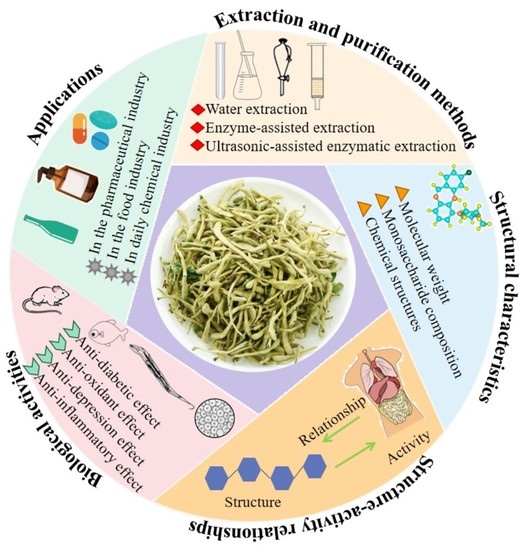
Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review
Abstract
1. Introduction
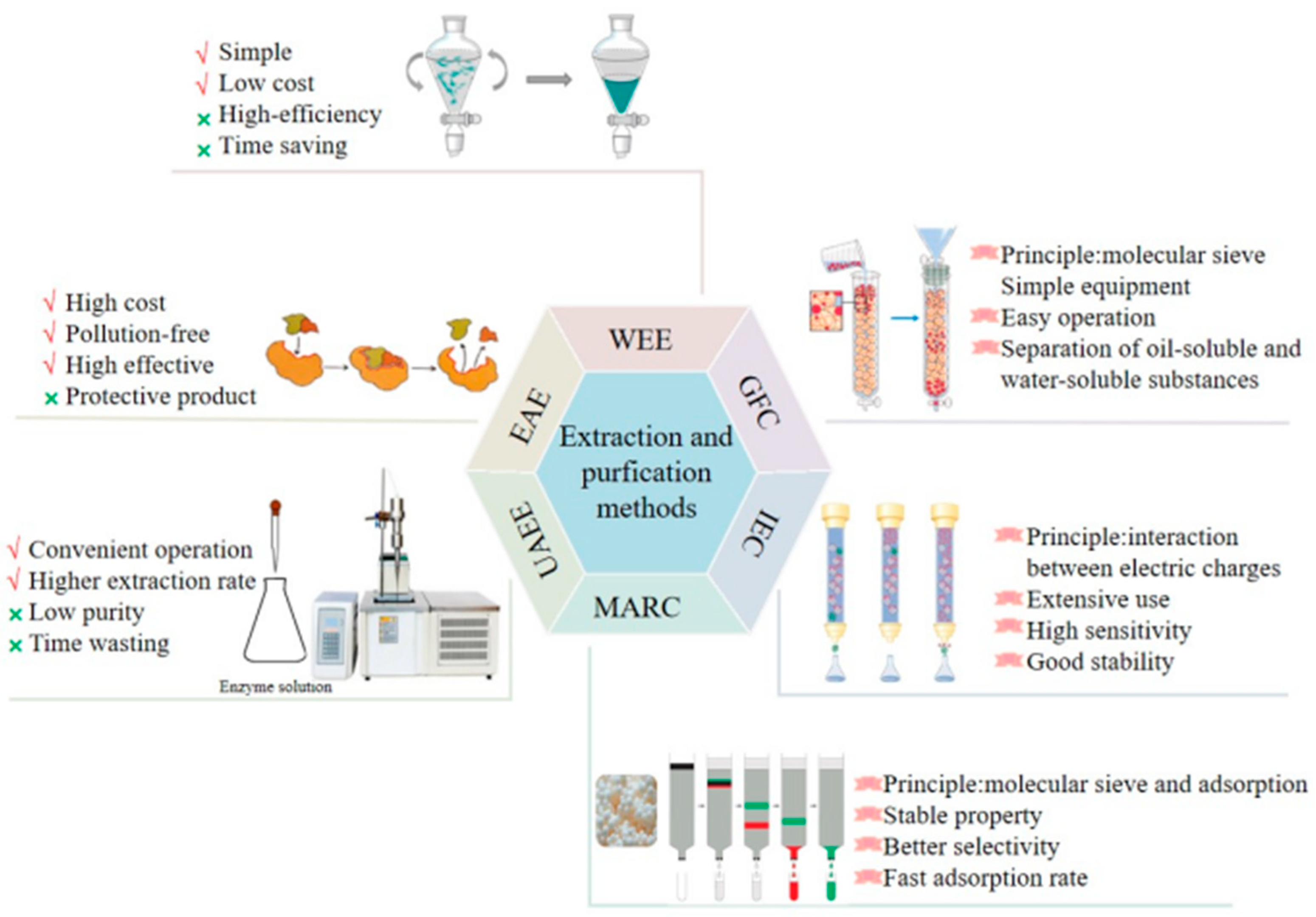
2. Extraction and Purification Methods of L. japonica Polysaccharides
3. Structural Characteristics of L. japonica Polysaccharides
3.1. Molecular Weight
3.2. Monosaccharide Composition
3.3. Chemical Structures
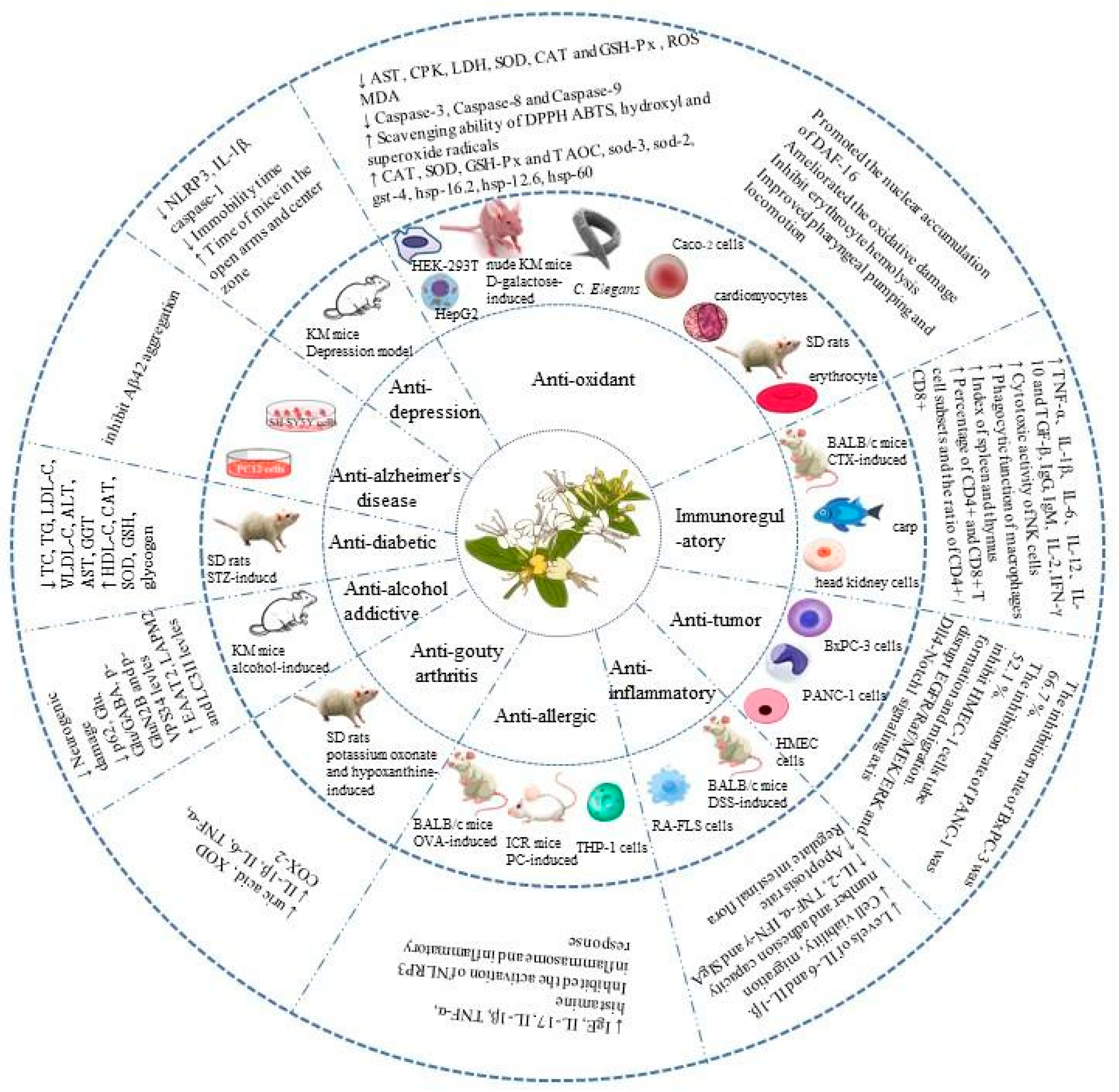
4. Health Benefits of L. japonica Polysaccharides
4.1. Anti-Diabetic Effects
4.2. Anti-Alzheimer’s Effects
4.3. Anti-Depressant Effects
4.4. Antioxidant Effects
4.5. Immunoregulatory Effects
4.6. Anti-Tumor Effects
4.7. Anti-Inflammatory Effects
4.8. Anti-Allergic Effects
4.9. Anti-Gout Effects
4.10. Anti-Alcohol-AddictionEffects
5. Structure–Activity Relationship and Structural Modifications
6. Practical and Potential Applications of L. japonica Polysaccharides
6.1. In the Food Industry
6.2. In the Pharmaceutical Industry
6.3. In the Daily Chemical Industry
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology; phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Liu, S.; Hou, A.; Wang, S.; Na, Y.; Hu, J.; Jiang, H.; Yang, L. Systematic review of Lonicerae Japonicae Flos: A significant food and traditional Chinese medicine. Front. Pharmacol. 2022, 13, 1013992. [Google Scholar] [CrossRef]
- Ge, L.; Xie, Q.; Jiang, Y.; Xiao, L.; Wan, H.; Zhou, B.; Wu, S.; Tian, J.; Zeng, X. Genus Lonicera: New drug discovery from traditional usage to modern chemical and pharmacological research. Phytomedicine 2022, 96, 153889. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, L.; Zhang, Y.; Huang, X.; Zhao, F.; Cui, X.; Shi, L.; Xu, L. Study on anti-inflammatory efficacy and correlative ingredients with pharmacodynamics detected in acute inflammation rat model serum from Caulis Lonicerae japonicae. Phytomedicine 2016, 23, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, Y.; Fu, C.; Xiang, J.; Gan, J.; Wu, G.; Jia, H.; Yu, L.; Li, M. Different Gene Expression Patterns between Leaves and Flowers in Lonicera japonica Revealed by Transcriptome Analysis. Front. Plant. Sci. 2016, 7, 637. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, X.; Yu, Y.; Wu, Y.; Xie, L.; Chang, C. Lonicerae Japonicae Flos extract and chlorogenic acid attenuates high-fat-diet- induced prediabetes via CTRPs-AdipoRs-AMPK/PPARα axes. Front. Nutr. 2022, 9, 1007679. [Google Scholar] [CrossRef]
- Su, X.; Zhu, Z.H.; Zhang, L.; Wang, Q.; Xu, M.M.; Lu, C.; Zhu, Y.; Zeng, J.; Duan, J.A.; Zhao, M. Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. J. Ethnopharmacol. 2021, 267, 113502. [Google Scholar] [CrossRef]
- Li, J.; Ye, C.; Chang, C. Comparative transcriptomics analysis revealing flower trichome development during flower development in two Lonicera japonica Thunb. cultivars using RNA-seq. BMC. Plant. Biol. 2020, 20, 341. [Google Scholar] [CrossRef]
- Xie, X.; Gu, L.; Xu, W.; Yu, X.; Yin, G.; Wang, J.; Jin, Y.; Wang, L.; Wang, B.; Wang, T. Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos. Molecules 2022, 27, 5789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qi, F. Herbal medicines exhibit a high affinity for ACE2 in treating COVID-19. Biosci. Trends. 2023, 17, 14–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, S.; Wang, W.; Chen, Y.; Zhao, C.; Song, Y.; Yan, D.; Zhang, L.; Tang, Y. Potential Role of Gut Microbiota in Traditional Chinese Medicine against COVID-19. Am. J. Chin. Med. 2021, 49, 785–803. [Google Scholar] [CrossRef]
- Su, H.X.; Yao, S.; Zhao, W.F.; Li, M.J.; Liu, J.; Shang, W.J.; Xie, H.; Ke, C.Q.; Hu, H.C.; Gao, M.N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta. Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, C.X.; Tang, Y.; Ma, G.L.; Tong, Y.P.; Jin, Z.X.; Zang, Y.; Osman, E.E.A.; Li, J.; Xiong, J.; et al. Structurally diverse glycosides of secoiridoid; bisiridoid; and triterpene-bisiridoid conjugates from the flower buds of two Caprifoliaceae plants and their ATP-citrate lyase inhibitory activities. Bioorg. Chem. 2022, 120, 105630. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water; ethanol and supercritical fluid extraction techniques. Ind. Crop. Prod. 2016, 89, 543–549. [Google Scholar]
- Lee, H.L.; Kim, J.M.; Go, M.J.; Kim, T.Y.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Kim, H.J.; Heo, H.J. Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway. Antioxidants 2023, 12, 968. [Google Scholar] [CrossRef]
- Ma, A.; Zou, F.; Zhang, R.; Zhao, X. The effects and underlying mechanisms of medicine and food homologous flowers on the prevention and treatment of related diseases. J. Food. Biochem. 2022, 46, e14430. [Google Scholar] [CrossRef] [PubMed]
- The Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China: Volume 1; Chinese Medical Science and Technology Press: Beijing, China, 2020.
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.S.; et al. The analgesic and anti-inflammatory effect of WIN-34B.; a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine 2020, 68, 153173. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, J.; Wang, P.; Du, Z.; Li, Y.; Wang, S.; Ding, K. Characterization of a pectin from Lonicera japonica Thunb. and its inhibition effect on Aβ42 aggregation and promotion of neuritogenesis. Int. J. Biol. Macromol. 2018, 107, 112–120. [Google Scholar] [CrossRef]
- Su, D.; Li, S.; Zhang, W.; Wang, J.; Wang, J.; Lv, M. Structural elucidation of a polysaccharide from Lonicera japonica flowers.; and its neuroprotective effect on cerebral ischemia-reperfusion injury in rat. Int. J. Biol. Macromol. 2017, 99, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef]
- Wang, S.; He, F.; Wu, H.; Xiang, F.; Zheng, H.; Wu, W.; Li, S. Health-Promoting Activities and Associated Mechanisms of Polygonati Rhizoma Polysaccharides. Molecules 2023, 28, 1350. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Fu, R.; Ou, J.; Wang, B. Structural characterization and anti-inflammatory activity of a novel polysaccharide PKP2-1 from Polygonatum kingianum. Front. Nutr. 2023, 10, 1156798. [Google Scholar] [CrossRef]
- Hao, R.; Zhou, X.; Zhao, X.; Lv, X.; Zhu, X.; Gao, N.; Jiang, Y.; Wu, M.; Sun-Waterhouse, D.; Li, D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total. Environ. 2023, 877, 162910. [Google Scholar] [CrossRef]
- Hu, W.; Yu, A.; Wang, S.; Bai, Q.; Tang, H.; Yang, B.; Wang, M.; Kuang, H. Extraction, Purification, Structural Characteristics, Biological Activities, and Applications of the Polysaccharides from Zingiber officinale Roscoe. (Ginger): A Review. Molecules 2023, 28, 3855. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Correia, A.; Silva, A.M.S.; Wessel, D.F.; Cardoso, S.M.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of pectic polysaccharides from broccoli by-products with in vitro B lymphocyte stimulatory activity. Carbohydr. Polym. 2023, 303, 120432. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, Z.C.; Liu, Y. Structural characterisation and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int. J. Biol. Macromol. 2020, 154, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, C.; Zhang, Y.; Yang, D. The prebiotic properties of polysaccharides obtained by differentiated deproteinization methods from Flos Sophorae Immaturus on Lactobacillus fermentum. Front. Microbiol. 2022, 13, 1007267. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y.; Zhuang, Y.; Wang, N.; Jin, J.; Zhan, Z. Purification, Structural Analysis and Cardio-Protective Activity of Polysaccharides from Radix Astragali. Molecules 2023, 28, 4167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Lee, W.Y. Anti-glycation effect of Ecklonia cava polysaccharides extracted by combined ultrasound and enzyme-assisted extraction. Int. J. Biol. Macromol. 2021, 180, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Wang, Y.D.; Li, G.R.; Liu, Z.Q.; Feng, J.; Qiao, C.L.; Zhang, H.F. Ultrasound-Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules 2023, 28, 3880. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Wang, X.; Yu, S.; Na, Z.; Li, C.; Yang, M.; Ma, Y. Structural Characterization, In Vitro Digestion Property, and Biological Activity of Sweet Corn Cob Polysaccharide Iron (III) Complexes. Molecules 2023, 28, 2961. [Google Scholar] [CrossRef]
- Gao, D.; Chen, H.; Liu, H.; Yang, X.; Guo, P.; Cao, X.; Cai, Y.; Xu, H.; Yang, J. Structure characterization and antioxidant activity analysis of polysaccharides from Lanzhou Lily. Front. Nutr. 2022, 9, 976607. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Ma, P.; Huang, J.; Bai, X.; Liu, P.; Zhu, L.; Min, X. Immunomodulatory effect of polysaccharides isolated from Lonicera japonica Thunb. in cyclophosphamide-treated BALB/c mice. Heliyon 2022, 8, e11876. [Google Scholar] [CrossRef]
- Liu, P.; Shen, H.; Zhang, Q.; Zhou, L.; Bai, X.; Zhang, T. Inhibition of reinstatement of alcohol-induced conditioned place preference in mice by Lonicera japonica polysaccharide. Food. Funct. 2022, 13, 8643–8651. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2020, 151, 1058–1066. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Q.; Kang, X.; Tian, G.; Ming, D.; Yang, J. Protective Role of a New Polysaccharide Extracted from Lonicera japonica Thunb in Mice with Ulcerative Colitis Induced by Dextran Sulphate Sodium. Biomed. Res. Int. 2021, 2021, 8878633. [Google Scholar] [CrossRef]
- Zhou, X.; He, G.; Ma, J.; Tang, M.; Tian, G.; Gong, X.; Zhang, H.; Kui, L. Protective Effect of a Novel Polysaccharide from Lonicera japonica on Cardiomyocytes of Mice Injured by Hydrogen Peroxide. Biomed. Res. Int. 2020, 2020, 5279193. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, e0204152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Q.; Deng, W.; Sun, C.; Wei, Q.; Adu-Frimpong, M.; Shi, J.; Yu, J.; Xu, X. Anti-hyperuricemic and anti-gouty arthritis activities of polysaccharide purified from Lonicera japonica in model rats. Int. J. Biol. Macromol. 2019, 123, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yu, Y.; Wang, X.; Xu, J.; Wang, X.; Feng, Z.; Zhou, Y.; Xiao, H.; Sun, L. Structural characterization and anti-oxidation activity evaluation of pectin from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 998462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jia, Y.; Wang, C.; Zhou, W.; Shu, Y.; Zhang, K.; Zeng, X.; Guo, R. Lonicera japonica polysaccharides improve longevity and fitness of Caenorhabditis elegans by activating DAF-16. Int. J. Biol. Macromol. 2023, 229, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, P.; Du, Z.; Wang, W.; Cong, Q.; Zheng, C.; Jin, C.; Ding, K.; Shao, C. Structural elucidation of a pectin from flowers of Lonicera japonica and its antipancreatic cancer activity. Int. J. Biol. Macromol. 2016, 88, 130–137. [Google Scholar] [CrossRef]
- Wang, P.; Liao, W.; Fang, J.; Liu, Q.; Yao, J.; Hu, M.; Ding, K. A glucan isolated from flowers of Lonicera japonica Thunb. inhibits aggregation and neurotoxicity of Aβ42. Carbohydr. Polym. 2014, 110, 142–147. [Google Scholar] [CrossRef]
- Liao, W.; Hu, X.; Du, Z.; Wang, P.; Ding, K. A homogalacturonan from Lonicera japonica Thunb. disrupts angiogenesis via epidermal growth factor receptor and Delta-like 4 associated signaling. Glycoconj. J. 2022, 39, 725–735. [Google Scholar] [CrossRef]
- An, F.; Ren, G.; Wu, J.; Cao, K.; Li, M.; Liu, Y.; Liu, Y.; Hu, X.; Song, M.; Wu, R. Extraction, purification, structural characterization, and antioxidant activity of a novel polysaccharide from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 1035760. [Google Scholar] [CrossRef]
- Bai, X.; Liu, P.; Shen, H.; Zhang, Q.; Zhang, T.; Jin, X. Water-extracted Lonicera japonica polysaccharide attenuates allergic rhinitis by regulating NLRP3-IL-17 signaling axis. Carbohydr. Polym. 2022, 297, 120053. [Google Scholar] [CrossRef]
- Feng, J.; Chang, X.; Zhang, Y.; Lu, R.; Meng, X.; Song, D.; Yan, X.; Zhang, J.; Nie, G. Characterization of a polysaccharide HP-02 from Honeysuckle flowers and its immunoregulatory and anti-Aeromonas hydrophila effects in Cyprinus carpio L. Int. J. Biol. Macromol. 2019, 140, 477–483. [Google Scholar] [CrossRef]
- Liu, P.; Bai, X.; Zhang, T.; Zhou, L.; Li, J.; Zhang, L. The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 2019, 7, 811. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, X.; Liu, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Che, H.; Ha, D.; Wei, Y.; Zheng, S. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr. Polym. 2012, 90, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, T.; Xiang, F. Polyethylene glycol-based ultrasonic-assisted enzymatic extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Lonicerae japonica leaves. Food. Sci. Nutr. 2019, 7, 3452–3462. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, Y.; Hu, J.; Ding, J.; Yang, P.; Liu, Y.; Lu, Y.; Jin, Y.; Tang, H.; Liu, Y.; et al. A novel polysaccharide from Lonicerae Japonicae Caulis: Characterization and effects on the function of fibroblast-like synoviocytes. Carbohydr. Polym. 2022, 292, 119674. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Chen, H.; Yu, Q.; Yan, C. Structural characterization and osteogenic activity in vitro of novel polysaccharides from the rhizome of Polygonatum sibiricum. Food. Funct. 2021, 12, 6626–6636. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Wang, Q.; Wu, Z.; Liu, X.; Chen, S.; Zhou, A. Structural characterization and immunomodulatory activity of a polysaccharide from finger citron extracted by continuous phase-transition extraction. Int. J. Biol. Macromol. 2023, 240, 124491. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Mei, N.; Li, W.; Yang, T.; Xie, J. Three acidic polysaccharides derived from sour jujube seeds protect intestinal epithelial barrier function in LPS induced Caco-2 cell inflammation model. Int. J. Biol. Macromol. 2023, 240, 124435. [Google Scholar] [CrossRef]
- Sun, W.; Kou, X.H.; Wu, C.E.; Fan, G.J.; Li, T.T.; Cheng, X.; Xu, K.; Suo, A.; Tao, Z. Low-temperature plasma modification, structural characterization and anti-diabetic activity of an apricot pectic polysaccharide. Int. J. Biol. Macromol. 2023, 240, 124301. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, C.; Liu, X.; Zhao, Y.; Ding, Q.; Sun, S.; Zhang, J.; Yang, J.; Liu, W.; Li, W. Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules 2023, 28, 3733. [Google Scholar] [CrossRef]
- Cai, J.; Liang, Z.; Li, J.; Manzoor, M.F.; Liu, H.; Han, Z.; Zeng, X. Variation in physicochemical properties and bioactivities of Morinda citrifolia L. (Noni) polysaccharides at different stages of maturity. Front. Nutr. 2023, 9, 1094906. [Google Scholar] [CrossRef]
- Sun, H.; Shu, F.; Guan, Y.; Kong, F.; Liu, S.; Liu, Y.; Li, L. Study of anti-fatigue activity of polysaccharide from fruiting bodies of Armillaria gallica. Int. J. Biol. Macromol. 2023, 241, 124611. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics.; antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Wang, L.; Lian, J.; Zheng, Q.; Wang, L.; Wang, Y.; Yang, D. Composition analysis and prebiotics properties of polysaccharides extracted from Lepista sordida submerged cultivation mycelium. Front. Microbiol. 2023, 13, 1077322. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Fang, H.; Chen, Z.; Liu, Z.; Yu, X.; Liang, C. Ectopic Expression of a R2R3-MYB Transcription Factor Gene LjaMYB12 from Lonicera japonica Increases Flavonoid Accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 4494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gautam, A.; Rohatgi, S.; Kundu, P.P. Synthesis of vildagliptin loaded acrylamide-g-psyllium/alginate-based core-shell nanoparticles for diabetes treatment. Int. J. Biol. Macromol. 2022, 218, 82–93. [Google Scholar] [CrossRef]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2022, 8, e10367. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.L.; Lawler, P.E.; Bollinger, J.G.; Li, Y.; Schindler, S.E.; Li, M.; Lopez, S.; Ovod, V.; Nakamura, A.; Shaw, L.M.; et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: A literature review. Alzheimers. Res. Ther. 2022, 14, 195. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Ippati, S.; Watling, M.; Imbimbo, C. Role of monomeric amyloid-β in cognitive performance in Alzheimer’s disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacol. Res. 2023, 187, 106631. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef]
- Kim, G.; Han, D.W.; Lee, J.H. The Cytoprotective Effects of Baicalein on H2O2-Induced ROS by Maintaining Mitochondrial Homeostasis and Cellular Tight Junction in HaCaT Keratinocytes. Antioxidants 2023, 12, 902. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Liang, X.; Wu, K.; Cao, Y.; Ma, T.; Guo, S.; Chen, P.; Yu, S.; Ruan, Q.; et al. Defensing against oxidative stress in Caenorhabditis elegans of a polysaccharide LFP-05S from Lycii fructus. Carbohydr. Polym. 2022, 289, 119433. [Google Scholar] [CrossRef]
- Xie, C.; Ya Likun, M.M.; Luo, Q.L.; Dong, J.C. Role of cellular senescence in inflammatory lung diseases. Cytokine. Growth. Factor. Rev. 2023, 70, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Du, M.; Yang, X.; Wang, Q.; Huang, S.; Ma, Y.; Sun, Y. Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers. Molecules 2023, 28, 2885. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, A.; Yi, M.; Yu, S.; Zhang, M.; Wu, K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Koroukian, S.M.; Booker, B.D.; Vu, L.; Schumacher, F.R.; Rose, J.; Cooper, G.S.; Selfridge, J.E.; Markt, S.C. Receipt of Targeted Therapy and Survival Outcomes in Patients with Metastatic Colorectal Cancer. JAMA Netw. Open 2023, 6, e2250030. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.; Chu, P.Y.; Lin, H.Y.; Huang, K.W.; Hung, W.C.; Shan, Y.S.; Chen, L.T.; Tsai, H.J. PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation. J. Biomed. Sci. 2022, 29, 92. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Feagan, B.G.; Peyrin-Biroulet, L.; Vermeire, S.; Faes, M.; Harris, K.; Oortwijn, A.; Daniele, P.; Patel, H.; Danese, S. Filgotinib Improved Health-Related Quality of Life and Led to Comprehensive Disease Control in Individuals with Ulcerative Colitis: Data from SELECTION. J. Crohns. Colitis. 2023; online ahead of print. [Google Scholar]
- Stavre, Z.; Kim, J.M.; Yang, Y.S.; Nündel, K.; Chaugule, S.; Sato, T.; Park, K.H.; Gao, G.; Gravallese, E.M.; Shim, J.H. Schnurri-3 inhibition suppresses bone and joint damage in models of rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2023, 120, e2218019120. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M.; Boyd, S.D.; Sampath, V.; Galli, S.J.; Nadeau, K.C. Allergy: Mechanistic insights into new methods of prevention and therapy. Sci. Transl. Med. 2023, 15, eadd2563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, F.; Zhang, L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef]
- Yu, W.; Xie, D.; Yamamoto, T.; Koyama, H.; Cheng, J. Mechanistic insights of soluble uric acid-induced insulin resistance: Insulin signaling and beyond. Rev. Endocr. Metab. Disord. 2023, 24, 327–343. [Google Scholar] [CrossRef]
- Egervari, G.; Siciliano, C.A.; Whiteley, E.L.; Ron, D. Alcohol and the brain: From genes to circuits. Trends. Neurosci. 2021, 44, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Pachito, D.V.; Pega, F.; Bakusic, J.; Boonen, E.; Clays, E.; Descatha, A.; Delvaux, E.; De Bacquer, D.; Koskenvuo, K.; Kröger, H.; et al. The effect of exposure to long working hours on alcohol consumption.; risky drinking and alcohol use disorder: A systematic review and meta-analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 2021, 146, 106205. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Guo, S.; Wen, L.; Yu, M.; Feng, L.; Jia, X. Structural Elucidation.; Modification.; and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 2022, 9, 908175. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.; Wu, J.; Zhao, J. Modifications of polysaccharide-based biomaterials under structure-property relationship for biomedical applications. Carbohydr. Polym. 2021, 266, 118097. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Shen, M.; Yi, C.; Yu, Q.; Chen, X.; Xie, J.; Xie, M. Acetylated polysaccharides: Synthesis.; physicochemical properties.; bioactivities.; and food applications. Crit. Rev. Food. Sci. Nutr. 2022, 16, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Shi, L.; Li, Y.; Tuerhong, M.; Abudukeremu, M.; Cui, J.; Li, Y.; Jin, D.Q.; Xu, J.; et al. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Carbohydr. Polym. 2021, 273, 118496. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Shan, Q.; Shi, Z.; Li, J.; Zhao, X.; Chang, C.; Yu, J. Integrated volatile metabolomic and transcriptomic analysis provides insights into the regulation of floral scents between two contrasting varieties of Lonicera japonica. Front. Plant. Sci. 2022, 13, 989036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ji, W.; Liu, S.; Fan, J.; Lu, H.; Wang, X. Metabolomics Analysis of Different Tissues of Lonicera japonica Thunb. Based on Liquid Chromatography with Mass Spectrometry. Metabolites 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhao, Y.; Yang, L.; Du, M.; Li, Q.; Ren, Z.; Li, X. A new perspective to improve the treatment of Lianhuaqingwen on COVID-19 and prevent the environmental health risk of medication. Environ. Sci. Pollut. Res. Int. 2022, 29, 74208–74224. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Li, C.; Liang, Y.T.; Lv, J.; Yang, L.F.; Zhao, B.N. Study on the Antipyretic and Anti-inflammatory Mechanism of Shuanghuanglian Oral Liquid Based on Gut Microbiota-Host Metabolism. Front. Pharmacol. 2022, 13, 843877. [Google Scholar] [CrossRef]
- Mokgehle, T.M.; Madala, N.; Gitari, W.M.; Tavengwa, N.T. Advances in the development of biopolymeric adsorbents for the extraction of metabolites from nutraceuticals with emphasis on Solanaceae and subsequent pharmacological applications. Carbohydr. Polym. 2021, 264, 118049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Sheng, L.; Zhang, D.; Zheng, X.; Pan, Y.; Yu, X.; Liang, X.; Wang, Q.; Wang, B.; et al. Effect of ultrasonic degradation on the structural feature, physicochemical property and bioactivity of plant and microbial polysaccharides: A review. Int. J. Biol. Macromol. 2023, 236, 123924. [Google Scholar] [CrossRef]

| Part | Extraction | Purification | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polysaccharide Fraction | Extraction Methods | Time (h/min) | Temperature (°C) | Solid–Liquid Ratio | Total Yield (%) | Polysaccharide Fraction | Purification Methods | ||
| L. japonica | LJP-h | Water extraction | N/A | 100 °C | N/A | N/A | LJP-N | DEAE-cellulose, Sepharose CL-6B column | [37] |
| L. japonica | LJP-h | Water extraction | N/A | 100 °C | N/A | N/A | LJP-A | DEAE-cellulose, Sepharose CL-6B column | [37] |
| L. japonica | LJP-s | Water extraction | 8 h | 100 °C | 1:20 | N/A | N/A | N/A | [38] |
| L. japonica | LJP-b | Water extraction | 8 h | 100 °C | 1:28 | 5.3% | LJP-N | DEAE-cellulose column, Sepharose CL-6B column | [39] |
| L. japonica | LJP-b | Water extraction | 8 h | 100 °C | 1:28 | 5.3% | LJP-A-1 | DEAE-cellulose column, Sepharose CL-6B column | [39] |
| L. japonica | LJP-b | Water extraction | 8 h | 100 °C | 1:28 | 5.3% | LJP-A-2 | DEAE-cellulose column, Sepharose CL-6B column | [39] |
| L. japonica | LJP-b | Water extraction | 8 h | 100 °C | 1:28 | 5.3% | LJP-A-3 | DEAE-cellulose column, Sepharose CL-6B column | [39] |
| L. japonica | LJP-b | Water extraction | 8 h | 100 °C | 1:28 | 5.3% | LJP-A-4 | DEAE-cellulose column, Sepharose CL-6B column | [39] |
| L. japonica | LJP-k | Enzyme-assisted extraction | 50 min | 45 °C | 1:20 | N/A | NA | N/A | [40] |
| L. japonica | LJP-z | Enzyme-assisted extraction | 50 min | 45 °C | 1:20 | N/A | N/A | N/A | [41] |
| L. japonica | LJP-d | Enzyme-assisted extraction | 50 min | 45 °C | 1:20 | N/A | N/A | N/A | [42] |
| L. japonica | CLJP-y | Water extraction | 12 h | 100 °C | 1:20 | 4.32% | LJP-1-y | Macroporous adsorbent resin column D315 Macroporous adsorbent resin column D101, DEAE-cellulose 52 | [43] |
| L. japonica | WLJP | Water extraction | 6 h | 100 °C | 1:16 | 7.6% | WLJP-A0.2b | DEAE-cellulose column, Sepharose CL-6B column | [44] |
| L. japonica | CLJP-z | Water extraction | 6 h | 90 °C | 1:30 | 5.4% | N/A | N/A | [45] |
| L. japonica | CLJP-z | Water extraction | 6 h | 90 °C | 1:30 | N/A | LJP-2-1 | DEAE-cellulose column, Sepharose G-100 column | [45] |
| L. japonica flowers | LFA | Water extraction | 16 h | N/A | N/A | 3.60% | LFA03-a | DEAE-cellulose column, Sephacryl S-100 HR column | [21] |
| L. japonica flowers | LJ | Water extraction | 24 h | N/A | 1:20 | 4.6% | LJ-02-1 | DEAE-cellulose 52 column, Sephacryl S-200HR column | [46] |
| L. japonica flowers | LJW | Water extraction | 25 h | N/A | N/A | 6.5% | LJW0F2 | DEAE-cellulose column, Sephadex G150 column | [47] |
| L. japonica flowers | LJW | Water extraction. | 25 h | N/A | N/A | N/A | LJW2F2 | DEAE-cellulose column, Sephadex G150 column | [48] |
| L. japonica flowers | LJP-sd | Water extraction | 16 h | N/A | N/A | 5.5% | LJPB2 | DEAE-cellulose 52 column, Sephacryl S-300 column | [22] |
| L. japonica flowers | Crude polysaccharide | Water extraction | 3 h | 80 °C | 1:10 | N/A | HEP-4 | DEAE-52 cellulose column, Sephadex G-75 column | [49] |
| L. japonica flowers | LJP-bx | Water extraction | N/A | N/A | N/A | 5.1% | WLJP-025p | DEAE-cellulose, Sepharose CL-6B column | [50] |
| L. japonica flowers | HP | Water extraction | 6 h | 100 °C | 1:20 | 7.11% | HP-02 | DEAE-cellulose 32 column, Sephacryl S-200HR column | [51] |
| L. japonica flowers | LJP-l | Water extraction | 12 h | 100 °C | 1:20 | 5.1% | N/A | N/A | [52] |
| L. japonica flower buds | LJPs | Water extraction | 9 h | 80 °C | 1:20 | 6.9% | LJP-w | DEAE-52 cellulose anion exchange chromatography column | [53] |
| L. japonica flower buds | CLJP-t | Water extraction | 12 h | 100 °C | 1:20 | 4.7% | LJP-1-t | DEAE-cellulose column, Sephacryl S-300 column | [54] |
| L. japonica leaves | LJLP | Ultrasound-assisted enzymatic extraction | 33 min | 60 °C | 1:20 | 14.76% | N/A | N/A | [55] |
| L. japonica caulis | LJCP | Water extraction | 6 h | 90 °C | 1:30 | 1.8% | LJCP-2b | DEAE cellulose-52, Sephadex G75 column | [56] |
| Source | Compound Name | Molecular Weights | Monosaccharide Composition | Structures | Analytical Techniques | Ref. |
|---|---|---|---|---|---|---|
| L. japonica | LJP-N | 5.4 kDa | Glc:Gal:Ara = 43.7:25.1:31.2 | LJP-N is a starch-like glucan with some arabinogalactan and/or arabinan domains. | N/A | [37] |
| L. japonica | LJP-A | 400 kDa | GalA:Gal:Ara = 82.1:7.1:10.8 | LJP-A is a pectic polysaccharide, mainly containing galacturonan with some galactan and/or arabinan domains. | N/A | [37] |
| L. japonica | LJP-s | 18.5 kDa. | GalA:Rha:Gal:Ara:Glc:Xyl = 13.7:6.0:28.3:22.6:21.1:3.5 | LJP-s may contain some RG-I pectin domains with galactan, arabinan, and arabinogalactan side chains, and some starch-like glucan domains. | HPLC, NMR | [38] |
| L. japonica | LJP-A-1 | 19.0 kDa | GalA:GlcA:Gal:Ara:Rha = 18.6:5.5:19.9:54.8:1.2 | LJP-A-1 is mainly composed of Gal and Ara (>70%) with some GalA and GlcA residues. | HPLC, FT-IR, HPGPC | [39] |
| L. japonica | LJP-A-2 | 47.6 kDa | GalA:Gal:Ara:Rha = 53.8:17.4:26.4:1.1 | LJP-A-2 is a HG domain-rich pectic polysaccharide, mainly composed of GalA (>50%) with some Gal and Ara residues. | HPLC, FT-IR, HPGPC | [39] |
| L. japonica | LJP-A-3 | 200.5 kDa | GalA:Gal:Ara:Rha = 55.4:11.5:31.9:1.2 | LJP-A-3 is defined as a backbone mainly made up of (1→4)-linked α-D-GalpA, with a trace of→)4-α-D-GalpA-(1→2)-α-L-Rhap-(1→. The substituent were composed of (1→4)-linked Gal, (1→5)-linked Ara and (1→3,5)-linked Ara, which are substituted partly at C4 of Rha. | HPLC, FT-IR, HPGPC | [39] |
| L. japonica | LJP-A-4 | 383.8 kDa | GalA:Gal:Ara = 76.2:6.5:17.3 | LJP-A-4 is an HG-domain-rich pectic polysaccharide mainly composed of GalA (>50%) with some Gal and Ara residues. | HPLC, FT-IR, HPGPC | [39] |
| L. japonica | LJP-k | N/A | Glu:Gal:Man:Rha:Xyl:Ara = 61.37:8.29:2.73:5.39:2.58:19.64 | N/A | HPLC, FT-IR | [40] |
| L. japonica | LJP-z | N/A | N/A | N/A | FT-IR | [41] |
| L. japonica | LJP-d | N//A | Glu:Gal:Man:Rha:Xyl:Ara = 58.62:9.17:2.89:5.33:3.26:20.73 | N/A | FT-IR, HPLC | [42] |
| L. japonica | LJP-1-y | 17.5 kDa | GlcA:Glc:Gal:Ara:Xyl = 2.43:1:2.09:1.95:1.96 | LJP-1-y has a spatial triple helix structure. | FT-IR, NMR, HPGPC, UV | [43] |
| L. japonica | WLJP-A0.2b | 40.6 kDa | GalA:Rha:Gal:Ara:Glc:GlcA:Xyl:Man = 72.2:2.8:5.8:15.9:2.0:0.5:0.6:0.2 | WLJP-A0.2b is shown to be dominated by HG domains and covalently linked with RG-I and RG-II domains. The RG-I domain contains α-L-1,5-arabinan; β-D-1,4-galactan; and AG-II side chains. | FT-IR, NMR, ESI-MS | [44] |
| L. japonica | CLJP | 1450 kDa | Man:GluUA:GalUA:Glu:Gal:Ara:Rha:Fuc = 2.93:3.63:23.57:11.72:23.45:23.45:4.83:6.43 | N/A | FT-IR, HPLC | [45] |
| L. japonica | LJP-2-1 | 1280 kDa | Man:GluUA:GalUA:Glu:Gal:Ara:Rha = 2.01:2.80:51.25:8.03:9.04:22.89:3.97 | N/A | FT-IR, HPLC | [45] |
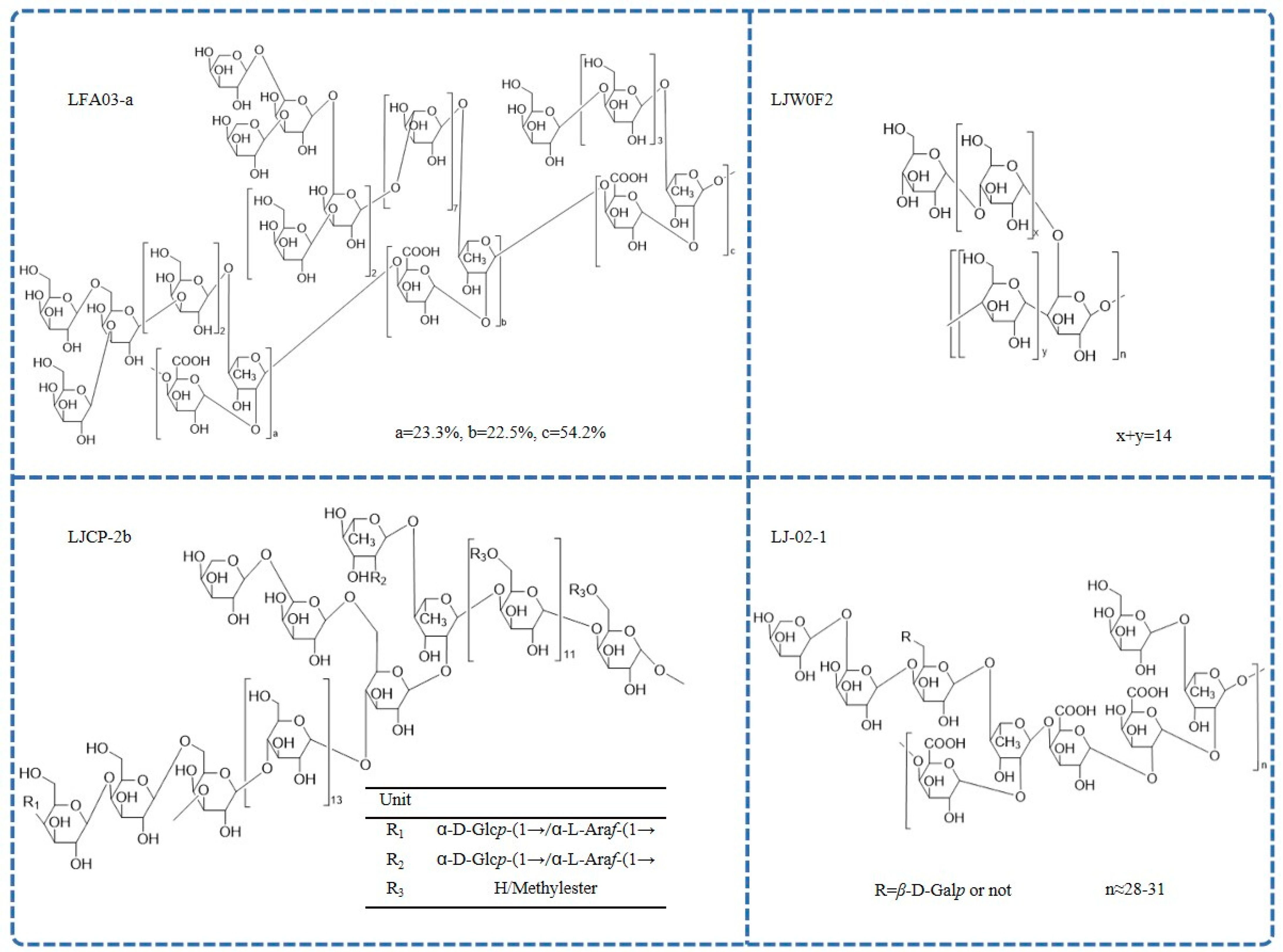
| L. japonica flowers | LFA03-a | 67 kDa | Rha:Ara:Gal:GalA = 18.1:25.3:36.8:19.5 | LFA03-a is proposed to have a backbone consisting of repeating unit →4)-α-D-GalpA-1-(1→2)-α-L-Rhap-(1→, with a substitution at C4 of rhamnose. The branches are mainly composed of T/1,5/1,3,5-linked Ara and T/1,4-/1,3/1,3,6-linked Gal. | GC-MS, FT-IR, NMR | [21] |
| L. japonica flowers | LJ-02-1 | 54 kDa | Rha:GalA:Gal:Ara = 10.77:7.88:15.45:65.89 | LJ-02-1 is composed of a repeat unit of →4)-α-D-GalpA-1-(1→2)-α-L-Rhap-(1→ and is partly substituted at C4 of rhamnose. The branches contain T- and 1,4,6-linked β-D-Galp, T- and 1,5-linked α-L-Araf. | HPGPC, HPLC, GC-MS, NMR | [46] |
| L. japonica flowers | LJW0F2 | 37.1 kDa | glucose (99.7%) | LJW0F2 is elucidated to be an α-D-(1→4) glucan with an α-(1→4)-linked branch attached to the C6 position. | HPLC, FT-IR, NMR | [47] |
| L. japonica flowers | LJW2F2 | 7.2 kDa. | galacturonic acid (98.2%) | LJW2F2 is a homogalacturonan consisting of α-1,4-D-galacturonic acid. | FT-IR, NMR | [48] |
| L. japonica flowers | LJPB2 | 8.9 kDa | Ara:Man:Glc:Gal = 1.8:1.0:3.6:3.7 | LJPB2 mainly contains 1, 4, 6-linked mannose; 1, 4-linked glucose; and 1, 4-linked galactose, with a highly branched structure of arabinan and terminal glucose. | GC-MS, FT-IR | [22] |
| L. japonica flowers | HEP-4 | 198 kDa | Man:Rha:GalA;Glc:Gal:Ara = 6.74:1.56:1.04:14.21:4.31:5.4 | The backbone of HEP-4 is 1,4-β-D-Glcp; 1-α-D-Glcp, and other rhamnose residues branched at 1-β-D-Arap; 1,3,4-β-D-Arap; or 1,3,6-β-D-Manp. | HPLC, FT-IR, NMR | [56] |
| L. japonica flowers | WLJP-025p | 23 kDa. | GalA:Gal:Ara:Glc:Man = 66.8:14.6:8.2:10.0:0.4 | The primary structure of WLJP- 025p may be defined as an α-(1→4)-D-GalpA main chain, with β-(1→4)-galactan, α-(1→5)-linked, (1→3,5)-linked arabinan/arabinogalactan, and α-(1→4)-glucan side chains. | HPLC, UV, NMR | [50] |
| L. japonica flowers | HP-02 | 3.8 kDa | Ara:Rha:Man:Glc:Gal = 2.5:1.8:3.6:3.7:1.9 | N/A | HPGPC, GC | [51] |
| L. japonica flowers | LJP-l | <1000 kDa | GalA:Rha:Gal:Ara:Glc:Man = 8.7:8.2:16.2:19.5:26.9:20.5 | LJP-l is a heterogenous polysaccharide and may contain some of RG-I pectin domains. | HPLC, UV | [52] |
| L. japonica flower buds | LJP-w | N/A | Gal:Glc:Man:Rha:Xyl = 0.46:0.32:0.25:3.71:0.27. | N/A | GC | [53] |
| L. japonica flower buds | LJP-1-t | 180 kDa | N/A | The main backbone chain of LJP-1-t is predominantly composed of Residue A and Residue B and is branched at O-3 position of Residue B with Residue C. | GC, FT-IR | [54] |
| L. japonica leaves | LJLP | N/A | Gal:Glc:Rib = 32.3:20.9:15.2 | There are pyranose rings in the structure of LJLP polysaccharides. | FT-IR, HPLC | [55] |
| L. japonica caulis | LJCP-2b | 7.0 kDa | Glc:GalA:Ara:Gal:Rha:Xyl:Man:GlcA = 3.89:2.49:1.87:1.51:1.00:0.40:0.21:0.19 | LJCP-2b is α homogeneous heteropolysaccharide mainly composed of 1,3,6-β-D-Galp; 1,4-α-D-Glcp; 1,4,6-α-D-Glcp; 1,4-β-D-Galp; 1,2,4-α-L-Rhap; and 1,4-α-D-GalpA. | IR, NMR, ICS | [49] |
| Biological Activities | Source | Polysaccharide Name | In Vitro or In Vivo | Indicated Concentrations | Models/Test System | Action or Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Anti-diabetic effect | L. japonica flower buds | LJP-w | In vitro and in vivo | 200, 400, and 800 mg/kg | α-Amylase and α-Glucosidase and male SD rats (200 ± 20 g) | In vitro: Inhibition of α-amylase with IC50 values of 61.2 ± 3.1 μg/mL and inhibition of α-glucosidase with IC50 values of 45.6 ± 1.9 μg/mL In vivo: ↓ TC, TG, LDL-C, VLDL-C, ALT, AST, and GGT ↑ HDL-C, CAT, SOD, and GSH ↑ Contents of liver and skeletal muscle glycogen ↑ Concentrations of hepatic pyruvate kinase and hexokinase | [53] |
| Anti-Alzheimer’s effect | L. japonica flowers | LFA03-a | In vitro | 0.2 and 1 mg/mL | PC12 cells | Inhibition of Aβ42 aggregation and induction of neurite outgrowth | [21] |
| L. japonica flowers | LJW0F2 | in vitro | 0.1, 1, and 10 g/mL | SH-SY5Y cells | Blockage of Aβ42 aggregation and reduction in its toxicity in SH-SY5Y cells | [47] | |
| Anti-depression effect | L. japonica flowers | LJP-l | In vivo | 30 and 100 mg/kg | Male KM mice (20 ± 2 g) | ↓ NLRP3, IL-1β, and caspase-1 ↓ Immobility time ↑ Time spent of mice in the open arms and center zone | [52] |
| Antioxidant effect | L. japonica flowers | HEP-4 | In vitro | 200, 400, and 800 µg/mL | HepG2 cells | ↑ Scavenging effects on DPPH and ABTS radicals ↑ CAT and GSH-Px activity ↓ Levels of ROS and MDA | [49] |
| L. japonica | WLJP-A0.2b | In vitro | 0, 0.5, 1.0, 2.0, 5.0, and 10.0 mg/mL | HEK-293T cells | ↓ Level of ROS ↑ Scavenging ability of ABTS, hydroxyl, and DPPH radicals | [44] | |
| L. japonica leaves | LJLP | In vitro and in vivo | 100, 200, 400, and 800 mg/kg | Male nude KM mice | ↑ Effect on scavenging superoxide radicals ↑ Activities of CAT, SOD, GSH-Px, and TAOC in serum and liver ↓ Levels of MDA in serum and liver | [55] | |
| L. japonica | LJP-N, LJP-A-1, LJP-A-2, LJP-A-3 and LJP-A-4 | In vitro | 0, 0.25, 0.5, 1.0, 2.0, and 4.0 mg/mL | Erythrocyte | ↑ Scavenging ability of DPPH ABTS, hydroxyl, and superoxide radicals Inhibition of erythrocyte hemolysis Alleviation of oxidative stress | [39] | |
| L. japonica | CLJP and LJP-2-1 | In vitro and in vivo | CLJP: 0, 100, 200, and 400 μg/mL LJP-2-1: 0 and 200 μg/mL | C. Elegans and Caco-2 cells | In vivo: ↓ Lipofuscin and MDA contents ↑ Sod-3, sod-2, gst-4, hsp-16.2, hsp-12.6, and hsp-60 levels ↑ SOD and CAT activities Promotion of the nuclear accumulation of DAF-16 Improvement of pharyngeal pumping and locomotion In vitro: Amelioration of the oxidative damage | [45] | |
| L. japonica | LJP-z | In vitro | 10, 20, and 40 μg/mL | Male mice cardiomyocytes | ↑ Cardiomyocyte oxidative-stress survival rate ↑ Cardiomyocyte apoptosis rate ↓ AST, CPK, LDH, SOD, CAT, and GSH-Px activities ↓ ROS and MDA content ↓ Caspase-3, Caspase-8, and Caspase-9 activities | [41] | |
| L. japonica flowers | LJPB2 | In vivo | 50, 100, and 200 mg/kg/d | Male SD rats (200-250 g) | ↓ MDA and NO levels ↑ SOD and GSH-Px activities ↑ Scavenging ability of DPPH | [22] | |
| Immunoregulatory effect | L. japonica | LJP-N and LJP-A | In vivo | 50 and 200 mg/kg | Female BALB/c mice (20–22 g) | ↑ Index of spleen and thymus ↑ Phagocytic function of macrophages ↑ Secretion of IL-2, IL-6, TNF-α, IgG, IgM ↑ Cytotoxic activity of NK cells | [37] |
| L. japonica | LJP-d | In vivo | 100 and 150 mg/kg | Male BALB/c mice (20.8 ± 2.3 g) | ↑ Index of spleen and thymus ↑ Phagocytic function of macrophages ↑ Levels of IL-2, TNF-α, and IFN-γ ↑ Percentage of CD4+ and CD8+ T cell subsets and ratio of CD4+/CD8+ ↑ Cytotoxic activity of NK cells | [42] | |
| L. japonica flowers | HP-02 | In vitro and in vivo | 250, 500, and 1000 μg/mL | Head kidney cells and common carp (Cyprinus carpio L.) | In vitro: ↑ Contents of TNF-α, IL-1β, IL-6, IL-12, IL-10, and TGF-β In vivo: ↑ Contents of TNF-α, IL-1β, IL-6, IL-10, and TGF-β | [51] | |
| Anti-tumor effect | L. japonica flowers | LJ-02-1 | In vitro | 0.016, 0.031, 0.063, 0.125, 0.250, 0.500, and 1 mg/mL | BxPC-3 and PANC-1 pancreatic tumors cells | Inhibition rate of BxPC-3 was 66.7% Inhibition rate of PANC-1 was 52.1% | [46] |
| L. japonica flowers | LJW2F2 | In vitro | 0.9, 1.8, 3.5, 7.0, and 13.9 µM | HMEC-1 cells | Inhibition of HMEC-1 cell tube formation and migration Disruption of EGFR/Raf/MEK/ERK and Dll4-Notch1 signaling axis | [48] | |
| Anti-inflammatory effect | L. japonica | LJP-k | In vivo | 50, 100, and 150 mg/kg | Male BALB/c mice | ↑ IL-2, TNF-α, IFN-γ, and SIgA concentrations ↑ Apoptosis rate of spleen lymphocytes Regulation of intestinal flora | [40] |
| L. japonica Caulis | LJCP-2b | In vitro | 50, 100, and 200 μg/mL | RA-FLS cells | ↓ Cell viability, migration number, and adhesion capacity of RA-FLS cells ↓ Levels of IL-6 and IL-1β ↑ Apoptosis rate of RA-FLS cells | [56] | |
| Anti-allergic effect | L. japonica flower buds | LJP-1-t | In vivo | 20, 40, and 80 mg/kg | Female ICR strain mice (25–30 g) | ↓ IgE, TNF-α, and histamine levels | [54] |
| L. japonica flowers | WLJP-025p | In vivo and in vitro | In vivo: 30 and 60 mg/kg In vitro: 400 and 800 μg/mL | Female BALB/c mice (21 ± 2 g) and THP-1 cells | In vivo: ↓ Concentrations of IgE, IL-17 and IL-1β In vitro: Inhibition of the activation of NLRP3 inflammasome and inflammatory response | [50] | |
| Anti-gouty arthritis effect | L. japonica | LJP-1-y | In vivo | 100, 200, and 300 mg/kg | Male SD rats (200 ± 20 g) | ↓ Uric acid level and XOD activity ↓ IL-1β, IL-6, TNF-α, and COX-2 | [43] |
| Anti-alcohol-addiction effect | L. japonica | LJP-s | In vivo | 50 and 100 mg/kg | Male KM mice (20 ± 2 g) | ↓ Neurogenic damage ↓ p62, Glu, Glu/GABA, p-GluN2B, and p-VPS34 levels ↑ EAAT2, LAPM2, and LC3II levels Inhibition of reinstatement of CPP Inhibition of autophagy pathway activation ↑ | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, A.; Hu, W.; Zhang, Z.; Ruan, Y.; Kuang, H.; Wang, M. Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review. Molecules 2023, 28, 4828. https://doi.org/10.3390/molecules28124828
Yang X, Yu A, Hu W, Zhang Z, Ruan Y, Kuang H, Wang M. Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review. Molecules. 2023; 28(12):4828. https://doi.org/10.3390/molecules28124828
Chicago/Turabian StyleYang, Xinpeng, Aiqi Yu, Wenjing Hu, Zhaojiong Zhang, Ye Ruan, Haixue Kuang, and Meng Wang. 2023. "Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review" Molecules 28, no. 12: 4828. https://doi.org/10.3390/molecules28124828
APA StyleYang, X., Yu, A., Hu, W., Zhang, Z., Ruan, Y., Kuang, H., & Wang, M. (2023). Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review. Molecules, 28(12), 4828. https://doi.org/10.3390/molecules28124828