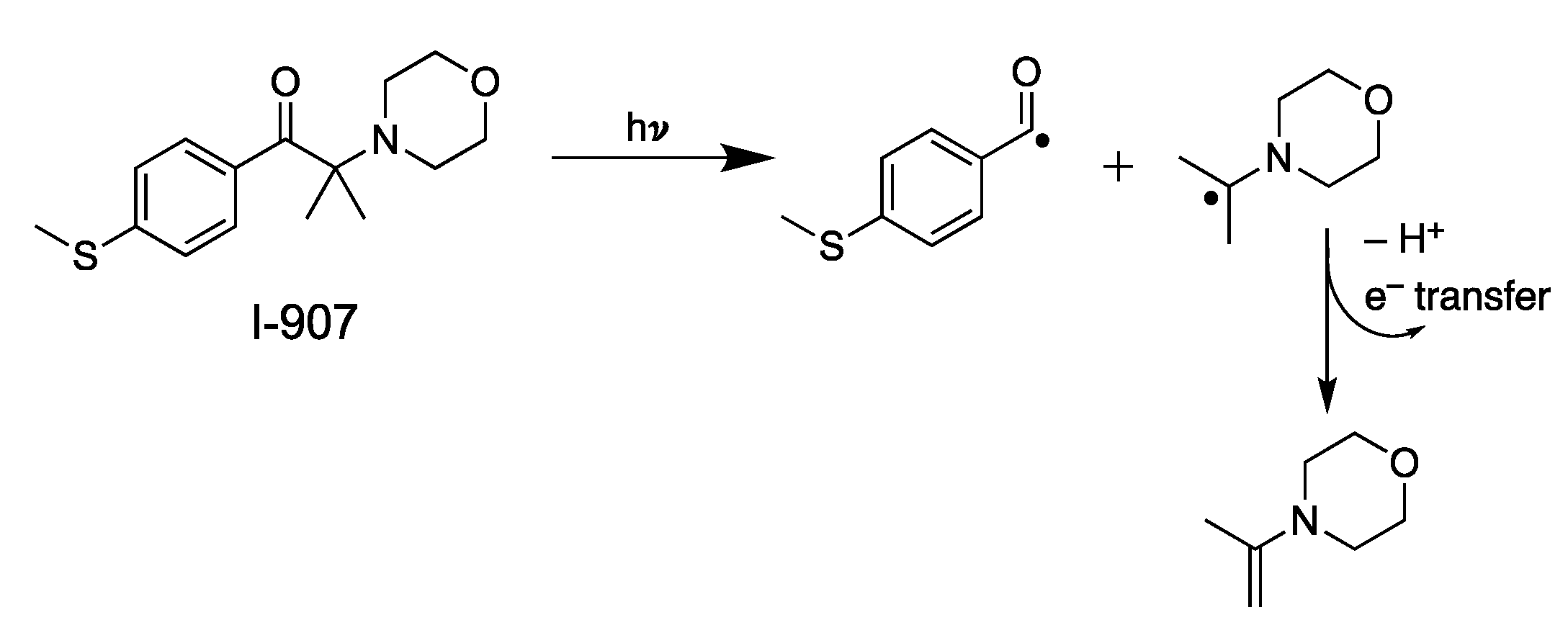
2.2. Batch Experiments and Controls
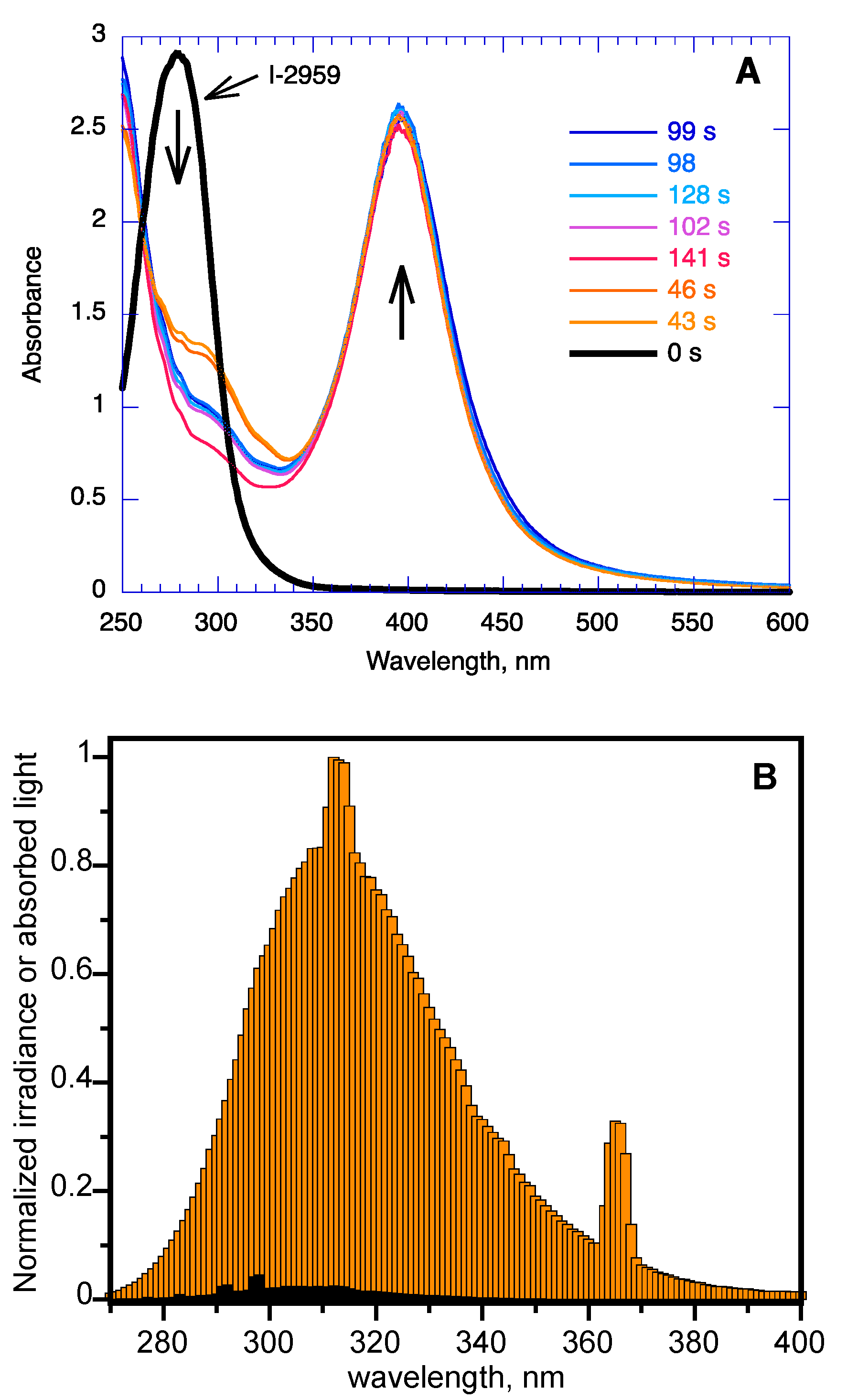
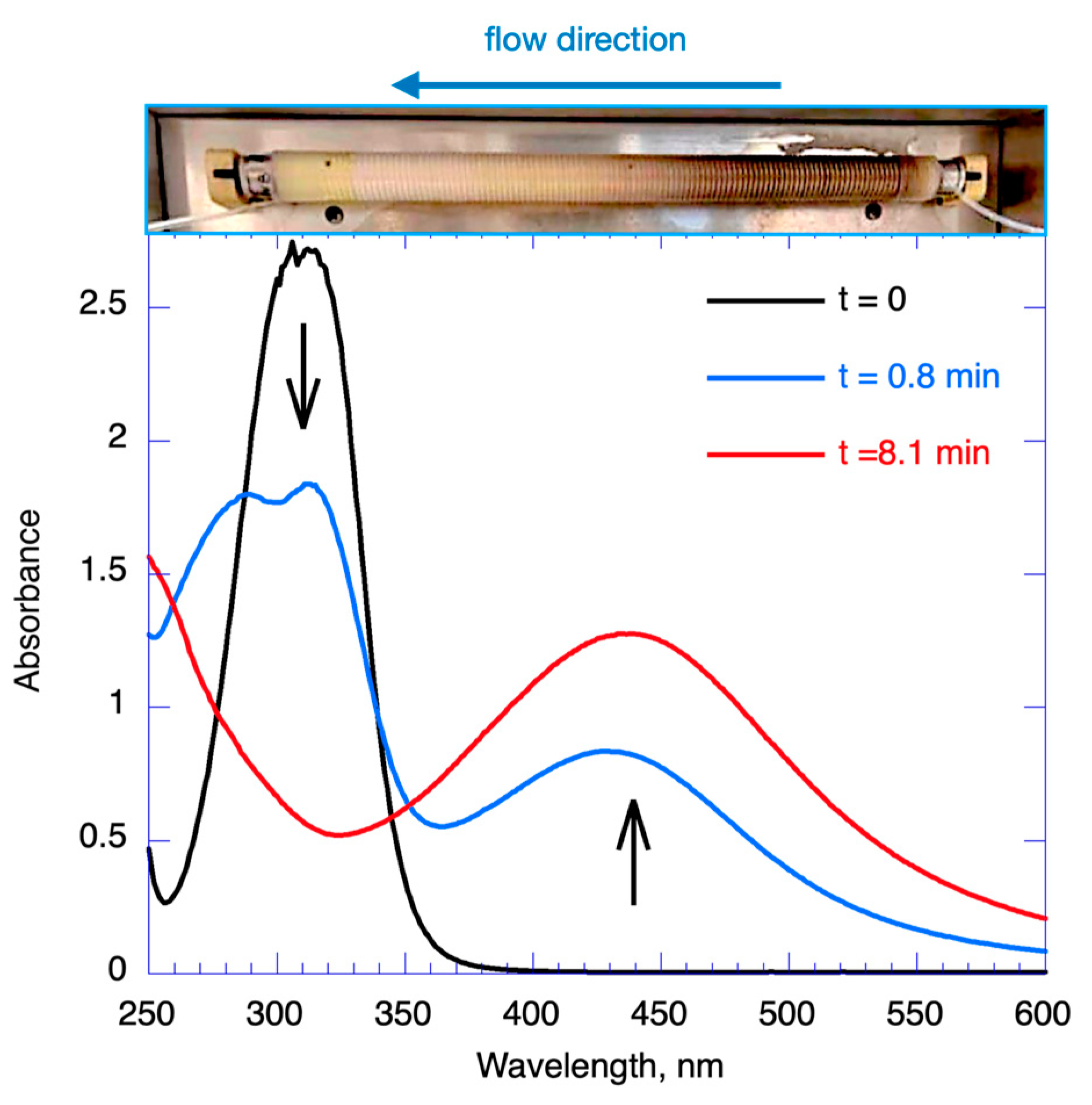
2.3. Nanoparticle Synthesis Using Flow Strategies
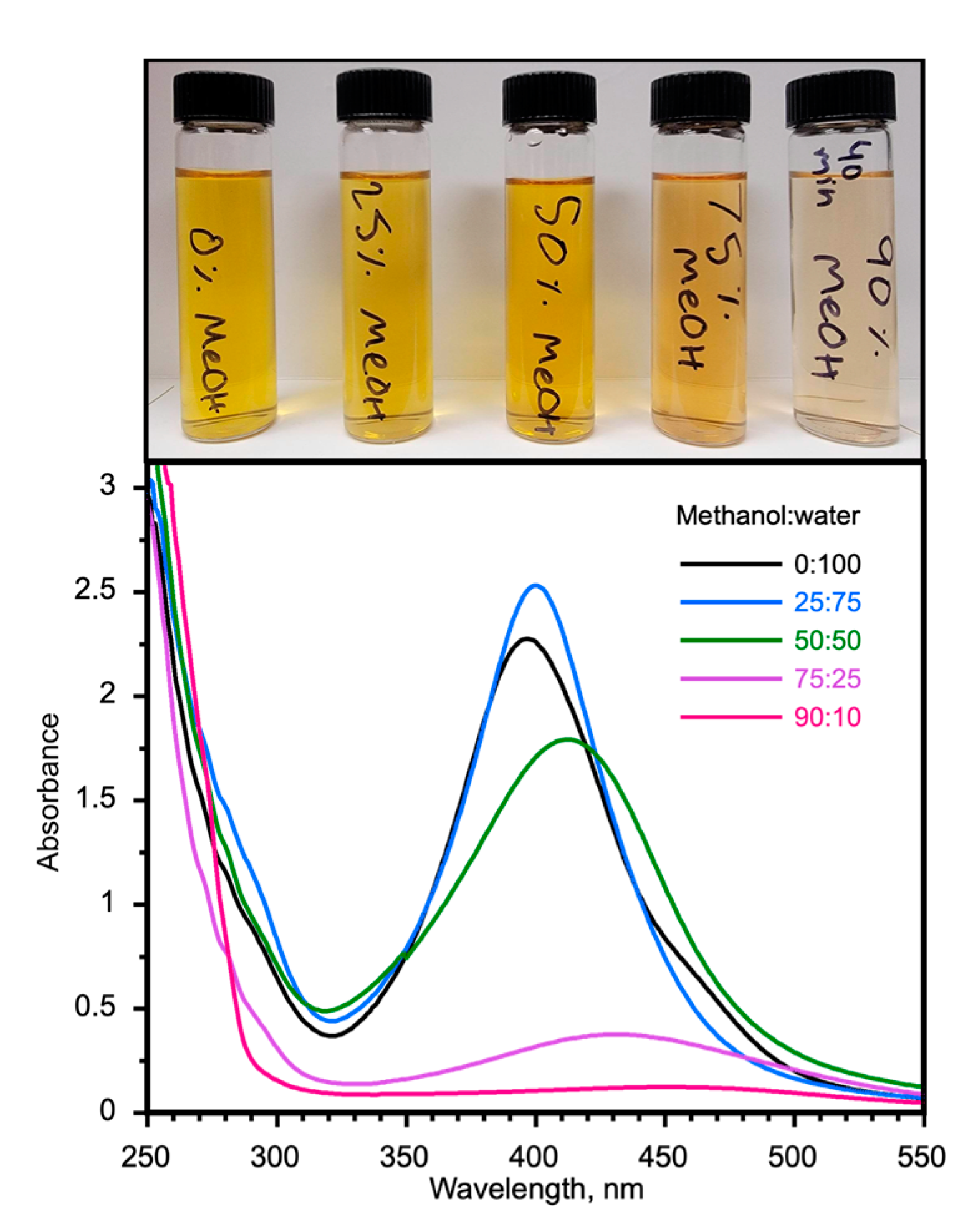
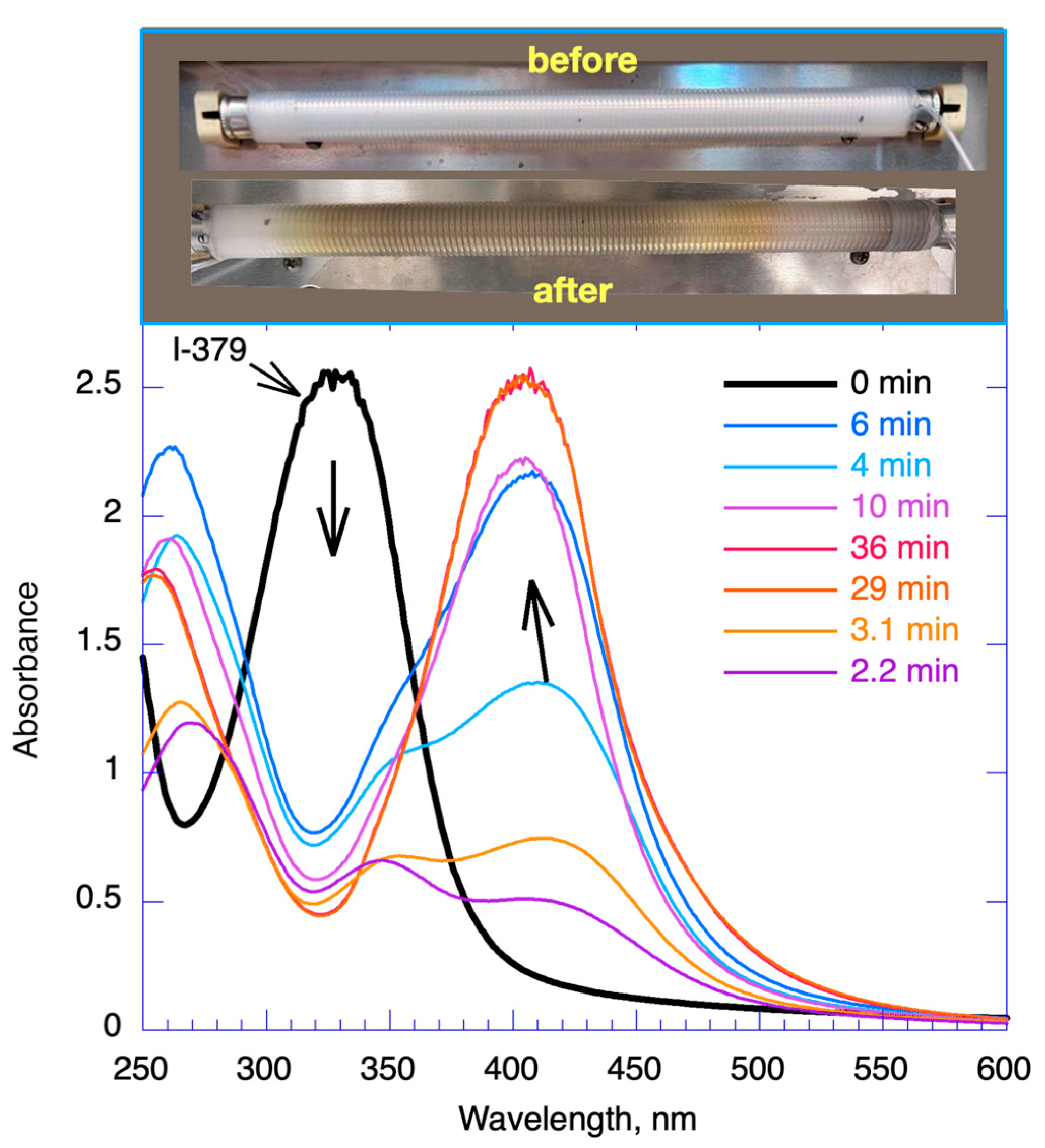
2.4. Concentration Tolerance and Stability during Long Fabrication Times
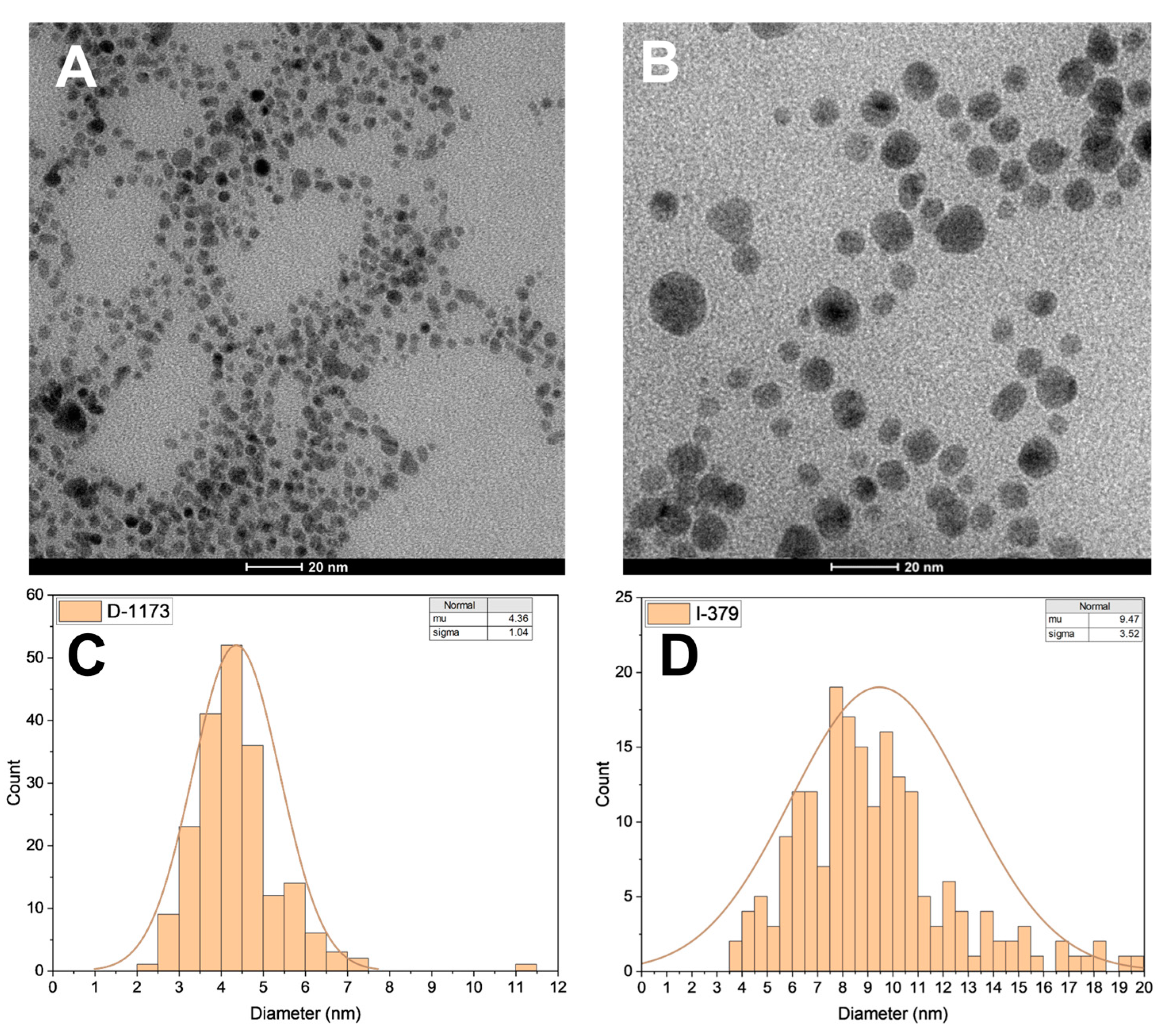
2.5. Characterization of the Nanostructures Prepared
3. Materials and Methods
3.1. Materials
3.2. Spectroscopy
3.3. Batch Reactions
3.4. Flow Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scaiano, J.C.; Stamplecoskie, K.G.; Hallett-Tapley, G.L. Photochemical Norrish type I reaction as a tool for metal nanoparticle synthesis: Importance of proton coupled electron transfer. Chem. Commun. 2012, 48, 4798–4808. [Google Scholar] [CrossRef] [PubMed]
- Lipson, M.; Turro, N.J. Picosecond investigation of the effect of solvent on the photochemistry of benzoin. J. Photochem. Photobiol. A Chem. 1996, 99, 93–96. [Google Scholar] [CrossRef]
- Jockusch, S.; Landis, M.S.; Freiermuth, B.; Turro, N.J. Photochemistry and Photophysics of a-Hydroxy Ketones. Macromolecules 2001, 34, 1619–1626. [Google Scholar] [CrossRef]
- Esen, D.S.; Arsu, N.; Da Silva, J.P.; Jockusch, S.; Turro, N.J. Benzoin type photoinitiator for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1865–1871. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Decan, M.R.; Wang, D.; Scaiano, J.C. Facile Photochemical Synthesis of Unprotected Aqueous Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 15980–15981. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; McGilvray, K.L.; Scaiano, J.C. Photochemical Strategies for the Synthesis of Gold Nanoparticles from Au(III) and Au(I) Using Photoinduced Free Radical Generation. J. Am. Chem. Soc. 2008, 130, 16572–16584. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Billone, P.; Gonzalez, C.M.; Maretti, L.; Marin, M.L.; McGilvray, K.L.; Yuan, N. Photochemical routes to silver and gold nanoparticles. Pure Appl. Chem. 2009, 81, 635–647. [Google Scholar] [CrossRef]
- Yaghmaei, M.; Scaiano, J.C. A simple Norrish Type II actinometer for flow photoreactions. Photochem. Photobiol. Sci. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.R.; Ahmadi, T.S. Effects of Intensity and Energy of CW UV Light on the Growth of Gold Nanorods. J. Phys. Chem. B 2005, 109, 15724–15734. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Kinetics of the Formation of Silver Dimers: Early Stages in the Formation of Silver Nanoparticles. J. Am. Chem. Soc. 2011, 133, 3913–3920. [Google Scholar] [CrossRef]
- Cambié, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Rehm, T.H. Flow Photochemistry as a Tool in Organic Synthesis. Chem. Eur. J. 2020, 26, 16952–16974. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Scaiano, J.C. Light Emitting Diode Irradiation Can Control the Morphology and Optical Properties of Silver Nanoparticles. J. Am. Chem. Soc. 2010, 132, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Chen, C.Y.; Chen, W.M.; Yu, A.B. Role of Citric Acid in the Formation of Silver Nanoplates through a Synergistic Reduction Approach. Langmuir 2010, 26, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study in the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Cruickshank, F.R.; Benson, S.W. Carbon-hydrogen bond dissociation energy in methanol. J. Phys. Chem. 1969, 73, 733–737. [Google Scholar] [CrossRef]
- Belloni, J. The role of silver clusters in photography. Comptes Rendus Physique 2002, 3, 381–390. [Google Scholar] [CrossRef]
- Lewis, F.D.; Lauterback, R.J.; Heine, H.G.; Hartmann, W.; Rudolph, H. Photochemical a-cleavage of Benzoin Derivatives. Polar Transition States for Free Radical Formation. J. Am. Chem. Soc. 1975, 97, 1519–1525. [Google Scholar] [CrossRef]
- Mahy, J.G.; Kiendrebeogo, M.; Farcy, A.; Drogui, P. Enhanced Decomposition of H2O2 Using Metallic Silver Nanoparticles under UV/Visible Light for the Removal of p-Nitrophenol from Water. Catalysts 2023, 13, 842. [Google Scholar] [CrossRef]
- Bourgonje, C.R.; Regis Correa da Silva, D.; McIlroy, E.; Calvert, N.D.; Shuhendler, A.J.; Scaiano, J.C. Silver Nanoparticles with exceptional near-infrared absorbance for photo-enhanced antimicrobial applications. J. Mat. Chem B 2023, 11. [Google Scholar] [CrossRef]
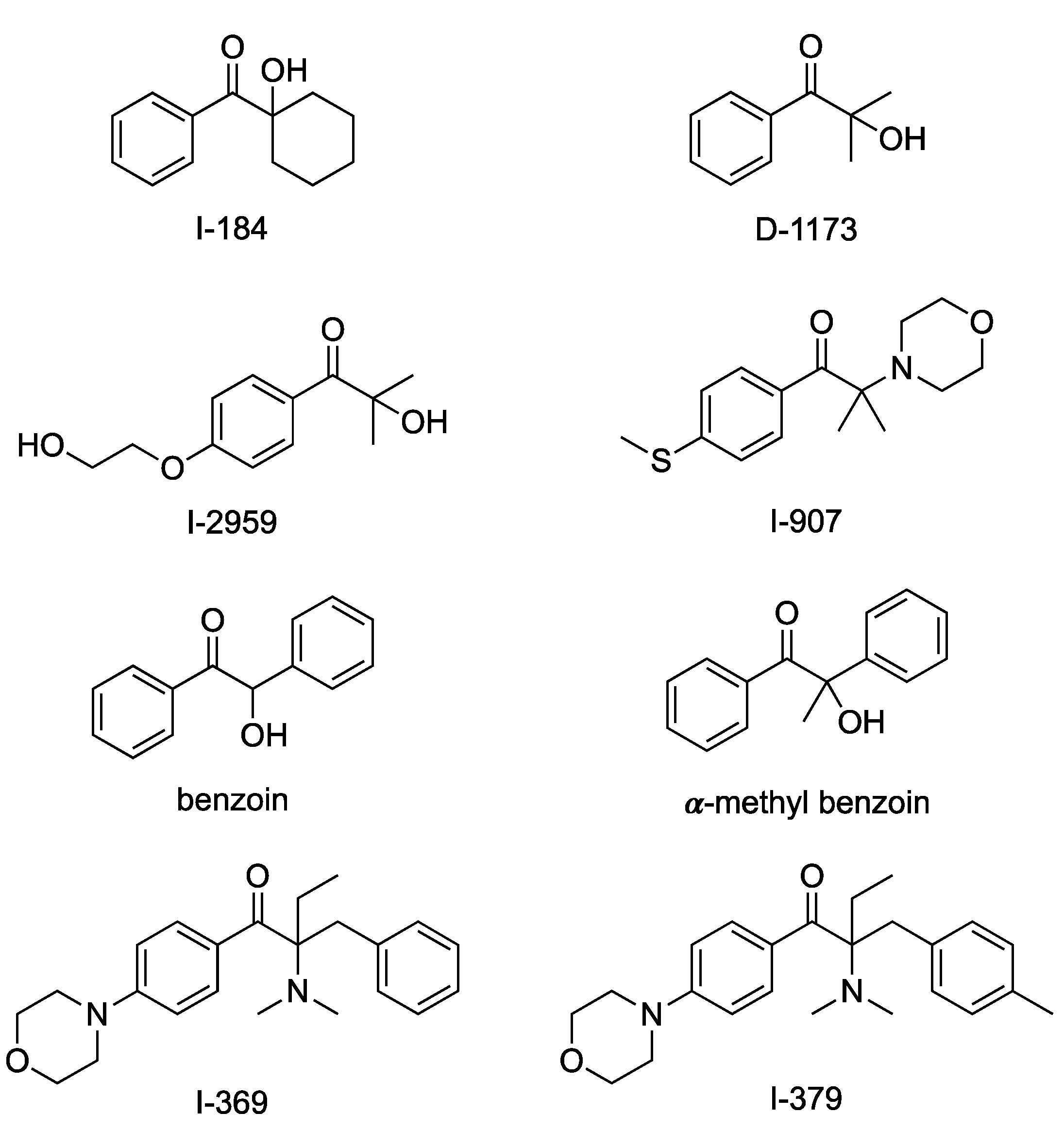



| Initiator | Solvent | 𝜆max FWHM (nm) | Residence Time (min) | Notes |
|---|---|---|---|---|
| I-184 | Water | 400 50 | 0.8 | Clean no deposits in the flow tube |
| 1:1 CH3OH:Water | 420 100 | 4 | ||
| D-1173 | Water | 400 60 | 0.8 | Clean no deposits in the flow tube |
| 1:1 CH3OH:Water | 415 95 | 0.8 | ||
| I-2959 | Water | 400 50 | 0.7 | Clean no deposits in the flow tube |
| 1:1 CH3OH:Water | 415 80 | 0.8 | ||
| I-907 | 1:9 CH3OH:Water | 445 160 | 4 | Heavy black deposits in the flow tube |
| 1:1 CH3OH:Water | 445 150 | 8 | ||
| Benzoin | Water | 400 85 | 30 | Very weak signals |
| 1:1 CH3OH:Water | 410 80 | 0.8 | Yellow deposits in the flow tube | |
| α-Me Benzoin | Water | 400 70 | 0.8 | Yellow deposits in the flow tube |
| 1:1 CH3OH:Water | 405 80 | 0.8 | Faint yellow deposits in flow tube | |
| I-369 a | 1:1 CH3OH:Water | 410 90 | 4 | Heavy black deposits in the flow tube |
| I-379 | 1:1 CH3OH:Water | 410 90 | 6 | Heavy black deposits in the flow tube |
| 2:3 CH3OH:Water | 410 80 | 10 |
| Initiator Used | Average Diameter (nm) | Standard Deviation (nm) |
|---|---|---|
| I-2959 | 4.6 | 1.8 |
| D-1173 | 4.4 | 1.0 |
| I-184 | 7.6 | 2.6 |
| I-907 | 5.3 | 1.5 |
| I-369 | 8.6 | 4.2 |
| I-379 | 9.5 | 3.5 |
| benzoin | 6.1 | 2.2 |
| α-methylbenzoin | 8.3 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghmaei, M.; Bourgonje, C.R.; Scaiano, J.C. Facile Scale-Up of the Flow Synthesis of Silver Nanostructures Based on Norrish Type I Photoinitiators. Molecules 2023, 28, 4445. https://doi.org/10.3390/molecules28114445
Yaghmaei M, Bourgonje CR, Scaiano JC. Facile Scale-Up of the Flow Synthesis of Silver Nanostructures Based on Norrish Type I Photoinitiators. Molecules. 2023; 28(11):4445. https://doi.org/10.3390/molecules28114445
Chicago/Turabian StyleYaghmaei, Mahzad, Connor R. Bourgonje, and Juan C. Scaiano. 2023. "Facile Scale-Up of the Flow Synthesis of Silver Nanostructures Based on Norrish Type I Photoinitiators" Molecules 28, no. 11: 4445. https://doi.org/10.3390/molecules28114445
APA StyleYaghmaei, M., Bourgonje, C. R., & Scaiano, J. C. (2023). Facile Scale-Up of the Flow Synthesis of Silver Nanostructures Based on Norrish Type I Photoinitiators. Molecules, 28(11), 4445. https://doi.org/10.3390/molecules28114445