Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process
Abstract
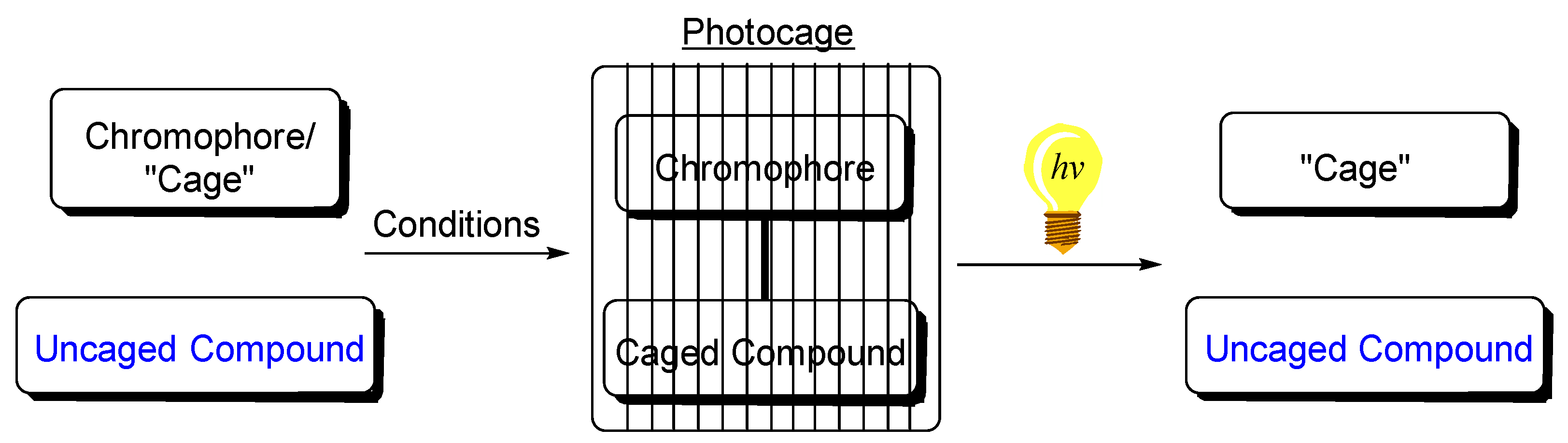
1. Introduction
2. Results and Discussion
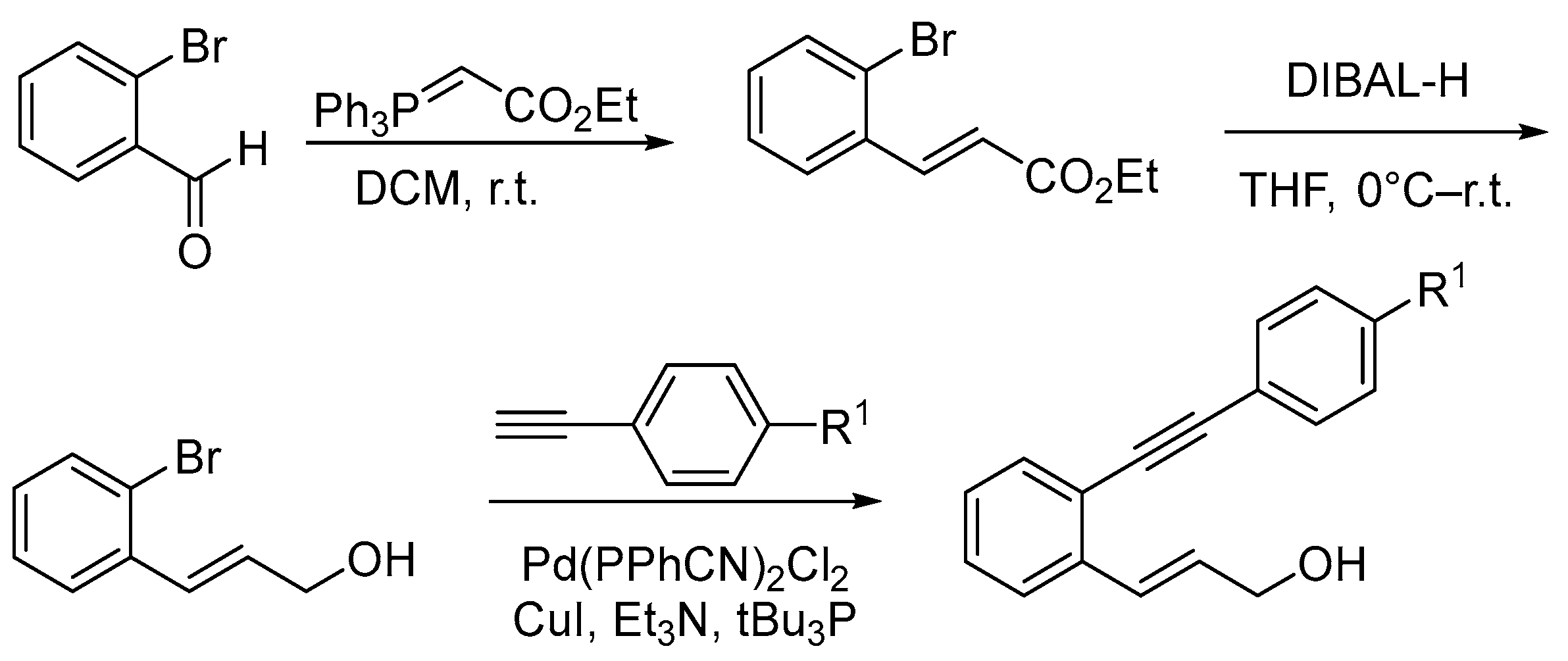
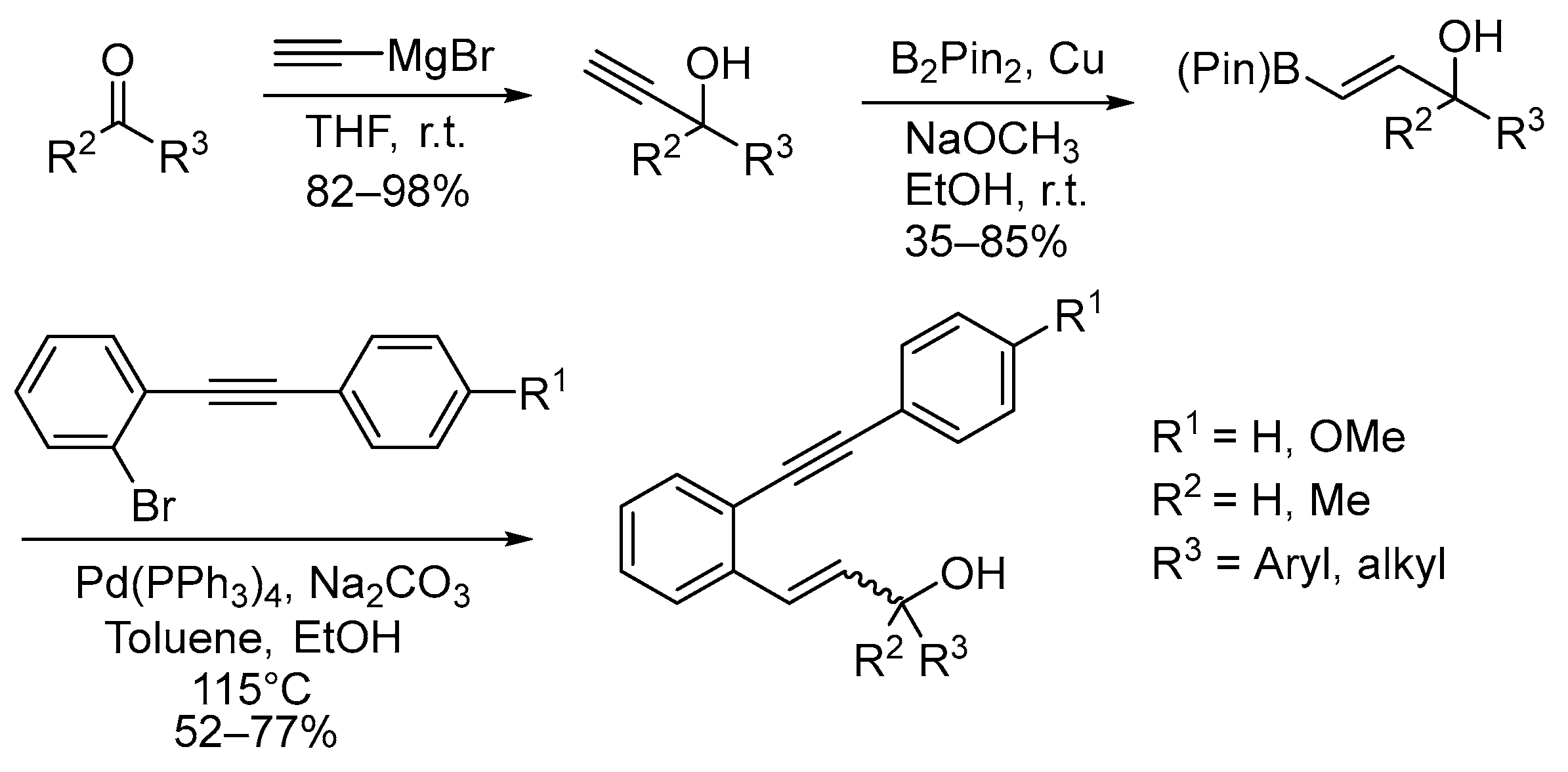
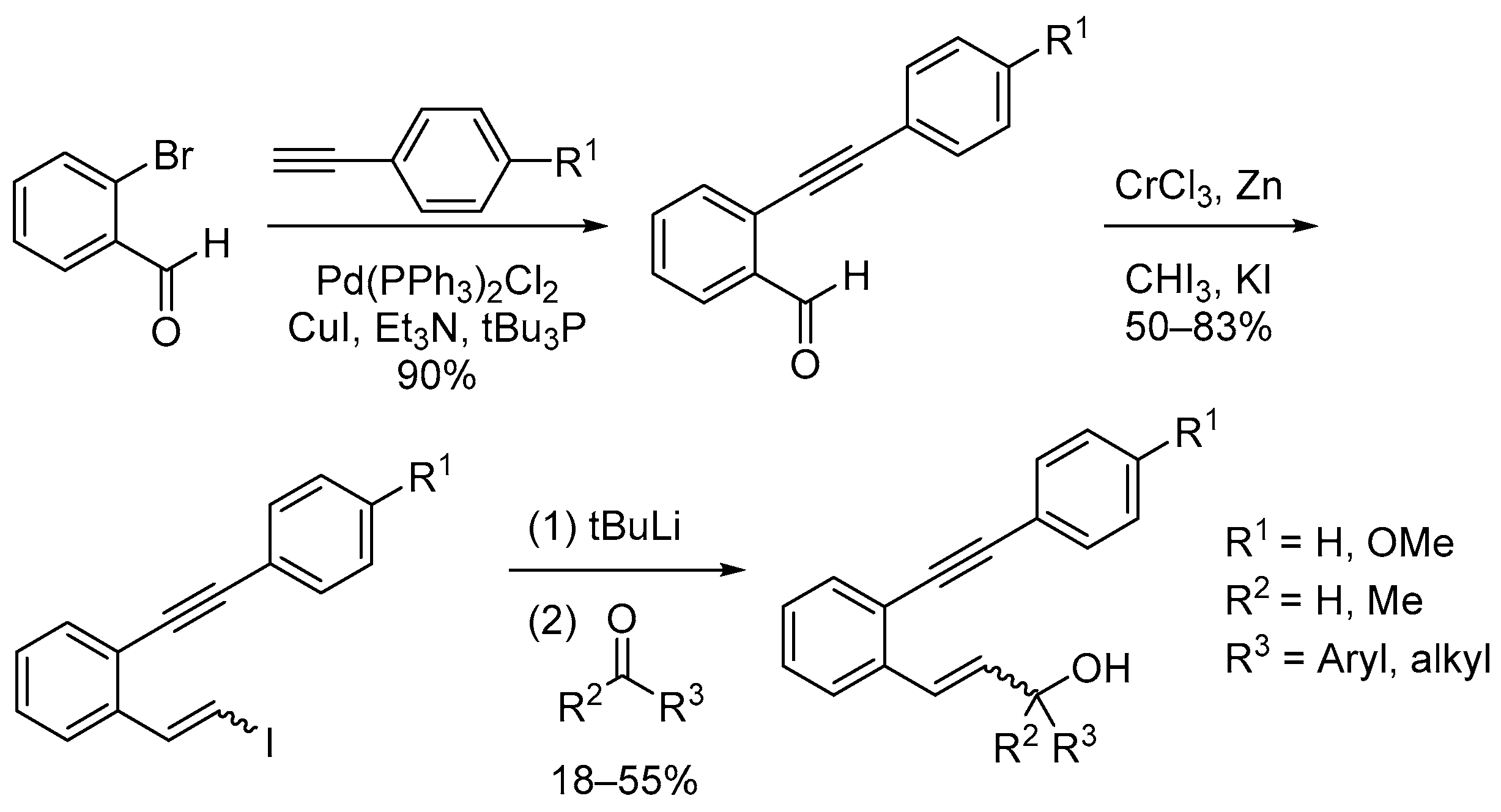
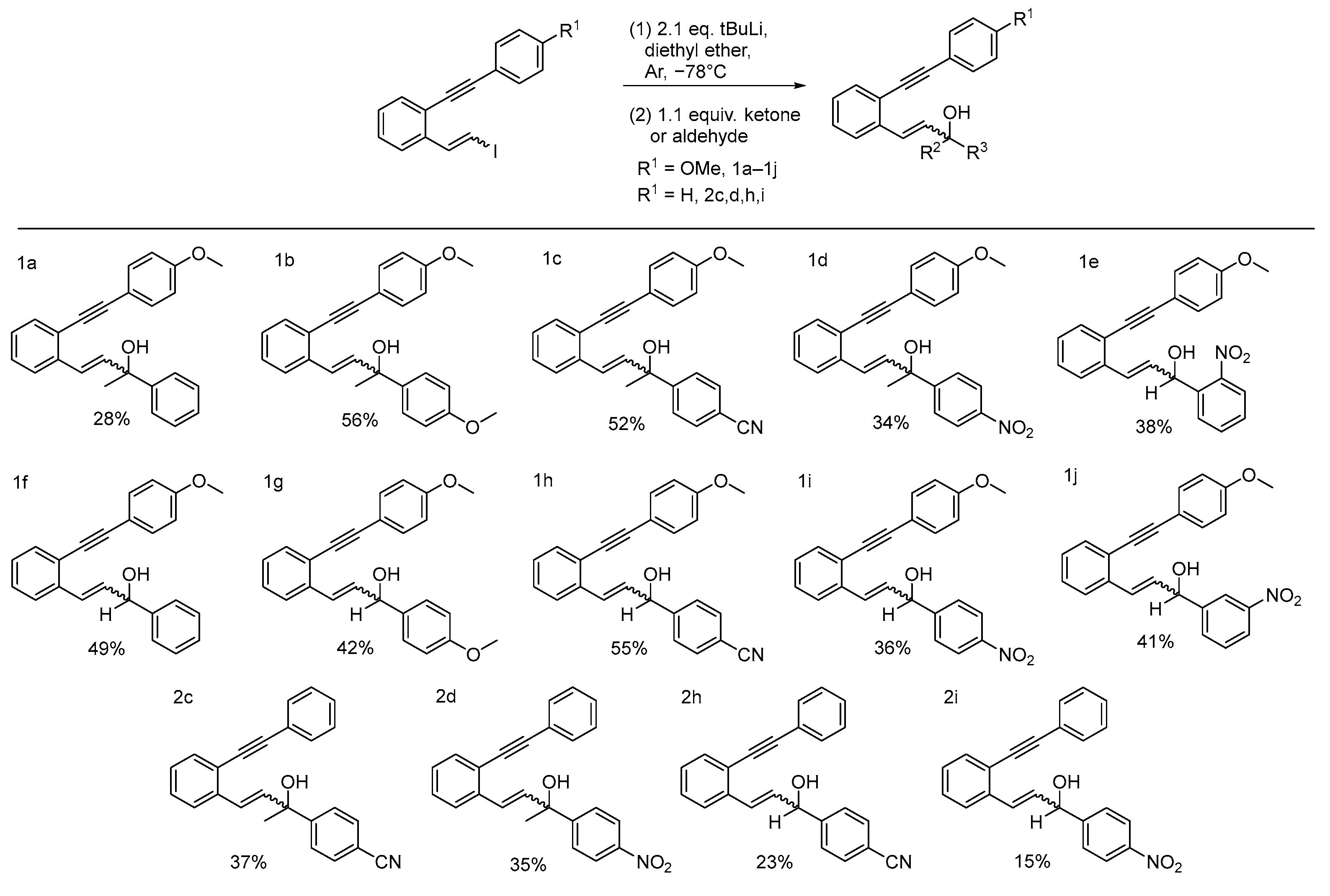
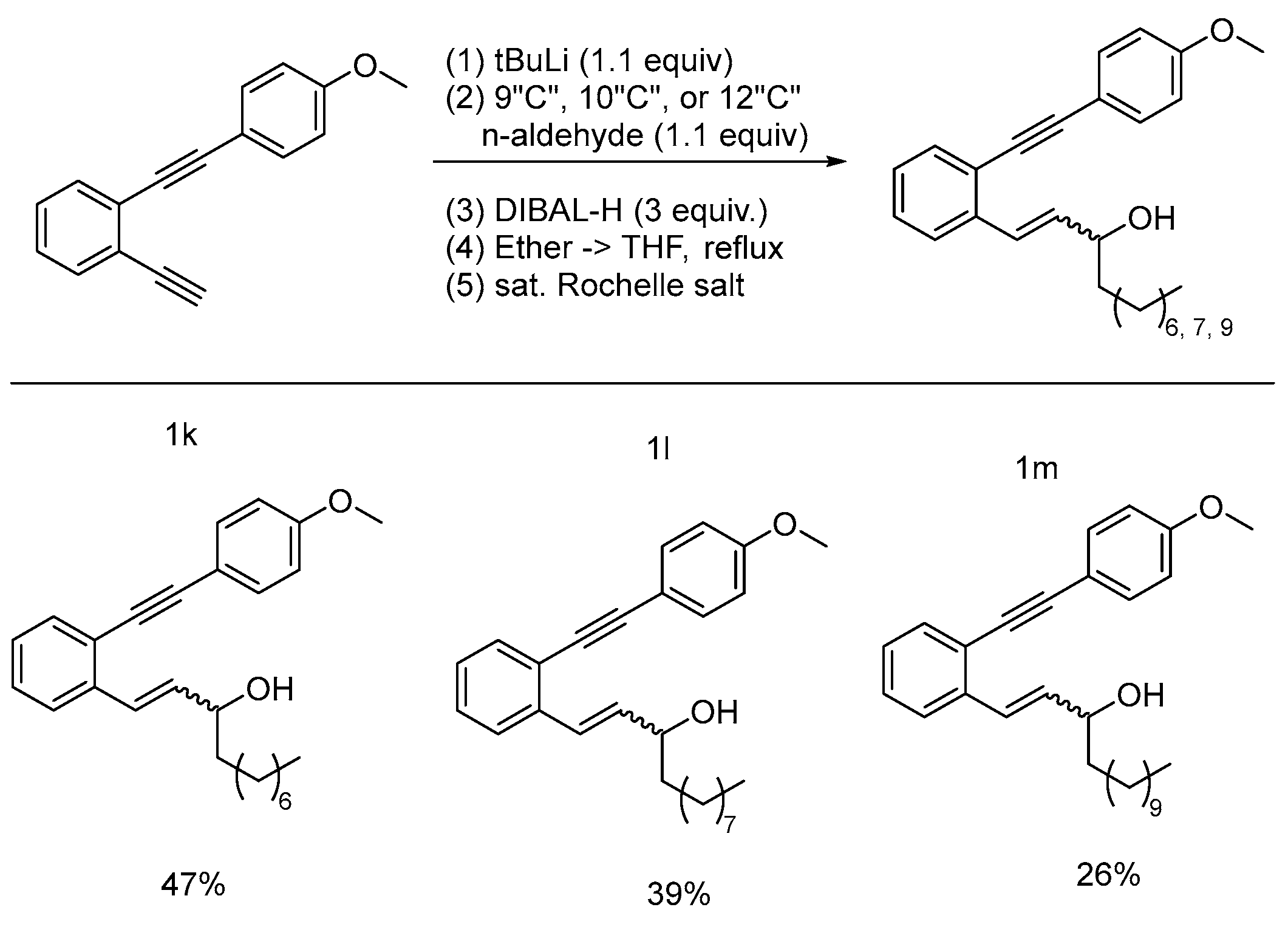
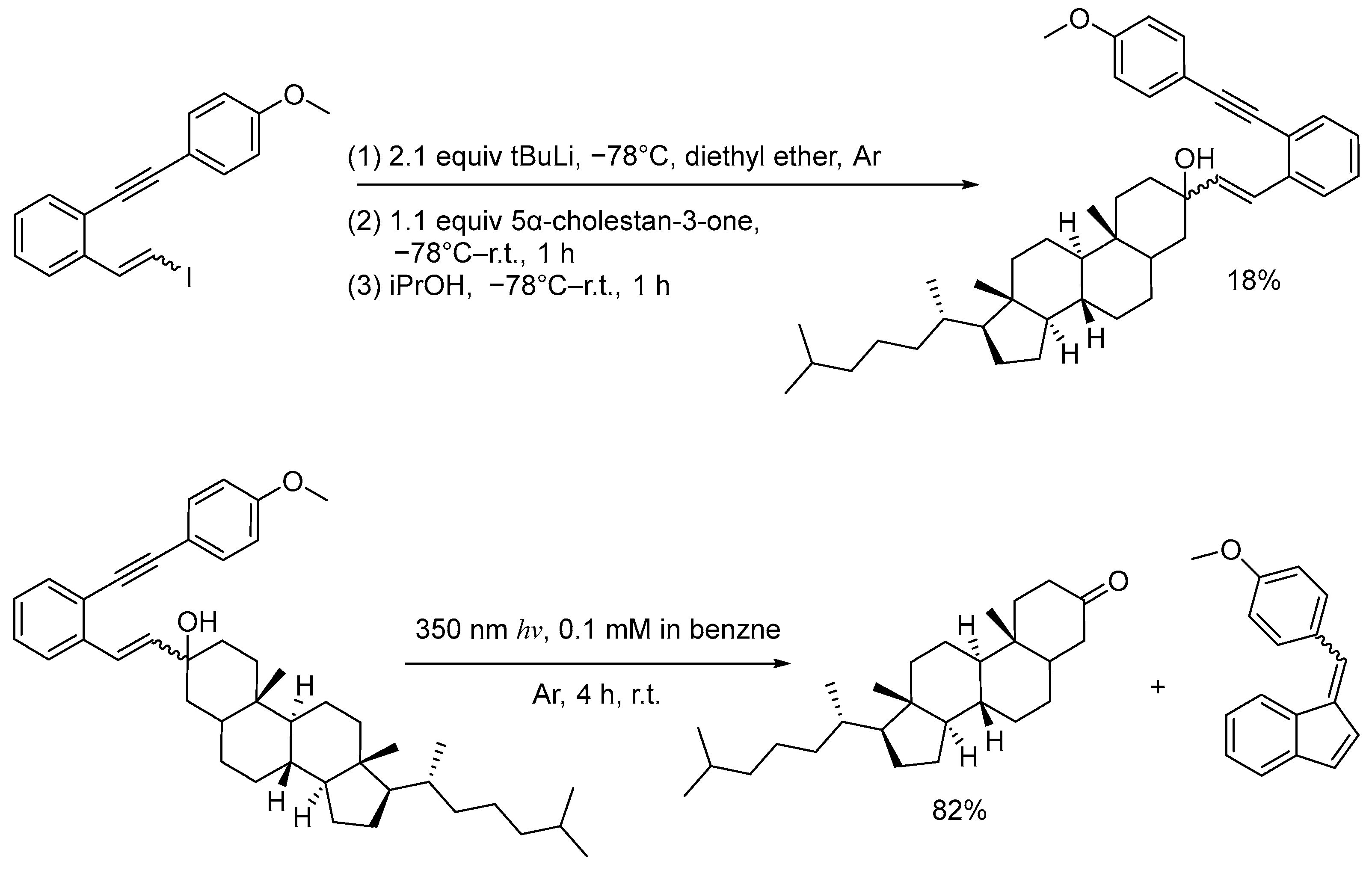
2.1. Synthesis
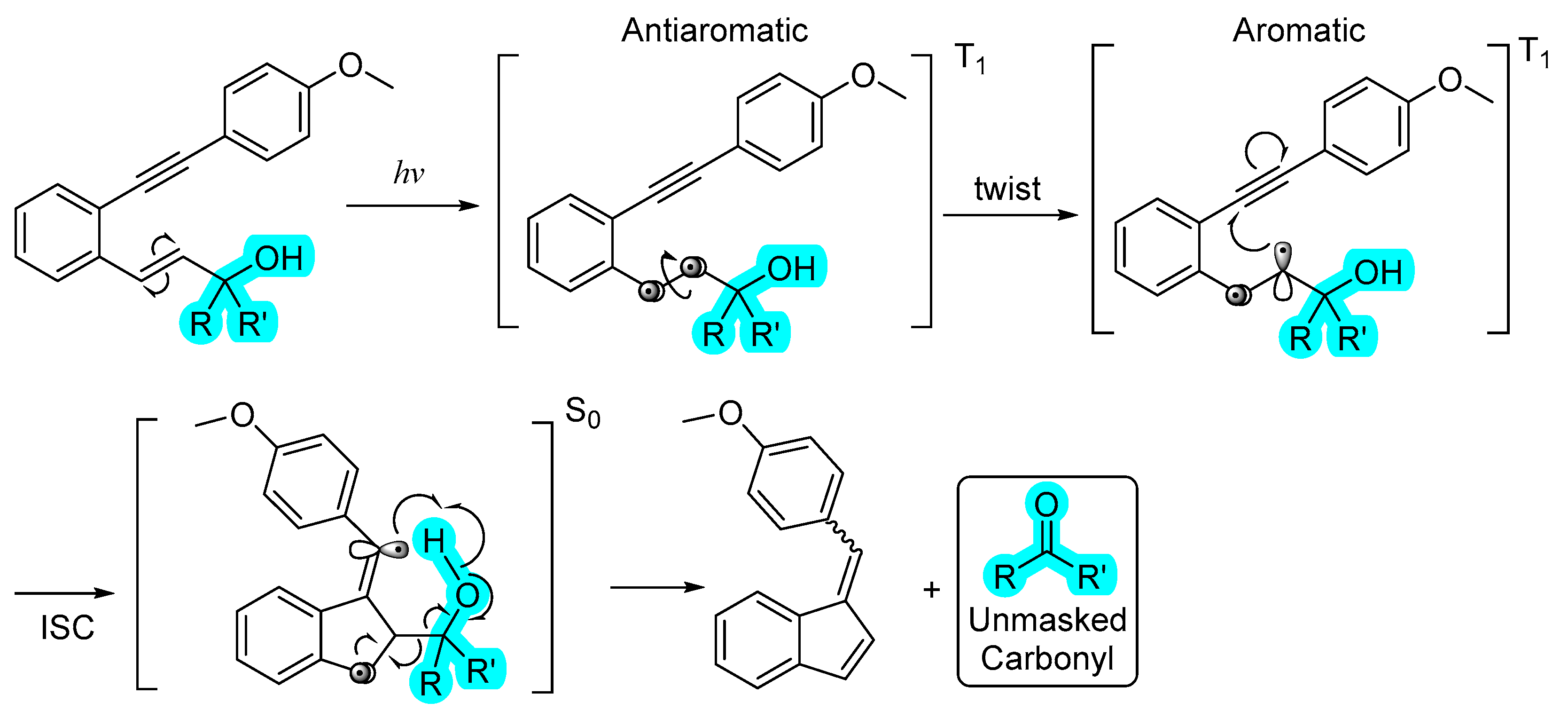
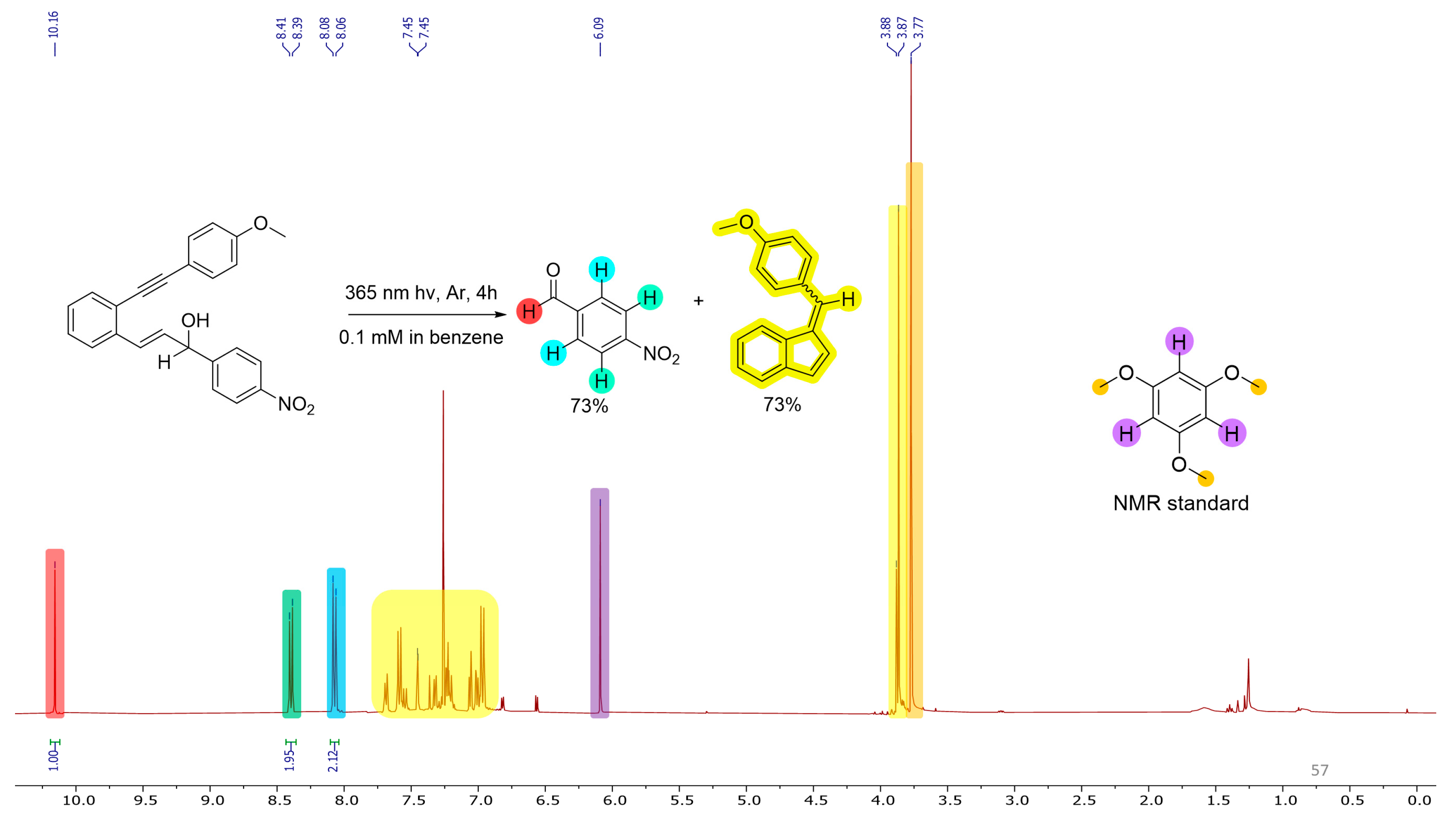
2.2. Photochemistry
3. Materials and Methods
3.1. 2-((4-Methoxyphenyl)ethynyl)benzaldehyde
3.2. 2-(Phenylethynyl)benzaldehyde
3.3. Vinyl Iodides
3.4. Lithium-Halogen Exchange Addition Reactions for Synthesis of Caged Compounds
3.5. Photouncaging of Enynols
- (1a) 4-(2-((4-methoxyphenyl)ethynyl)phenyl)-2-phenylbut-3-en-2-ol
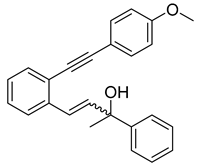
- (1b) 2-(4-methoxyphenyl)-4-(2-((4-methoxyphenyl)ethynyl)phenyl)but-3-en-2-ol
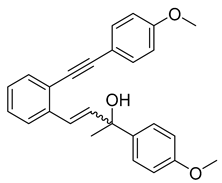
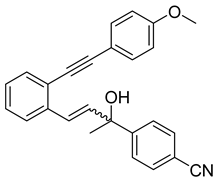
- (1c) (2-hydroxy-4-(2-((4-methoxyphenyl)ethynyl)phenyl)but-3-en-2-yl)benzonitrile
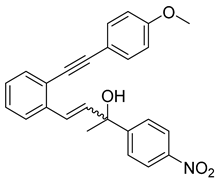
- (1d) 4-(2-((4-methoxyphenyl)ethynyl)phenyl)-2-(4-nitrophenyl)but-3-en-2-ol
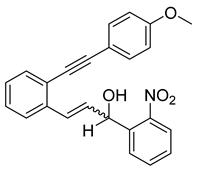
- (1e) 3-(2-((4-methoxyphenyl)ethynyl)phenyl)-1-(2-nitrophenyl)prop-2-en-1-ol
- (1f) 3-(2-((4-methoxyphenyl)ethynyl)phenyl)-1-phenylprop-2-en-1-ol

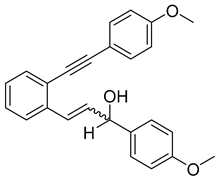
- (1g) 1-(4-methoxyphenyl)-3-(2-((4-methoxyphenyl)ethynyl)phenyl)prop-2-en-1-ol
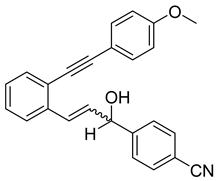
- (1h) 4-(1-hydroxy-3-(2-((4-methoxyphenyl)ethynyl)phenyl)allyl)benzonitrile
- (1i) 3-(2-((4-methoxyphenyl)ethynyl)phenyl)-1-(4-nitrophenyl)prop-2-en-1-ol

- (1j) 3-(2-((4-methoxyphenyl)ethynyl)phenyl)-1-(3-nitrophenyl)prop-2-en-1-ol

- (2c) 4-(2-hydroxy-4-(2-(phenylethynyl)phenyl)but-3-en-2-yl)benzonitrile
- (2d) 2-(4-nitrophenyl)-4-(2-(phenylethynyl)phenyl)but-3-en-2-ol
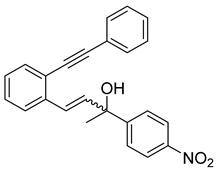
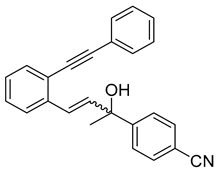
- (2h) 4-(1-hydroxy-3-(2-(phenylethynyl)phenyl)allyl)benzonitrile
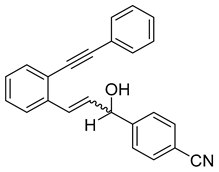
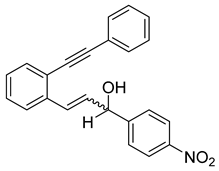
- (2i) 1-(4-nitrophenyl)-3-(2-(phenylethynyl)phenyl)prop-2-en-1-ol
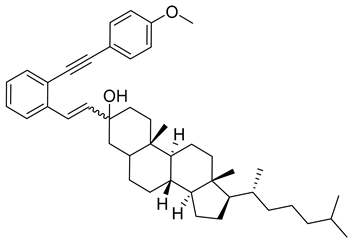
- (1n) (8R,9S,10S,13R,14S,17R)-3-(2-((4-methoxyphenyl)ethynyl)styryl)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthren-3-ol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sheehan, J.C.; Umezawa, K. Phenacyl Photosensitive Blocking Groups. J. Org. Chem. 1973, 38, 3771–3774. [Google Scholar] [CrossRef]
- Literák, J.; Dostálová, A.; Klán, P. Chain Mechanism in the Photocleavage of Phenacyl and Pyridacyl Esters in the Presence of Hydrogen Donors. J. Org. Chem. 2006, 71, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Patchornik, A.; Amit, B.; Woodward, R.B. Photosensitive Protecting Groups. J. Am. Chem. Soc. 1970, 92, 6333–6335. [Google Scholar] [CrossRef]
- Walker, J.W.; Reid, G.P.; McCray, J.A.; Trentham, D.R. Photolabile 1-(2-Nitrophenyl)Ethyl Phosphate Esters of Adenine Nucleotide Analogs. Synthesis and Mechanism of Photolysis. J. Am. Chem. Soc. 1988, 110, 7170–7177. [Google Scholar] [CrossRef]
- Givens, R.S.; Matuszewski, B. Photochemistry of Phosphate Esters: An Efficient Method for the Generation of Electrophiles. J. Am. Chem. Soc. 1984, 106, 6860–6861. [Google Scholar] [CrossRef]
- Lin, W.; Lawrence, D.S. A Strategy for the Construction of Caged Diols Using a Photolabile Protecting Group. J. Org. Chem. 2002, 67, 2723–2726. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Schofield, P. Photosensitive Protecting Groups. Tetrahedron Lett. 1962, 3, 697–699. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Hu, H.; Spencer, C.; Liang, X.; Pan, L. Sequential Removal of Photolabile Protecting Groups for Carbonyls with Controlled Wavelength. J. Org. Chem. 2008, 73, 6152–6157. [Google Scholar] [CrossRef]
- Karas, L.J.; Campbell, A.T.; Alabugin, I.V.; Wu, J.I. Antiaromaticity Gain Activates Tropone and Nonbenzenoid Aromatics as Normal-Electron-Demand Diels–Alder Dienes. Org. Lett. 2020, 22, 7083–7087. [Google Scholar] [CrossRef]
- Wu, C.-H.; Karas, L.J.; Ottosson, H.; Wu, J.I.-C. Excited-State Proton Transfer Relieves Antiaromaticity in Molecules. Proc. Natl. Acad. Sci. USA 2019, 116, 20303–20308. [Google Scholar] [CrossRef]
- Slanina, T.; Ayub, R.; Toldo, J.; Sundell, J.; Rabten, W.; Nicaso, M.; Alabugin, I.; Galván, I.F.; Gupta, A.K.; Lindh, R.; et al. Impact of Excited-State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]Hexenes. J. Am. Chem. Soc. 2020, 142, 10942–10954. [Google Scholar] [CrossRef] [PubMed]
- Alabugin, I.V.; Campbell, A.T. Aromaticity—Modern Computational Methods and Applications; Fernández, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Lage Robles, J.; Bochet, C.G. Photochemical Release of Aldehydes from α-Acetoxy Nitroveratryl Ethers. Org. Lett. 2005, 7, 3545–3547. [Google Scholar] [CrossRef] [PubMed]
- Gravel, D.; Murray, S.; Ladouceur, G. O-Nitrobenzyl Alcohol, a Simple and Efficient Reagent for the Photoreversible Protection of Aldehydes and Ketones. J. Chem. Soc. Chem. Commun. 1985, 24, 1828–1829. [Google Scholar] [CrossRef]
- Wang, P.; Hu, H.; Wang, Y. Novel Photolabile Protecting Group for Carbonyl Compounds. Org. Lett. 2007, 9, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- McHale, W.A.; Kutateladze, A.G. An Efficient Photo-SET-Induced Cleavage of Dithiane−Carbonyl Adducts and Its Relevance to the Development of Photoremovable Protecting Groups for Ketones and Aldehydes. J. Org. Chem. 1998, 63, 9924–9931. [Google Scholar] [CrossRef]
- Mohamed, R.K.; Mondal, S.; Jorner, K.; Delgado, T.F.; Lobodin, V.V.; Ottosson, H.; Alabugin, I.V. The Missing C1–C5 Cycloaromatization Reaction: Triplet State Antiaromaticity Relief and Self-Terminating Photorelease of Formaldehyde for Synthesis of Fulvenes from Enynes. J. Am. Chem. Soc. 2015, 137, 15441–15450. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Manoharan, M. Radical-Anionic Cyclizations of Enediynes: Remarkable Effects of Benzannelation and Remote Substituents on Cyclorearomatization Reactions. J. Am. Chem. Soc. 2003, 125, 4495–4509. [Google Scholar] [CrossRef]
- Baird, N.C. Quantum Organic Photochemistry. II. Resonance and Aromaticity in the Lowest 3.Pi..Pi.* State of Cyclic Hydrocarbons. J. Am. Chem. Soc. 1972, 94, 4941–4948. [Google Scholar] [CrossRef]
- Augé, J.; Boucard, V.; Gil, R.; Lubin-Germain, N.; Picard, J.; Uziel, J. An Alternative Procedure in the Takai Reaction Using Chromium(III) Chloride Hexahydrate as a Convenient Source of Chromium(II). Synth. Commun. 2003, 33, 3733–3739. [Google Scholar] [CrossRef]
- Neumann, H.; Seebach, D. Stereospecific Preparation of Terminal Vinyllithium Derivatives by Br/Li-Exchange with t-Butyllithium. Tetrahedron Lett. 1976, 17, 4839–4842. [Google Scholar] [CrossRef]
- Palei, B.A.; Gavrilenko, V.V.; Zakharkin, L.I. Comparative Reactivity of Alkyl, Vinyl, and Acetylene Derivatives of Aluminum. Bull. Acad. Sci. USSR Div. Chem. Sci. 1969, 18, 2590–2595. [Google Scholar] [CrossRef]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.W.; Mohamed, R.K.; Alabugin, I.V. How to Lose a Bond in Two Ways: The Diradical/Zwitterion Dichotomy in Cycloaromatization Reactions. Eur. J. Org. Chem. 2013, 13, 2505–2527. [Google Scholar] [CrossRef]


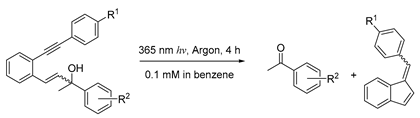
| R1 | R2 | Ketone | Fulvene |
|---|---|---|---|
| H | p-CN | 54% | 62% |
| H | p-NO2 | 66% | 62% |
| OCH3 | p-OCH3 | 84% | 87% |
| OCH3 | H | 76% | 79% |
| OCH3 | p-CN | 95% | 85% |
| OCH3 | p-NO2 | 92% | 41% |
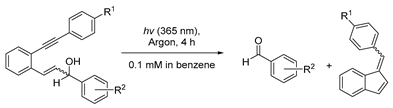
| R1 | R2 | Aldehyde | Fulvene |
|---|---|---|---|
| H | p-CN | 76% | 98% |
| H | p-NO2 | 44% | 48% |
| OCH3 | p-OCH3 | 82% | 85% |
| OCH3 | H | 7% | 70% |
| OCH3 | p-CN | 75% | 75% |
| OCH3 | p-NO2 | 73% | 73% |
| OCH3 | m-NO2 | 57% | 72% |
| OCH3 | o-NO2 | 0% | 0% |
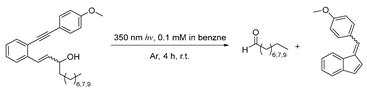
| Compound | Aldehyde | Fulvene |
|---|---|---|
| Nonanal | 51% | 75% |
| Decanal | 71% | 84% |
| Dodecanal | 68% | 68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.; Dos Santos, N.R.; Alabugin, I. Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process. Molecules 2023, 28, 5704. https://doi.org/10.3390/molecules28155704
Campbell A, Dos Santos NR, Alabugin I. Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process. Molecules. 2023; 28(15):5704. https://doi.org/10.3390/molecules28155704
Chicago/Turabian StyleCampbell, Adam, Nikolas R. Dos Santos, and Igor Alabugin. 2023. "Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process" Molecules 28, no. 15: 5704. https://doi.org/10.3390/molecules28155704
APA StyleCampbell, A., Dos Santos, N. R., & Alabugin, I. (2023). Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process. Molecules, 28(15), 5704. https://doi.org/10.3390/molecules28155704







